Abstract
The endocannabinoid arachidonoyl ethanolamide (AEA) is a potent inducer of tumor cell apoptosis however its mechanism of cytotoxicity is unclear. A previous report from our laboratory showed that AEA induced cell death in a COX-2-dependent manner and in this report our data indicate that AEA-induced apoptosis is mediated by COX-2 metabolic products of the J-series. In experiments conducted with JWF2 keratinocytes which overexpress COX-2, AEA caused a concentration-regulated increase in J-series prostaglandin production and apoptosis. Similarly, cell treatment with exogenously added J-series prostaglandins (15-deoxy, Δ12,14 PGJ2 and PGJ2) induced apoptosis. AEA-induced apoptosis was inhibited by the antioxidant, N-acetyl cysteine, indicating that reactive oxygen species generation was required for apoptosis. Using antagonists of cannabinoid receptor 1, cannabinoid receptor 2, or TRPV1, it was observed that cannabinoid receptor inhibition did not block AEA-mediated cell death. In contrast, an inhibitor of fatty acid amide hydrolase (FAAH) potentiated AEA-induced J-series PG synthesis and apoptosis. These results suggest that the metabolism of AEA to J-series PGs regulates the induction of apoptosis in cells with elevated COX-2 levels. Our data further indicate that the proapoptotic activity of AEA can be enhanced by combining it with an inhibitor of FAAH. As such, AEA may be an effective agent to eliminate tumor cells that overexpress COX-2.
Keywords: anandamide, cannabinoid, keratinocyte, COX-2, bioactive lipids
INTRODUCTION
Cannabinoids are being investigated for their potential as cancer chemotherapeutic agents [2]. Many of the physiologic and pathologic effects of cannabinoids including the modulation of pain and inflammation and the promotion of drug addiction were identified with the prototypic phytocannabinoid, Δ9-tetrahydrocannabinol (Δ9-THC) which is isolated from Cannabis sativa (marijuana). Synthetic cannabinoids including WIN55,212-2 and JWH-133 have also been extensively studied and are critical tools in the characterization of this class of molecules [3;4]. Interestingly, mammalian cells also produce cannabinoids endogenously (endocannabinoids) that mimic many of the effects of the phytocannabinoids and synthetic cannabinoids. Several endocannabinoids have been identified including arachidonoyl ethanolamide (AEA; also known as anandamide), 2-arachidonoyl glycerol (2-AG), oleoyl ethanolamide (OEA) and palmitoyl ethanolamide (PEA) however 2-AG and AEA are the most extensively characterized [1].
Cannabinoids are components of the endocannabinoid system (ECS) which also include cannabinoid receptors, molecular transporters and the enzymes involved in cannabinoid synthesis and degradation. Binding of the endocannabinoid AEA to G-protein-coupled cannabinoid receptor 1 (CB1R) or cannabinoid receptor 2 (CB2R) modulates cellular signaling through pathways that include cAMP, MAPK and Akt [5;6]. AEA can also bind to the TRPV1 channel or the GPR55 receptor causing an increase in intracellular Ca2+ [7;8]. AEA enters the cell via the anandamide membrane transporter (AMT) and the activity of AEA is then terminated by fatty acid amide hydrolase (FAAH) which degrades AEA to arachidonic acid plus ethanolamine [9].
Several studies have shown that AEA induces tumor cell toxicity both in vivo and in vitro however it is unclear whether cell death occurs in a ECS-dependent or ECS-independent manner. The use of selective antagonist of the CB1R, CB2R or TRPV1 receptors have revealed that AEA-induced cell death can occur by a receptor-mediated mechanism [10–12]. In addition, FAAH inhibitors and other agents that block endocannabinoid degradation enhance AEA cytotoxicity by regulating the endocannabinoid tone [13;14]. On the other hand, cellular molecules which are not components of the ECS are also implicated as primary mediators of AEA-induced cell death. For example, AEA recruits the death receptor/ligand, Fas/FasL to lipid rafts activating the apoptotic cascade by a cannabinoid-receptor independent mechanism [15]. Another study also showed that cyclooxygenase-2 (COX-2) regulates AEA-induced cell death independent of components of the ECS [16]. COX-2 is an enzyme that converts arachidonic acid to PGH2 which is further metabolized to prostaglandins (PGs) of the E-, F-, and D- series by prostaglandin synthases. Several recent investigations show that COX-2 also converts the endocannabinoid AEA to PGH2-EA which is then metabolized to prostaglandin-ethanolamides of the E-, F-, and D-series by prostaglandin synthases [17;18]. These findings have led to observations by our group and others that the E-, D- or J-series metabolic products of AEA (or AEA analogues) are cytotoxic [19–21]. J-series PGs (PGJ2, 12Δ-PGJ2 and 15-deoxy, 12,14Δ PGJ2) are cyclopentanone PGs that are formed readily from D-series PGs through chemical rearrangement and dehydration reactions [22;23]. The cytotoxic activity of both the D- and J-series PGs is well known and has been described extensively in many cell types [24–26].
Studies have been initiated in our laboratory to determine the mechanism by which AEA induces apoptosis in non-melanoma skin cancer (NMSC) cells. It is projected that more than 2 million individuals will have developed NMSC in the United States in the year 2010 [27] and tumors of this type typically contain elevated levels of COX-2. In this communication, we show that AEA-induced apoptosis in tumorigenic keratinocytes which overexpress COX-2 is regulated by the production of J-series PGs and also by FAAH. Our findings shed greater light on the mechanism of AEA cytotoxicity in cells with elevated COX-2 expression and suggest that an effective strategy for treatment of NMSC may include the administration of AEA with an inhibitor of FAAH.
MATERIALS AND METHODS
Antibodies and reagents
Arachidonoyl ethanolamide (AEA), URB597, capsazepine, anti-CB1R antibody, PGD2, PGD2-EA, PGJ2 and 15-deoxy, 12,14Δ PGJ2 (15-d-PGJ2) were purchased from Cayman Chemical Company (Ann Arbor, MI). Anti-FAAH was a kind gift from Dr. Benjamin Cravatt (Scripps Institute, LaJolla, CA). Anti-COX-2, anti-CB2R, anti-Nrf2, anti-Keap1 and anti-lamin B1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Full-length (FL) caspase 3 was from Invitrogen (Carlsbad, California), anti-TRPV1 was from Millipore (Billerica, MA) and anti-GAPDH was from Trevigen (Gaithersburg, MD). Antibody directed towards cleaved caspase-3 and FL/cleaved PARP were from Cell Signaling Technologies (Beverley, MA). SR141716 (CB1R antagonist) and SR144528 (CB2R antagonist) were kind gifts from Sanofi-aventis (Paris, France).
Cell line and cell culture conditions
The murine squamous carcinoma cell line JWF2 was cultured in Eagle's Minimal Essential Medium (USBiological, Swampscott, Massachusetts) containing 5% heat-inactivated fetal bovine serum (FBS), penicillin (100 mg/mL), streptomycin (100 mg/mL), nonessential amino acids, and glutamine. JWF2 cells were a kind gift from Dr. Susan Fischer (University of Texas, MD Anderson Cancer Center, Smithville, TX).
Western blot analysis
JWF2 cells were incubated in medium containing the indicated agents. For Western blot analysis the protein concentration of cell lysates were determined with BCA reagents (Pierce, Rockford, IL). Equal concentrations of each sample were loaded onto SDS-PAGE gels and protein bands transferred to PVDF membranes (BioRad, Hercules, CA). Blocked membranes were incubated in 5% nonfat dry milk containing cleaved caspase 3 (1:500), FL/cleaved PARP (1:1000), anti-COX-2 (1:1000), anti-CB1R (1:500), anti-CB2R (1:500), anti-TRPV1 (1:500), anti-FAAH (1:500), anti-Nrf2 (1:500), anti-Keap1 (1:500), anti-lamin B1(1:1000) or anti-GAPDH (1:5000) antibody. Protein bands were visualized by Enhanced Chemi-luminescence-Plus (ECL-Plus) (GE Healthcare Life Sciences; Piscataway, NJ).
Cell viability assays
JWF2 cells were plated in 96-well dishes and cultured for 48 h. Serum-free medium containing various concentrations of AEA, SR141716, SR144528, capsazepine or URB597 were the added to fresh, serum-free cell culture medium. MTS reagent, [3-(4, 5-dimethylthiazol-2-yl) -5- (3 carboxymethoxyphenyl) -2- (4-sulfophenyl)-2H-tetrazolium, inner salt] (Promega; Madison, WI) was then added to each well after 18 hours of incubation and absorbance measured at 495 nm as directed by the manufacturer.
15-deoxy, Δ12,14 PGJ2 ELISA assays
The culture medium from treated JWF2 cells was collected and J-series PGs measured as described by the manufacturer (Assay Designs; Ann Arbor, MI). This ELISA kit also measures other J-series prostaglandins but not prostaglandins of the E-, F-, or D-series.
TUNEL Assays
JWF2 cells were cultured in chamber slides and treated as indicated. Cells were then washed with PBS and fixed in 4% paraformaldehyde before measurement of DNA fragmentation. TUNEL assay was performed as described by the manufacturer (Roche; Indianapolis, IN) and cells visualized by fluorescent microscopy.
RESULTS
AEA-induced apoptosis correlates with increased J-series prostaglandin synthesis
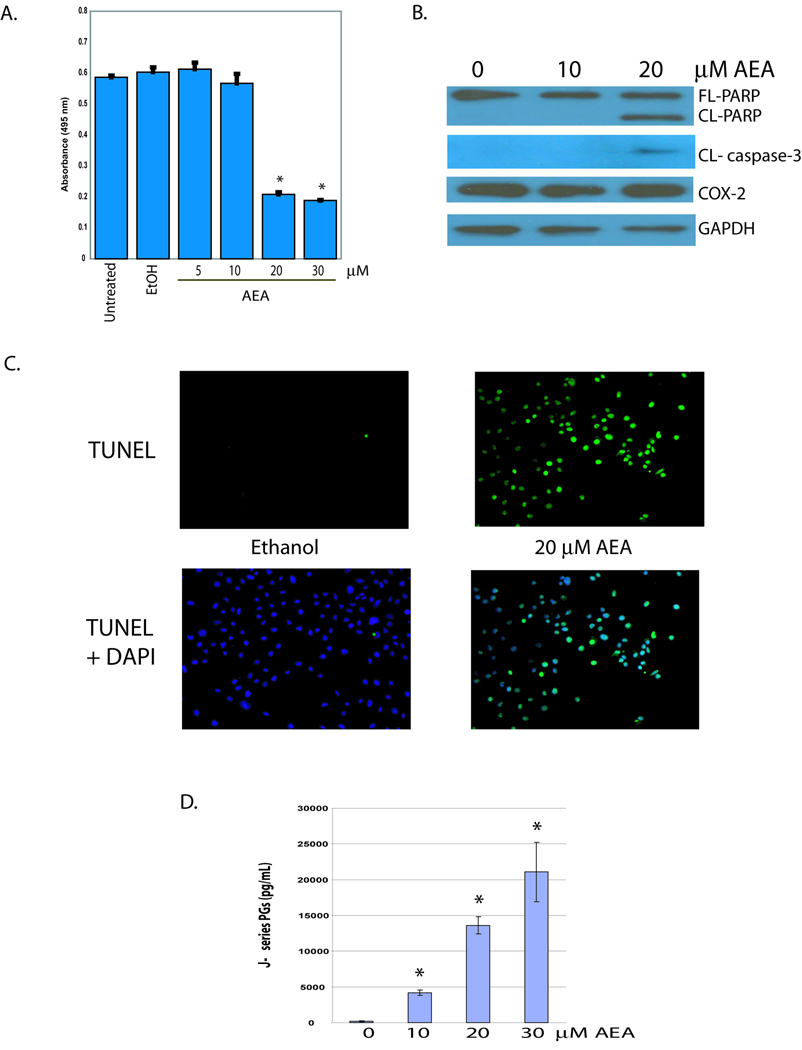
To confirm our previous finding that AEA induces cell death in JWF2 keratinocytes the cells were exposed to various concentrations of AEA or vehicle (ethanol) and viability measured with MTS experiments (Figure 1A). A significant decrease in survival was observed in cells treated with AEA in the range of 20 to 30 µM whereas exposure to 10 µM AEA or less had little impact on cell viability. Western blot analysis was then conducted to examine the cleavage of caspase-3 and PARP as a measure of the induction of apoptosis. In cells treated with 20 µM AEA caspase-3 and PARP cleavage was evident although 10 µM AEA or vehicle did not induce apoptosis (Figure 1B). We then confirmed that AEA induces apoptosis by measuring DNA fragmentation, another hallmark of apoptosis. Figure 1C shows that 20µM AEA significantly increases TUNEL positive cells compared to cells treated with ethanol verifying that AEA induces apoptosis.
Figure 1. AEA induces apoptosis and J-series PG synthesis in JWF2 keratinocytes.
(A). AEA decreases JWF2 cell viability. AEA was included in the culture medium of the cells at a final concentration of 5, 10, 20 or 30 µM AEA. As a negative control, cells remained untreated or were treated with the solvent for AEA (ethanol-EtOH). Cells were incubated for 18 hours and absorbance at 495 nm measured after the addition of MTS reagent to each well. (B). AEA induces apoptosis in COX-2 overexpressing, JWF2 cells. Full length PARP (FL-PARP), cleaved PARP (CL-PARP), and cleaved caspase-3 (CL-caspase-3) were measured in cells treated with 10 or 20 µM AEA for 6 hours. GAPDH expression was assessed as a loading control. (C). AEA induces DNA fragmentation in JWF2 keratinocytes. Cells were treated with 20 µM AEA or ethanol and TUNEL positive cells (TUNEL) or TUNEL positive cells with corresponding DAPI stain (TUNEL + DAPI) recorded (D). AEA-induces J-series PG production. J-series PG levels were measured in the culture medium of cells treated with various concentrations of AEA. An asterisks indicates that the amount of J-series PGs obtained from AEA-treated cells is statistically significantly different from the amount of J-series PGs obtained from ethanol-treated cells (* = P< 0.05).
In a previous study we showed that AEA induced the production of D-, E-, and F- series PGs in JWF2 cells which overexpress COX-2 [19]. However to the best of our knowledge, the induction of J-series PG synthesis by AEA, has not been demonstrated. Treatment of JWF2 keratinocytes with various concentrations of AEA produced a concentration-related increase in J-series PG production (Figure 1D). In contrast, J-series PGs were not detectible in cells treated with ethanol. These findings demonstrate that the induction of J-series PGs synthesis correlates with the induction of apoptosis by AEA.
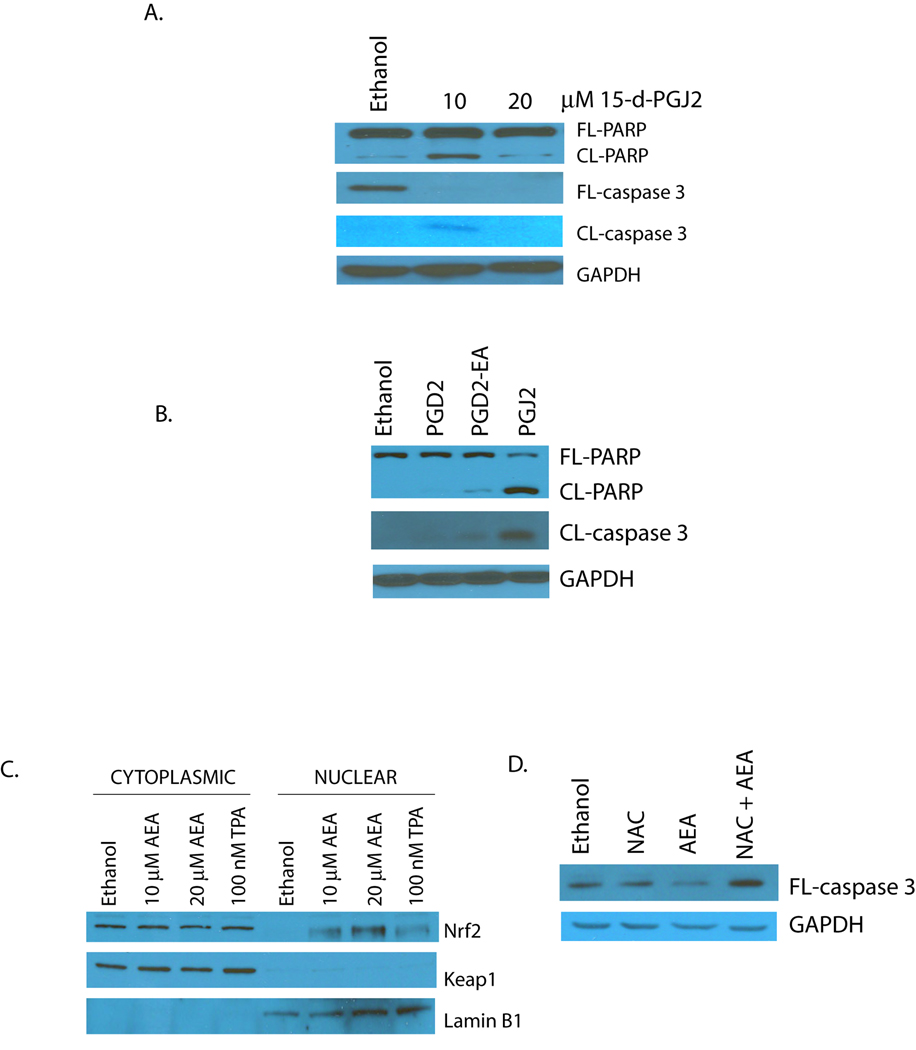
The bioactive lipids, 15-deoxy, Δ12,14 PGJ2 (15-d-PGJ2), PGJ2, PGD2 and PGD2-EA induce cell death in JWF2 keratinocytes [19]. To determine if these D- and J-series PGs induce cell death via apoptosis, the PGs were added to cultured JWF2 cells and cleavage of caspase-3 and PARP measured. As shown in Figure 2A, cell exposure to 15-d-PGJ2 at a final concentration of 10 and 20 µM induced a concentration-dependent increase in apoptosis. PGJ2 also induced apoptosis however little apoptosis was seen in cells treated with the D-series PGs (Figure 2B). This finding suggests that the predominant mechanism of cell death for J-series PGs is apoptosis while D-series PGs primarily initiate cell death through a distinct cellular pathway.
Figure 2. ROS regulates AEA-induced apoptosis.
(A). 15-d-PGJ2 induces apoptosis in JWF2 cells in a concentration-regulated manner. Cells were treated with 10 or 20 µM 15-d-PGJ2 for 3 hours and apoptosis assessed by measuring full-length PARP (FL-PARP), cleaved PARP (CL-PARP), full-length caspase-3 (FL-caspase-3) and cleaved caspase-3 (CL-caspase-3). (B). J-series PGs induce apoptosis in tumor-derived keratinocytes. PGD2, PGD2-EA, and PGJ2 were included in the cell culture medium of JWF2 cells at a final concentration of 20 µM and apoptosis measured by examining PARP and caspase-3 cleavage in Western blot experiments. (C). AEA-induces ROS production. JWF2 cells were treated with various concentrations of AEA, 100 nM TPA (positive control) or ethanol (negative control) and nuclear and cytoplasmic cellular fractions isolated. The subcellular content of Nrf-2 and Keap1 were examined by Western blot analysis. Expression level of the nuclear marker lamin B1 was also monitored. (D). AEA-induced apoptosis is dependent upon ROS generation. JWF2 cells were pretreated with 50 mM N-acetylcysteine (NAC) followed by cell exposure to 20 µM AEA or ethanol. Apoptosis was then measured by evaluating the level of FL-caspase-3.
Numerous studies have determined that J-series PG-mediated apoptosis is regulated by its ability to promote ROS production [25;28] therefore, we were interested in determining if ROS was required for AEA-induced apoptosis. The generation of ROS is often detected by measuring activation of Nrf-2, a transcription factor which translocates from the cytoplasm to the nucleus in the presence of ROS in order to induce transcription of neutralizing antioxidant genes [29]. To determine if AEA induced ROS production in JWF2 keratinocytes the cells were treated with AEA, subcellular fractionation conducted, and nuclear Nrf-2 expression examined. In Figure 2C we show that AEA induced Nrf2 nuclear localization whereas Nrf-2 was sequestered in the cytoplasm of ethanol treated cells. As a positive control, the cells were exposed to TPA, a known inducer of ROS and Nrf2 nuclear localization was noted as well. Next, we exposed AEA-treated cells to the thiol antioxidant N-acetyl cysteine (NAC) to determine whether ROS production was required for the induction of apoptosis. As shown in Figure 2D, NAC blocked the initiation of apoptosis mediated by AEA. These results suggest that the J-series PGs derived from AEA induce apoptosis via the generation of oxidative stress.
Cannabinoid receptors are not required for AEA-induced cell death
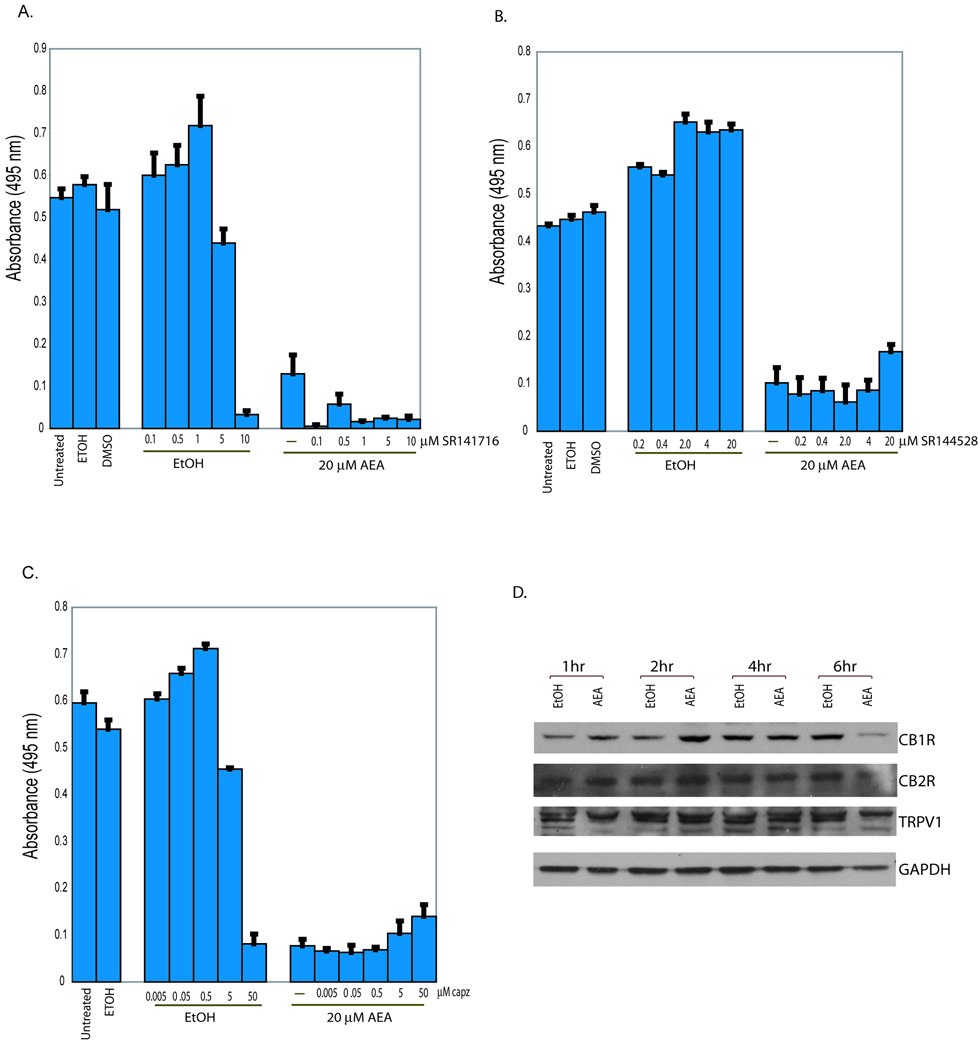
The cytotoxic effects of AEA are reported to be mediated by ECS-dependent and ECS-independent mechanisms [10–12;16]. To determine if the ECS was required for AEA-mediated cytotoxicity in JWF2 keratinocytes, cells were treated with selective CB1R (SR141716), CB2R (SR144528) or TRPV1 (capsazepine) antagonists and then exposed to AEA (Figure 3). As we observed previously, 20 µM AEA dramatically decreased JWF2 cell viability. Concurrent treatment with AEA and SR141716 produced a further decrease in cell viability (Figure 3A). We then examined whether CB2R was required for AEA cytotoxicity and observed a slight, but statistically insignificant reversal of AEA cytotoxicity induced by SR144528 (Fig. 3B). The consequence of TRPV1 channel inactivation on AEA-induced cell death was then investigated using capsazepine (Fig. 3C). Similar to our observation with the CB2R antagonist, an insignificant reversal in AEA-induced cytotoxicity was seen. Next, we examined the expression of CB1R, CB2R and TRPV1 to determine if the lack of reversal of AEA-induced cell death could be explained by alterations in receptor protein expression. Compared to time-matched vehicle treated cells, AEA induced an increase in CB1R expression within 2 hours of exposure followed by a leveling of protein expression at 4 hours (Fig. 3D). The expression of CB2R or TRPV1 in cells treated with AEA was not changed compared to cells treated with ethanol. The cellular levels of CB1R, CB2R and TRPV in AEA-exposed cells were decreased after 6 hours of exposure most likely due to the induction of cell death and the accompanying decrease in total cellular protein content. Since AEA did not decrease cannabinoid receptor expression and selective antagonist were unable to prevent AEA-induced cell death our results suggest that CB1R, CB2R or TRPV1 receptors are not required for AEA-mediated cell death in tumorigenic keratinocytes.
Figure 3. Cannabinoid receptors do not regulate AEA-induced cell death in JWF2 keratinocytes.
(A). AEA-induces cell death in a cannabinoid receptor-independent manner. Cells were pretreated with of 0.1, 0.5. 1.0, 5.0 or 10 µM SR141716 in the presence (20µM AEA) or absence (EtOH) of AEA. (B) Cells were pretreated with the CB2R antagonist, SR144528 at a final concentration of 0.2, 0.4, 2.0, 4.0, or 20 µM in the presence (20µM AEA) or absence (EtOH) of AEA. (C) Cells were pretreated with the TRPV1 channel inhibitor, capsazepine at a final concentration of 0.005, 0.05, 0.5, 5, and 50 µM in the presence (20µM AEA) or absence (EtOH) of AEA. As a negative control, cells remained untreated or were treated with the appropriate vehicle (ethanol or DMSO). Cells were incubated for 18 hours and viability measured in MTS experiments. The viability of cells treated with AEA plus the receptor antagonists was not statistically significantly different from cells treated with AEA alone. (D). Effect of AEA on cannabinoid receptor expression. JWF2 keratinocytes were treated with 20 µM AEA or ethanol and cells harvested at 1, 2, 4, and 6 hours. Cellular levels of CB1R, CB2R and TRPV1 were determined by conducting Western blot analysis.
Inhibition of fatty acid amide hydrolase (FAAH) increases AEA-induced apoptosis
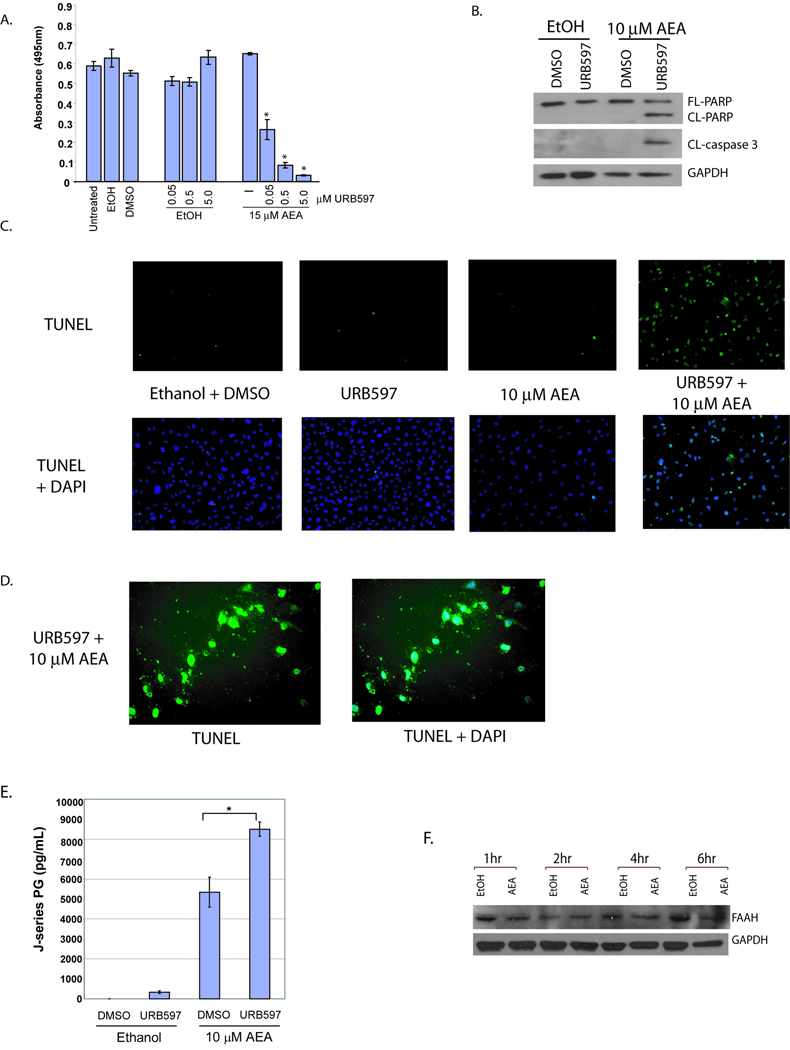
AEA is metabolized by FAAH to arachidonic acid plus ethanolamine [30] and AEA is also metabolized by COX-2 to prostaglandin-ethanolamides [17;18]. Therefore, the inhibition of AEA degradation with a FAAH inhibitor should increase the amount of AEA that is available for metabolism by COX-2 thus increasing J-series PGs synthesis and apoptosis. To test this, FAAH activity was blocked with URB597, cells treated with AEA, and the effect on cell viability observed (Figure 4A). Inclusion of various amounts of URB597 in the culture medium of cells treated with a sublethal concentration of AEA induced a concentration-dependent increase in cytotoxicity. In contrast, a decrease in viability was not observed in cells treated with URB597 alone. Next, we sought to determine if the URB597-mediated enhancement of AEA-induced cell death occurred via the apoptotic pathway. As shown in Figure 4B, FAAH inhibition enhances apoptosis when combined with a sublethal concentration of AEA. We also conducted TUNEL analysis and show in Figure 4C that concurrent treatment with a sublethal concentration of AEA and URB597 induced DNA fragmentation (Figure 4C & 4D). To determine if J-series PG production correlated with the FAAH-regulated enhancement in AEA-induced apoptosis J-series PG levels were measured in cells treated with AEA or AEA plus URB597. A statistically significant difference in J-series PG levels was seen in JWF2 keratinocytes treated with AEA plus URB597 compared to cells exposed to AEA alone (Figure 4E). Finally, we examined FAAH protein levels in AEA-treated cells and determined that AEA had little effect on its expression indicating that the enhancement of AEA-mediated toxicity was not due to alterations in FAAH expression (Figure 4F). Because the inhibition of FAAH significantly increases the production of J-series PGs and apoptosis in the presence of AEA, our findings suggest that the co-administration of these agents may produce potent antitumor activity.
Figure 4. FAAH inhibition enhances AEA-induced J-series PG synthesis and apoptosis.
(A). FAAH inhibition increases AEA-induced cell death. JWF2 keratinocytes were pretreated with 0.05, 0.5 or 5 µM URB597 or DMSO (URB597 vehicle) for 30 minutes and the cells exposed to 15 µM AEA or ethanol. Cell viability was measured by MTS assay as described previously. Asterisk indicates that the viability of URB597 + AEA-treated cells is statistically significantly different from the viability of cells treated with AEA alone (* = P ≤ 0.05). (B). FAAH inhibition increases AEA-induced apoptosis. Cells were pretreated with 5 µM URB and then exposed to 10 µM AEA or ethanol (AEA vehicle). Protein levels of FL-PARP, CL-PARP, CL-caspase-3 and GAPDH were measured by Western analysis. (C). Co-administration of AEA with the FAAH inhibitor URB597 induces DNA fragmentation. Cells were treated with vehicle (Ethanol + DMSO), URB597, sublethal AEA, or URB597 + sublethal AEA and TUNEL positive cells or TUNEL with corresponding DAPI stain visualized by fluorescence microscopy at 10X (C) or 40X (D) magnification. (E). URB597 enhances AEA-induced J-series PG synthesis. J-series PG levels were measured by ELISA in the culture medium of cells pretreated with 5 µM URB597 or DSMO followed by cell exposure to 10 µM AEA or ethanol. (F). JWF2 cells were treated with 20 µM AEA or an equivalent volume of ethanol for 1, 2, 4, or 6 hours and FAAH protein expression measured The multiple TRPV1 bands likely represent slice variants as reported previously [41].
DISCUSSION
AEA induces apoptosis in many cell types however the mechanism underlying this effect is unclear. In the present communication, we determined that cell exposure to AEA increases J-series prostaglandin production and that J-series PGs induce apoptosis (Figures 1 & 2). By pretreating cells with the antioxidant NAC we found that the proapoptotic activity of AEA was dependent upon the generation of oxidative stress (Figure 2). We also showed that AEA-mediated J-series PG production and apoptosis was regulated by FAAH (Figure 4). In contrast, cell surface cannabinoid receptors were not required for AEA-induced cell death (Figure 3). These results indicate that AEA is metabolized to J-series PGs which induce apoptosis in a ROS-dependent manner (see Figure 5 for proposed mechanism). Our data also suggest that AEA or other endocannabinoids could be exploited for therapeutic use.
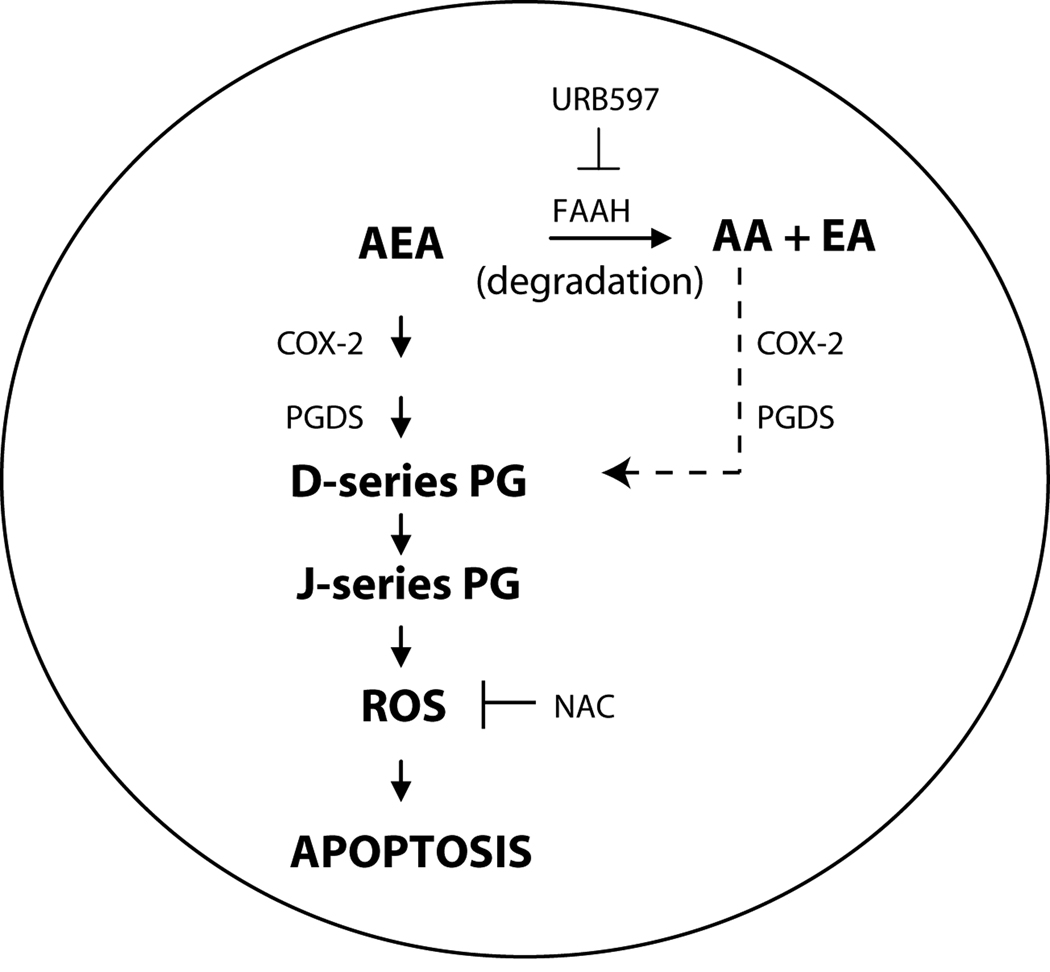
Figure 5. Proposed mechanism of AEA-induced apoptosis in tumorigenic keratinocytes.
We propose that in tumor cells which overexpress COX-2, AEA is metabolized by COX-2 followed by prostaglandin D synthase (PGDS) to D-series PGs which are subsequently dehydrated to J-series PGs. J-series prostaglandins then induce the generation of ROS which promotes the induction of apoptosis. Also, the arachidonic acid released from the metabolism of AEA by FAAH may contribute to J-series PG production and apoptosis (dashed arrowhead). In addition, our data show that inhibiting AEA degradation with the FAAH inhibitor URB597 enhances the apoptotic activity of AEA by increasing the synthesis of J-series PGs. Because COX-2 is not overexpressed in normal keratinocytes, we anticipate that AEA will selectively induce apoptosis in tumor cells.
As a class, cannabinoids have shown tremendous potential to inhibit tumor growth [2]. The development of cannabinoids for clinical use has been limited partly because cannabinoids are best known as the active component in marijuana. However, several cannabinoids are used clinically with minimal adverse effects reported. For example, the cannabinoids nabilone and dronabinol are currently approved for use in the US for chemotherapy-induced emesis [31]. In Canada and Europe, the cannabinoid receptor agonist Sativex® is approved for treatment of neuropathic pain associated with multiple sclerosis [32]. In addition, the synthetic prostaglandin-ethanolamide analog, Bimatoprost, is one the most potent drugs available for treatment of glaucoma [33]. Therefore, we propose that the endocannabinoid AEA (or AEA analogues) may also be useful for treatment of tumors that overexpress COX-2. However, caution must be observed in developing cannabinoids as therapeutic agents in light of the withdrawal of the CB1R antagonist Rimonabant from the market due to adverse neurological effects [34]. Nevertheless, cannabinoids are slowly gaining acceptance as bona fide pharmacological agents and our goal in this study was to determine the mechanism of AEA’s cytotoxic activity in order to begin to assess the feasibility of developing endocannabinoids as novel chemotherapeutic agents.
The endocannabinoid AEA is metabolized by COX-2 and the role of these metabolic products in AEA-induced apoptosis is a subject of increasing interest. For example, the combined effects of PGE2-EA and PGD2-EA were reported to regulate AEA-induced cell death in colorectal carcinoma cells [20]. Using cervical carcinoma cells Hinz and associates show that the AEA analogue, R(+)-Methanandamide [R(+)-MA] increased COX-2 expression and the production of cytotoxic PGD2 and PGE2 derived from arachidonic acid [21;35]. Our studies which were carried out in tumorigenic keratinocytes suggest that the J-series PGs are critical regulators of AEA-induced apoptosis. Although J-series PGs are synthesized from D-series PGs it is difficult to determine which of these PG(s) cause AEA-induced apoptosis because specific tools to inhibit J-series PGs or to distinguish between the activity of D- and J- PGs are currently not available. In order to determine which of these prostaglandins might initiate apoptosis we treated cells with exogenous D- and J-series PGs and observed that the primary mechanism of cell death for the J- but not the D-series prostaglandins was apoptotic. We also examined the regulation of AEA-induced apoptosis by ROS since J-series PGs are known to be potent inducers of oxidative stress compared to D-series PGs. Our data show that AEA induces ROS and that the antioxidant NAC blocked AEA-induced apoptosis (Figure 2). These combined data suggest that J-series PGs rather than the D-series PGs are the primary mediators of AEA-induced apoptosis.
The identity of the specific J-series PG(s) involved in AEA-regulated apoptosis is uncertain. To the best of our knowledge, ethanolamide-conjugated J-series PGs (PGJ-EAs) have not been characterized or described in the literature previously. The existence of PGJ-EA(s) is plausible because Kozak et. al., showed that AEA is metabolized by COX-2 to PGE2-EA, PGF2α-EA and PGD2-EA [17] and PGD2 is readily converted to J-series PGs [22;23]. In this study, J-series PGs were measured by ELISA analysis but because PGJ-EAs have not been described previously, it is unclear whether J-series PGs (PGJ2, 12Δ-PGJ2 or 15-d-PGJ2) or J-series PG-EAs (PGJ2-EA, 12Δ-PGJ2-EA or 15-d-PGJ2-EA) are being measured. ELISA kits designed to detect PGE2, PGF2α and PGD2 also detect PGE2-EA, PGF2α-EA and PGD2-EA respectively, therefore it is probable that the J-series ELISA kit used here also detects PGJ-EAs. Alternatively, the ELISA kit could be measuring the products of PGJ-EA hydrolysis to PGJ although studies by Matias et. al. [36] argue against this scenario. The use more powerful analytical tools to identify the specific J-series PGs involved in AEA-induced apoptosis is currently being used by our group to fully address this important issue.
Although the identity of the specific J-series PG(s) produced in this study is unclear numerous studies have determined that the cytotoxic activity of the J-series PGs is mediated by its reactive group. Ciucci et al., determined that the reactive cyclopentanone ring in 15-d-PGJ2 inhibits IKB kinase (IKK beta) thus blocking NF-κB signaling and inducing apoptosis in ER-negative breast cancer cells [37]. Cocca and associates showed that the reactive group in 15-d-PGJ2 interacts with α- and β- tubulin subunits inducing microtubule depolymerization and cell cycle arrest in MCF-7 breast cancer cells [24]. Neutralization of the reactive group in J-series PGs with antioxidants also abolished its pro-apoptotic activity in HL-60 leukemia cells [25]. Further, Kondo et. al., determined that the J-series PGs induced the production of oxidation products such as acrolein and 4-hydroxy-2-noneal (HNE) and decreased the activity of antioxidants including glutathione and glutathione peroxidase [28]. Unfortunately, few studies have examined the effect of the J-series PGs on tumor development and the reports that are available contain contradictory findings. For example, Clay et. al., showed that 15-d-PGJ2 induced apoptosis and blocked tumorigenesis of breast cancer cells [38]. In another report from Millan and colleagues, apoptosis was not induced by 15-d-PGJ2 and an increase in DMBA/TPA-mediated skin tumorigenesis occurred when 15-d-PGJ2 was applied topically during the tumor initiation phase [39]. The differences in these reports are likely to be due to the concentration and source of J-series PGs used. Levonen et. al. found that low concentrations of 15-d-PGJ2 were cytoprotective while high concentrations of 15-d-PGJ2 were pro-apoptotic [40]. These findings emphasize the importance of achieving high levels of J-series PG production and suggest that co-administration of FAAH inhibitors and AEA will be important for achieving tumor cell death.
CB1R, CB2R or the TRPV channel have been shown to regulate AEA-induced apoptosis in many studies. A recent report indicated that CB1R controls AEA-induced apoptosis by modulating the expression of BiP (GRP78) in neuroblastoma cells [11]. In hyperplastic cholangiocytes, AEA-induced apoptosis required CB2R-dependent activation of thioredoxin 1/ redox factor1 and AP-1 [15]. In neuroblastoma cells, the TRPV1 channel was also reported to regulate apoptosis induced by AEA in a process that required an increase in intracellular Ca2+ [10]. In contrast, we observed that CB1R, CBR2 and TRPV1 were not required for AEA-induced cell death. Our measurement of the receptor levels in AEA-treated cells showed that AEA transiently increased CB1R but had little impact on the expression of CB2R or TRPV1. These data suggest that the inability to inhibit AEA-induced cell death with selective receptor antagonists was not caused by receptor down-regulation but that these receptors are not required for the induction of cell death by AEA. Thus, AEA-induced cell death is a complex process that likely occurs in a cell-type and context-specific manner.
Another ECS component FAAH metabolizes AEA to arachidonic acid plus ethanolamine and thus is a major regulator of the cellular effects of AEA. Studies by Weber et al. demonstrated that the levels and effects of AEA were elevated in FAAH−/− but not in FAAH +/+ mice [30]. Inhibitors of endocannabinoid catabolism also blocked tumor cell growth in vivo and in vitro [14]. In addition, Siegmund and colleagues showed that AEA-induced apoptosis was enhanced by FAAH inhibition and inhibited by FAAH overexpression in hepatic stellate cells [13]. We also examined the consequence of blocking AEA degradation and our evidence show that FAAH blockade leads to increased J-series PG production and cellular apoptosis (Figure 4B & 4C). These studies suggest that a reasonable pharmacological strategy to eliminate tumor cells could include the co-administration of AEA and FAAH inhibitors.
Acknowledgements
This work was supported by NIH grant CA098685 (to RVD).
Abbreviations
- Δ9-THC
Δ9-tetrahydrocannabinol
- AEA
arachidonoyl ethanolamide
- 2-AG
2-arachidonoyl glycerol
- AMT
anandamide membrane transporter
- CB1R
cannabinoid receptor 1
- CB2R
cannabinoid receptor 2
- COX-2
cyclooxygenase-2
- FAAH
fatty acid amide hydrolase
- GPR55
G-protein-coupled receptor 55
- NMSC
non-melanoma skin cancer
- PGDS
prostaglandin D synthase
- TPA
12-O-tetradecanoyl phorbol-13-acetate
- TRPV1
transient receptor potential cation channel, subfamily V, member 1
REFERENCES
- 1.Di MV. 'Endocannabinoids' and other fatty acid derivatives with cannabimimetic properties: biochemistry and possible physiopathological relevance. Biochim.Biophys.Acta. 1998;1392:153–175. doi: 10.1016/s0005-2760(98)00042-3. [DOI] [PubMed] [Google Scholar]
- 2.Oesch S, Gertsch J. Cannabinoid receptor ligands as potential anticancer agents--high hopes for new therapies? J.Pharm.Pharmacol. 2009;61:839–853. doi: 10.1211/jpp/61.07.0002. [DOI] [PubMed] [Google Scholar]
- 3.Montecucco F, Lenglet S, Braunersreuther V, Burger F, Pelli G, Bertolotto M, Mach F, Steffens S. CB(2) cannabinoid receptor activation is cardioprotective in a mouse model of ischemia/reperfusion. J.Mol.Cell Cardiol. 2009;46:612–620. doi: 10.1016/j.yjmcc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Martin WJ, Lai NK, Patrick SL, Tsou K, Walker JM. Antinociceptive actions of cannabinoids following intraventricular administration in rats. Brain Res. 1993;629:300–304. doi: 10.1016/0006-8993(93)91334-o. [DOI] [PubMed] [Google Scholar]
- 5.Melck D, Rueda D, Galve-Roperh I, De PL, Guzman M, Di MV. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999;463:235–240. doi: 10.1016/s0014-5793(99)01639-7. [DOI] [PubMed] [Google Scholar]
- 6.Gomez del PT, Velasco G, Guzman M. The CB1 cannabinoid receptor is coupled to the activation of protein kinase B/Akt. Biochem.J. 2000;347:369–373. doi: 10.1042/0264-6021:3470369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc.Natl.Acad.Sci.U.S.A. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans RM, Scott RH, Ross RA. Multiple actions of anandamide on neonatal rat cultured sensory neurones. Br.J.Pharmacol. 2004;141:1223–1233. doi: 10.1038/sj.bjp.0705723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 10.Maccarrone M, Lorenzon T, Bari M, Melino G, Finazzi-Agro A. Anandamide induces apoptosis in human cells via vanilloid receptors. Evidence for a protective role of cannabinoid receptors. J.Biol.Chem. 2000;275:31938–31945. doi: 10.1074/jbc.M005722200. [DOI] [PubMed] [Google Scholar]
- 11.Pasquariello N, Catanzaro G, Marzano V, Amadio D, Barcaroli D, Oddi S, Federici G, Urbani A, Finazzi AA, Maccarrone M. Characterization of the endocannabinoid system in human neuronal cells and proteomic analysis of anandamide-induced apoptosis. J.Biol.Chem. 2009;284:29413–29426. doi: 10.1074/jbc.M109.044412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casanova ML, Blazquez C, Martinez-Palacio J, Villanueva C, Fernandez-Acenero MJ, Huffman JW, Jorcano JL, Guzman M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J.Clin.Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siegmund SV, Seki E, Osawa Y, Uchinami H, Cravatt BF, Schwabe RF. Fatty acid amide hydrolase determines anandamide-induced cell death in the liver. J.Biol.Chem. 2006;281:10431–10438. doi: 10.1074/jbc.M509706200. [DOI] [PubMed] [Google Scholar]
- 14.Bifulco M, Laezza C, Valenti M, Ligresti A, Portella G, Di MV. A new strategy to block tumor growth by inhibiting endocannabinoid inactivation. FASEB J. 2004;18:1606–1608. doi: 10.1096/fj.04-1754fje. [DOI] [PubMed] [Google Scholar]
- 15.DeMorrow S, Glaser S, Francis H, Venter J, Vaculin B, Vaculin S, Alpini G. Opposing actions of endocannabinoids on cholangiocarcinoma growth: recruitment of Fas and Fas ligand to lipid rafts. J.Biol.Chem. 2007;282:13098–13113. doi: 10.1074/jbc.M608238200. [DOI] [PubMed] [Google Scholar]
- 16.Patsos HA, Greenhough A, Hicks DJ, Al KM, Collard TJ, Lane JD, Paraskeva C, Williams AC. The endogenous cannabinoid, anandamide, induces COX-2-dependent cell death in apoptosis-resistant colon cancer cells. Int.J.Oncol. 2010;37:187–193. doi: 10.3892/ijo_00000666. [DOI] [PubMed] [Google Scholar]
- 17.Kozak KR, Crews BC, Morrow JD, Wang LH, Ma YH, Weinander R, Jakobsson PJ, Marnett LJ. Metabolism of the endocannabinoids, 2-arachidonylglycerol and anandamide, into prostaglandin, thromboxane, and prostacyclin glycerol esters and ethanolamides. J.Biol.Chem. 2002;277:44877–44885. doi: 10.1074/jbc.M206788200. [DOI] [PubMed] [Google Scholar]
- 18.Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J.Biol.Chem. 1997;272:21181–21186. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- 19.Van Dross RT. Metabolism of Anandamide by COX-2 Is Necessary for Endocannabinoid-induced Cell Death in Tumorigenic Keratinocytes. Mol.Carcinog. 2009;48:724–732. doi: 10.1002/mc.20515. [DOI] [PubMed] [Google Scholar]
- 20.Patsos HA, Hicks DJ, Dobson RR, Greenhough A, Woodman N, Lane JD, Williams AC, Paraskeva C. The endogenous cannabinoid, anandamide, induces cell death in colorectal carcinoma cells: a possible role for cyclooxygenase 2. Gut. 2005;54:1741–1750. doi: 10.1136/gut.2005.073403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eichele K, Ramer R, Hinz B. R(+)-methanandamide-induced apoptosis of human cervical carcinoma cells involves a cyclooxygenase-2-dependent pathway. Pharm.Res. 2009;26:346–355. doi: 10.1007/s11095-008-9748-3. [DOI] [PubMed] [Google Scholar]
- 22.Fukushima M, Kato T, Ota K, Arai Y, Narumiya S, Hayaishi O. 9-deoxy-delta 9-prostaglandin D2, a prostaglandin D2 derivative with potent antineoplastic and weak smooth muscle-contracting activities. Biochem.Biophys.Res Commun. 1982;109:626–633. doi: 10.1016/0006-291x(82)91986-6. [DOI] [PubMed] [Google Scholar]
- 23.Uchida K, Shibata T. 15-Deoxy-delta(12, 14)-prostaglandin J2: An electrophilic trigger of cellular responses. Chem.Res.Toxicol. 2007;21:138–144. doi: 10.1021/tx700177j. [DOI] [PubMed] [Google Scholar]
- 24.Cocca C, Dorado J, Calvo E, Lopez JA, Santos A, Perez-Castillo A. 15-Deoxi-Delta(12, 14)-prostaglandin J(2) is a tubulin-binding agent that destabilizes microtubules and induces mitotic arrest. Biochem.Pharmacol. 2009 doi: 10.1016/j.bcp.2009.06.100. [DOI] [PubMed] [Google Scholar]
- 25.Chen YC, Shen SC, Tsai SH. Prostaglandin D(2) and J(2) induce apoptosis in human leukemia cells via activation of the caspase 3 cascade and production of reactive oxygen species. Biochim.Biophys.Acta. 2005;1743:291–304. doi: 10.1016/j.bbamcr.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Ward C, Dransfield I, Murray J, Farrow SN, Haslett C, Rossi AG. Prostaglandin D2 and its metabolites induce caspase-dependent granulocyte apoptosis that is mediated via inhibition of I kappa B alpha degradation using a peroxisome proliferator-activated receptor-gamma-independent mechanism. J.Immunol. 2002;168:6232–6243. doi: 10.4049/jimmunol.168.12.6232. [DOI] [PubMed] [Google Scholar]
- 27.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J.Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 28.Kondo M, Oya-Ito T, Kumagai T, Osawa T, Uchida K. Cyclopentenone prostaglandins as potential inducers of intracellular oxidative stress. J.Biol.Chem. 2001;276:12076–12083. doi: 10.1074/jbc.M009630200. [DOI] [PubMed] [Google Scholar]
- 29.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J.Biol.Chem. 2000;275:16023–16029. doi: 10.1074/jbc.275.21.16023. [DOI] [PubMed] [Google Scholar]
- 30.Weber A, Ni J, Ling KH, Acheampong A, Tang-Liu DD, Burk R, Cravatt BF, Woodward D. Formation of prostamides from anandamide in FAAH knockout mice analyzed by HPLC with tandem mass spectrometry. J.Lipid Res. 2004;45:757–763. doi: 10.1194/jlr.M300475-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.McQuaid Kenneth R. Drugs Used in the Treatment of Gastrointestinal Diseases. 2010 p http://www.accessmedicine.com/context/aspx?aID=4514056.
- 32.Health Canada Drug Product Database. 2010 http://www.hc-sc.gc.ca.
- 33.Woodward DF, Carling RW, Cornell CL, Fliri HG, Martos JL, Pettit SN, Liang Y, Wang JW. The pharmacology and therapeutic relevance of endocannabinoid derived cyclo-oxygenase (COX)-2 products. Pharmacol.Ther. 2008;120:71–80. doi: 10.1016/j.pharmthera.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Boekholdt SM, Peters RJ. Rimonabant: obituary for a wonder drug. Lancet. 2010;376:489–490. doi: 10.1016/S0140-6736(10)61080-X. [DOI] [PubMed] [Google Scholar]
- 35.Hinz B, Ramer R, Eichele K, Weinzierl U, Brune K. Up-regulation of cyclooxygenase-2 expression is involved in R(+)-methanandamide-induced apoptotic death of human neuroglioma cells. Mol.Pharmacol. 2004;66:1643–1651. doi: 10.1124/mol.104.002618. [DOI] [PubMed] [Google Scholar]
- 36.Matias I, Chen J, De PL, Bisogno T, Ligresti A, Fezza F, Krauss AH, Shi L, Protzman CE, Li C, Liang Y, Nieves AL, Kedzie KM, Burk RM, Di MV, Woodward DF. Prostaglandin ethanolamides (prostamides): in vitro pharmacology and metabolism. J.Pharmacol.Exp.Ther. 2004;309:745–757. doi: 10.1124/jpet.103.061705. [DOI] [PubMed] [Google Scholar]
- 37.Ciucci A, Gianferretti P, Piva R, Guyot T, Snape TJ, Roberts SM, Santoro MG. Induction of apoptosis in estrogen receptor-negative breast cancer cells by natural and synthetic cyclopentenones: role of the IkappaB kinase/nuclear factor-kappaB pathway. Mol.Pharmacol. 2006;70:1812–1821. doi: 10.1124/mol.106.025759. [DOI] [PubMed] [Google Scholar]
- 38.Clay CE, Namen AM, Atsumi G, Willingham MC, High KP, Kute TE, Trimboli AJ, Fonteh AN, Dawson PA, Chilton FH. Influence of J series prostaglandins on apoptosis and tumorigenesis of breast cancer cells. Carcinogenesis. 1999;20:1905–1911. doi: 10.1093/carcin/20.10.1905. [DOI] [PubMed] [Google Scholar]
- 39.Millan O, Rico D, Peinado H, Zarich N, Stamatakis K, Perez-Sala D, Rojas JM, Cano A, Bosca L. Potentiation of tumor formation by topical administration of 15-deoxy-delta12, 14-prostaglandin J2 in a model of skin carcinogenesis. Carcinogenesis. 2006;27:328–336. doi: 10.1093/carcin/bgi213. [DOI] [PubMed] [Google Scholar]
- 40.Levonen AL, Dickinson DA, Moellering DR, Mulcahy RT, Forman HJ, rley-Usmar VM. Biphasic effects of 15-deoxy-delta(12, 14)-prostaglandin J(2) on glutathione induction and apoptosis in human endothelial cells. Arterioscler.Thromb.Vasc.Biol. 2001;21:1846–1851. doi: 10.1161/hq1101.098488. [DOI] [PubMed] [Google Scholar]
- 41.Schumacher MA, Eilers H. TRPV1 splice variants: structure and function. Front Biosci. 2010;15:872–882. doi: 10.2741/3651. [DOI] [PMC free article] [PubMed] [Google Scholar]