Abstract
The process of erythropoiesis in the fetal liver and adult bone marrow is regulated by the hormone erythropoietin (Epo), which is produced in the kidney at low levels under homeostatic conditions. Defects in Epo production result in severe anemia; use of recombinant hormone has improved the lives of patients suffering from renal failure or anemia due to bone marrow suppression. Deletion of the Epo gene in mice leads to embryonic lethality at day 13–15, coincident with the establishment of definitive (adult-type) erythropoiesis and underscoring the absolute necessity of Epo function in vivo. Epo has proven to be a successful pharmaceutical agent, one of the early triumphs of recombinant protein technology. Due to its clinical importance, a great deal of attention has focused upon the molecular mechanisms of Epo-regulated erythropoiesis. This review highlights basic concepts of Epo signal transduction within the hematopoietic system, the major site of Epo action in vivo.
INTRODUCTION
Epo is present in low amounts in the circulation under homeostatic conditions while erythropoietic stress such as hypoxia or anemia can stimulate a dramatic increase in Epo production in the kidney, leading to a significant rise in circulating hormone amounts and subsequently increased erythropoiesis (Koury et al., 1989; Lacombe et al., 1988; reviewed in Krantz, 1991). Epo stimulates red blood cell production by binding and activating a high affinity receptor (EpoR) that is expressed predominantly on the surface of immature erythroid cells (Broudy et al., 1991). The EpoR is a member of the type I cytokine receptor superfamily, sharing specific structural motifs with other members of this receptor family including two extracellular immunoglobulin-like domains, four similarly spaced cysteine residues and the sequence WSXWS (Bazan, 1990; Cosman et al., 1990). Signal transduction through the EpoR is initiated by ligand binding, which induces a dimerization and/or reorientation of EpoR monomers within a dimeric receptor structure (Livnah et al., 1999; Philo et al., 1996; Syed et al., 1998; Watowich et al., 1994; Watowich et al., 1992), a process that remains poorly understood despite its clear importance in terms of developing Epo agonists. The general mechanisms used by EpoR to activate intracellular signal transduction pathways are shared by other members of the Type I and Type II cytokine receptor families, namely ligand-dependent oligomerization and/or structural reorientation of clustered receptor molecules (Hibi and Hirano, 1998). The predominant pathway activated by EpoR and other cytokine receptors is the Jak/STAT signaling cascade (Darnell et al., 1994; Darnell Jr., 1997; Hibi and Hirano, 1998). Jak tyrosine kinases are constitutively associated with the membrane-proximal regions of cytokine receptor intracellular domains and are activated upon ligand binding and receptor reorientation. The EpoR associates selectively with the Jak2 kinase (Witthuhn et al., 1993). Following EpoR activation, Jak2 phosphorylates tyrosine residues in the intracellular region of the EpoR, providing docking sites for signaling molecules with phosphotyrosine binding motifs, including the signal transducer and activator of transcription protein STAT5, which mediates the principal intracellular signaling pathway elicited by the EpoR (Cui et al., 2004; Socolovsky et al., 1999) (Fig. 1). Here, I will discuss EpoR structural features and mechanisms of EpoR signal transduction via Jak2 and STAT5 that regulate erythropoiesis.
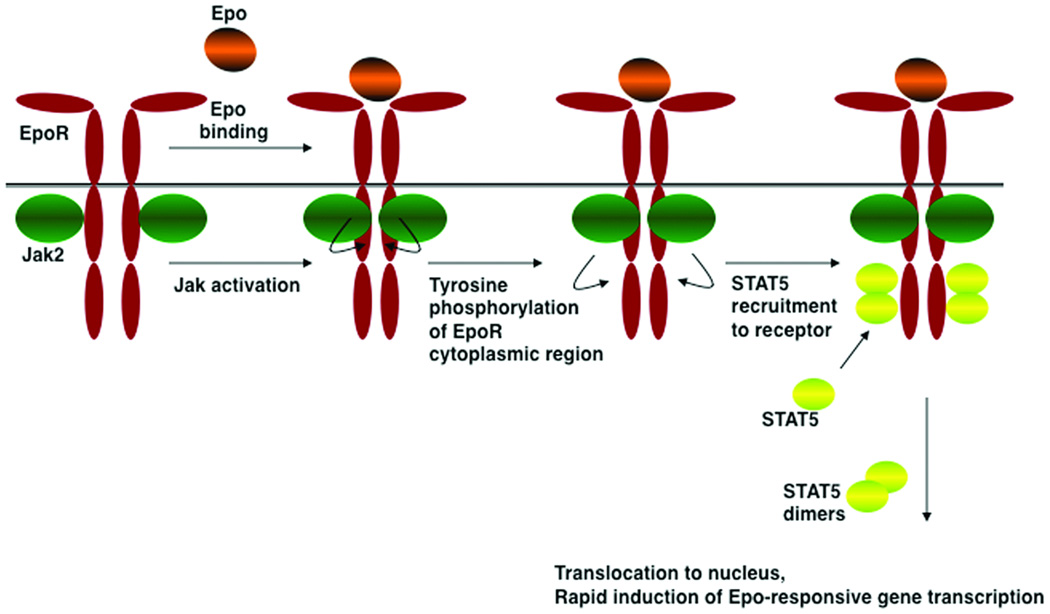
Figure 1. Model for EpoR activation mechanism.
The EpoR is thought to be expressed at the cell surface as a dimer in the absence of ligand. Epo binding proceeds by an asymmetrical association, first via the high affinity Epo site 1 followed by the lower affinity site 2 interaction (not shown), which stimulates a reorientation of EpoR monomers within the dimeric complex and subsequent activation of the receptor-associated Jak2 kinase, presumably by a transphosphorylation mechanism. Jak2 phosphorylates tyrosine residues in the EpoR cytoplasmic domain and itself, providing docking sites for molecules with phosphotyrosine binding motifs. STAT5 proteins are principal signaling molecules for EpoR, undergoing homodimerization after recruitment and phosphorylation by the receptor-Jak2 complex. STAT5 mediates erythroid survival, proliferation and differentiation signals. Additional signaling cascades are activated by the EpoR (not shown), however their role in erythropoiesis in vivo remains less understood relative to STAT5.
Role of dimerization in EpoR activation
The activation mechanism for EpoR was elucidated first through investigation of a constitutively acting (hormone-independent) form of the EpoR, which was isolated from a retroviral transduction screen in the interleukin-3-dependent murine cell line Ba/F3 by virtue of its ability to support cytokine-independent proliferation (Yoshimura et al., 1990). Initially, the biochemical basis for the constitutive activity was unclear, however a point mutation at residue 129 in the extracellular region that rendered an arginine to cysteine substitution (EpoR R129C) (Yoshimura et al., 1990) suggested the possibility that aberrant disulfide bond formation was involved. The constitutive activity of EpoR R129C is attributed to acquisition of cysteine and not loss of arginine (Watowich et al., 1992). Moreover, EpoR R129C but not the wild type receptor forms disulfide-linked homodimers in the absence of Epo (Watowich et al., 1994; Watowich et al., 1992). These data collectively implicate dimerization as an important feature of the EpoR activation mechanism. Studies with the constitutive EpoR provided a paradigm for the process of Type I and Type II cytokine receptor activation as it was subsequently recognized that oligomerization or structural reorientation of receptor subunits was a common activation mechanism within the cytokine receptor family (reviewed in Foxwell et al., 1992; Hibi and Hirano, 1998; Ihle et al., 1995).
Equilibrium binding experiments with iodinated Epo demonstrated that Epo:receptor complexes containing either wild type EpoR or EpoR R129C were governed by a single affinity (dissociation constant ∼100–700pM) (Broudy et al., 1991; Mayeux et al., 1987; Watowich et al., 1992), indicating that the three-dimensional structure of EpoR R129C is similar to the wild type receptor, at least within the Epo-binding domain. This suggested that covalent dimerization of EpoR R129C mimics the Epo/EpoR conformation, further supporting a role for receptor dimerization in the activation process. To determine whether the signals elicited by EpoR R129C are similar to or distinct from the ligand-occupied wild type receptor, the ability of EpoR R129C to support erythropoiesis was assessed. EpoR R129C stimulates Epo-independent colony forming unit-erythroid (CFU-E) development, as judged by ex vivo assays (Longmore et al., 1994; Pharr et al., 1993), indicating it supports erythroid proliferation and differentiation. Moreover, mice infected with retrovirus carrying EpoR R129C develop erythrocytosis and splenomegaly, and show increased amounts of circulating red blood cells (Longmore and Lodish, 1991; Longmore et al., 1993a), demonstrating that EpoR R129C stimulates expansion of the erythroid compartment in vivo and indicating deregulation of homeostatic mechanisms most likely due to constitutive signaling of EpoR R129C. Thus, EpoR R129C appears to mimic the biological activity of the Epo:EpoR complex in terms of directing proliferation and differentiation of red blood cell precursors, without perturbing the erythroid developmental program. Interesting, overexpression of EpoR R129C in hematopoietic progenitor cells can enhance the generation of other myeloid lineages (Longmore et al., 1993a; Longmore et al., 1994; Pharr et al., 1993), early evidence that suggested redundancy in the signal pathways between blood cell growth factors.
Several methods have been employed to test the hypothesis that the wild type EpoR is active as a dimer at the cell surface, upon ligand binding. Biochemical assays to probe for EpoR dimers using covalent cross-linking agents were largely unsuccessful (Watowich and Hilton, 1992). This is most likely due to the low cell surface expression of the receptor and specific features of the EpoR extracellular region such as the presence of only 3 lysine residues (K10, K14, K65 in the human; K10, K14, K64 in the murine EpoR) located distal to all binding interfaces (Livnah et al., 1996; Syed et al., 1998), which would be substrates for many of the cross-linkers available. However, studies with co-expressed wild type and carboxy-terminally truncated EpoRs demonstrated that mutant EpoRs exhibited dominant inhibitory activity, suggestive of interactions with the wild type EpoR that interfere with receptor signaling (Barber et al., 1994; Watowich et al., 1994). Moreover, EpoR dimerization by bivalent antibodies, analysis of chimeric receptor molecules or biochemical studies of the purified EpoR extracellular region further supported the idea that receptor clustering is an important step in the activation process (Elliot et al., 1996; Philo et al., 1996; Schneider et al., 1997; Watowich et al., 1999a). The most definitive evidence for receptor dimerization was obtained by three dimensional structure analyses of the EpoR extracellular region bound to Epo or erythropoietic peptide agonists (Livnah et al., 1996; Syed et al., 1998). The EpoR:Epo complex is a dimeric receptor occupied by a single Epo molecule in a 2:1 EpoR:Epo ratio (Syed et al., 1998). These data indicate the strong likelihood for a dimeric receptor structure as the principal EpoR signaling complex. Evidence from EpoR R129C metabolic pulse-chase labeling experiments, showing intracellular disulfide-linked dimers, as well as structural analyses of the EpoR extracellular region without bound ligand, suggest the EpoR dimer forms during receptor synthesis within the cell (Livnah et al., 1999; Watowich et al., 1992). Hence, dimeric EpoRs appear to be transported to the plasma membrane, where ligand activation induces a conformational change in the orientation of receptor subunits within the dimer.
The quaternary structure of the EpoR has important implications for hematopoiesis in humans as it suggests EpoR-mediated signal transduction could be altered in individuals with heterozygous mutation of the EpoR gene resulting from inherited or acquired events (Arcasoy et al., 1997; Arcasoy et al., 2002; de la Chapelle et al., 1993a; de la Chapelle et al., 1993b; Kralovics et al., 1997; Watowich et al., 1999b). Individuals from an extended Finnish family with dominant benign familial erythrocytosis provide such an example; fortunately in this instance the phenotype is mild. Certain individuals within this Finnish family were found to have enhanced erythrocytosis, accompanied by increased hematocrits and hemoglobin amounts (de la Chapelle et al., 1993a; de la Chapelle et al., 1993b). Erythroid progenitor cells isolated from these individuals demonstrate hypersensitivity to Epo in culture, as judged by their ability to undergo effective proliferation and differentiation in reduced Epo amounts ex vivo, compared to progenitor cells from unaffected individuals (de la Chapelle et al., 1993a; de la Chapelle et al., 1993b). The individuals exhibiting erythrocytosis possess a mutation in one copy of the EpoR gene, which generates a premature stop codon and truncated receptor isoform lacking approximately 70 amino acids from the carboxy-terminus (de la Chapelle et al., 1993b). This truncated EpoR is missing an important negative regulatory region in the cytoplasmic domain that is responsible for recruitment of hematopoietic cell phosphatase 1, which has been shown to suppress signaling from the EpoR as well as other cytokine receptors upon its association with activated receptor complexes (D'Andrea et al., 1991; Jiao et al., 1997; Klingmuller et al., 1995; Tapley et al., 1997; Yi et al., 1993). Assuming both wild type and mutant EpoR alleles are co-expressed in individuals with erythrocytosis, these individuals may express different EpoR complexes versus those with only wild type EpoR. The EpoR complexes may include homodimers of the truncated EpoR and heterodimers of wild type and mutant EpoRs, which may alter receptor signal transduction resulting in hypersensitivity to Epo and mild erythrocytosis. Co-expression of wild type and truncated EpoRs in tissue culture cells validated this model. Cytokine-dependent cells that were engineered to express both wild type and truncated EpoRs mimicking the Finnish mutation, or a similarly truncated EpoR expressed in affected members of a Swedish family with dominant erythrocytosis, exhibited enhanced Epo-mediated signal transduction and cellular proliferation compared to cells expressing only wild type EpoR (Watowich et al., 1999b). In both Finnish and Swedish families, the EpoR mutation appears to be inherited with Mendelian frequencies (de la Chapelle et al., 1993a; de la Chapelle et al., 1993b; Watowich et al., 1999b). Moreover, the EpoR mutation functions in a dominant manner relative to the wild type allele of the EpoR gene (de la Chapelle et al., 1993a; de la Chapelle et al., 1993b; Watowich et al., 1999b). These data are collectively indicative of association between co-expressed mutant and wild type EpoRs and subsequent enhancement of EpoR signal transduction.
Molecular structure of the EpoR
The three-dimensional structure of the EpoR extracellular region was first determined at 2.8Å resolution in complex with the agonist peptide EMP1 (Livnah et al., 1996), and later in complex with Epo at 1.9Å resolution (Syed et al., 1998). Both approaches showed the EpoR extracellular region comprises two immunoglobulin-like domains, each formed by a β-sandwich-like structure containing 7 β-strands. The membrane-distal (D1) domain and membrane-proximal (D2) domain are linked by a short hinge, and are oriented at approximately 90° to one another (Fig. 1). The D1 domain contains the four conserved cysteine residues, which form two intramolecular disulfide bridges that stabilize D1 (Livnah et al., 1996; Syed et al., 1998), while D2 contains the conserved WSXWS motif. As predicted from saturation mutagenesis experiments of the EpoR WSXWS motif, this motif appears to stabilize the EpoR tertiary structure (Hilton et al., 1994; Livnah et al., 1996; Syed et al., 1998).
The EpoR:Epo complex revealed that Epo has two discrete binding sites for the EpoR (Syed et al., 1998). One binding interface of the ligand governs a high affinity interaction with the receptor, comprising a hydrophobic core surrounded by hydrophilic residues, a motif that has been referenced as a “hot-spot” in terms of directing cytokine:cytokine receptor interactions (Clackson and Wells, 1995). The high affinity site exhibits a dissociation constant of approximately 1nM and is thought to contribute the majority of the ligand binding energy (Syed et al., 1998). A second binding site that utilizes a distinct set of determinant residues on Epo as well as EpoR has an affinity approximately 1000-fold lower (dissociation constant of ∼1µM) (Philo et al., 1996). Thus a sequential binding model for ligand-mediated activation of EpoR has been proposed, in which ligand interacts first via the high affinity site with one receptor chain and then through the low affinity interaction with the second EpoR monomer (Matthews et al., 1996). Despite the asymmetry of this complex, both monomers appear to be functionally similar in terms of activating signal transduction (Zhang et al., 2009). Moreover, interactions between the transmembrane and membrane-proximal cytoplasmic domains of EpoR monomers facilitate dimerization and/or stabilization of the EpoR dimeric complex (Constantinescu et al., 2001; Gurezka et al., 1999; Kubatzky et al., 2001; Seubert et al., 2003). The importance of the asymmetric complex and the dimerization model of receptor activation is supported by the observation that an Epo molecule mutated in the “site 2” region (R103A) or EpoRs mutated at the binding region for Epo “site 1” or “site 2” fail to elicit receptor signaling in hematopoietic cells (Matthews et al., 1996; Zhang et al., 2009). The Epo R103A mutant has also provided an opportunity to characterize the EpoR complex on non-hematopoietic cells. Cytoprotective activities of Epo on the differentiated neuroblastoma SH-SY5Y cell line were suppressed considerably with Epo R103A or via RNA-mediated interference of EpoR expression (Um et al., 2007), indicating that survival signaling elicited by Epo on neuronal cells is most likely due to low levels of the EpoR expressed in the configuration of the hematopoietic receptor (i.e., EpoR homodimer).
The dimeric EpoR structures formed by interaction with Epo or the EMP1 agonist differ in several aspects, likely explaining the fact that EMP1 exhibits reduced potency in terms of EpoR activation, as EMP1 is required at significantly higher concentrations than Epo to elicit erythropoietic responses (Livnah et al., 1996; Syed et al., 1998). The angle between the D1 domains differs in each EpoR:ligand complex (∼120° in the EpoR:Epo complex versus ∼180° in the EpoR:EMP1 complex). Moreover, the EpoR:Epo complex shows the D2 domains positioned within the same plane while they are twisted at an approximate 45° angle in the EpoR:EMP1 structure (Livnah et al., 1996; Syed et al., 1998). This distinction may affect the ability to activate the associated Jak2 kinase, as a particular orientation may be favored for full Jak2 activation via autophosphorylation. Hence, efficient Epo agonists will likely need to more closely mimic the ligand-occupied receptor orientation.
Roles for EpoR-mediated Jak2 and STAT5 signaling in erythropoiesis
One of the earliest detectable signaling events elicited upon EpoR activation is tyrosine phosphorylation of several intracellular proteins (Miura et al., 1991; Yoshimura and Lodish, 1992). Since the receptor lacks a kinase domain within its cytoplasmic region, these results indicated that protein tyrosine kinase function is carried out by a distinct factor. Subsequently, the Jak2 protein tyrosine kinase was identified as associating with the EpoR and serving as the principal kinase involved in mediating Epo-responsive signal transduction (Miura et al., 1994; Witthuhn et al., 1993; Yoshimura and Lodish, 1992). Jak2 is constitutively bound to the EpoR intracellular region (Fig. 1), and appears to provide a chaperone function for newly assembled EpoR molecules, aiding their transit through the secretory pathway from the endoplasmic reticulum to the plasma membrane (Huang et al., 2001). Deletion of the Jak2 gene in mice causes embryonic lethality at d 12–13 accompanied by severe anemia (Parganas et al., 1998). The phenotype of Jak2−/− animals closely resembles the Epo−/− or Epor−/− mice (Parganas et al., 1998; Wu et al., 1995); hematopoietic progenitors from Jak2−/− fetal livers are deficient in responses to Epo, indicating Jak2 is essential for Epo-dependent definitive erythropoiesis (Parganas et al., 1998). Jak2−/− hematopoietic progenitors also fail to respond to other myeloid cytokines such as GM-CSF and interleukin-3 (Parganas et al., 1998), hence the embryonic lethality of Jak2−/− animals most likely represents the first essential role for Jak2 during development but not all aspects of Jak2 function in vivo.
Significantly, Jak2 function is important in human erythropoiesis. A mutation within the Jak2 pseudokinase domain rendering a valine to phenylalanine substitution at residue 617 (V617F) and hormone-independent kinase activity was identified in individuals with a spectrum of myeloproliferative disorders (MPDs) including polycythemia vera (Baxter et al., 2005; James et al., 2005; Kralovics et al., 2005). In vitro and in vivo approaches to study Jak2 V617F show this mutant protein mediates Epo-independent erythroid progenitor growth and development as well as erythroid cell expansion in vivo (James et al., 2005; Zaleskas et al., 2006). Jak2 is therefore a feasible target for pharmacological intervention as a new approach for treatment of Jak2 V617F-positive MPDs.
The EpoR contains eight tyrosine residues within the membrane-distal portion of cytoplasmic tail; upon phosphorylation, several of these serve as docking sites for intracellular signaling molecules, including the transcription factors STAT5A and STAT5B, the p85 subunit of phosphoinositol 3'-kinase (PI3K), the cytokine suppressor CIS and the phosphatase SHP-1 (Chin et al., 1996; Damen et al., 1995a; Damen et al., 1993; Damen et al., 1995b; Gobert et al., 1996; Iwatsuki et al., 1997; Klingmuller et al., 1996; Klingmuller et al., 1995; Klingmuller et al., 1997; Matsumoto et al., 1997; Wakao et al., 1995). Tyrosine phosphorylated Jak2 also appears to interact directly with STAT5A and STAT5B (Fujitani et al., 1997), indicating it can serve as a scaffold for signal protein activation in addition to its enzymatic role in EpoR signal transduction. Recruitment of signaling molecules in proximity to Jak2 in the EpoR complex enables their tyrosine phosphorylation, which is an important step in subsequent activation of their respective signaling cascades (Fig. 1). The STAT5A and STAT5B proteins are predominant signal transducers for EpoR (Cui et al., 2004; Damen et al., 1995b; Klingmuller et al., 1996; Socolovsky et al., 1999; Wakao et al., 1995); these proteins are activated within seconds of Epo binding and accumulate in the nucleus to mediate Epo-responsive transcription.
The first report of mice deficient in both STAT5A and STAT5B indicated that STAT5 activity was non-essential for adult erythropoiesis in homeostatic conditions, evidenced by a normal hematocrit in mice with targeted gene disruption (Teglund et al., 1998). However, STAT5 deficiency was found to cause a severe effect upon fetal erythropoiesis, which proceeds at a high rate in the liver during gestation (Socolovsky et al., 1999), as well as adult red blood cell formation during erythropoietic stress (Socolovsky et al., 2001), indicating a critical role for STAT5 in demand-driven erythropoiesis. These studies showed that STAT5 operates by regulating erythroid survival, and highlighted a key mechanism via STAT5-mediated control of the survival gene BclXL (Socolovsky et al., 1999; Socolovsky et al., 2001). It was later determined that the original STAT5-deficient animals corresponded to a hypomorphic STAT5 model (Stat5abΔN/ΔN), in which an N-terminally-truncated STAT5 protein is detectable within certain cell types (Moriggl et al., 2005). The Stat5abΔN/ΔN mice have been a valuable resource for understanding STAT5 structure-function relationships in vivo and have revealed critical roles for the STAT5 N-domain in mechanisms such as leukemia progression (Moriggl et al., 2005). However, since Stat5abΔN/ΔN mice are not completely deficient in STAT5 function, work in this model could not definitively delineate the role for STAT5 in red blood cell development. Fortunately a complete Stat5a−/− Stat5b−/− knockout mouse model was developed within Dr. Lothar Hennighausen’s lab (Cui et al., 2004). Stat5a−/− Stat5b−/− mice exhibit severe anemia, as judged by significantly reduced hematocrits compared to wild type littermates (Cui et al., 2004). Stat5a−/− Stat5b−/− mice have a perinatal lethality, although this is not thought to be a consequence of the severe anemia as a small proportion of Stat5a−/− Stat5b−/− are able to survive despite low red blood cell numbers. Thus, STAT5 serves an essential role in controlling erythropoiesis in vivo.
Summary and future perspectives
The EpoR has provided a paradigm for understanding cytokine receptor structure and signal transduction, as well as cytokine function in normal and aberrant hematopoiesis (Goyal and Longmore, 1999; Longmore et al., 1993b; Socolovsky, 2007; Watowich et al., 1996; Wojchowski et al., 1999). Functioning as a dimeric molecule, the ligand-occupied receptor activates the Jak2 protein tyrosine kinase, which in turn phosphorylates tyrosine residues on the receptor and associated intracellular signaling molecules (Fig. 1). STAT5 is a principal signaling protein activated upon receptor ligation, and its function is essential for normal red blood cell development in vivo. While mutations in Jak2 have been identified in MPDs and leukemia, it is not yet clear if STAT5 mutations are associated with human disease. However, persistently activated STAT5 is found in many hematological cancers (Benekli et al., 2003; Benekli et al., 2009; Sternberg and Gilliland, 2004) and thus a greater understanding of STAT5-mediated signaling cascades is necessary to reveal molecular mechanisms that may contribute to disease. Moreover, other signaling cascades such as the PI3K/Akt and Ras/MAPK pathways are elicited upon EpoR activation and appear to play a role in erythropoiesis as judged by studies with primary red cell progenitors ex vivo or hematopoietic cell lines (Carroll et al., 1991; Ghaffari et al., 2006; Gobert et al., 1995; Haseyama et al., 1999; Klingmuller et al., 1997; Nagata et al., 1997a; Nagata et al., 1997b; Nagata and Todokoro, 1999; Shan et al., 1999; Zhao et al., 2006), however, we have limited knowledge of the function of these signaling responses during steady state and stress erythropoiesis due to the lack of appropriate genetic mouse models. Future work should include dissection of non-Jak-STAT signaling cascades in the erythropoietic response.
ACKNOWLEDGEMENTS
I would like to thank Hoainam Nguyen-Jackson for her critical review of the manuscript and members of the Watowich lab at M. D. Anderson for their advice and discussion. Unrelated work in my laboratory is supported by an Investigator-initiated Preclinical Research Agreement with Amgen, Inc., grants from the NIH (AI073587, AR059010) and a seed grant from the Center for Stem Cell and Developmental Biology at UT M. D. Anderson Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arcasoy MO, Degar BA, Harris KW, Forget BG. Familial erythrocytosis associated with a short deletion in the erythropoietin receptor gene. Blood. 1997;89:4628–4635. [PubMed] [Google Scholar]
- Arcasoy MO, Karayal AF, Segal HM, Sinning JG, Forget BG. A novel mutation in the erythropoietin receptor gene is associated with familial erythrocytosis. Blood. 2002;99:3066–3069. doi: 10.1182/blood.v99.8.3066. [DOI] [PubMed] [Google Scholar]
- Barber DL, DeMartino JC, Showers MO, D’Andrea AD. A dominant negative erythropoietin (epo) receptor inhibits epo-dependent growth and blocks F-gp55-dependent transformation. Mol. Cell. Biol. 1994;14:2257–2265. doi: 10.1128/mcb.14.4.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, Vassiliou GS, Bench AJ, Boyd EM, Curtin N, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365:1054–1061. doi: 10.1016/S0140-6736(05)71142-9. [DOI] [PubMed] [Google Scholar]
- Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benekli M, Baer MR, Baumann H, Wetzler M. Signal transducer and activator of transcription proteins in leukemias. Blood. 2003;101:2940–2954. doi: 10.1182/blood-2002-04-1204. [DOI] [PubMed] [Google Scholar]
- Benekli M, Baumann H, Wetzler M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J Clin Oncol. 2009;27:4422–4432. doi: 10.1200/JCO.2008.21.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broudy VC, Lin N, Brice M, Nakamoto B, Papayannopoulou T. Erythropoietin receptor characteristics on primary human erythroid cells. Blood. 1991;77:2583–2590. [PubMed] [Google Scholar]
- Carroll MP, Spivak JL, McMahon M, Weich N, Rapp UR, May WS. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991;266:14964–14969. [PubMed] [Google Scholar]
- Chin H, Nakamura N, Kamiyama R, Miyasaka N, Ihle JN, Miura O. Physical and functional interactions between Stat5 and the tyrosine-phosphorylated receptors for erythropoietin and interleukin-3. Blood. 1996;88:4415–4425. [PubMed] [Google Scholar]
- Clackson T, Wells JA. A hot spot of binding energy in a hormone-receptor interface. Science. 1995;267:383–386. doi: 10.1126/science.7529940. [DOI] [PubMed] [Google Scholar]
- Constantinescu SN, Keren T, Socolovsky M, Nam H, Henis YI, Lodish HF. Ligand-independent oligomerization of cell-surface erythropoietin receptor is mediated by the transmembrane domain. Proc Natl Acad Sci U S A. 2001;98:4379–4384. doi: 10.1073/pnas.081069198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosman D, Lyman SD, Idzerda RL, Beckmann MP, Park LS, Goodwin RG, March CJ. A new cytokine receptor superfamily. Trends Biochem Sci. 1990;15:265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Tang W, Li C, Deng C-X, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea AD, Yoshimura A, Youssoufian H, Zon LI, Koo JW, Lodish HF. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991;11:1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damen Je, Cutler RL, Jiao H, Yi T, Krystal G. Phosphorylation of tyrosine 503 in the erythropoietin receptor (EpR) is essential for binding the P85 subunit of phosphatidylinositol (PI) 3-kinase and for EpR-associated PI 3-kinase activity. Chem. J. Biol. 1995a;270:23402–23408. doi: 10.1074/jbc.270.40.23402. [DOI] [PubMed] [Google Scholar]
- Damen JE, Mui AL, Puil L, Pawson T, Krystal G. Phosphatidylinositol 3-kinase associates, via its Src homology 2 domains, with the activated erythropoietin receptor. Blood. 1993;81:3204–3210. [PubMed] [Google Scholar]
- Damen JE, Wakao H, Miyajima A, Krosl J, Humphries RK, Cutler RL, Krystal G. Tyrosine 343 in the erythropoietin receptor positively regulates erythropoietin-induced cell proliferation and Stat5 activation. EMBO J. 1995b;14:5557–5568. doi: 10.1002/j.1460-2075.1995.tb00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Darnell JE., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A, Sistonen P, Lehvaslaiho H, Ikkala E, Juvonen E. Familial erythrocytosis genetically linked to erythropoietin receptor gene. Lancet. 1993a;341:82–84. doi: 10.1016/0140-6736(93)92558-b. [DOI] [PubMed] [Google Scholar]
- de la Chapelle A, Traskelin AL, Juvonen E. Truncated erythropoietin receptor causes dominantly inherited benign human erythrocytosis. Proc Natl Acad Sci U S A. 1993b;90:4495–4499. doi: 10.1073/pnas.90.10.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot S, Lorenzini T, Yanagihara D, Chang D, Elliot G. Activation of the erythropoietin (EPO) receptor by bivalent anti-EPO receptor antibodies. J. Biol Chem. 1996;271:24691–24697. doi: 10.1074/jbc.271.40.24691. [DOI] [PubMed] [Google Scholar]
- Foxwell BM, Barrett K, Feldmann M. Cytokine receptors: structure and signal transduction. Clin Exp Immunol. 1992;90:161–169. doi: 10.1111/j.1365-2249.1992.tb07922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani Y, Hibi M, Fukada T, Takahashi-Tezuka M, Yoshida H, Yamaguchi T, Sugiyama K, Yamanaka Y, Nakajima K, Hirano T. An alternative pathway for STAT activation that is mediated by the direct interaction between JAK and STAT. Oncogene. 1997;14:751–761. doi: 10.1038/sj.onc.1200907. [DOI] [PubMed] [Google Scholar]
- Ghaffari S, Kitidis C, Zhao W, Marinkovic D, Fleming MD, Luo B, Marszalek J, Lodish HF. AKT induces erythroid-cell maturation of JAK2-deficient fetal liver progenitor cells and is required for Epo regulation of erythroid-cell differentiation. Blood. 2006;107:1888–1891. doi: 10.1182/blood-2005-06-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert S, Chretien S, Gouilleux F, Muller O, Pallard C, Dusanter-Fourt I, Groner B, Lacombe C, Gisselbrecht S, Mayeux P. Identification of tyrosine residues within the intracellular domain of the erythropoietin receptor crucial for Stat5 activation. EMBO J. 1996;15:2434–2441. [PMC free article] [PubMed] [Google Scholar]
- Gobert S, Duprez V, Lacombe C, Gisselbrecht S, Mayeux P. The signal transduction pathway of erythropoietin involves three forms of mitogen-activated protein (MAP) kinase in UT7 erythroleukemia cells. Eur J Biochem. 1995;234:75–83. doi: 10.1111/j.1432-1033.1995.075_c.x. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Longmore GD. Abnormalities of cytokine receptor signalling contributing to diseases of red blood cell production. Ann Med. 1999;31:208–216. doi: 10.3109/07853899909115980. [DOI] [PubMed] [Google Scholar]
- Gurezka R, Laage R, Brosig B, Langosch D. A heptad motif of leucine residues found in membrane proteins can drive self-assembly of artificial transmembrane segments. J Biol Chem. 1999;274:9265–9270. doi: 10.1074/jbc.274.14.9265. [DOI] [PubMed] [Google Scholar]
- Haseyama Y, Sawada K, Oda A, Koizumi K, Takano H, Tarumi T, Nishio M, Handa M, Ikeda Y, Koike T. Phosphatidylinositol 3-kinase is involved in the protection of primary cultured human erythroid precursor cells from apoptosis. Blood. 1999;94:1568–1577. [PubMed] [Google Scholar]
- Hibi M, Hirano T. Signal transduction through cytokine receptors. Int Rev Immunol. 1998;17:75–102. doi: 10.3109/08830189809084488. [DOI] [PubMed] [Google Scholar]
- Hilton DJ, Watowich SS, Katz L, Lodish HF. Saturation mutagenesis of the WSXWS motif of the erythropoietin receptor. J. Biol. Chem. 1994 doi: 10.1074/jbc.271.9.4699. submitted. [DOI] [PubMed] [Google Scholar]
- Huang LJ, Constantinescu SN, Lodish HF. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol Cell. 2001;8:1327–1338. doi: 10.1016/s1097-2765(01)00401-4. [DOI] [PubMed] [Google Scholar]
- Ihle JN, Witthuhn BA, Quelle FW, Yamamoto K, Silvennoinen O. Signaling through the hematopoietic cytokine receptors. Annu Rev Immunol. 1995;13:369–398. doi: 10.1146/annurev.iy.13.040195.002101. [DOI] [PubMed] [Google Scholar]
- Iwatsuki K, Endo T, Misawa H, Yokouchi M, Matsumoto A, Ohtsubo M, Mori KJ, Yoshimura A. STAT5 activation correlates with erythropoietin receptor-mediated erythroid differentiation of an erythroleukemia cell line. J Biol Chem. 1997;272:8149–8152. doi: 10.1074/jbc.272.13.8149. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C, Garcon L, Raslova H, Berger R, Bennaceur-Griscelli A, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- Jiao H, Yang W, Berrada K, Tabrizi M, Shultz L, Yi T. Macrophages from motheaten and viable motheaten mutant mice show increased proliferative responses to GM-CSF: detection of potential HCP substrates in GM-CSF signal transduction. Exp Hematol. 1997;25:592–600. [PubMed] [Google Scholar]
- Klingmuller U, Bergelson S, Hsiao JG, Lodish HF. Multiple tyrosine residues in the cytosolic domain of the erythropoietin receptor promote activation of STAT5. Proc Natl Acad Sci U S A. 1996;93:8324–8328. doi: 10.1073/pnas.93.16.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–738. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Klingmuller U, Wu H, Hsiao JG, Toker A, Duckworth BC, Cantley LC, Lodish HF. Identification of a novel pathway important for proliferation and differentiation of primary erythroid progenitors. Proc Natl Acad Sci U S A. 1997;94:3016–3021. doi: 10.1073/pnas.94.7.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koury ST, Koury MJ, Bondurant MC, Caro J, Graber SE. Quantitation of erythropoietin-producing cells in kidneys of mice by in situ hybridization: correlation with hematocrit, renal erythropoietin mRNA, and serum erythropoietin concentration. Blood. 1989;74:645–651. [PubMed] [Google Scholar]
- Kralovics R, Indrak K, Stopka T, Berman BW, Prchal JF, Prchal JT. Two new EPO receptor mutations: truncated EPO receptors are most frequently associated with primary familial and congenital polycythemias. Blood. 1997;90:2057–2061. [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, Tichelli A, Cazzola M, Skoda RC. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005;352:1779–1790. doi: 10.1056/NEJMoa051113. [DOI] [PubMed] [Google Scholar]
- Krantz SB. Erythropoietin. Blood. 1991;77:419–434. [PubMed] [Google Scholar]
- Kubatzky KF, Ruan W, Gurezka R, Cohen J, Ketteler R, Watowich SS, Neumann D, Langosch D, Klingmuller U. Self assembly of the transmembrane domain promotes signal transduction through the erythropoietin receptor. Curr Biol. 2001;11:110–115. doi: 10.1016/s0960-9822(01)00018-5. [DOI] [PubMed] [Google Scholar]
- Lacombe C, Da Silva JL, Bruneval P, Fournier JG, Wendling F, Casadevall N, Camilleri JP, Bariety J, Varet B, Tambourin P. Peritubular cells are the site of erythropoietin synthesis in the murine hypoxic kidney. J Clin Invest. 1988;81:620–623. doi: 10.1172/JCI113363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnah O, Stura EA, Johnson DL, Middleton SA, Mulcahy LS, Wrighton NC, Dower WJ, Jolliffe LK, Wilson IA. Functional mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 A. Science. 1996;273:464–471. doi: 10.1126/science.273.5274.464. [DOI] [PubMed] [Google Scholar]
- Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–990. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- Longmore GD, Lodish HF. An activating mutation in the murine erythropoietin receptor induces erythroleukemia in mice: a cytokine receptor superfamily oncogene. Cell. 1991;67:1089–1102. doi: 10.1016/0092-8674(91)90286-8. [DOI] [PubMed] [Google Scholar]
- Longmore GD, Pharr P, Neumann D, Lodish HF. Both megakaryocytopoiesis and erythropoiesis are induced in mice infected with a retrovirus expressing an oncogenic erythropoietin receptor. Blood. 1993a;82:2386–2395. [PubMed] [Google Scholar]
- Longmore GD, Pharr PN, Lodish HF. A constitutively activated erythropoietin receptor stimulates proliferation and contributes to transformation of multipotent, committed nonerythroid and erythroid progenitor cells. Mol. Cell. Biol. 1994;14:2266–2277. doi: 10.1128/mcb.14.4.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmore GD, Watowich SS, Hilton DJ, Lodish HF. The erythropoietin receptor: its role in hematopoiesis and myeloproliferative diseases. J Cell Biol. 1993b;123:1305–1308. doi: 10.1083/jcb.123.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto A, Masuhara M, Mitsui K, Yokouchi M, Ohtsubo M, Misawa H, Miyajima A, Yoshimura A. CIS, a cytokine inducible SH2 protein, is a target of the JAK-STAT5 pathway and modulates STAT5 activation. Blood. 1997;89:3148–3154. [PubMed] [Google Scholar]
- Matthews DJ, Topping RS, Cass RT, Giebel LB. A sequential dimerization mechanism for erythropoietin receptor activation. Proc. Natl. Acad. Sci. USA. 1996;93:9471–9476. doi: 10.1073/pnas.93.18.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux P, Billat C, Jacquot R. Murine erythroleukaemia cells (Friend cells) possess high-affinity binding sites for erythropoietin. Febs Lett. 1987;211:229–233. doi: 10.1016/0014-5793(87)81442-4. [DOI] [PubMed] [Google Scholar]
- Miura O, D’Andrea AD, Kabat D, Ihle JN. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991;11:4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura O, Nakamura N, Quelle FW, Witthuhn BA, Ihle JN, Aoki N. Erythropoietin induces association of the JAK2 protein tyrosine kinase with the erythropoietin receptor in vivo. Blood. 1994;84:1501–1507. [PubMed] [Google Scholar]
- Moriggl R, Sexl V, Kenner L, Duntsch C, Stangl K, Gingras S, Hoffmeyer A, Bauer A, Piekorz R, Wang D, et al. Stat5 tetramer formation is associated with leukemogenesis. Cancer Cell. 2005;7:87–99. doi: 10.1016/j.ccr.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Nagata Y, Moriguchi T, Nishida E, Todokoro K. Activation of p38 MAP kinase pathway by erythropoietin and interleukin-3. Blood. 1997a;90:929–934. [PubMed] [Google Scholar]
- Nagata Y, Nishida E, Todokoro K. Activation of JNK signaling pathway by erythropoietin, thrombopoietin, and interleukin-3. Blood. 1997b;89:2664–2669. [PubMed] [Google Scholar]
- Nagata Y, Todokoro K. Requirement of activation of JNK and p38 for environmental stress-induced erythroid differentiation and apoptosis and of inhibition of ERK for apoptosis. Blood. 1999;94:853–863. [PubMed] [Google Scholar]
- Parganas E, Wang D, Stravopodis D, Topham DJ, Marine J-C, Teglund S, Vanin EF, Bodner S, Colamonici OR, van Deursen JM, et al. Jak2 is essential for signaling through a variety of cytokine receptors. Cell. 1998;93:385–395. doi: 10.1016/s0092-8674(00)81167-8. [DOI] [PubMed] [Google Scholar]
- Pharr PN, Hankins D, Hofbauer A, Lodish HF, Longmore GD. Expression of a constitutively active erythropoietin receptor in primary hematopoietic progenitors abrogates erythropoietin dependence and enhances erythroid colony-forming unit, erythroid burst-forming unit, and granulocyte/macrophage progenitor growth. Proc. Natl. Acad. Sci. USA. 1993;90:938–942. doi: 10.1073/pnas.90.3.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philo JS, Aoki KH, Arakawa T, Owers Narhi L, Wen J. Dimerization of the extracellular domain of the erythropoietin (EPO) receptor by EPO: one high-affinity and one low-affinity interaction. Biochemistry. 1996;35:1681–1691. doi: 10.1021/bi9524272. [DOI] [PubMed] [Google Scholar]
- Schneider H, Chaovapong W, Matthews DJ, Karkaria C, Cass RT, Zhan H, Boyle M, Lorenzini T, Elliot SG, Giebel LB. Homodimerization of erythropoietin receptor by a bivalent monoclonal antibody triggers cell proliferation and differentiation of erythroid precursors. Blood. 1997;89:473–482. [PubMed] [Google Scholar]
- Seubert N, Royer Y, Staerk J, Kubatzky KF, Moucadel V, Krishnakumar S, Smith SO, Constantinescu SN. Active and inactive orientations of the transmembrane and cytosolic domains of the erythropoietin receptor dimer. Mol Cell. 2003;12:1239–1250. doi: 10.1016/s1097-2765(03)00389-7. [DOI] [PubMed] [Google Scholar]
- Shan R, Price JO, Gaarde WA, Monia BP, Krantz SB, Zhao ZJ. Distinct roles of JNKs/p38 MAP kinase and ERKs in apoptosis and survival of HCD-57 cells induced by withdrawal or addition of erythropoietin. Blood. 1999;94:4067–4076. [PubMed] [Google Scholar]
- Socolovsky M. Molecular insights into stress erythropoiesis. Curr Opin Hematol. 2007;14:215–224. doi: 10.1097/MOH.0b013e3280de2bf1. [DOI] [PubMed] [Google Scholar]
- Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a−/−5b−/− mice: a direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. doi: 10.1016/s0092-8674(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Socolovsky M, Nam H, Fleming MD, Haase VH, Brugnara C, Lodish HF. Ineffective erythropoiesis in Stat5a(−/−)5b(−/−) mice due to decreased survival of early erythroblasts. Blood. 2001;98:3261–3273. doi: 10.1182/blood.v98.12.3261. [DOI] [PubMed] [Google Scholar]
- Sternberg DW, Gilliland DG. The role of signal transducer and activator of transcription factors in leukemogenesis. J Clin Oncol. 2004;22:361–371. doi: 10.1200/JCO.2004.10.124. [DOI] [PubMed] [Google Scholar]
- Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, et al. Efficiency of signaling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:511–516. doi: 10.1038/26773. [DOI] [PubMed] [Google Scholar]
- Tapley P, Shevde NK, Schweitzer PA, Gallina M, Christianson SW, Lin IL, Stein RB, Shultz LD, Rosen J, Lamb P. Increased G-CSF responsiveness of bone marrow cells from hematopoietic cell phosphatase deficient viable motheaten mice. Exp Hematol. 1997;25:122–131. [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, van Deursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Um M, Gross AW, Lodish HF. A "classical" homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Wakao H, Harada N, Kitamura T, Mui AL, Miyajima A. Interleukin 2 and erythropoietin activate STAT5/MGF via distinct pathways. Embo J. 1995;14:2527–2535. doi: 10.1002/j.1460-2075.1995.tb07250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich SS, Hilton DJ. 1992 unpublished results. [Google Scholar]
- Watowich SS, Hilton DJ, Lodish HF. Activation and inhibition of erythropoietin receptor function: role of receptor dimerization. Mol. Cell. Biol. 1994;14:3535–3549. doi: 10.1128/mcb.14.6.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich SS, Liu KD, Xie X, Lai SY, Mikami A, Longmore GD, Goldsmith MA. Oligomerization and scaffolding functions of the erythropoietin receptor cytoplasmic tail. J. Biol. Chem. 1999a;274:5415–5421. doi: 10.1074/jbc.274.9.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich SS, Wu H, Socolovsky M, Klingmuller U, Constantinescu SN, Lodish HF. Cytokine receptor signal transduction and the control of hematopoietic cell development. Annu Rev Cell Dev Biol. 1996;12:91–128. doi: 10.1146/annurev.cellbio.12.1.91. [DOI] [PubMed] [Google Scholar]
- Watowich SS, Xie X, Klingmuller U, Kere J, Lindlof M, Berglund S, de la Chapelle A. Erythropoietin receptor mutations associated with familial erythrocytosis cause hypersensitivity to erythropoietin in the heterozygous state. Blood. 1999b;94:2530–2532. [PubMed] [Google Scholar]
- Watowich SS, Yoshimura A, Longmore GD, Hilton DJ, Yoshimura Y, Lodish HF. Homodimerization and constitutive activation of the erythropoietin receptor. Proc Natl Acad Sci U S A. 1992;89:2140–2144. doi: 10.1073/pnas.89.6.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Wojchowski DM, Gregory RC, Miller CP, Pandit AK, Pircher TJ. Signal transduction in the erythropoietin receptor system. Exp Cell Res. 1999;253:143–156. doi: 10.1006/excr.1999.4673. [DOI] [PubMed] [Google Scholar]
- Wu H, Liu X, Jaenisch R, Lodish HF. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 1995;83:59–67. doi: 10.1016/0092-8674(95)90234-1. [DOI] [PubMed] [Google Scholar]
- Yi T, Mui AL, Krystal G, Ihle JN. Hematopoietic cell phosphatase associates with the interleukin-3 (IL-3) receptor beta chain and down-regulates IL-3-induced tyrosine phosphorylation and mitogenesis. Mol Cell Biol. 1993;13:7577–7586. doi: 10.1128/mcb.13.12.7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Lodish HF. In vitro phosphorylation of the erythropoietin receptor and an associated protein, pp130. Mol Cell Biol. 1992;12:706–715. doi: 10.1128/mcb.12.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A, Longmore G, Lodish HF. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990;348:647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]
- Zaleskas VM, Krause DS, Lazarides K, Patel N, Hu Y, Li S, Van Etten RA. Molecular pathogenesis and therapy of polycythemia induced in mice by JAK2 V617F. PLoS One. 2006;1:e18. doi: 10.1371/journal.pone.0000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YL, Radhakrishnan ML, Lu X, Gross AW, Tidor B, Lodish HF. Symmetric signaling by an asymmetric 1 erythropoietin: 2 erythropoietin receptor complex. Mol Cell. 2009;33:266–274. doi: 10.1016/j.molcel.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Kitidis C, Fleming MD, Lodish HF, Ghaffari S. Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood. 2006;107:907–915. doi: 10.1182/blood-2005-06-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]