Abstract
Background
The carbohydrate Sialyl Lewis X (sLeX) is expressed on leukocytes and carcinoma cells and binds to selectins during inflammatory processes and early metastasis. Synthesis of sLeX is dependent on activity of enzymes including α(1,3/1,4) fucosyltransferase (FucT-III). Tumor necrosis factor alpha (TNF-α) up-regulates FucT-III resulting in increased sLeX in the airways of patients with respiratory disease, however, the mechanisms that regulate sLeX in the inflammatory tumor microenvironment are not well understood.
Methods
We stably transfected human lung carcinoma cell lines with the FucT-III gene and exposed them to TNF-α to investigate its role in regulation of sLeX expression and selectin binding ability by semi-quantitative real-time polymerase chain reaction and flow cytometry. We examined cytokine expression of transfected cells using chemiluminescent arrays and ELISA, invasion using Matrigel assays, and alterations in morphology. Human lung tissue arrays were analyzed for immunohistochemical detection of sLeX and neutrophils.
Results
Stimulation of FucT-III transfected cells with recombinant human (rh) TNF-α upregulated sLeX expression and increased E-selectin binding. Transfected cells secreted high levels of IL-8, GRO-α and MCP-1. Cells exposed to rhTNF-α, neutrophil conditioned media, and 5:1 ratio cultures of neutrophils to cancer cells significantly increased sLeX expression and invasiveness, and underwent non-adherent morphological changes. In lung carcinomas but not in normal lung tissues, 71% of tumors were highly positive for sLeX expression in areas of increased neutrophil infiltration.
Conclusions
Neutrophils may be recruited to areas of FucT-III activity and sLeX expression in lung carcinomas to enhance the invasive and metastatic potential of lung cancer cells.
Keywords: cytokines, inflammation, neoplasms, neoplasm invasiveness, neoplasm metastasis, neutrophils, oligosaccharides, selectins
Introduction
Lung cancer is the leading cause of cancer-related deaths in the USA and worldwide. Of these cancers, 80% are non-small cell lung cancer (NSCLC) and mortality commonly is caused by locally invasive or recurrent metastatic disease.1 Alterations in tumor-associated carbohydrates occurs in many cancers and are highly correlated with decreased survival time because of metastasis.2 Specifically, the carbohydrate sialyl Lewis X (sLeX) is often associated with advanced malignancies and a poor prognosis in many carcinomas.3 Increased post-operative distant metastasis and mortality are linked to higher expression of sLeX on NSCLC cells.4 SLeX is a ligand for the selectin adhesion molecules (E-selectin, P-selectin, and L-selectin) of which endothelial E-selectin is the major target. Selectins modulate the binding of circulating inflammatory cells and tumor cells to blood vessels during inflammatory processes and metastasis.5
The contribution of inflammation to metastasis in NSCLC is not well-understood. Tumor-associated macrophages are the predominant myeloid cell thought to promote tumor progression, 6 but controversial results have been obtained for the role of macrophages in NSCLC. High numbers of tumor-infiltrating macrophages were reported to be associated with shorter relapse-free survival of patients.7 Alternatively, others did not find an association between macrophage count and outcome in NSCLC.8 Our focus was to investigate the influence of tumor-associated neutrophils on sLeX expression and metastatic properties of NSCLC cells because these factors could predict tumor advancement. Recent evidence suggests that neutrophils are pro-tumorigenic but their role in tumor progression is not well-defined.9 Infiltration of neutrophils in lung cancers is associated with a poor outcome.10 Tumor-elicited neutrophils have been found to increase tumor metastatic potential and invasiveness in a rat mammary adenocarcinoma model,11 and may regulate invasion of tumors by activation of matrix metalloproteinases that remodel the extracellular matrix.12, 13 Tumor-associated macrophages and neutrophils may promote a metastatic phenotype through secretion of the pro-inflammatory cytokine TNF-α.14, 15 In contrast to its better-known anti-cancer effects, TNF-α also has pro-tumorigenic activity in many cancers.16 In lung cancer, the role of TNF-α is not well-understood: it has been shown to up-regulate sLeX expression in a lung cancer cell line which may be associated with increased metastatic potential.17
Our study focused on NSCLC disease progression from a novel perspective. We investigated the interactions of neutrophils with NSCLC cells involving the tumor cell-surface carbohydrate sLeX and the contribution to malignant cellular invasion. Our results indicated that neutrophils induced sLeX expression on NSCLC cells and promoted their non-adherent growth and invasiveness by a TNF-α dependent mechanism. Our findings are applicable to the development of therapies to reduce NSCLC invasion and ultimately metastasis.
Materials and Methods
Cell Culture and Stable Transfections
H1299 and A549 NSCLC cell lines were obtained from American Type Culture Collection (Manassas, VA) and were authenticated and cultured in recommended medium under standard conditions. The human FucT-III gene was removed from a pRc/RSV vector (a gift from Dr. Rodger P. McEver, Oklahoma Medical Research Foundation, Oklahoma City, OK) and cloned into a pcDNA3.1(+)neo expression vector (Invitrogen) using HindIII and XbaI restriction enzymes to create the FucT-III/ pcDNA3.1(+)neo plasmid. The FucT-III/ pcDNA3.1(+)neo and pcDNA 3.1(+)neo plasmids were linearized by digestion with the Mfe I restriction enzyme. H1299 and A549 lung carcinoma cells were cultured in a 6-well plate (3.5×105 cells/well) for 24 hours. The cells were then transfected with 4 µg of each linearized plasmid using Lipofectamine 2000 (Invitrogen) and incubated for 48 hours. Heterogeneous populations of stably FucT-III transfected and mock-transfected cells were selected with 1 mg/ml G418 (Sigma-Aldrich, St. Louis, MO) for 14 days before use in the experiments. Multiple aliquots of selected, stable transfectants were also stored in liquid nitrogen. Transfected cells were maintained in medium containing G418 for up to one month after which time cells were discarded and new aliquots thawed for use. FucT-III gene activity was verified by analysis of mRNA levels by semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR) and by analysis of sLeX expression by flow cytometry as described below.
Flow Cytometric Analysis
The sLeX epitope on cells was detected using the monoclonal antibodies (mAbs) (10 µg/ml) mouse anti-human IgM CSLEX-1 (generously provided by Dr. Bruce Walcheck, University of Minnesota) or an IgM isotype control (Caltag Laboratories, Burlingame, CA). The secondary antibody was phycoerythrin (PE) conjugated F(ab’)2 goat anti-mouse IgM (Jackson Immunoresearch Laboratories, West Grove, PA). For the adhesion assays, calcium-dependent cell binding was tested using 10 µg/ml of a mouse E-selectin/human Fc chimera (R & D Systems, Minneapolis, MN) in buffer containing 2mM CaCl2 with or without 2mM EDTA (a calcium ion chelator), or with 10 mM EDTA alone as controls. PE conjugated F(ab’)2 goat anti-human IgG (Jackson Immunoresearch Laboratories) was used to detect bound E-selectin chimera. Data acquisition and analysis were performed using a FACSCanto instrument and BD FACSDiva software (Becton-Dickinson Biosciences, San Jose, CA).
Cytokine Secretion Analysis
Cellular supernatants were collected after 72 hours of culture in serum-free media and cytokine secretion was detected with the Human Cytokine Antibody Array 1 system (Ray Biotech, Inc., Norcross, GA) using chemiluminescence. The data were quantified by densitometry using Image J software (National Institutes of Health, Bethesda, MD). Secreted IL-8 levels were analyzed with the human IL-8 ELISA kit (Ray Biotech, Inc.) using a SpectraMax Plus 385 microplate reader at an optical density at 450nm and SoftMax Pro 3.1.1 software (Molecular Devices, Sunnyvale, CA).
Stimulation Assays with Neutrophils, Neutrophil Conditioned Media (NCM), or Recombinant Human TNF-α (rhTNF-α)
Neutrophils were isolated from peripheral blood of human healthy donors as previously described,18 and with the approval of the Institutional Review Board: Human Subjects Committee at the University of Minnesota. Cell viability was determined by trypan blue exclusion. NCM was obtained from neutrophils (1.25×106 cells/ml) by culturing in opti-MEM with penicillin/streptomycin for 2 hours. After serum-starvation of H1299 cells for 24 hours, cells were cultured with neutrophils, NCM, or rhTNF-α for 48 hours. An optimal concentration of 5 ng/ml rhTNF-α was used for stimulation of sLeX expression on H1299 cells. Neutrophils were labeled with carboxyfluorescein succinimidyl ester (CFSE) fluorescent dye (Molecular Probes, Eugene, Oregon) and then added to H1299 cells in the following neutrophil to cancer cell ratios: H1299 cells alone, 5:1 (1.25 × 106 neutrophils), 1:1 (2.5 × 105 neutrophils), and 1:5 (5 × 104 neutrophils) and an optimal 1:1 ratio was used in the experiments.
Cells were also cultured with a LEAF purified TNF-α blocking antibody (10 µg/ml, BioLegend, San Diego, CA) or an isotype-matched control mAb (Invitrogen) for 48 hours. H1299 cells were analyzed for cell-membrane expression of sLeX using CSLEX1 mAb and for TNF-receptor 1 (TNF-R1) using an anti-human TNF-RI antibody (10µg/ml, R & D Systems, Minneapolis, MN) and neutrophils were distinguished from cancer cells based on their morphology by forward and side scatter flow cytometric analysis. Changes in cellular morphology of cultured cells were assessed by light and fluorescent microscopic analysis at 100X magnifications.
RNA Isolation and Semi-Quantitative RT-PCR Analysis
FucT-III gene expression in H1299 cells either un-stimulated or stimulated with 5 ng/ml rhTNF-α or NCM for 24 and 48 hours was verified by reverse transcriptase polymerase chain reaction (RT-PCR) by amplification for 35 cycles as previously described.19 FucT-III primers were sense: 5’-CCT CCT GAT CCT GCT ATG GA-3’ and antisense: 5’-GCG GTA GGA CAT GGT GAG AT-3') (Genbank # NM_000149), and β-actin primers were sense: 5’- GCT CGT CGT CGA CAA CGG CT-3' and antisense: 5’- CAA ACA TGA TCT GGG TCA TCT TCT C - 3') (Genbank # NM_001101).
Histopathology
Duplicate cases of 36 common human lung cancers, 4 normal, and 8 inflamed lung tissues were obtained from the Tissue Procurement Facility, University of Minnesota or a human lung cancer tissue array (Pantomics, Inc., San Francisco, CA). Tissues were used in accordance with the regulations of the University of Minnesota's Institutional Review Board. Sections were of 4 µm thickness, fixed in 10% neutral buffered formalin, and embedded in paraffin. Specimens were stained with hematoxylin & eosin (H&E) by the University of Minnesota’s Comparative Pathology Core Resource Facility.
Immunohistochemistry
Tissue sections were analyzed for the presence of the sLeX epitope using CSLEX1 mAb and for cytokeratins using a mouse IgG1 mAb Ab-3, clone Lu-5 that reacts with epithelial cells (Thermo Fisher Scientific, Fremont, CA) as previously described.19 The staining procedure was modified to detect neutrophil elastase using a rabbit polyclonal primary antibody (0.5 µg/ml, Abcam, Cambridge, MA). All antibody incubations were performed at 30°C. Pepsin Solution Digest-All 3 ready-to-use reagent (Invitrogen) was used for antigen retrieval. The secondary antibody was horseradish peroxidase (HRP)-conjugated donkey anti-rabbit IgG (BioFX Laboratories, Owings Mills, MD) at a 1:500 dilution.
Histologic Grading System
Lung tissues were evaluated semi-quantitatively for reactivity with CSLEX-1 mAb to indicate sLeX positive cells and quantitatively for reactivity with the neutrophil elastase antibody to determine the number of neutrophils present in tissues. For individual lung tissues, sections were evaluated in five random fields at 100X magnification. For the tissue array, the entire tissue core was examined. Positive staining was defined as cells in which cytoplasmic and/or cell membrane labeling with the relevant antibody was detected. For both antibodies, the presence of positive staining was scored from 0–3. For CSLEX-1 mAb reactivity, score 0, no visible immunostaining; score 1, less than 25% of the total tissue area was positive; score 2, 26–50% of the total tissue area was positive; and score 3, greater than 50% of the total tissue area was positive. For reactivity with neutrophil elastase antibody, score 0, cells were not visibly immunostained; score 1, 1–10 cells in the total tissue area were positive; score 2, 11–35 cells in the total tissue area were positive; score 3, more than 35 cells in the total tissue area were positive.
Invasion Assays
Twenty-four well BioCoat™ Matrigel™ Invasion Chambers (Becton-Dickinson, Bedford, MA, USA) were used for the tumor cell invasion assays according to the manufacturer’s instructions. Chambers were cultured for 24 hours under standard conditions. The invading cells were stained and counted in five random fields at 100X magnification. Digital images were captured using a Zeiss Axiovert 200 inverted microscope and an AxioCam MRc camera using AxioVision 4.1 software (Carl Zeiss Inc., Germany).
Statistical Analyses
Differences between groups were analyzed using a two-tailed Student’s t test, with two-sample unequal variance where appropriate. Reported p-values were considered significant at p ≤ 0.05.
Results
Characteristics of Transfected H1299 Cells and Response to rhTNF-α
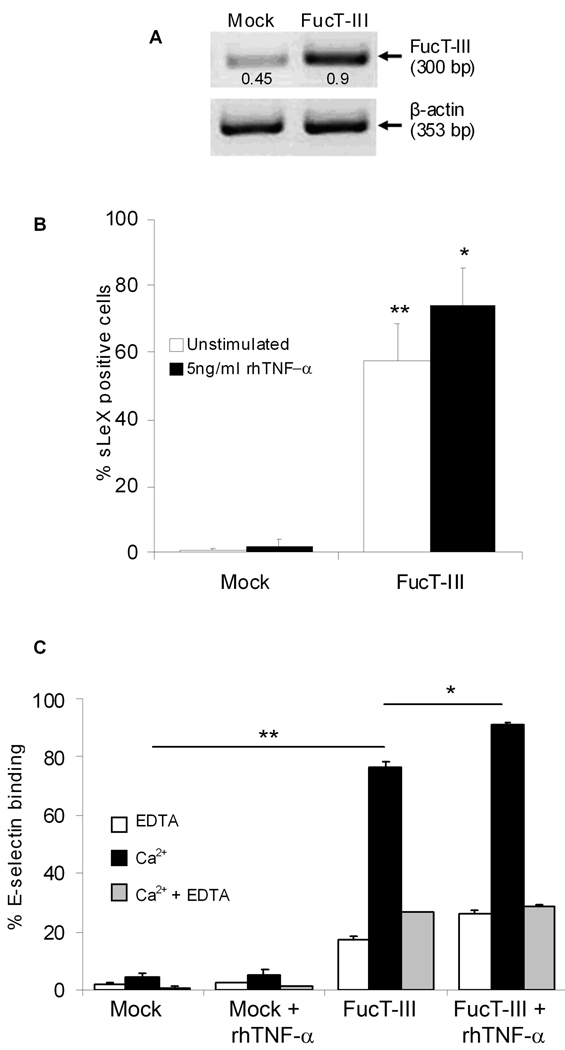
Stably transfected H1299 and A549 cells but not mock-transfected cells highly expressed FucT-III mRNA by RT-PCR and were sLeX positive by flow cytometry using CSLEX-1 mAb. Data are shown only for transfected H1299 cells that were 57% sLeX positive compared to mock-transfected cells (p < 0.01) (Figure 1A, B). Cell viability after transfection was 98–99%. We investigated the effects of TNF-α on sLeX expression because it is one of the major cytokines produced in a tumor microenvironment. We stimulated cells with rhTNF-α between 1 – 100 ng/ml concentrations for 24 and 48 hours. We found that exposure to 5 ng/ml rhTNF-α for 48 hours was the lowest dose that produced significant up-regulation of sLeX expression on FucT-III transfected H1299 cells (74%), compared to mock-transfected cells (p < 0.01) and expression was higher than un-stimulated, transfected cells (74% versus 57%, p < 0.05, Figure 1B). All groups of cells expressed similar levels of TNF-R1 (57–63%, data not shown).
Figure 1. H1299 α(1,3/1,4) fucosyltransferase (FucT-III) transfected lung carcinoma cells express sLeX and bind to E-selectin.
(A) FucT-III transfected cells express 2-fold higher mRNA levels of FucT-III than mock-transfected cells by reverse transcriptase-polymerase chain reaction. Experiments were repeated 5 times and 1 representative example is shown. Band intensities were normalized to β-actin by densitometry (bp indicates base pairs). (B) The percentage of sLeX positive cells is increased after FucT-III transfection and is up-regulated further after stimulation with 5 ng/ml rhTNF-α for 48 hours. Significant differences are observed between un-stimulated, mock-transfected cells and un-stimulated, FucT-III transfected cells, (double asterisks; p < 0.01) and between un-stimulated, FucT-III transfected cells and cells stimulated with rhTNF-α (single asterisk; p < 0.05). (C) FucT-III transfected H1299 cells substantially bind to E-selectin in the presence of calcium ions (Ca2+) compared to mock-transfected cells(double asterisk; p < 0.01), and binding is further enhanced after stimulation with rhTNF-α, (asterisk; p < 0.05) compared with un-stimulated, transfected cells. EDTA indicates ethylene diamine tetraacetic acid. Mean values of up to 5 experiments are shown.
The E-selectin binding ability of mock-transfected and FucT-III transfected H1299 and A549 cells expressing the sLeX epitope that were either un-stimulated or stimulated with 5 ng/ml rhTNF-α was investigated by flow cytometry using a mouse E-selectin chimera. Mock-transfected H1299 cells did not appreciably bind to E-selectin regardless of whether or not they were stimulated with rhTNF-α whereas un-stimulated, FucT-III transfected H1299 cells bound to E-selectin in a calcium-dependent manner (80%, p < 0.01, Figure 1C). After stimulation with rhTNF-α, E-selectin binding of transfected H1299 cells was further significantly increased (92%, p < 0.05). As expected, appreciable binding did not occur in the presence of EDTA. Similar results were obtained for FucT-III transfected and mock-transfected A549 cells (data not shown). These results indicate that NSCLC cells expressing sLeX but not those cells lacking sLeX specifically bound to E-selectin and that binding was responsive to stimulation with TNF-α.
FucT-III Transfected H1299 Cells Secrete Neutrophil Chemoattractants
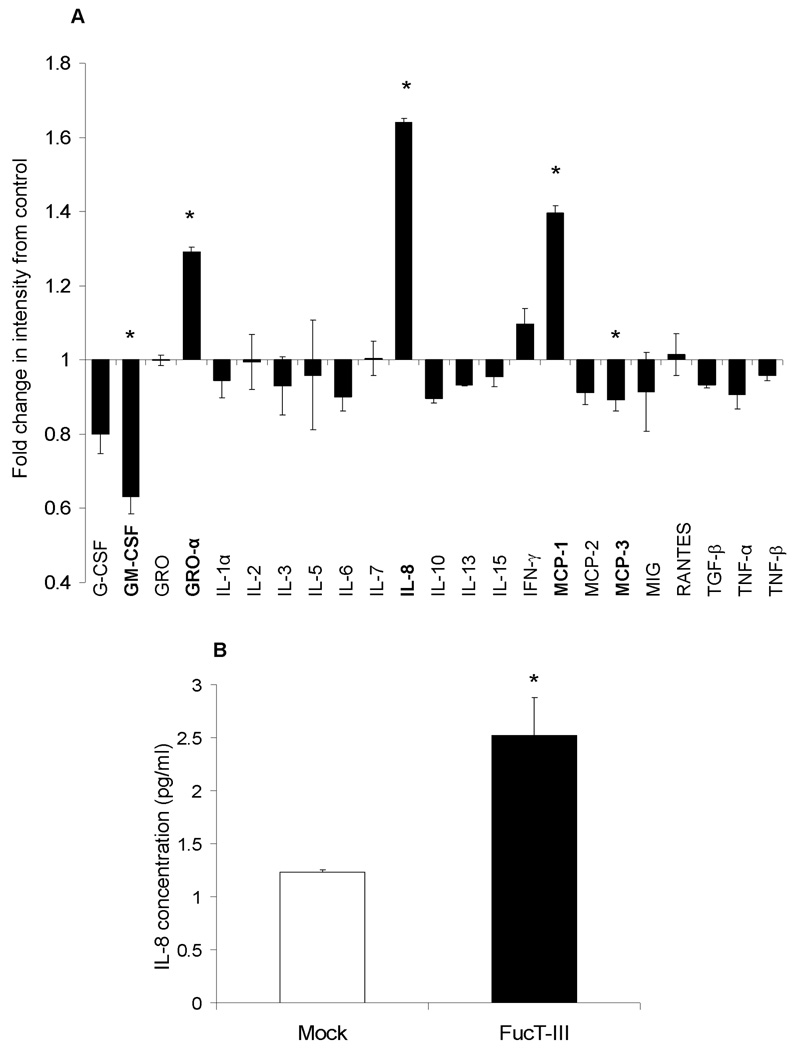
To investigate whether FucT-III gene expression influenced characteristics that might impact invasion and metastasis, we compared the cytokine expression profile of FucT-III transfected H1299 cells to that of un-manipulated cells after 72 hours in culture using a human anti-cytokine antibody membrane array. Transfected cells secreted increased levels of GRO-α, IL-8, and MCP-1 but levels of GM-CSF and MCP-3 were down-regulated (p < 0.05, Figure 2A). Because IL-8 is a potent neutrophil chemoattractant, we quantified IL-8 secretion using a human IL-8 ELISA kit. FucT-III transfected cells secreted 2-fold higher levels of IL-8 than mock-transfected cells (p < 0.05, Figure 2B). Our results suggest that sLeX expression on NSCLC cells is associated with up-regulated secretion of neutrophil chemoattractants.
Figure 2. H1299 cells transfected with α(1,3/1,4) fucosyltransferase (FucT-III) secrete neutrophil chemoattractants.
(A) FucT-III–transfected cells secrete high levels of growth-regulated oncogene-alpha (GRO-α), interleukin-8 (IL-8), and mast cell proteinase-1 (MCP-1) but decreased levels of granulocyte macrophage–colony-stimulating factor (GM-CSF) and MCP-3 compared with un-manipulated cells (asterisks; p < 0.05). GCSF indicates granulocyte–colony-stimulating factor; MIG, monokine induced by interferon-c; RANTES, regulated upon activation, normal T-cell expressed and secreted; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α. (B) FucT-III–transfected H1299 cells secreted 2-fold higher levels of IL-8 than mock-transfected cells when quantified by an enzyme-linked immunosorbent assay (asterisk; p < 0.05). The average of 2 independent experiments is shown and each experiment was repeated in duplicate.
FucT-III Transfected H1299 Cells Cultured with Neutrophils or with Neutrophil Conditioned Media (NCM) Up-regulate sLeX
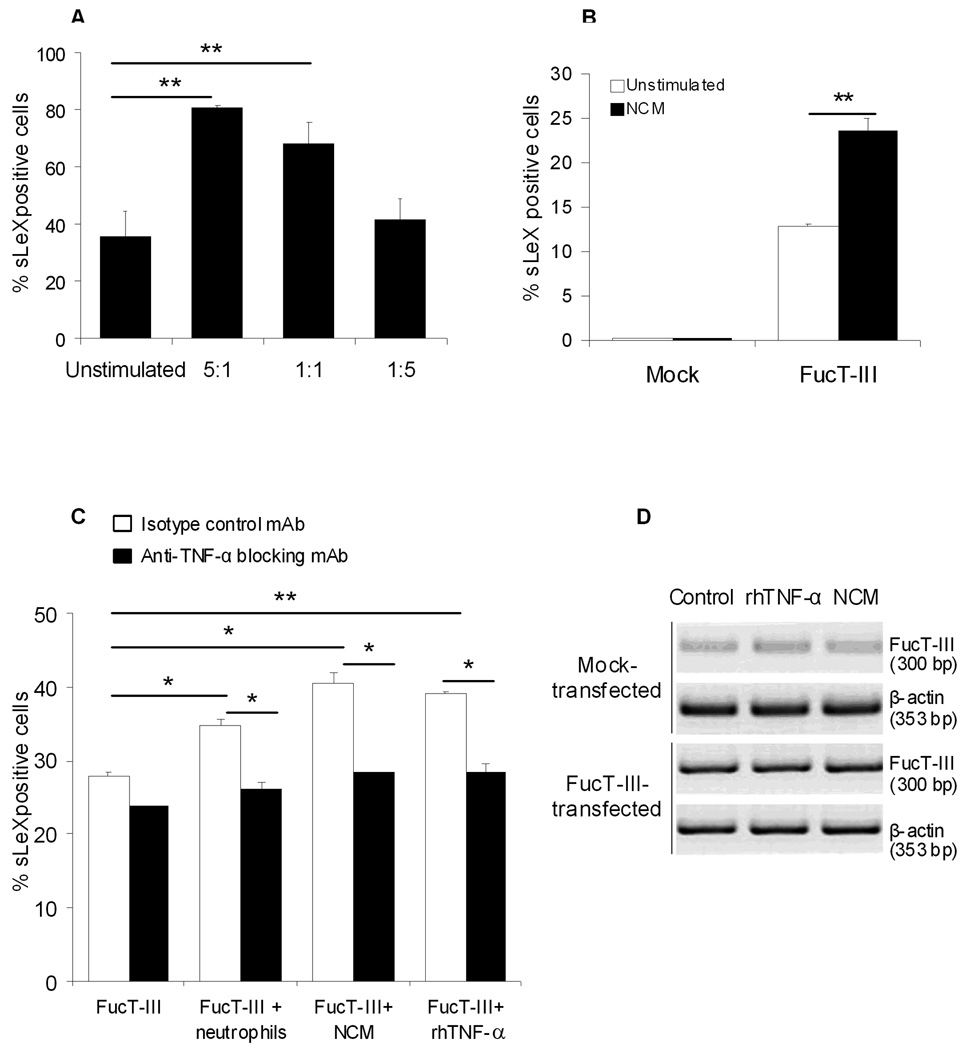
We investigated whether neutrophils regulated sLeX expression on H1299 cells. Serum-starved cells were cultured for 48 hours with neutrophils or with neutrophil conditioned media (NCM) and the effects on sLeX expression were determined by flow cytometry. After culture of neutrophils with FucT-III transfected cells at 5:1 and 1:1 ratios, the latter cells were 81% and 68% sLeX positive respectively compared to un-stimulated FucT-III transfected cells (36%, p < 0.01, Figure 3A). After stimulation with NCM, 24% of FucT-III transfected cells were sLeX positive compared to un-stimulated FucT-III transfected cells (13%, Figure 3B). Our results suggested that neutrophils and their secreted factors stimulated sLeX expression on NSCLC cells.
Figure 3. Tumor necrosis factor-α (TNF-α) up-regulates sialyl Lewis X (sLeX) expression.
(A) At ratios of 5:1 and 1:1 of neutrophils to FucT-III–transfected cells in coculture, significantly higher percentages of cells were positive for sLeX compared with un-stimulated cells (double asterisks; p < 0.01). (B) A significantly higher percentage of α (1,3/1,4) fucosyltransferase (FucT-III)-transfected cells were positive for sLeX after culture with neutrophil-conditioned media (NCM) compared with unstimulated, FucT-III–transfected cells (double asterisks; p < 0.01). (C) Increased percentages of sLeX-positive cells were observed when FucT-III–transfected H1299 cells treated with an isotype control monoclonal antibody (mAb) were cultured with a 1:1 ratio of neutrophils or NCM (single asterisk; p < 0.05), or 5 ng/mL recombinant human TNF-α (rhTNF-α) (double asterisks; p < 0.01) for 48 hours. In the presence of an anti-TNF-α –blocking mAb, sLeX-positive cells were down-regulated compared with control mAb-treated cells when cultured with neutrophils, NCM, or rhTNF-α (single asterisk; p < 0.05). Mean values from 5 experiments are shown for A through C. (D) FucT-III messenger RNA levels are unchanged in H1299 mock-transfected and FucT-III–transfected cells after stimulation with rhTNF-α or NCM for 48 hours. A representative experiment with 2 repetitions is shown (bp indicates base pairs).
Up-regulation of sLeX by Neutrophils and NCM is TNF-α Dependent
We treated FucT-III transfected H1299 cells with an anti-TNF-α blocking mAb or an isotype-matched control mAb (10 µg/ml) in the presence of a 1:1 ratio of neutrophils, NCM, or 5 ng/ml rhTNF-α for 48 hours to determine whether TNF-α could be responsible for the up-regulation of sLeX. SLeX expression was significantly increased on control mAb treated, FucT-III transfected cells when cultured with neutrophils or NCM, (p < 0.05), or with rhTNF-α (p < .01) compared to cells exposed to control mAb alone (Figure 3C). In the presence of an anti-TNF-α blocking mAb, sLeX expression was significantly down-regulated on FucT-III transfected cells cultured with neutrophils, NCM, or rhTNF-α compared to similarly cultured cells treated with the control mAb (p < 0.05). Antibody treatment did not affect basal sLeX expression. Our findings suggest that neutrophil-derived TNF-α stimulates sLeX production on NSCLC cells expressing the FucT-III gene.
Steady State FucT-III mRNA levels are not Altered by rhTNF-α and NCM
To determine whether the up-regulation of sLeX that we observed after stimulation of FucT-III transfected H1299 cells with rhTNF-α or NCM was because of increased steady state mRNA levels of the FucT-III gene, we compared FucT-III mRNA levels in un-stimulated cells and cells stimulated with 5ng/ml rhTNF-α or NCM for 24 or 48 hours by RT-PCR. We did not examine mRNA from co-cultures of H1299 cells and neutrophils because they were indistinguishable. FucT-III mRNA levels were similar between un-stimulated cells and those stimulated with rhTNF-α or NCM for 24 or 48 hours. The 48 hour time point is shown in Figure 3D. Although not conclusive, the results suggest that molecular events downstream of synthesis of primary transcript mRNA may be regulated by neutrophil-derived TNF-α.
Neutrophils, Neutrophil Conditioned Media (NCM), and rhTNF-α induce morphological changes in H1299 cells
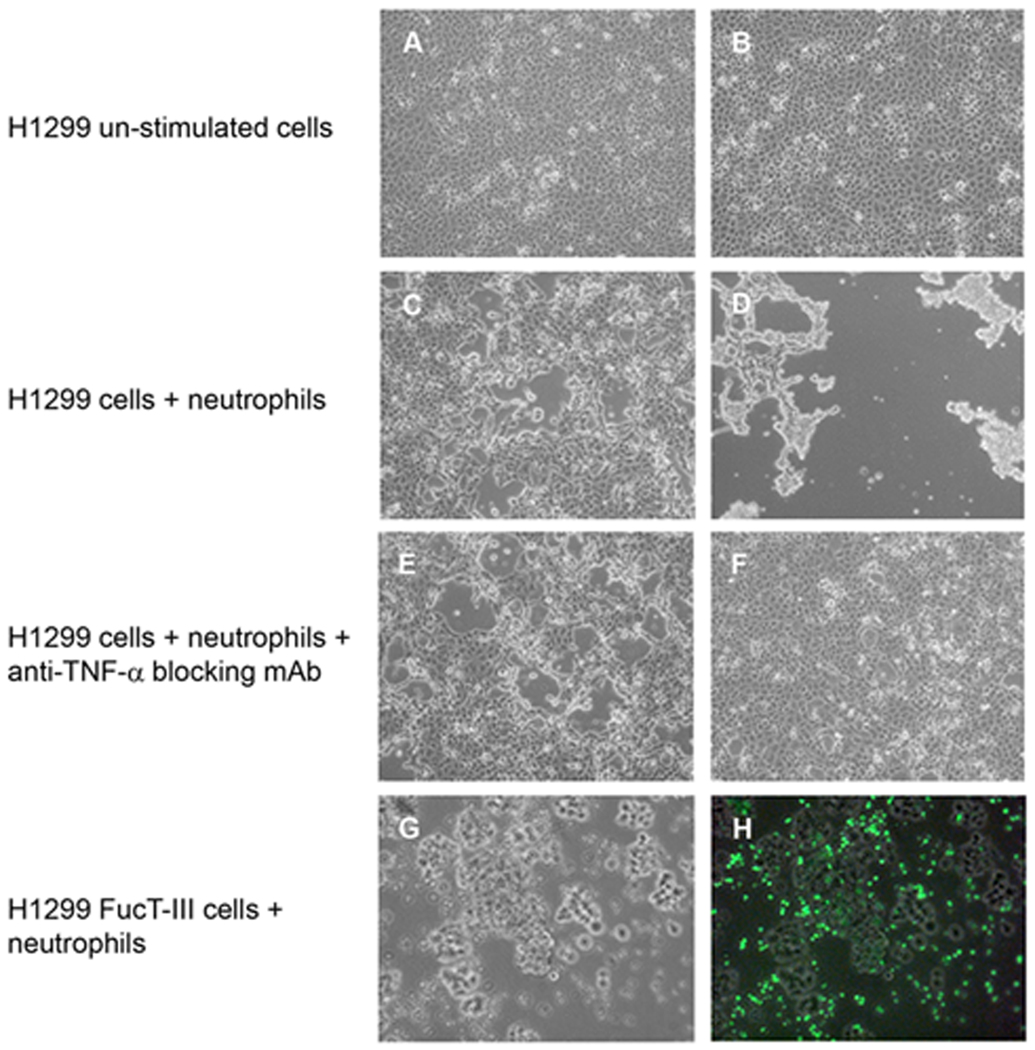
We assessed whether FucT-III transfected H1299 cells or mock-transfected cells co-cultured with neutrophils, NCM, or rhTNF-α for 48 hours underwent morphological changes indicative of a more metastatic phenotype. At 100X magnification, the morphology of un-stimulated FucT-III transfected H1299 cells was not altered from that of un-stimulated mock-transfected cells (Figure 4A, B) and was similar to that of a 1:1 ratio of neutrophils co-cultured with mock-transfected H1299 cells in the absence or presence of 10 µg/ml of an anti-TNF-α blocking mAb (Figure 4C, E). FucT-III transfected H1299 cells co-cultured with neutrophils formed viable non-adherent aggregates of elongated cells (Figure 4D). When co-cultures were exposed to the anti-TNF-α blocking mAb, the cancer cells reverted to their original adherent morphology (Figure 4F). In co-cultures, CFSE-labeled neutrophils were viable but distinct from FucT-III transfected cell aggregates when examined at 100X magnification under bright-field (Figure 4G) and fluorescent (Figure 4H) microscopy. In the absence of cancer cells, most neutrophils died in culture after 48 hours (data not shown).
Figure 4. H1299 cells cultured with neutrophils have TNF-α-dependent morphological alterations.
Photomicrographs depicting the morphology of (A) H1299 mock-transfected cells and (B) FucT-III transfected cells under un-stimulated conditions are shown. The appearance of (C) H1299 mock-transfected cells cultured for 48 hours with a 1:1 ratio of neutrophils to cancer cells resembles that of un-stimulated cells; however (D), FucT-III transfected cells under the same conditions as in (C) form viable, non-adherent aggregates of elongated, dendritic-like cells. H1299 mock-transfected cells cultured with neutrophils and an anti-TNF-α function blocking mAb (E) are morphologically similar to the cells in (A) and (C), whereas (F) FucT-III transfected cells under the same conditions as in (E) revert to a morphology that is similar to un-stimulated cells shown in (B) (original magnification, 100X in A-F). FucT-III transfected cells that were cultured with CFSE-labeled neutrophils are seen under (G) bright-field microscopy and (H) fluorescent microscopy (original magnification, 100X). Neutrophils are not present in the aggregates of cancer cells. Representative images from 3 independent experiments are shown.
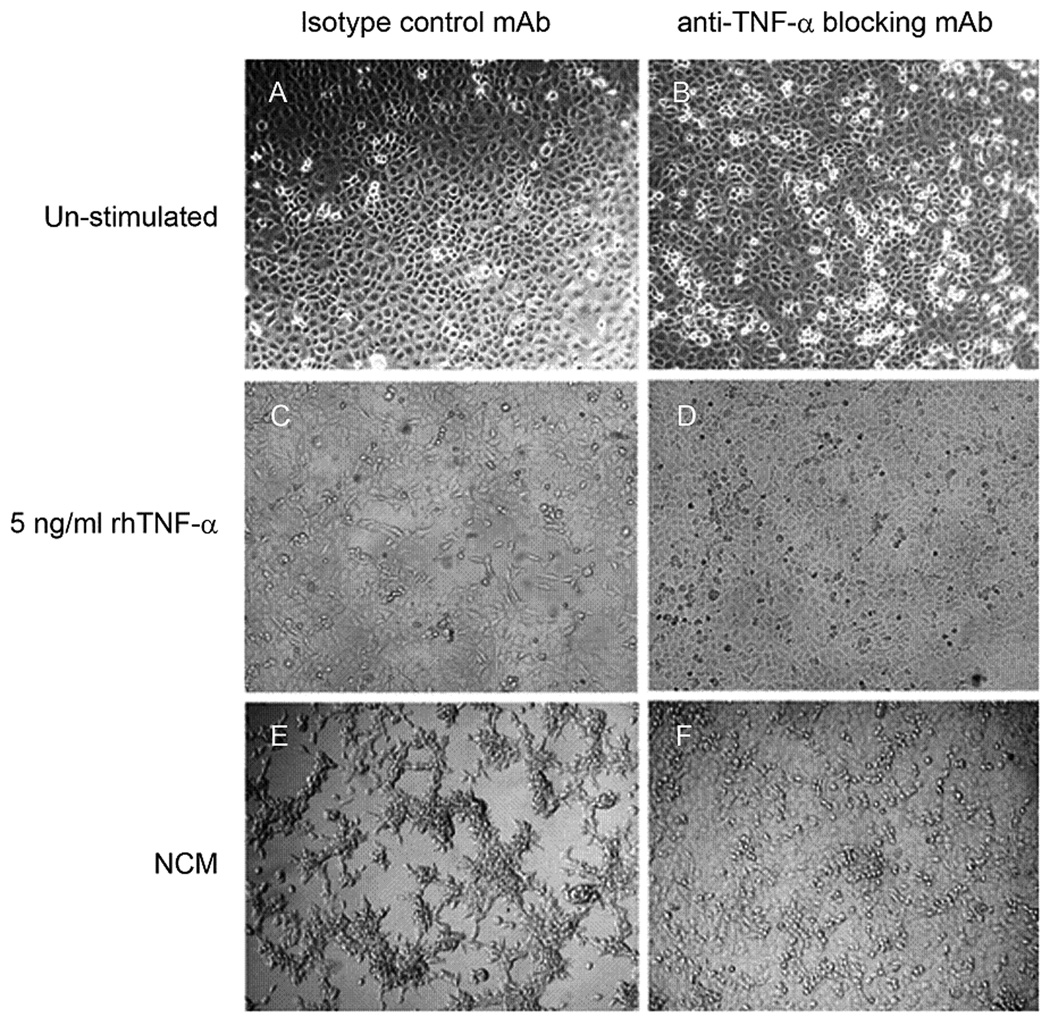
After 48 hours of culture, we did not observe morphological differences between un-stimulated FucT-III transfected H1299 cells treated with the isotype control mAb and those cells treated with the anti-TNF-α blocking mAb (10 µg/ml), (Figure 5A, B). Control mAb treated, FucT-III transfected cells cultured with 5 ng/ml rhTNF-α formed an adherent monolayer of elongated cells and similarly treated cells cultured with NCM grew as adherent, elongated colonies (Figure 5C, E). In contrast, FucT-III transfected cells treated with the anti-TNF-α blocking mAb and cultured with 5 ng/ml rhTNF-α or with NCM grew as a monolayer with a similar appearance to un-stimulated cells (Figure 5B, D, F). Our results suggest that TNF-α produced by neutrophils caused the altered morphology of cancer cells.
Figure 5. H1299 α(1,3/1,4) fucosyltransferase (FucT-III)-transfected cells cultured with recombinant human tumor necrosis factor-α (rhTNF-α) or neutrophil-conditioned media (NCM) have TNF-α–dependent morphologic alterations.
The morphology of unstimulated FucT-III–transfected H1299 cells cultured with (A) an isotype-matched control monoclonal antibody (mAb) or (B) or with an anti-TNF-α function-blocking mAb is similar. (C) Control mAb-treated cells that were cultured with 5 ng/mL rhTNF-a became elongated but reverted back to the appearance of un-stimulated cells after exposure to 10 μg/mL of an anti-TNF-α function-blocking mAb (D). (E) Transfected cells exposed to NCM formed aggregates of elongated cells, and this morphology was reversible after treatment with anti-TNF-α mAb (F). A representative experiment from 3 repetitions is shown (original magnification, 100X).
FucT-III regulates the invasiveness of H1299 cells which is enhanced by rhTNF-α stimulation
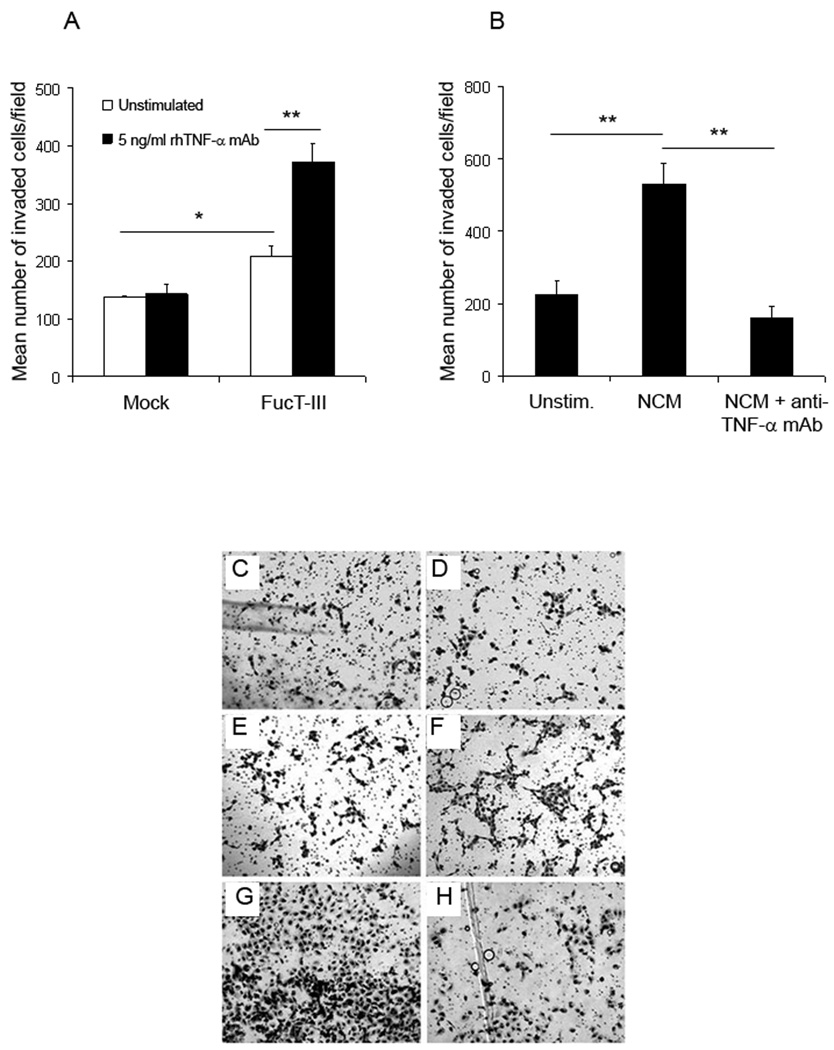
In order to determine whether the morphological changes that we observed in H1299 cells were indicative of alterations in their metastatic behavior, we examined the invasiveness of cells, which is a key metastatic property, using in vitro Matrigel invasion assays. Un-stimulated FucT-III transfected H1299 cells were more invasive than mock-transfected cells (p < 0.05, Figure 6A, C, E). After stimulation with 5 ng/ml rhTNF-α for 24 hours, FucT-III transfected cells but not mock-transfected cells were more invasive than un-stimulated cells (p < 0.01, Figure 6A, C, D, E, F). FucT-III transfected cells stimulated with NCM for 24 hours were more invasive than un-stimulated cells (Figure 6B, G, p < 0.01). Cells that were treated with the anti-TNF-α blocking mAb and cultured with NCM were less invasive than those cultured with NCM alone (Figure 6B, H, p < 0.01). The results indicate that sLeX expression on H1299 cells enhanced their invasiveness which was further augmented by stimulation with TNF-α.
Figure 6. The invasiveness of H1299 α(1,3/1,4) fucosyltransferase (FucT-III)-transfected cells stimulated with recombinant human tumor necrosis factor-α (rhTNF-α) or neutrophil-conditioned media (NCM) is up-regulated.
(A,E) Un-stimulated, H1299 FucT-III–transfected cells are more invasive than unstimulated, mock-transfected cells (C) after 24 hours in Matrigel assays (single asterisk; p < 0.05). The invasiveness of FucT-III–transfected cells (A,F), but not of mock-transfected cells (A,D), is up-regulated further after exposure to 5 ng/mL rhTNF-a (double asterisks; p < 0.01; A). (B,G) Exposure to NCM increases the invasiveness of FucT-III–transfected cells compared with un-stimulated, FucT-III transfected cells (E), and treatment with an anti-TNF-a monoclonal antibody (H) abrogates this effect compared with cells that were cultured with NCM alone (double asterisks; p < 0.01; B). Mean values of 3 experiments are shown in A and B, and representative photomicrographs of cells are shown in C–H (original magnification, 100X).
Neutrophils co-localize with sLeX positive lung adenocarcinoma and squamous cell carcinoma cells
We examined 36 duplicate patient samples of human lung cancers and 4 normal lung tissues for sLeX expression and neutrophil infiltration by immunohistochemistry. Of these malignant lung tumors, 12 were adenocarcinomas and 12 were squamous cell carcinomas. The remaining 12 malignant tumors consisted of 3 adenosquamous carcinomas, 3 small cell carcinomas, 1 each of a bronchioalveolar and a large cell carcinoma, and 4 papillary carcinomas. We also examined an additional 8 inflamed tissues. We found that 78% of the malignant lung cancers were reactive with CSLEX1 mAb indicating that these tissues were sLeX positive (sLeX staining scores of 1–3). Of these malignant tumors, 61% were tumors in which more than 25% of the total tissue area examined was sLeX positive (scores of 2–3). Higher numbers of neutrophils were stained with neutrophil elastase antibody (neutrophil scores of 2–3) in 71% of highly sLeX positive tumor tissue samples with scores of 2–3. All of the inflamed tissues contained high numbers of stained neutrophils and 63% were sLeX positive.
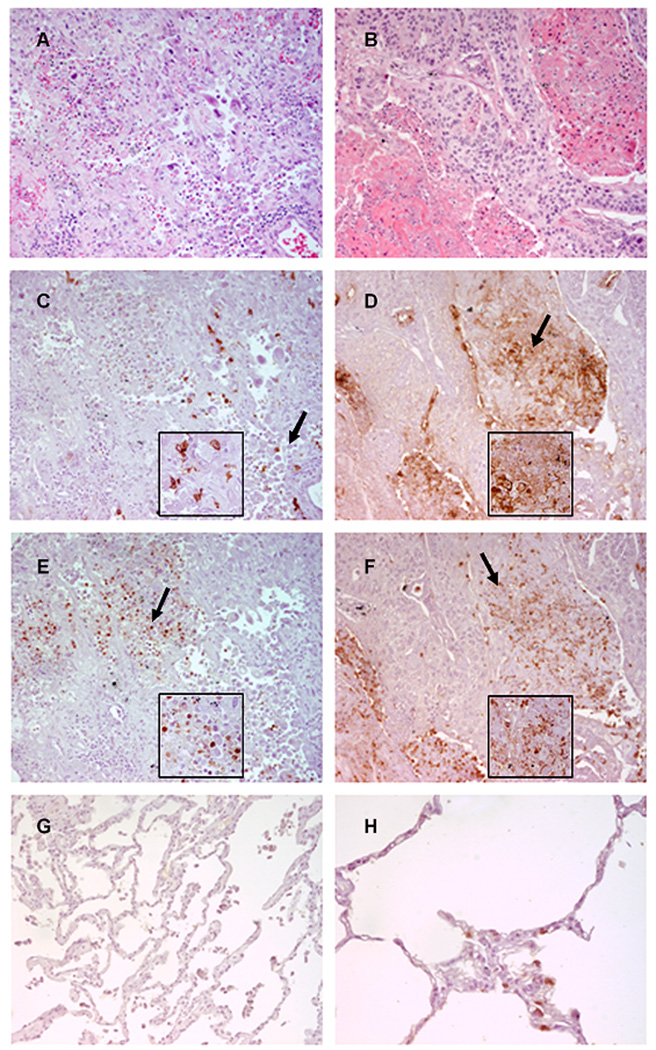
We focused on adenocarcinomas and squamous cell carcinomas because they are common tumor types. All 12 adenocarcinomas were sLeX positive and 83% of these sLeX positive tumors were infiltrated with neutrophils. All 12 squamous cell carcinomas contained neutrophils and 50% had high neutrophil scores of 2–3. Of these squamous cell carcinomas, 83% were sLeX positive. In contrast, all 4 normal lung tissues were sLeX negative but contained variable numbers of neutrophils. Although our sample size was too small to draw statistical conclusions, we observed that in 75% of adenocarcinoma samples, and 58% of squamous cell carcinoma samples, areas of positive sLeX expression were similar to areas of neutrophil infiltration. Representative examples of these results are shown in Figure 7.
Figure 7. Neutrophils are recruited to sialyl Lewis X (sLeX)-positive areas of human lung carcinomas.
These are representative photomicrographs of serial sections from (A,C,E) a lung adenocarcinoma and (B,D,F) a squamous cell carcinoma (original magnification, 200X). Insets show the areas of tissue indicated by the arrows (original magnification, 400X). Sections were stained with (A,B) H&E, (C,D) CSLEX-1 monoclonal antibody (mAb), and (E,F) neutrophil elastase antibody. Positively stained cells are brown. (G) This normal lung tissue sample was stained with the CSLEX-1 mAb (original magnification, 200X). Note the absence of brown color indicating positive staining. (H) This normal lung tissue sample was stained with neutrophil elastase antibody (original magnification, 400X). Positively stained neutrophils are brown.
Discussion
Despite recent advances made in earlier diagnosis and treatment, NSCLC continues to have a poor prognosis and survival and these outcomes are commonly because of locally invasive or metastatic disease.1 We expect that identification of novel molecular and cellular determinants that contribute to disease progression of NSCLC will lead to the development of strategies to inhibit or limit cancer growth, invasion, and metastasis and improve therapeutic efficacy. We investigated the influence of neutrophils and TNF-α on regulation of sLeX expression in NSCLC and the importance of these interactions in promoting metastatic properties of tumor cells.
The cytokine TNF-α is produced predominantly by macrophages and T-cells but also by neutrophils and regulates diverse cellular functions including inflammation and immunity, homeostasis, and apoptosis. TNF-α also participates in cell-mediated killing and necrosis of certain tumors.20 Recent evidence suggests that tumor-associated neutrophils promote a metastatic phenotype through secretion of TNF-α.14,15 TNF-α regulates expression of sLeX epitopes on human lung carcinomas.17, 21 NSCLCs with high expression of sLeX have an increased metastatic potential.4 Using NSCLC cell lines, we show here that FucT-III gene expression resulted in production of cell membrane sLeX, release of neutrophil chemoattractants, and increased invasiveness of tumor cells. Neutrophil-derived TNF-α stimulated sLeX expression on tumor cells, caused them to be non-adherent, and further enhanced their invasiveness. These changes were indicative of increased metastatic behavior of NSCLC cells. In human lung specimens of NSCLC, we found that neutrophils were recruited to tumor areas of sLeX expression. Thus, carbohydrate-mediated interactions between NSCLC cells and neutrophils may facilitate invasion and possibly metastasis of tumor cells.
Using an in vivo rat model, tumor-elicited neutrophils but not normal neutrophils, were shown to enhance metastatic potential and invasiveness of tumor cells.11 We are currently testing the potential mechanisms involving sLeX and neutrophils that could be responsible for the increased invasiveness of NSCLC cells. It is well established that invasion and metastasis of malignant cells requires degradation of basement membranes and the extracellular matrix involving the matrix metalloproteinases MMP-2 and MMP-9. Increased levels of MMP-2 and MMP-9 as well as sLeX in NSCLC have been correlated with an advanced stage of disease, distant metastasis, and poor survival of patients.4,22,23 SLeX is synthesized by members of the family of alpha 2,3-sialyltransferases and alpha-1,3/4 fucosyltransferases (FucTs),24, 25 FucT-III is up-regulated in NSCLC and determines the amount of sLeX antigens in lung cancer tissues.26 We speculate that in NSCLC, the FucT-III enzyme may glycosylate and up-regulate the expression or activity of MMP-2, MMP-9, or other matrix metalloproteinases resulting in more invasive behavior of tumor cells. In our study, steady state FucT-III mRNA levels were unchanged after stimulation of FucT-III transfected H1299 cells with TNF-α or NCM although sLeX expression was increased. It is conceivable that post-transcriptional modifications of FucT-III mRNA in these cells may have occurred after exposure to TNF-α or NCM such as alterations in mRNA stability or in mRNA processing and transport. Alternatively, FucT-III enzyme function may be changed by post-translational modifications of the protein such as glycosylation or phosphorylation in response to TNF-α stimulation that may result in up-regulation of sLeX. These possibilities require further investigation.
Many cancers are able to recruit neutrophils that release substances to modify tumor growth and invasiveness.27 The presence of tumor-associated neutrophils in bronchioloalveolar carcinoma has been linked to poor clinical outcomes including decreased survival, and these tumor cells produce IL-8 which is a chemoattractant for neutrophils.10 In agreement with these studies, we found that H1299 FucT-III transfected cells expressing sLeX produced significantly increased levels of the neutrophil chemoattractants GRO-α, MCP-1, and IL-8. In addition, sLeX expression and invasiveness of these lung carcinoma cells was augmented by neutrophil-derived TNF-α. Thus, our study indicates that a novel mechanism of tumor progression of NSCLC exists whereby TNF-α secreted by neutrophils present in NSCLC may up-regulate FucT-III gene expression and thus sLeX on tumor cells that promotes invasiveness. Tumor cells expressing sLeX secrete higher amounts of IL-8 that may recruit neutrophils to tumors to continue to drive the invasive process, ultimately resulting in metastasis. We observed significant down-regulation of GM-CSF and MCP-3 in H1299 transfected cells. These chemokines promote eosinophil recruitment to tumors which may also facilitate NSCLC progression.28
In summary, our findings point to a unique role of neutrophils producing TNF-α in NSCLC tumor progression: enhancement of sLeX expression on NSCLC cells, alterations in morphology consistent with increased invasiveness, and together with sLeX, induction of increased invasive behavior of NSCLC cells. These data support the identification of a novel mechanism of lung tumor progression which may be manipulated to inhibit the invasiveness and metastasis of lung cancers. Thus, our findings lay the groundwork for a promising new approach to lung cancer therapy.
Acknowledgements
The authors would like to thank the following resources at the University of Minnesota’s Masonic Cancer Center: members of the Comparative Pathology Shared Resource core facility for assistance with immunohistochemistry and the Tumor Biology and Progression Research Program for discussion of the data.
Financial Disclosures: This work was supported in part by funds from the National Institutes of Health, National Cancer Institute grant 5KO8CA111829-04.
References
- 1.Coello MC, Luketich JD, Litle VR, et al. Prognostic significance of micrometastasis in non-small-cell lung cancer. Clin Lung Cancer. 2004;5:214–225. doi: 10.3816/CLC.2004.n.002. [DOI] [PubMed] [Google Scholar]
- 2.Nakamori S, Kameyama M, Imaoka S, et al. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res. 1993;53:3632–3637. [PubMed] [Google Scholar]
- 3.Izumi Y, Kawamura YJ, Irimura T. Carbohydrate antigens in carcinoma invasion and metastasis. Nippon Geka Gakkai Zasshi. 1996;97:140–144. [PubMed] [Google Scholar]
- 4.Ogawa JI, Inoue H, Koide S. alpha-2,3-Sialyltransferase type 3N and alpha-1,3-fucosyltransferase type VII are related to sialyl Lewis(x) synthesis and patient survival from lung carcinoma. Cancer. 1997;79:1678–1685. doi: 10.1002/(sici)1097-0142(19970501)79:9<1678::aid-cncr7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Foxall C, Watson SR, Dowbenko D, et al. The three members of the selectin receptor family recognize a common carbohydrate epitope, the sialyl Lewis(x) oligosaccharide. J. Cell Biol. 1992;117:895–902. doi: 10.1083/jcb.117.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 7.Chen JJ, Yao PL, Yuan A, et al. Upregulation of tumor interleukin-8 expression by infiltrating macrophages: Its correlation with tumor angiogenesis and patient survival in non-small cell lung cancer. Clin Cancer Res. 2003;9:729–737. [PubMed] [Google Scholar]
- 8.Toomey D, Smyth G, Condron C, et al. Infiltrating immune cells, but not tumour cells, express FasL in non-small cell lung cancer: No association with prognosis identified in 3-year follow-up. Int J Cancer. 2003;103:408–412. doi: 10.1002/ijc.10836. [DOI] [PubMed] [Google Scholar]
- 9.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bellocq A, Antoine M, Flahault A, et al. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 11.Welch DR, Schissel DJ, Howrey RP, et al. Tumor-elicited polymorphonuclear cells, in contrast to "normal" circulating polymorphonuclear cells, stimulate invasive and metastatic potentials of rat mammary adenocarcinoma cells. Proc Natl Acad Sci U S A. 1989;86:5859–5863. doi: 10.1073/pnas.86.15.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwartz JD, Monea S, Marcus SG, et al. Soluble factor(s) released from neutrophils activates endothelial cell matrix metalloproteinase-2. J Surg Res. 1998;76:79–85. doi: 10.1006/jsre.1998.5294. [DOI] [PubMed] [Google Scholar]
- 13.Muhs BE, Plitas G, Delgado Y, et al. Temporal expression and activation of matrix metalloproteinases-2, -9, and membrane type 1-matrix metalloproteinase following acute hindlimb ischemia. J Surg Res. 2003;111:8–15. doi: 10.1016/s0022-4804(02)00034-3. [DOI] [PubMed] [Google Scholar]
- 14.Tazawa H, Okada F, Kobayashi T, et al. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wislez M, Antoine M, Rabbe N, et al. Neutrophils promote aerogenous spread of lung adenocarcinoma with bronchioloalveolar carcinoma features. Clin Cancer Res. 2007;13:3518–3527. doi: 10.1158/1078-0432.CCR-06-2558. [DOI] [PubMed] [Google Scholar]
- 16.Leibovich SJ, Polverini PJ, Shepard HM, et al. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 17.Kuninaka S, Yano T, Yokoyama H, et al. Direct influences of pro-inflammatory cytokines (IL-1beta, TNF-alpha, IL-6) on the proliferation and cell-surface antigen expression of cancer cells. Cytokine. 2000;12:8–11. doi: 10.1006/cyto.1998.0504. [DOI] [PubMed] [Google Scholar]
- 18.St. Hill CA, Alexander SR, Walcheck B. Indirect capture augments leukocyte accumulation on P-selectin in flowing whole blood. J Leukoc Biol. 2003;73:464–471. doi: 10.1189/jlb.1002491. [DOI] [PubMed] [Google Scholar]
- 19.St. Hill CA, Farooqui M, Mitcheltree G, et al. The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglucosaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer. 2009;9:79. doi: 10.1186/1471-2407-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balkwill F. Tumor necrosis factor and cancer. Nat Rev Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 21.Ishibashi Y, Inouye Y, Okano T, et al. Regulation of sialyl-Lewis x epitope expression by TNF-alpha and EGF in an airway carcinoma cell line. Glycoconj J. 2005;22:53–62. doi: 10.1007/s10719-005-0292-7. [DOI] [PubMed] [Google Scholar]
- 22.Pinto CA, Carvalho PE, Antonângelo L, et al. Morphometric evaluation of tumor matrix metalloproteinase 9 predicts survival after surgical resection of adenocarcinoma of the lung. Clin Cancer Res. 2003;9:3098–3104. [PubMed] [Google Scholar]
- 23.Guo CB, Wang S, Deng C, et al. Relationship between matrix metalloproteinase 2 and lung cancer progression. Mol Diagn Ther. 2007;11:183–192. doi: 10.1007/BF03256240. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki K, Kurata K, Funayama K, et al. Expression cloning of a novel α(1,3)-fucosyltransferase that is involved in biosynthesis of the sialyl Lewis x carbohydrate determinants in leukocytes. J Biol Chem. 1994;269:14730–14737. [PubMed] [Google Scholar]
- 25.Sasaki K, Watanabe E, Kawashima K, et al. Expression cloning of a novel Gal beta (1–3/1–4) GlcNAc alpha 2,3-sialyltransferase using lectin resistance selection. J Biol Chem. 1993;268:22782–22787. [PubMed] [Google Scholar]
- 26.Togayachi A, Kudo T, Ikehara Y, et al. Up-regulation of Lewis enzyme (Fuc-TIII) and plasma-type a1,3fucosyltransferase (Fuc-TVI) expression determines the augmented expression of sialyl Lewis X antigen in non-small cell lung cancer. Int J Cancer. 1999;83:70–79. doi: 10.1002/(sici)1097-0215(19990924)83:1<70::aid-ijc14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 27.Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9:1732–1737. doi: 10.4161/cc.9.9.11297. [DOI] [PubMed] [Google Scholar]
- 28.Takanami I, Takeuchi K, Gika M. Immunohistochemical detection of eosinophilic infiltration in pulmonary adenocarcinoma. Anticancer Res. 2002;22:2391–2396. [PubMed] [Google Scholar]