Abstract
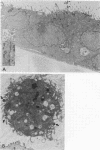
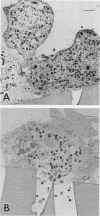
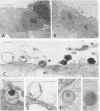
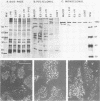
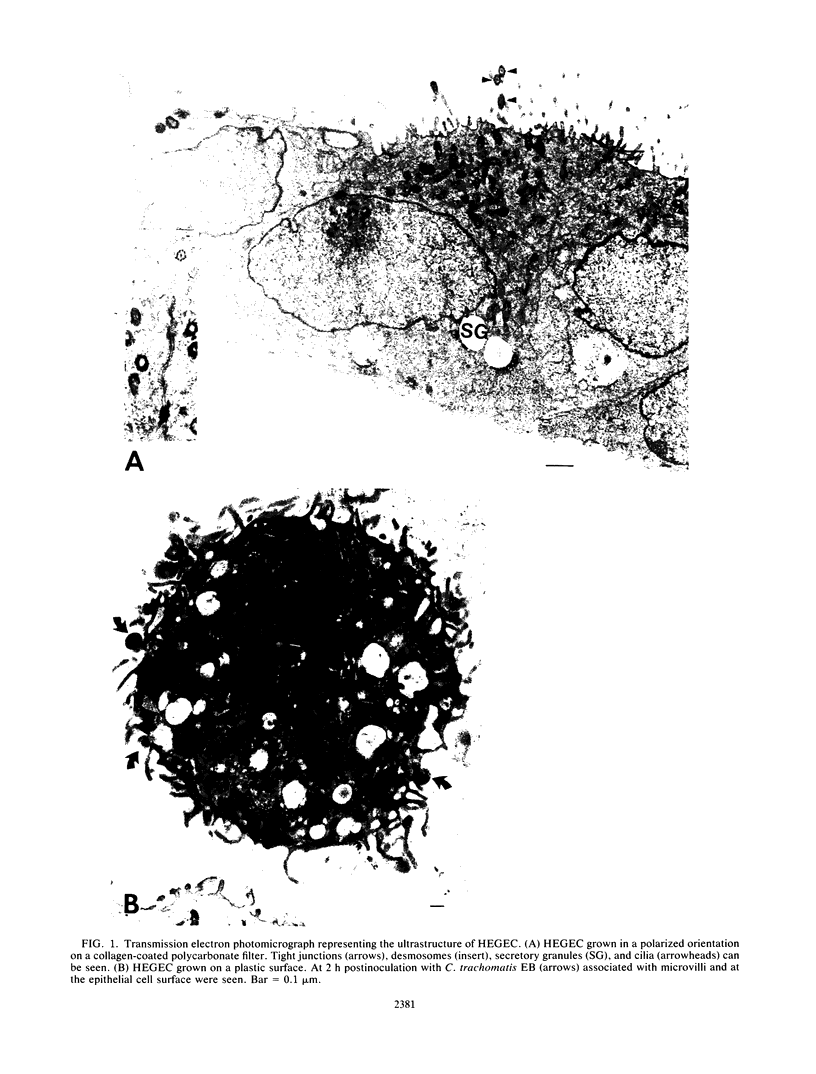
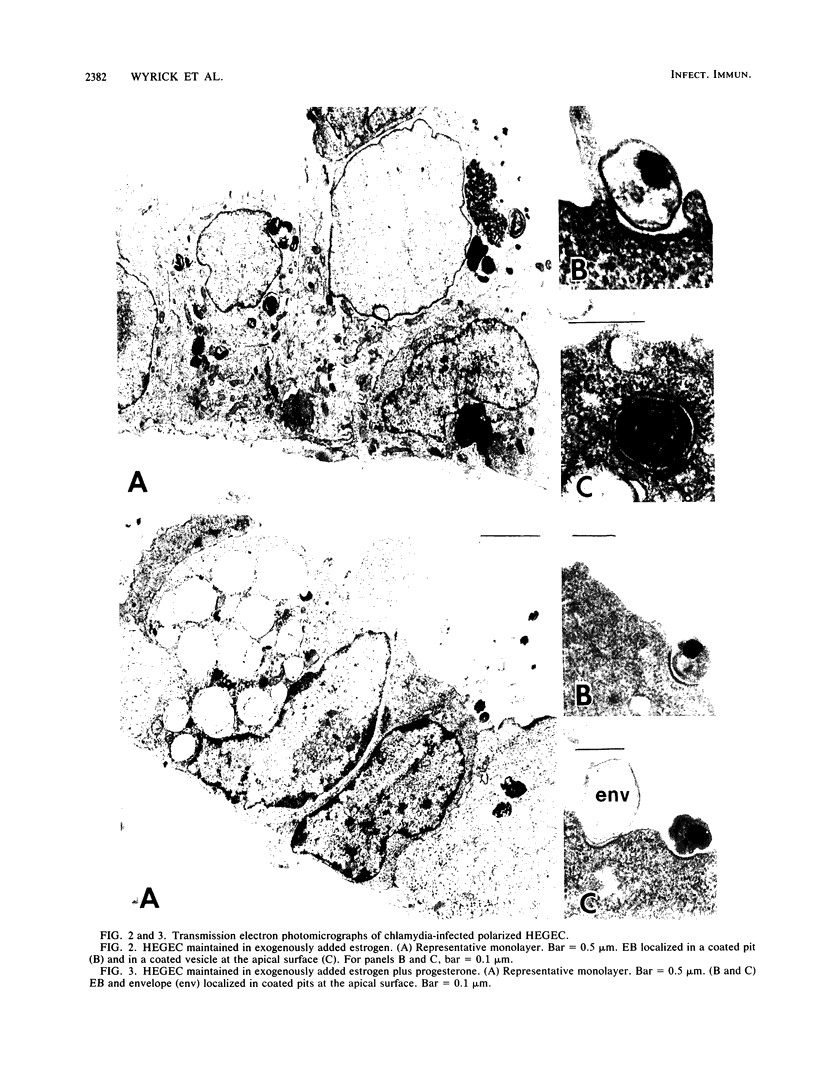
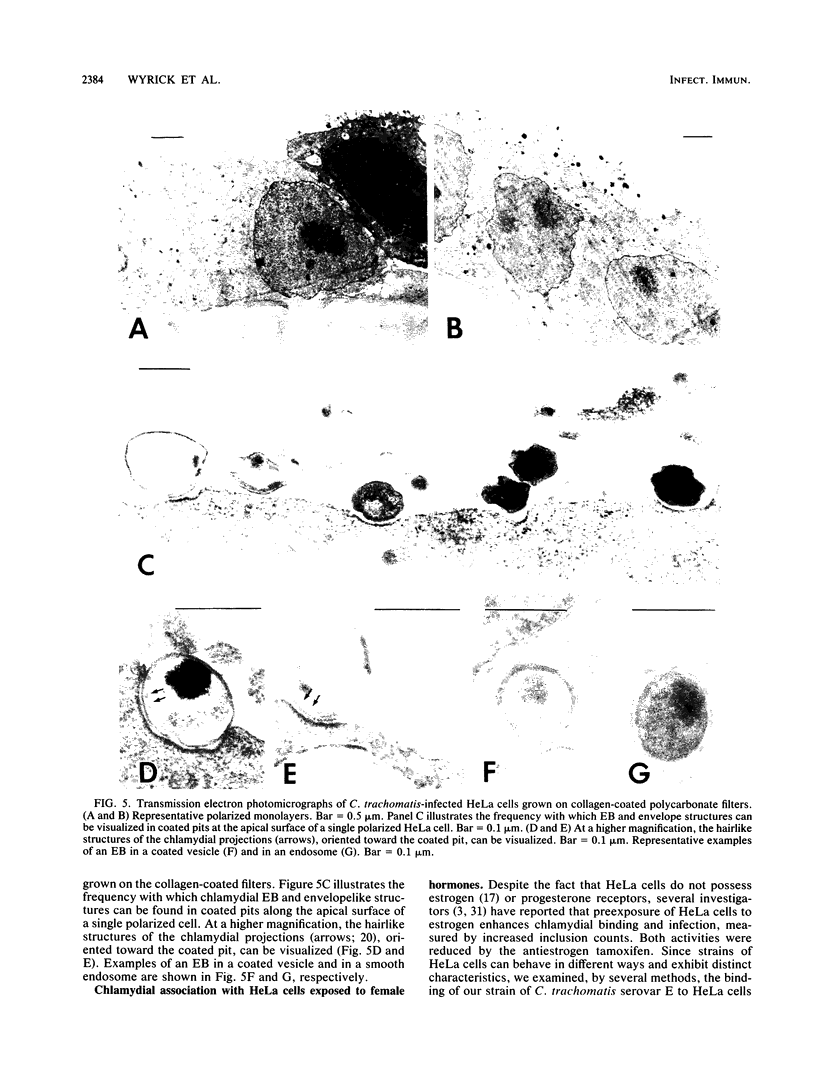
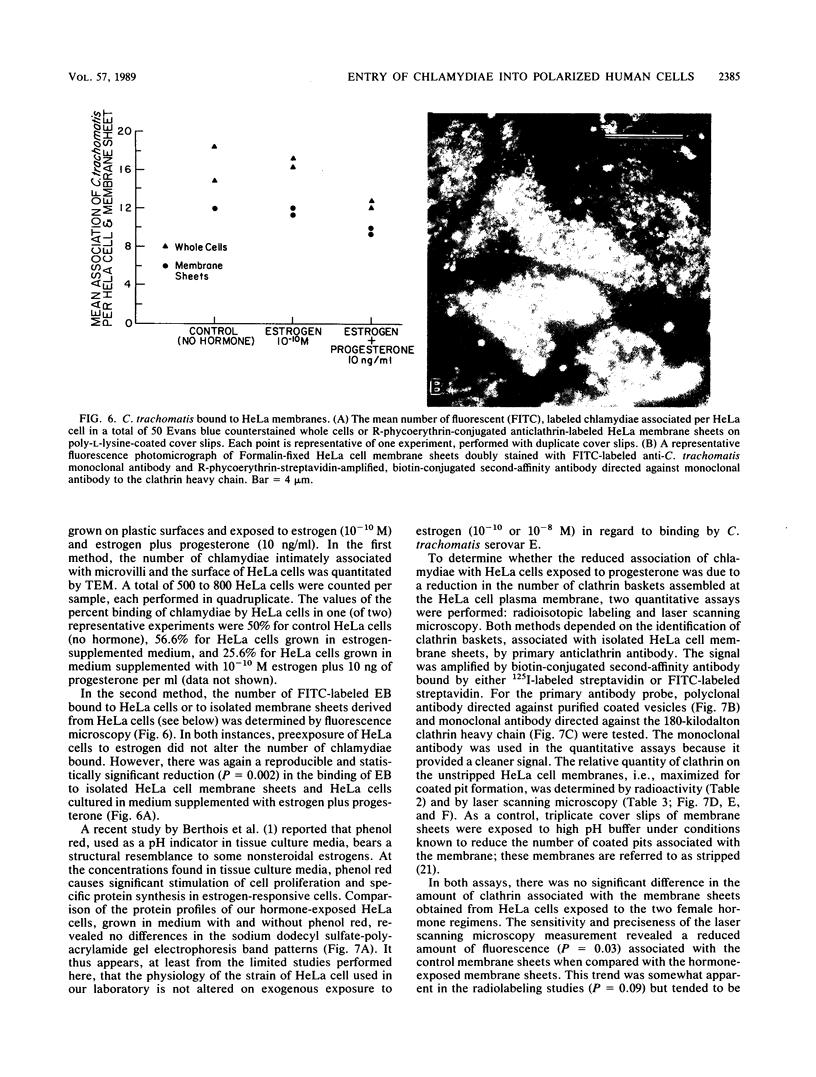
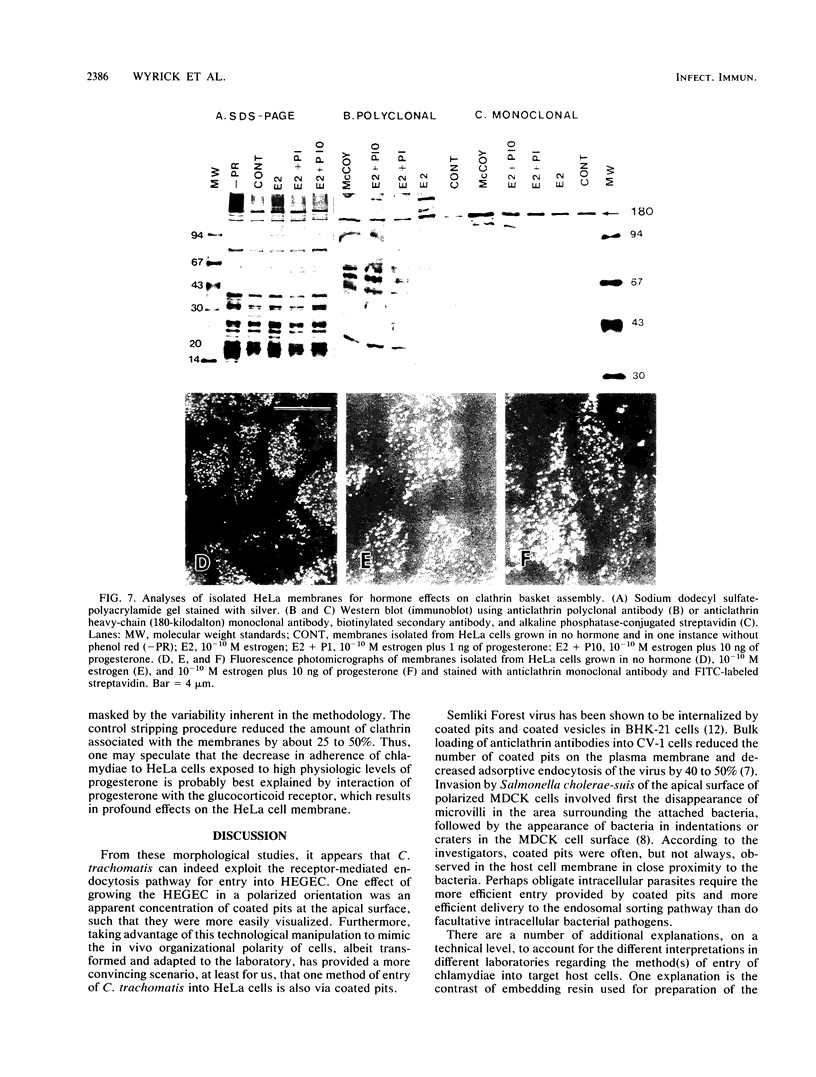
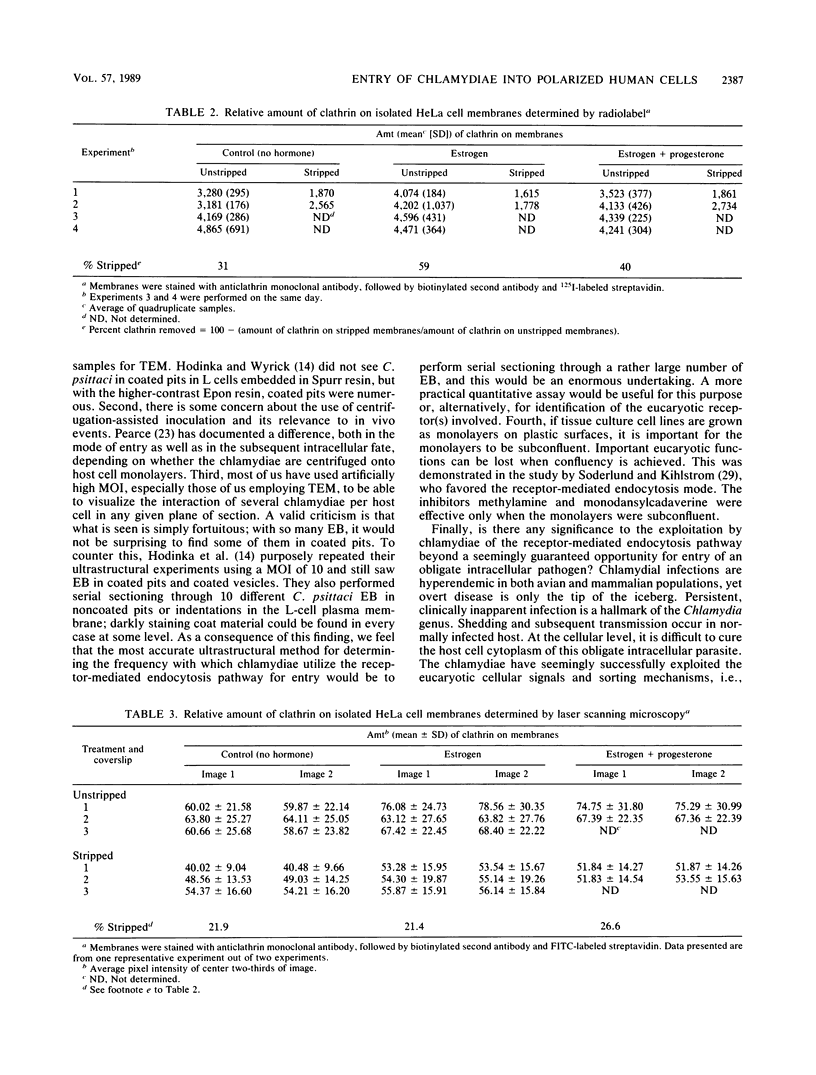
To study the initial invasion process(es) of genital chlamydiae, a model system consisting of hormonally maintained primary cultures of human endometrial gland epithelial cells (HEGEC), grown in a polarized orientation on collagen-coated filters, was utilized. After Chlamydia trachomatis inoculation of the apical surface of polarized HEGEC, chlamydiae were readily visualized, by transmission electron microscopy, in coated pits and coated vesicles. This was true for HEGEC maintained in physiologic concentrations of estrogen (proliferative phase) and of estrogen plus progesterone (secretory phase), despite the finding that association of chlamydiae with secretory-phase HEGEC is significantly reduced (P = 0.025; A.S. Maslow, C.H. Davis, J. Choong, and P.B. Wyrick, Am. J. Obstet. Gynecol. 159:1006-1014, 1988). In contrast, chlamydiae were rarely observed in the clathrin-associated structures if the HEGEC were cultured on plastic surfaces. The same pattern of coated pit versus noncoated pit entry was reproducible in HeLa cells. The quantity of coated pits associated with isolated membrane sheets derived from HeLa cells, grown on poly-L-lysine-coated cover slips in medium containing the female hormones, was not significantly different as monitored by radiolabeling studies and by laser scanning microscopy. These data suggest that culture conditions which mimic in vivo cellular organization may enhance entry into coated pits for some obligate intracellular pathogens.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besterman J. M., Low R. B. Endocytosis: a review of mechanisms and plasma membrane dynamics. Biochem J. 1983 Jan 15;210(1):1–13. doi: 10.1042/bj2100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S. K., Goswami P. C. Enhancement of adherence and growth of Chlamydia trachomatis by estrogen treatment of HeLa cells. Infect Immun. 1986 Sep;53(3):646–650. doi: 10.1128/iai.53.3.646-650.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M. Living with clathrin: its role in intracellular membrane traffic. Science. 1988 Dec 9;242(4884):1396–1402. doi: 10.1126/science.2904698. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Moulder J. W. Parasite-specified phagocytosis of Chlamydia psittaci and Chlamydia trachomatis by L and HeLa cells. Infect Immun. 1978 Feb;19(2):598–606. doi: 10.1128/iai.19.2.598-606.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doxsey S. J., Brodsky F. M., Blank G. S., Helenius A. Inhibition of endocytosis by anti-clathrin antibodies. Cell. 1987 Jul 31;50(3):453–463. doi: 10.1016/0092-8674(87)90499-5. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Gumbiner B., Falkow S. Penetration of Salmonella through a polarized Madin-Darby canine kidney epithelial cell monolayer. J Cell Biol. 1988 Jul;107(1):221–230. doi: 10.1083/jcb.107.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Gregory W. W., Byrne G. I., Gardner M., Moulder J. W. Cytochalasin B does not inhibit ingestion of Chlamydia psittaci by mouse fibroblasts (L cells) and mouse peritoneal macrophages. Infect Immun. 1979 Jul;25(1):463–466. doi: 10.1128/iai.25.1.463-466.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler J. S., Preston A. S., Steele R. E. Factors affecting the differentiation of epithelial transport and responsiveness to hormones. Fed Proc. 1984 May 15;43(8):2221–2224. [PubMed] [Google Scholar]
- Helenius A., Kartenbeck J., Simons K., Fries E. On the entry of Semliki forest virus into BHK-21 cells. J Cell Biol. 1980 Feb;84(2):404–420. doi: 10.1083/jcb.84.2.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Davis C. H., Choong J., Wyrick P. B. Ultrastructural study of endocytosis of Chlamydia trachomatis by McCoy cells. Infect Immun. 1988 Jun;56(6):1456–1463. doi: 10.1128/iai.56.6.1456-1463.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodinka R. L., Wyrick P. B. Ultrastructural study of mode of entry of Chlamydia psittaci into L-929 cells. Infect Immun. 1986 Dec;54(3):855–863. doi: 10.1128/iai.54.3.855-863.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Voorhis D. L., Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987 Aug 28;50(5):769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Chi E. Y., Grayston J. T. Ultrastructural study of entry of Chlamydia strain TWAR into HeLa cells. Infect Immun. 1988 Jun;56(6):1668–1672. doi: 10.1128/iai.56.6.1668-1672.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow A. S., Davis C. H., Choong J., Wyrick P. B. Estrogen enhances attachment of Chlamydia trachomatis to human endometrial epithelial cells in vitro. Am J Obstet Gynecol. 1988 Oct;159(4):1006–1014. doi: 10.1016/s0002-9378(88)80189-3. [DOI] [PubMed] [Google Scholar]
- Moore M. S., Mahaffey D. T., Brodsky F. M., Anderson R. G. Assembly of clathrin-coated pits onto purified plasma membranes. Science. 1987 May 1;236(4801):558–563. doi: 10.1126/science.2883727. [DOI] [PubMed] [Google Scholar]
- Moorman D. R., Sixbey J. W., Wyrick P. B. Interaction of Chlamydia trachomatis with human genital epithelium in culture. J Gen Microbiol. 1986 Apr;132(4):1055–1067. doi: 10.1099/00221287-132-4-1055. [DOI] [PubMed] [Google Scholar]
- Pearce J. H. Early events in chlamydial infection. Ann Inst Pasteur Microbiol. 1986 May-Jun;137A(3):325–332. doi: 10.1016/s0769-2609(86)80043-6. [DOI] [PubMed] [Google Scholar]
- Richmond S. J., Stirling P. Localization of chlamydial group Antigen in McCoy cell monolayers infected with Chlamydia trachomatis or Chlamydia psittaci. Infect Immun. 1981 Nov;34(2):561–570. doi: 10.1128/iai.34.2.561-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Fuller S. D. Cell surface polarity in epithelia. Annu Rev Cell Biol. 1985;1:243–288. doi: 10.1146/annurev.cb.01.110185.001331. [DOI] [PubMed] [Google Scholar]
- Small P. L., Isberg R. R., Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect Immun. 1987 Jul;55(7):1674–1679. doi: 10.1128/iai.55.7.1674-1679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Mellman I. S., Muller W. A., Cohn Z. A. Endocytosis and the recycling of plasma membrane. J Cell Biol. 1983 Jan;96(1):1–27. doi: 10.1083/jcb.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugarman B., Epps L. R. Effect of estrogens on bacterial adherence to HeLa cells. Infect Immun. 1982 Feb;35(2):633–638. doi: 10.1128/iai.35.2.633-638.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderlund G., Kihlström E. Effect of methylamine and monodansylcadaverine on the susceptibility of McCoy cells to Chlamydia trachomatis infection. Infect Immun. 1983 May;40(2):534–541. doi: 10.1128/iai.40.2.534-541.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Murray A. Control mechanisms governing the infectivity of Chlamydia trachomatis for HeLa cells: mechanisms of endocytosis. J Gen Microbiol. 1984 Jul;130(7):1765–1780. doi: 10.1099/00221287-130-7-1765. [DOI] [PubMed] [Google Scholar]
- Yewdell J. W., Bennink J. R., Hosaka Y. Cells process exogenous proteins for recognition by cytotoxic T lymphocytes. Science. 1988 Feb 5;239(4840):637–640. doi: 10.1126/science.3257585. [DOI] [PubMed] [Google Scholar]