Abstract
Background
Research points to the importance of breastfeeding for promoting close mother-infant contact and social-emotional development. Recent functional magnetic resonance imaging (fMRI) studies have identified brain regions related to maternal behaviors. However, little research has addressed the neurobiological mechanisms underlying the relationship between breastfeeding and maternal behavior in human mothers. We investigated the associations between breastfeeding, maternal brain response to own infant stimuli, and maternal sensitivity in the early postpartum.
Methods
Seventeen biological mothers of healthy infants participated in two matched groups according to feeding method – exclusive breastfeeding and exclusive formula-feeding at 2-4 weeks postpartum. fMRI scanning was conducted in the first postpartum month to examine maternal brain activation in response to her own baby's cry versus control baby-cry. Dyadic interactions between mothers and infants at 3-4 months postpartum were videotaped in the home and blindly coded for maternal sensitivity.
Results
In the first postpartum month, breastfeeding mothers showed greater activations in the superior frontal gyrus, insula, precuneus, striatum, and amygdala while listening to their own baby-cry as compared to formula-feeding mothers. For both breastfeeding and formula-feeding mothers, greater activations in the right superior frontal gyrus and amygdala were associated with higher maternal sensitivity at 3-4 months postpartum.
Conclusions
Results suggest links between breastfeeding and greater response to infant cues in brain regions implicated in maternal-infant bonding and empathy during the early postpartum. Such brain activations may facilitate greater maternal sensitivity as infants enter their social world.
Keywords: breastfeeding, infancy, maternal sensitivity, mother-infant interaction, neuroimaging
Breastfeeding represents a set of maternal behaviors that enhance the emotional bond between mother and infant via close physical contact and affectionate dyadic interactions (Feldman & Eidelman, 2003; Strathearn, Mamun, Najman, & O'Callaghan, 2009). Research has demonstrated that breastfeeding may contribute to the development of a range of sensitive maternal behaviors during the early postpartum period. For instance, breastfeeding mothers exhibit more interactive behaviors toward their infants, including touching and gazing, as well as more affectionate responses during feeding at one and three months postpartum in comparison with formula-feeding mothers (Dunn & Richards, 1977; Kuzela, Stifter, & Worobey, 1990; Lavelli & Poli, 1998). Compared with formula-feeding mothers, breastfeeding mothers have increased parasympathetic nervous system modulation, greater vascular stress response, lower perceived stress levels, and fewer depressive symptoms (Groër, 2005; Mezzacappa & Katlin, 2002; Mezzacappa, Kelsey, & Katkin, 2005). Such close physical contact and protection from negative mood and stress may contribute to maternal attunement to the infant's physical and mental needs.
Indeed, sensitive maternal attunement to her infant is among the central determinants of positive parenting and contributes to children's physiological, cognitive, and social-emotional growth (Feldman, 2007a; Feldman & Eidelman, 2004). Despite various environmental, maternal, or genetic confounds, several longitudinal studies using large samples have shown that breastfeeding predicts better neurological and cognitive competence in the offspring, beginning in early childhood and continuing throughout adulthood (Golding, Rogers, & Emmett, 1997; Kramer et al., 2008). Several studies have similarly shown that breastfeeding may be associated with a lower risk for psychopathology in later life, including adult incidence of anxiety and alcoholism (Alati, Van Dooren, Najman, Williams, & Clavarino, 2009; Hughes & Bushnell, 1977). Furthermore, Jones and colleagues (2004) found that infants of depressed mothers who breastfed did not show the frontal EEG asymmetry patterns, i.e., left frontal hypoactivity, that had been reported for infants of depressed mothers associated with greater risk for depression in adulthood.
The neurobiological substrates that mediate the relationship between breastfeeding and maternal sensitivity toward their infants are being further studied with animals, and functional magnetic resonance imaging (fMRI) of human mothers. Extensive animal research across mammalian species has identified several brain regions important for parental responses to infant stimuli, highlighted by the hypothalamic region, including the medial preoptic area (MPOA) (Leckman & Herman, 2002; Numan & Insel, 2003). In addition, a rich set of limbic and cortical brain regions have been shown to regulate maternal behaviors in mammals, including the midbrain, amygdala, striatum (putamen, caudate, globus pallidus), anterior cingulate gyrus, and prefrontal cortex (Newman, 2007; Numan & Stolzenberg, 2009). Building on this, recent fMRI and volumetric MRI studies suggest that homologous brain regions in human mothers subserve maternal perceptions of infant stimuli as well as related human social cognition, affect and behavior (Barrett & Fleming, in press; Kim et al., 2010; Swain, in press; Swain, Lorberbaum, Kose, & Strathearn, 2007).
The current study examined the associations between breastfeeding, maternal brain activation in response to salient infant cues, and the mother's sensitivity to her infant. First, we investigated the relationship between breastfeeding and the neural correlates of mother-infant bonding by comparing maternal brain responses to own baby-cry versus a standard baby-cry in breastfeeding and formula-feeding mothers during the first postpartum month. Infant cry is a highly salient stimulus that elicits maternal behaviors (Bowlby, 1969) and related maternal brain responses (Swain, et al., 2007). We thus hypothesized that in response to own baby-cry, brain regions related to maternal behaviors including the hypothalamus, midbrain, amygdala, striatum, cingulate cortex, and prefrontal cortex would show greater activation in breastfeeding mothers relative to formula-feeding mothers.
Second, we examined whether the maternal brain responses to infant stimuli among breastfeeding and formula-feeding mothers in the first postpartum month would be associated with standardized observer ratings of maternal sensitivity during mother-infant dyadic interactions videotaped at 3-4 months postpartum. In the current study, we expected that breastfeeding mothers would show greater levels of maternal sensitivity at 3 months postpartum. We also hypothesized that the level of brain activity in response to own baby-cry at the first month postpartum would be associated with maternal sensitivity in dyadic interactions videotaped at 3-4 months postpartum.
Methods
Participants
Seventeen biological mothers with full-term, healthy infants were recruited in postpartum hospital rooms at the Yale-New Haven Hospital. All mothers were Caucasian and married or cohabiting. Exclusion criteria were birth complications, current psychiatric diagnosis, and history of prescription medications within 2 weeks of the experiment. Informed consent was obtained from each participant according to procedures approved by the Yale University School of Medicine Human Investigations Committee.
Nine of the mothers were categorized as breastfeeding mothers and they were breastfeeding exclusively at 2-4 weeks postpartum. The other eight mothers were categorized as formula-feeding mothers and they were exclusively feeding formula to their infant at 2-4 weeks postpartum. Mothers who chose to formula-feed because of their own medical conditions were excluded from the study. The non-medical reasons reported by the mothers for formula-feeding were: need to care for multiple children, problems with milk supply, or previous experience of pain while nursing. Among breastfeeding mothers, at 3-4 months postpartum, five out of the nine breastfeeding mothers were exclusively breastfeeding. Two mothers reported the use of formula supplementation less than 4 times per day and two mothers reported formula supplementation more than 7 times per day. All mothers in the formula-feeding group continued with formula feeding at 3-4 months postpartum.
Procedure
Study participants visited the research center to acquire brain imaging data between 2-4 weeks postpartum. Maternal sensitivity data was obtained between 3-4 months postpartum during a home visit. This entailed videotaping a mother and infant for five minutes, after being asked to interact with their infants in a natural way. Subsequently, the videotapes could be evaluated by an independent rater that had no direct interactions with the subjects and was blind to which mothers were breastfeeding their respective infants.
Maternal Sensitivity
Mother-infant interactions were coded using the Coding Interactive Behavior (CIB) Manual (Feldman, 1998). The CIB is a global rating system for adult-child interactions with versions for newborns, infants, children, and adolescents. It consists of 42 adult, child, and dyadic codes, each rated on a scale of 1 (a little) to 5 (a lot). These scales are then aggregated into six composites. The CIB has been used in multiple studies and has shown sensitivity to infant age, interacting partner, cultural variations, biological and social-emotional risk conditions, and the effects of interventions (Feldman, 2010; Feldman, Eidelman, & Rotenberg, 2004; Feldman & Klein, 2003; Feldman & Masalha, 2010; Feldman, Weller, Sirota, & Eidelman, 2003).
The Mother Sensitivity construct, used in this study, includes the following ten (averaged) codes (α = .91): mother acknowledgement of child communications, vocal clarity, positive affect, gaze, appropriate range of affect, affectionate touch, resourcefulness, consistency of style, adaptation to child signals, and supportive presence. The ten scales define the maternal sensitive-responsive style and include the typical post-partum human maternal behavior (gaze, affect, vocalizations, touch), a predictable style (consistency) and the adaptation of maternal behavior to the infant's cues (adaptation, resourcefulness when infant is distressed, appropriate range of affect [implying that mothers increase or decrease stimulation in accordance with infant signals] and supportive presence [assessing the degree to which mother's presence provides a “regulatory” context for the child]). Maternal sensitivity coded with the CIB has shown to be stable in multiple observations from 3 months to 13 years (Feldman, 2010) and to predict cognitive and social-emotional development across childhood and up to adolescence (Feldman, 2010; Feldman & Eidelman, 2009).
Brain imaging study
Image acquisition
High resolution T1-weighted anatomic magnetic resonance images (MRI) (3D MPRAGE; TR = 2530; TE = 3.66; matrix size 256×256; 176 slices) were obtained using a Siemens trio 3T full-body scanner (Erlangen, Germany). Anatomical T1-weighted echo-planar images (spin-echo; TR = 300ms; TE = 4ms; matrix size 64×64; 30 axial slices; 3.125mm in-plane resolution, 5mm thick) were acquired to be coplanar with the functional scans for spatial registration. Then, functional data were acquired (echo planar T2*-weighted gradient-echo, TR = 2000 ms, TE = 30ms, flip angle=80°, matrix size 64×64, 30 axial slices, 3.125mm in-plane resolution, 5mm thick).
fMRI stimuli
Following recruitment and consent to participate, mothers were asked to record their own baby-cry within the first 2 weeks postpartum using a portable miniature digital audio recorder provided by the researchers. For own baby-cry stimuli, mothers were asked to record them during diaper changes, but not while the baby was in pain or hungry. Samples of baby cries from mothers who did not participate in the current study were recorded, and were rated by seven adults on a 1-10 scale for emotional intensity. One with an average level of emotional intensity was selected for control baby-cry stimuli. For all stimuli, non-baby-cry sounds such as gurgles or grunts as well as any other background sounds were removed in order to generate blocks of 30 second baby-cry stimuli using sound editing software (Cool Edit Pro Version 2.0, Syntrillium Software, Phoenix, AZ). To ensure that differences in brain responses to cries between breastfeeding and formula-feeding mothers in this study were due to their different feeding practices and not due to the different levels of emotional intensity of cries, we asked ten adults to rate the emotional intensity of each baby-cry stimuli (1=none, 2= a little, 3= moderate, 4 = maximum). There were no differences of the emotional intensity levels between baby cries of breastfeeding mothers (M = 2.58, SD=.52) and formula-feeding mothers (M=2.69, SD = .53).
In the MRI scanner, mothers heard cries through padded headphones, organized into two functional runs, in which blocks of different cry stimuli lasted 30 seconds. Each run contained blocks of either (A) own baby-cry, or (B) control baby-cry. These stimulus blocks were arranged so participants heard each sound block a total of five times in the following order: ABBAB and then ABAAB. Each stimulus block was separated by a 10-second rest period during which only background scanner noise could be heard. Before scanning, participants listened to samples of all sounds through headphones. The volume of the sounds was adjusted so that the participants could hear the sounds clearly and so that the participants were familiarized to the sounds to minimize any possible surprise effects. During the scans, participants rated levels of emotional responses to each cry stimuli by using a button press (1=none, 2= a little, 3= moderate, 4 = maximum emotional response).
fMRI Data Preprocessing
Functional imaging data was preprocessed and statistical analysis was performed using standard algorithms of Brainvoyager QX Software versions 1.6 and 1.8 (Brain Innovation, Maastrict, The Netherlands). Functional runs were pre-processed using 3-dimentional motion correction based on trilinear interpolation by spatial alignment of all brain volumes to the first volume. One formula-feeding mother had a far greater than 3-mm translation in both runs; thus, data from this subject mother was excluded from further fMRI analyses. Slice scan-time correction was performed by sinc interpolation. After linear trend removal, a temporal high-pass filter was used to remove nonlinear drifts of 3 cycles or less per time course. Motion-corrected images were spatially smoothed using a Gaussian filter with a full-width half-maximum value of 6.25mm. Data from each subject were co-registered with each subject's high-resolution 3D anatomical data set, then transformed into standardized 3D Talairach coordinate space using piecewise linear warping. The functional data were resampled as 1 mm3 voxels.
Data analysis
The analysis of the blood-oxygen-level-dependent (BOLD) signal was based on the block design protocol in response to own baby-cry sound relative to control baby-cry sound; contrasts were analyzed using a random effects general linear model (GLM). Contrast images were computed for each condition for each participant separately. Group effects (breastfeeding vs. formula-feeding) were computed using the transformed contrast images in a conjunction of random effects. GLM analysis and the group t maps were generated for a specific contrast: brain difference activation in response to own baby-cry minus control baby-cry between breastfeeding and formula-feeding mothers at 2-4 weeks postpartum. The significance threshold for between-group differences was set at p < .01, and was corrected for multiple comparisons at the cluster level false-positive rate (α) of 0.05 (Forman et al., 1995; Goebel, Esposito, & Formisano, 2006). Thus, the uncorrected activated clusters were rejected if they included smaller than 44 contiguous voxels. Because we found that maternal education levels were significantly different between the two groups, we ran further analysis to address this issue. Once clusters with significant between-group differences were identified, signal value differences between own baby-cry vs. other baby-cry were extracted from each cluster, and entered into the SPSS (SPSS, Inc., Chicago, Ill). Then, ordinary least squares (OLS) regression was performed to test whether the group status (breastfeeding vs. formula-feeding) was significantly associated with signal differences between own baby-cry vs. other baby-cry in each region when maternal education was included as a covariate. Results from the clusters which had a significant effect of the feeding status at p<.05 were reported and included for the correlation analysis with maternal sensitivity at p<.05.
In order to test significant correlations of maternal sensitivity at 3-4 months postpartum with brain activations at 2-4 weeks postpartum, we performed a voxel-wise correlation between maternal sensitivity and brain responses to own minus control baby-cry sound at p<.01, corrected at the cluster level. A correction for multiple testing was not employed since there is only one primary analysis based on one hypothesis (Perneger, 1998). The hypothesis was that maternal sensitivity would be only positively correlated with the BOLD signals in response to own baby-cry in brain regions that were critical for maternal behaviors. Thus, in the hypothalamus, midbrain, amygdala, striatum, anterior cingulate gyrus and prefrontal regions, the correlation analysis was limited only to areas that were identified to exhibit significantly greater activations in the breastfeeding group relative to the formula-feeding group at 2-4 weeks postpartum after controlling for maternal education levels.
Results
Description of Breastfeeding and Formula-feeding groups
Mothers from the two groups were relatively well-matched in demographic background and included both first-time mothers as well as experienced mothers. Mothers in the breastfeeding and formula-feeding groups showed no mean differences in age, handedness, parenting experience, the number of children, and delivery method (see Table 1). However, since maternal education was higher in the breastfeeding group, maternal education level was included as a covariate in comparing the two groups' brain responses at 2-4 weeks postpartum.
Table 1. Comparison in Breastfeeding vs. Formula-feeding mothers.
| Breastfeeding | Formula-feeding | t(15) | p value | |
|---|---|---|---|---|
| n=9 | n=8 | |||
| Mean (SD) | Mean (SD) | |||
| Maternal Age | 33.23 (4.28) | 30.27 (8.02) | .97 | .35 |
| First-born/later-born | 3/6 | 2/6 | -.36 | .73 |
| # of older children | .78 (.67) | 1.88 (1.96) | -1.59 | .13 |
| Infant Gender ratio (M/F) | 5/4 | 4/4 | -.22 | .83 |
| Vaginal/C-section delivery | 7/2 | 7/1 | .50 | .63 |
| Maternal Handedness (L/R) | 1/8 | 1/7 | .18 | .86 |
| Maternal Educational level (in years) | 17.89 (1.76) | 14.75 (3.28) | 2.50 | .03 |
Behavioral Ratings
Mothers' subjective ratings of emotional response while listening to the cries in the scanner were analyzed. Repeated measure ANOVA with feeding status as a between-group variable, and cry type as a within-group variable and maternal education as a covariate was performed. The analysis revealed that at 2-4 weeks postpartum, neither group of mothers rated the two types of cries differently.
Brain responses to Own versus Control baby-cry at 2-4 weeks postpartum
The contrasts of own baby-cry versus control baby-cry between the two groups of mothers revealed that the breastfeeding mothers showed a greater BOLD signal in several brain regions in response to own baby-cry sound compared to formula-feeding mothers. Activations in right lateral globus pallidus/putamen, right putamen, left inferior frontal gyrus, and right superior prefrontal gyrus were greater among the breastfeeding mothers, p<.01 (corrected), p<.05 after controlling for maternal education (see Table 2, Figure 1). Bilateral insula, right fusiform gyrus, right precuneus, right cuneus as well as left superior and middle temporal gyrus were also activated in response to own baby-cry among breastfeeding mothers more than among formula-feeding mothers p<.01 (corrected), p<.05 after controlling for maternal education (see Table 2, Figure 1). No brain areas were found to exhibit significantly greater BOLD activations among the formula-feeding mothers in response to own baby-cry (minus control baby-cry) at the level of significance, <.01 (corrected).
Table 2.
Activation results of the group comparison in the contrast of ‘Own baby-cry’ minus ‘Control baby-cry’ at 2-4 weeks postpartum.
| Area of Activation | Brodmann area | Side | t score | cluster size | Talairach coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Breastfeeding > Formula-feeding mothers | |||||||
| Superior frontal gyrus | 9 | R | 3.04 | 170 | 23 | 50 | 28 |
| Inferior frontal gyrus | 47 | L | 3.25 | 200 | -50 | 22 | -2 |
| Superior temporal gyrus | 22 | L | 3.05 | 125 | -53 | 5 | -1 |
| 22 | L | 3.30 | 702 | -63 | -42 | 8 | |
| Middle temporal gyrus | 39 | L | 3.07 | 153 | -59 | -62 | 11 |
| Transverse temporal gyrus | 42 | L | 3.11 | 259 | -60 | -18 | 11 |
| Insula | 13 | R | 3.37 | 2963 | 45 | -21 | 12 |
| 13 | R | 3.25 | 2093 | 38 | -3 | -4 | |
| 13 | R | 2.99 | 175 | 33 | -69 | 37 | |
| 13 | L | 3.34 | 1589 | -44 | -17 | 2 | |
| Fusiform gyrus | 37 | R | 3.46 | 2701 | 54 | -47 | -4 |
| Precuneus | 31 | L | 3.07 | 3707 | -19 | -65 | 21 |
| Cuneus | 17 | R | 3.02 | 106 | 12 | -77 | 9 |
| 17 | L | 2.93 | 294 | -3 | -86 | 11 | |
| Putamen | -- | R | 3.18 | 676 | 21 | -1 | 14 |
| Lateral globus pallidus/Amygdala | -- | R | 3.23 | 446 | 20 | -5 | -6 |
| Cerebellum | -- | R | 3.03 | 253 | 46 | -57 | -28 |
| -- | L | 3.19 | 975 | -28 | -57 | -26 | |
p<.01: corrected for multiple comparisons; p<.05 after controlling for maternal education level x,y, and z coordinates refer to the location of the voxel with maximum signal intensity
Figure 1.
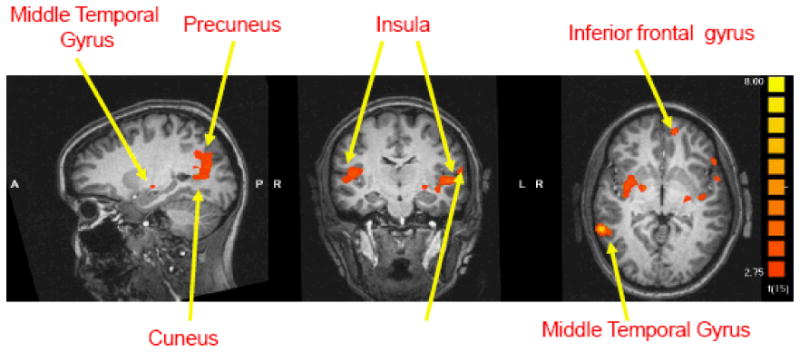
Areas of activation in the contrast (own baby-cry minus control baby-cry) at 2-4 weeks postpartum. Areas in red show greater activations in breastfeeding group relative to formula-feeding group (x, y, z = -22, -15, -2).
Relationships between neuroimaging and maternal sensitivity
The OLS regression analysis revealed that at 3-4 months postpartum, breastfeeding mothers (M=4.46, SD=0.42) had a trend toward higher levels of maternal sensitivity relative to formula-feeding mothers (M=3.54, SD=0.74), β = -2.13, p = .05. Maternal education was included as a covariate. Among breastfeeding mothers at 3-4 months postpartum, four mothers reported that they occasionally formula fed their infants. However, the OLS regression analysis revealed that the frequency of formula-feeding was not significantly associated with maternal sensitivity among the breastfeeding mothers, p >.10.
A voxel-wise correlation showed that maternal sensitivity at 3-4 months postpartum was positively correlated with activations in the right superior frontal gyrus (r=.62, p<.01), and right lateral globus pallidus/amygdala (r=.53, p<.05) at 2-4 weeks postpartum (see Figure 2). Correlations were not found in the left inferior frontal gyrus or right putamen.
Figure 2.
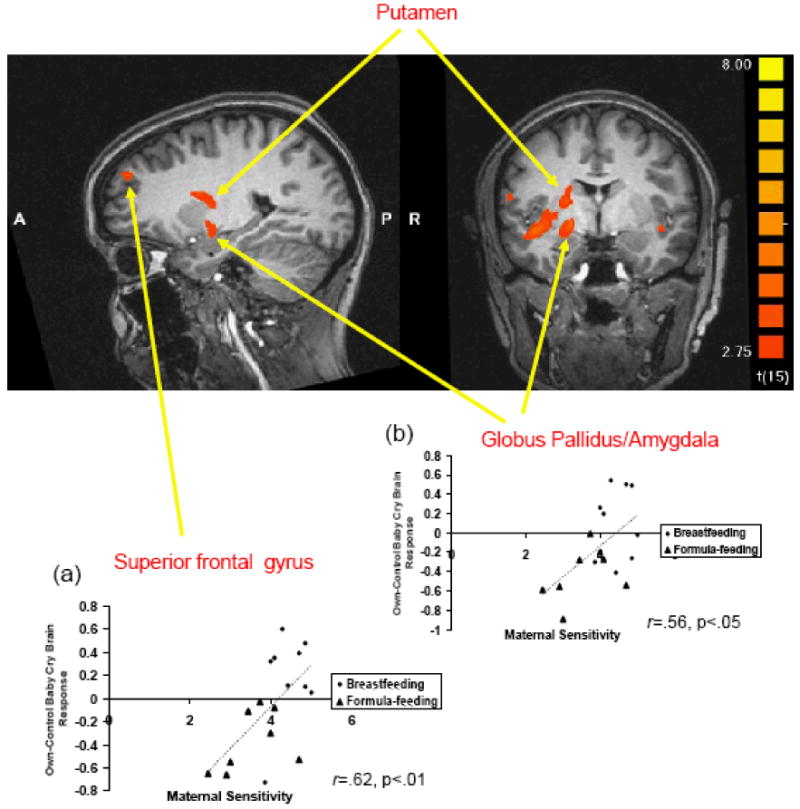
Areas of activation in the contrast (own baby-cry minus control baby-cry) at 2-4 weeks postpartum. Areas in red show greater activations in breastfeeding group relative to formula-feeding group (x, y, z = 23, -4, -9). Scatter plots showing the positive relationship between maternal sensitivity at 3-4 months postpartum and brain responses to own baby-cry (minus control baby-cry) at 2-4 weeks postpartum and (a) in right superior frontal gyrus and (b) in right lateral globus pallidus/amygdala.
Discussion
The current study demonstrates associations between breastfeeding and maternal brain responses to own infant stimuli as well as dyadic parenting behaviors in the early postpartum period. During the first postpartum month, several limbic and cortical brain regions previously shown to be important for caregiving behaviors and empathy, were more active among breastfeeding mothers compared to formula-feeding mothers while listening to their own baby versus a control baby-cry. Furthermore, for all the mothers in the study (breastfeeding and formula-feeding), the degree of activation in these brain regions in responses to own baby-cry at the first postpartum month was correlated with greater maternal sensitivity observed during dyadic mother-infant interactions that were videotaped at 3-4 months postpartum. These findings relate the neural processing of infant sensory cues by mothers and the development of maternal responsiveness as infants begin to take an active part in dyadic interactions.
As predicted, we found differences between breastfeeding and formula-feeding mothers in activations in the amygdala, putamen, globus pallidus, and superior frontal gyrus in response to their own baby's cry. Rodent studies have consistently shown that activations in these brain regions and the connections between them are correlated with the emergence of maternal behavior (Mello & Villares, 1997), and lesions in these circuits can severely impair the expression of maternal behavior (Numan, 2007; Numan & Smith, 1984). Similar findings have been reported in human studies when mothers were exposed to salient infant stimuli including baby cries, still photographs, or video clips (Noriuchi, Kikuchi, & Senoo, 2008; Ranote et al., 2004; Strathearn, Fonagy, Amico, & Montague, 2009; Strathearn, Li, Fonagy, & Montague, 2008; Swain et al., 2008). The brain areas implicated included portions of the mirror-neuron circuits (inferior frontal gyrus, superior temporal gyrus, insula, and fusiform gyrus). These circuits are important for processing the emotional states of others (Iacoboni & Dapretto, 2006; Völlm et al., 2005). Lenzi et al. (2009) found that parent responses to pictures of their own infant in these mirror-neuron circuits were related to parental reflective functioning. Thus, higher activations in these brain regions contribute to breastfeeding mother's ability to understand their own infant's emotional state and respond in an appropriate way to the infant's needs.
Breastfeeding mothers exhibited a trend toward higher maternal sensitivity during mother-infant interaction at 3-4 months postpartum as compared to formula-feeding mothers. The idea that the greater maternal brain activations among breastfeeding mothers may facilitate greater behavioral sensitivity to the infant is also supported by the finding of significant correlation between brain activations and maternal sensitivity in dyadic interactions observed 3 to 4 months postpartum. Importantly, sensitive maternal behavior observed in dyadic mother-child interactions at 3 months has been shown to be associated with improved cognitive, social, emotional, and moral development across childhood and up to adolescence (Feldman, 2007b; Feldman, Greenbaum, & Yirmiya, 1999; Isabella & Belsky, 1991).
It is important to note that the relationship of breastfeeding with greater maternal brain responses and more sensitive maternal behaviors may not be causal and confounded by other factors. For example, one possible mechanism underlying the relationships between breastfeeding, greater activations in the maternal circuits, and the mother's later sensitivity to infant stimuli could be due to the effects of the neurohormones involved in nursing, such as oxytocin. Oxytocin, which is synthesized in the hypothalamus and released from the posterior pituitary, stimulates milk release at the mammary glands. Oxytocin also facilitates other maternal behaviors in animal studies (Febo, Numan, & Ferris, 2005). Higher levels of peripheral oxytocin have been associated with sensitive and synchronous parental behaviors in human mothers and fathers (Feldman, Weller, Zagoory-Sharon, & Levine, 2007). In human fMRI research, brain responses to own baby-cry at the first month postpartum were increased in mothers who delivered vaginally compared with cesarean section (Swain, et al., 2008), which has been linked to decreased oxytocin (Kendrick, 2000). Peripheral maternal oxytocin levels, in response to dyadic interactions at 7 months postpartum, was associated with hypothalamic activation in the mother's brain in response to viewing pictures of their infant at 11 months postpartum (Strathearn, Fonagy, et al., 2009). The duration of breastfeeding may be related to the oxytocin levels and play a role in maternal sensitivity and infants' outcomes (Kramer, et al., 2008). It is possible that differences in regional brain activation seen in this study are due to the act of breastfeeding and the increased release of oxytocin. In future studies, it will be important to include measures of oxytocin and other related neurohormones such as prolactin directly.
Another potentially confounding issue concerns the factors which influence a mother's decision to breastfeed before the child is born. For example, Britton and colleagues (2006) found that intention to breastfeed prior to the child's birth predicted maternal sensitivity at three months postpartum. Likewise, mothers who experienced consistent and positive care in childhood are also more likely to provide warm and sensitive parenting to their children (Belsky, Jaffee, Sligo, Woodward, & Silva, 2005). In a related fMRI study, a mother's self-report of early experiences with her own mother has been associated with maternal brain responses to infant cries at the first postpartum period (Kim et al., 2010). Thus, these maternal factors that contribute to each mother's decision to breastfeed, include the strength of the emotional bond with the fetus as well as the sum of early life experiences with her own mother. These factors, in turn, may play a role in both the mother's brain activations and parenting behaviors. The relationship between breastfeeding and parenting may also be moderated by environmental factors such as cultural and socio-economic background as well as other maternal characteristics such as race, education level, and age. Studies have shown that breastfeeding mothers tend to be older, more educated, of higher socioeconomic status, and of greater social support (Dennis, 2002). In the current study, breastfeeding mothers tended to be more educated. As a result, we included maternal education as a covariate in the analyses. High levels of education, socioeconomic status and social support are also important factors for responsive parenting (Magnuson & Duncan, 2002; McLoyd & Wilson, 1990). Society's perception of breastfeeding could also moderate the link between breastfeeding and parental outcomes. In some societies, breastfeeding is the norm of infant feeding practice and breastfeeding may not necessarily predict the quality of maternal care, whereas in other societies breastfeeding may not be strongly encouraged. Thus, future studies are needed to assess whether these confounding maternal and environmental/contextual factors may have affected the choice of feeding styles and contribute to long-term differences in brain activations and parenting behaviors between breastfeeding and bottle-feeding mothers.
Limitations of the study are important to consider in the interpretation of the findings and for guiding future research. First, although our sample is considered sizable for a brain imaging study, the number of mothers who participated in this study is relatively small. Future studies with a larger sample of mothers may be able to tease apart changes related to specific parenting behaviors from those related to non-specific aspects of the early post-partum. Second, although infant cries from the two groups and the control infant cry were rated as having a similar level of emotional intensity by independent raters, because mothers selected samples of their own baby cries by themselves, it is possible that one group of mothers may have selected cry samples which they perceived as being more emotionally salient. Thus, future studies comparing brain responses to three groups of cry stimuli – own baby, other group's baby, and control baby – may help resolve this issue. Third, we did not measure maternal sensitivity at 2-4 weeks postpartum when the brain responses to infant stimuli were assessed. Future studies are needed to explore the relationship between patterns of maternal brain activations and concurrent mother-infant dyadic interaction.
In sum, our findings represent the first evidence using brain imaging that breastfeeding at the first month postpartum has a significant link to both enhanced maternal brain responses to infant stimuli and maternal behaviors. Consistent with these results, we found that the greater maternal brain response to own baby stimuli at the first few weeks of a child's life may be related to more positive and sensitive mothering during the early postpartum period. However, because factors related to a mother's decision to breastfeed may also be associated with these parental outcomes, future research is needed to understand whether breastfeeding moderate the relationship between a mother's decision to breastfeed and neurological responses to her infant with a pre- and post-intervention studies. More research is also required to examine whether the increase in brain response related to breastfeeding and the significant associations between early maternal brain responses and later parenting behaviors influence the infant's cognitive, social, and emotional development.
Acknowledgments
This work was supported by the Ellin Bloch and Pierre Ritchie Honorary Scholarship, the American Psychological Association of Graduate Students (APAGS) (PK); College of Human Ecology Graduate Research Grant, Cornell University (PK); the US-Israel binational science foundation (2005-273, RF, JFL), the Institute for the Study of Unlimited Love (JES, JFL); the National Alliance for Research on Schizophrenia and Depression (RF, JES), the National Institute of Mental Health (JFL: K05MH076273), the National Institute of Drug Abuse (LCM: 5K05DA020091), and the Associates of the Yale Child Study Center. We thank Drs. Gary W. Evans, Cindy Hazan, and Richard Depue at Cornell University for their helpful insights and suggestions.
Abbreviations
- ANOVA
analysis of variance
- BOLD
blood-oxygen-level-dependent
- CIB
Coding Interactive Behavior
- fMRI
functional magnetic resonance imaging
- GLM
general linear model
- MPOA
medial preoptic area
- OLS
ordinary least squares
References
- Alati R, Van Dooren K, Najman JM, Williams GM, Clavarino A. Early weaning and alcohol disorders in offspring: biological effect, mediating factors or residual confounding? Addiction. 2009;104:1324–1332. doi: 10.1111/j.1360-0443.2009.02643.x. [DOI] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry. doi: 10.1111/j.1469-7610.2010.02306.x. in press. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jaffee SR, Sligo J, Woodward L, Silva PA. Intergenerational transmission of warm-sensitive-stimulating parenting: a prospective study of mothers and fathers of 3-year-olds. Child Development. 2005;76:384–396. doi: 10.1111/j.1467-8624.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment. 2nd. Vol. 1. New York: Basic Books; 1969. [Google Scholar]
- Britton JR, Britton HL, Gronwaldt V. Breastfeeding, sensitivity, and attachment. Pediatrics. 2006;118:e1436–e1443. doi: 10.1542/peds.2005-2916. [DOI] [PubMed] [Google Scholar]
- Dennis CL. Breastfeeding initiation and duration: a 1990-2000 literature review. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 2002;31:12–32. doi: 10.1111/j.1552-6909.2002.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Dunn JB, Richards MPM. Observations on the developing relationship between mother and baby in the neonatal period. In: Schaffer HR, editor. Studies in mother-infant interaction. New York: Academic Press; 1977. pp. 427–455. [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. Journal of Neuroscience. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. Mother-newborn coding system manual. Tel Aviv, Israel: Bar-Ilan University University Press; 1998. [Google Scholar]
- Feldman R. Parent-infant synchrony: Biological foundations and developmental outcomes. Current Directions in Psychological Science. 2007a;16:340–345. [Google Scholar]
- Feldman R. Mother-infant synchrony and the development of moral orientation in childhood and adolescence: Direct and indirect mechanisms of developmental continuity. American Journal of Orthopsychiatry. 2007b;77:582–597. doi: 10.1037/0002-9432.77.4.582. [DOI] [PubMed] [Google Scholar]
- Feldman R. The relational basis of adolescent adjustment: Trajectories of mother-child interactive behaviors from infancy to adolescence shape adolescents' adaptation. Attachment & Human Development. 2010;12:173–192. doi: 10.1080/14616730903282472. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Direct and indirect effects of breast milk on the neurobehavioral and cognitive development of premature infants. Developmental Psychobiology. 2003;4:109–119. doi: 10.1002/dev.10126. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Parent-infant synchrony and the social-emotional development of triplets. Developmental Psychology. 2004;40:1133–1147. doi: 10.1037/0012-1649.40.6.1133. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Biological and environmental initial conditions shape the trajectories of cognitive and social-emotional development across the first years of life. Developmental Science. 2009;12:194–200. doi: 10.1111/j.1467-7687.2008.00761.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI, Rotenberg N. Parenting Stress, Infant Emotion Regulation, Maternal Sensitivity, and the Cognitive Development of Triplets: A Model for Parent and Child Influences in a Unique Ecology. Child Development. 2004;75:1774–1791. doi: 10.1111/j.1467-8624.2004.00816.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Greenbaum CW, Yirmiya N. Mother-infant affect synchrony as an antecedent of the emergence of self-control. Developmental Psychology. 1999;35:223–231. doi: 10.1037//0012-1649.35.1.223. [DOI] [PubMed] [Google Scholar]
- Feldman R, Klein PS. Toddlers' self-regulated compliance to mothers, caregivers, and fathers: Implications for theories of socialization. Developmental Psychology. 2003;39:680–692. doi: 10.1037/0012-1649.39.4.680. [DOI] [PubMed] [Google Scholar]
- Feldman R, Masalha S. Parent–child and triadic antecedents of children's social competence: Cultural specificity, shared process. Developmental Psychology. 2010;46:455–467. doi: 10.1037/a0017415. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Sirota L, Eidelman AI. Testing a family intervention hypothesis: The contribution of mother-infant skin-to-skin contact (kangaroo care) to family interaction, proximity, and touch. Journal of Family Psychology. 2003;17:94–107. [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychological Science. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Golding J, Rogers IS, Emmett PM. Association between breast feeding, child development and behaviour. Early Human Development. 1997;49(Suppl):S175–S184. doi: 10.1016/s0378-3782(97)00062-5. [DOI] [PubMed] [Google Scholar]
- Groër MW. Differences between exclusive breastfeeders, formula-feeders, and controls: a study of stress, mood, and endocrine variables. Biological Research For Nursing. 2005;7:106–117. doi: 10.1177/1099800405280936. [DOI] [PubMed] [Google Scholar]
- Hughes RN, Bushnell JA. Further relationships between IPATAnxiety Scale performance and infantile feeding experiences. Journal of Clinical Psychology. 1977;33:698–700. doi: 10.1002/1097-4679(197707)33:3<698::aid-jclp2270330318>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7:942–951. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Isabella RA, Belsky J. Interactional synchrony and the origins of infant-mother attachment: A replication study. Child Development. 1991;62:373–384. [PubMed] [Google Scholar]
- Jones N, McFall B, Diego M. Patterns of brain electrical activity in infants of depressed mothers who breastfeed and bottle feed: The mediating role of infant temperament. Biological Psychology. 2004;67:103–124. doi: 10.1016/j.biopsycho.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Kendrick KM. Oxytocin, motherhood and bonding. Experimental Physiology. 2000;85:111S–124S. doi: 10.1111/j.1469-445x.2000.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE. The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience. 2010;124:695–700. doi: 10.1037/a0020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Newman MA, Feldman R, Swain JE. Perceived quality of maternal care in childhood and structure and function of mothers' brain. Developmental Science. 2010;13:662–673. doi: 10.1111/j.1467-7687.2009.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Aboud F, Mironova E, Vanilovich I, Platt RW, Matush L, et al. Breastfeeding and child cognitive development: new evidence from a large randomized trial. Archives of general psychiatry. 2008;65:578–584. doi: 10.1001/archpsyc.65.5.578. [DOI] [PubMed] [Google Scholar]
- Kuzela A, Stifter C, Worobey J. Breastfeeding and mother–infant interactions. Journal of Reproductive and Infant Psychology. 1990;8:185–194. [Google Scholar]
- Lavelli M, Poli M. Early mother-infant interaction during breast- and bottle-feeding. Infant Behavior & Development. 1998;21:667–684. [Google Scholar]
- Leckman JF, Herman AE. Maternal behavior and developmental psychopathology. Biological Psychiatry. 2002;51:27–43. doi: 10.1016/s0006-3223(01)01277-x. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Trentini C, Pantano P, Macaluso E, Iacoboni M, Lenzi GL, et al. Neural basis of maternal communication and emotional expression processing during infant preverbal stage. Cereb Cortex. 2009;19:1124–1133. doi: 10.1093/cercor/bhn153. [DOI] [PubMed] [Google Scholar]
- Magnuson KA, Duncan GJ. Parents in poverty. In: Bronstein MH, editor. Handbook of Parenting. 2nd. Vol. 4. Mahwah, NJ: Erlbaum; 2002. pp. 95–121. [Google Scholar]
- McLoyd VC, Wilson L. Maternal behavior, social support, and economic conditions as predictors of distress in children. New Direction for Child Devlopment. 1990;46:49–69. doi: 10.1002/cd.23219904605. [DOI] [PubMed] [Google Scholar]
- Mello LE, Villares J. Neuroanatomy of the basal ganglia. Psychiatric Clinics of North America. 1997;20:691–704. doi: 10.1016/s0193-953x(05)70340-3. [DOI] [PubMed] [Google Scholar]
- Mezzacappa ES, Katlin ES. Breast-feeding is associated with reduced perceived stress and negative mood in mothers. Health Psychology. 2002;21:187–219. [PubMed] [Google Scholar]
- Mezzacappa ES, Kelsey RM, Katkin ES. Breast feeding, bottle feeding, and maternal autonomic responses to stress. Journal of Psychosomatic Research. 2005;58:351–365. doi: 10.1016/j.jpsychores.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Newman JD. Neural circuits underlying crying and cry responding in mammals. Behavioural Brain Research. 2007;182:155–165. doi: 10.1016/j.bbr.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biological Psychiatry. 2008;63:415–423. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Developmental Psychobiology. 2007;49:12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behavioral Neuroscience. 1984;98:712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. British Medical Journal. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranote S, Elliott R, Abel KM, Mitchell R, Deakin JF, Appleby L. The neural basis of maternal responsiveness to infants: an fMRI study. Neuroreport. 2004;15:1825–1829. doi: 10.1097/01.wnr.0000137078.64128.6a. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Mamun AA, Najman JM, O'Callaghan MJ. Does breastfeeding protect against substantiated child abuse and neglect? A 15-year cohort study. Pediatrics. 2009;123:483–493. doi: 10.1542/peds.2007-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE. The human parental brain: In vivo neuroimaging. Progress in Neuro-Psychopharmacology & Biological Psychiatry. doi: 10.1016/j.pnpbp.2010.10.017. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psycholology and Psychiatry. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völlm BA, Taylor AN, Richardson P, Corcoran R, Stirling J, McKie S, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2005;29:90–98. doi: 10.1016/j.neuroimage.2005.07.022. [DOI] [PubMed] [Google Scholar]


