Abstract
Background
Impulsive drive for immediate reward (IDIR) and delay aversion are dissociable elements of the preference for immediate over delayed rewards seen in Attention Deficit/Hyperactivity Disorder (ADHD). We hypothesized that IDIR would be associated with dopamine regulating genes and delay aversion with serotonin regulating genes.
Methods
IDIR and delay aversion were measured in 459 male children and adolescents (328 ADHD and 131 unaffected siblings) using a laboratory choice task. The sample was genotyped for the 5HTT (SLC6A4) promoter 5-HTTLPR polymorphism and a DAT1 (SLC6A3) 40-base pair VNTR located in the 3`-untranslated region of the gene.
Results
There was no effect of DAT1 on IDIR. As predicted 5-HTTLPR s-allele carriers were more delay averse. This effect was driven by the s/l genotype in the ADHD group. These results were not altered by taking account of the rs25531 A/G SNP and were independent of age, IQ and ODD symptoms.
Conclusions
The results support the genetic distinctiveness of IDIR and delay aversion in ADHD and implicate serotonin function in delay aversion. Possible explanations of the heterosis effect in the ADHD cases are presented.
Keywords: ATTENTION DEFICIT/HYPERACTIVITY DISORDER, IMPULSIVITY, DELAY AVERSION, 5-HTTLPR (SLC6A4), DAT1 (SLC6A3)
Introduction
The tendency to choose small-sooner over large delayed rewards is regarded as a signal marker of motivational dysfunction in ADHD (1). Effect sizes are moderate (Cohen’s d = .5 to .7 [2] with some between-study heterogeneity (e.g., [3] and [4] for non-significant findings). In a recent model this preference is explained as the product of two motivational components. The first component is an impulsive drive for immediate reward (IDIR; [1, 5]). IDIR manifests as a preference for small-sooner rewards in choice experiments where trial length is the same irrespective of which of the two options is chosen. This is achieved experimentally by arranging a period of post-reward delay (equal in length to the period of delay before the delayed-reward) after delivery of small-sooner rewards (i.e., a post reward delay condition). The second component is delay aversion which occurs when delay itself acquires a negative emotional valance, motivating actions allowing delay avoidance/escape. One model sees delay aversion as mediated by the experience of social censure associated with failures to perform effectively in delay settings in individuals with more fundamental IDIR-related deficits. In this model delay aversion exacerbates the effects of IDIR on small-sooner reward preference. Consistent with this formulation, in a recently reported choice delay experiment by Marco and colleagues, the preference for small-sooner rewards was significantly increased by removing the post-reward delay period so that choice of small-sooner rewards reduced overall trial delay (i.e., a no post reward delay condition;6). The difference between choices for small-sooner rewards in the post reward and no post reward delay conditions (an index of delay aversion) was significantly greater for individuals with ADHD than controls (6).
IDIR and delay aversion are postulated to be mediated by different brain systems. IDIR is hypothesized to be associated with dopamine function alterations within reward networks (7) that diminish signaling, and reduce the subjective value, of future rewards (8, 9). Consistent with this dopaminergic agents alter response to delayed reward in animal models (10), in healthy controls (11) and ADHD patients (12). Reward-related effects in the ventral striatum, a key component of the brain’s reward circuits, are altered in both pre-clinical (13) and clinical ADHD studies (3, 14, 15). The 40-base pair VNTR polymorphism located in the 3`-untranslated region (3`-UTR) of the DAT1 gene (SLC6A3; chromosome 5p15.3) contributes to the regulation of synaptic dopamine through altering its reuptake into pre-synaptic terminals. The DAT1 gene is differentially expressed in ventral striatum (16), and modulates reward-related activation there (17, 18) so that DAT1 genetic effects on impulsivity are thought to be moderated via alterations in reward circuits (18). Studies linking this polymorphism to ADHD give mixed results. Case-control and family association studies have shown inconsistent effects for the 10/10 genotype, and recent meta-analyses show significant but small effects(19, 20).
In contrast, delay aversion, regarded as a specific example of a more general response to negatively valenced environmental stimuli or experiences (21, 22) is hypothesized to be mediated by amygdala activation (23) and modulated by serotonin function (24). Supporting the notion that delay is negatively valenced for ADHD patients, an attentional bias towards cues of delay, similar to the response of anxious individuals to cues of threat, has been reported (25). Plichta et al. (15) found delay-related hyper-activation in amygdala in ADHD in response to delayed rewards. Serotonin function has been implicated in impulsivity and immediate over delayed reward choices (26–30). The 5HTT, encoded by genetic locus SLC6A4 (chromosome 17q11.2), is a key regulator of serotonin function in the amygdala. Transcriptional activity of the gene is modified by a polymorphic regulatory region, commonly known as 5-HTTLPR. The short allele (“s”) of the 5-HTTLPR is associated with lower transcription and functional capacity of the 5HTT; (31, 32). The 5-HTTLPR promoter polymorphism appears to influence functional (33, 34) and structural (35, 36) properties of the amygdala, in particular in moderating the response to threatening and aversive stimuli (31, 37). We are not aware of any studies of the effect of the 5-HTTLPR in determining delayed reward choices. However, Aluja et al. (38) found that the s-allele was associated with impulsiveness in a prison sample, while Oades et al. (39) demonstrated a potential link between another polymorphism in 5HTT, the intron 2 VNTR, and cognitive impulsivity but not motivational impulsivity in ADHD. The 5HTT gene has also been implicated in ADHD (19, 40, 41), although a recent multi-centre study was negative in this regard (42).
Here we test the hypothesis that IDIR and delay aversion will be differentially associated with polymorphisms in DAT1 and 5HTT genes in a secondary analysis of the sub-sample of male children and adolescents with ADHD and their sex-matched siblings using the Maudsley Index of Delay Aversion (6) data from the Marco et al. study. Our specific predictions were that the 10R/10R genotype of the DAT1 VNTR will be related to IDIR and the s-allele of the 5HTT promoter polymorphism associated with delay aversion.
Methods
Participants
Probands were from child psychiatry and specialist ADHD clinics in seven European countries (Belgium, Ireland, Germany, Spain, Switzerland, and UK) and Israel, and of European/Caucasian descent. The study was part of the neuropsychology component of the International Multi-centre ADHD Genetics (IMAGE) project (43). Each had a diagnosis DSM-IV ADHD-combined type and was between 6 and 16 years of age with at least one sibling in the same age range. The clinical diagnosis was validated against the Conners’ Rating Scales (44, 45) and the Parental Account of Children’s Symptoms (PACS; [46]) interview. Siblings were also screened for ADHD and if they met the inclusion threshold a PACS was administered in order to confirm the diagnosis. Exclusion criteria included pervasive developmental disorder, neurological diseases or other medical and genetic disorders. Parents gave written consent for the children to participate in the study.
To simplify and strengthen the current analysis males only were included because; (i) the number of girls with relevant data was too small (Nprobands=35) to allow analysis of possible interactions between gender and genotype (e.g., only 18 females probands with the relevant data carried the most common 10/10 DAT1 genotype compared to 168 male probands) and; (ii) there were markedly unequal male to female ratios for probands (35 v 285) compared to siblings (147 vs 158). MIDA data were available for 293 male probands (age range 6–16; mean= 10.78 years, sd= 2.61) and their 169 siblings (age range 5–17; mean= 10.73, sd=2.98). Genotype data for the DAT1 VNTR were available for 288 probands and 162 siblings, and for 291 probands and 168 siblings for the 5-HTTLPR. Seven cases with the DAT1 11-repeat allele were excluded from the analysis. Thirty five siblings had a diagnosis of ADHD (total ADHD cases N=328) and were designated so for the current analyses.
Tasks and measures
Clinical Evaluation
Symptom Rating Scales
Four scales were used to assess symptoms of ADHD and comorbid conditions: (the long versions of Conners’ Parent and Teacher Rating Scale and the parent and teacher Strengths and Difficulties Questionnaire (SDQ; [(47)]).
Research Diagnosis
This was carried out using the revised PACS interview (46), the Conners’ parent and teacher rating scales and the SDQ. The PACS is a semi-structured interview used to collect parent-based detailed information on children’s behaviour. The interviewer asks parents to describe their child’s behaviour in different settings, and then rate the severity and frequency of the behaviour according to previously defined criteria. The settings are chosen to represent common unstructured (watching TV, reading or playing alone), semi-structured (meals, outings or shopping) and structured (home tasks, homework or getting ready) daily life situations. In this study, parents were asked to focus on examples of their children’s behaviour during the most recent medication-free period. A standardized diagnostic algorithm based on the DSM-IV criteria was applied to the information from PACS and from the teacher rated ADHD subscale from Conners’ to derive a subtype diagnosis. In addition to the ADHD diagnosis, PACS also provides a Mood and an Anxiety score and a diagnosis of oppositional defiant disorder (ODD) based on the DSM-IV criteria. Previous studies have shown high inter-rater reliability (product-moment correlations between .76 and .96; [(46)]). The PACS has been validated against standardised questionnaires (such as the Conners’ scale) used to assess ADHD (48).
Intelligence
The vocabulary, similarities, picture completion and block designed subtests from the Wechsler Intelligence Scale for Children, 3rd edition (49) and the Wechsler Intelligence Scale for Adults, 3rd edition (50) were administered, and scores were prorated to provide a full estimate of IQ (51).
IDIR and delay aversion
These were derived from the MIDA (6, 52). The MIDA was one of three tests included in a battery implemented at eight IMAGE sites (see [53, 54] for description of the other two tasks). Participants were presented with a choice between small-sooner and large-delayed reward options in the context of a game-like space environment. Each trial involved a choice between firing at a single Cruiser that is presented first (the small-sooner option giving 1 point after 2 seconds) and waiting to fire at two Cruisers that come later (the large delayed option giving 2 points after 30 seconds).
There were two conditions. In the no post reward delay condition, each trial followed on immediately after the participant had received their reward so that trial length was determined by the length of the pre-reward delay for the chosen option. In the post reward delay condition, the trial length was equalized for the two reward options by including a period of post-reward delay (2 seconds for the large-delayed option or 30 seconds for the small-sooner option). Under this condition the length of trial was always 32 seconds. (See Marco, et al. (6) for a more detailed description of instructions and rewards). Our index of IDIR was the percentage of small-sooner choices on post-reward delay trials when choosing this could not reduce overall trial delay - i.e., was not an expression of delay aversion. The delay aversion index was the difference between the percentage of small-sooner choices in the post reward delay condition and the no post reward delay condition (where choosing the small-sooner reduces overall delay). For both IDIR and delay aversion high scores were more negative. Participants received instructions about the different options available in each condition.
Genotyping
DNA Extraction and Genotyping
DNA was extracted directly from blood samples or cell lines at Rutgers Cell line and DNA repository in the US. In a few cases we used a mouth swab sampling technique and extracted the DNA at the SGDP laboratories in London. For genotyping of the VNTR markers we used a standard PCR method according to previous optimized protocols for the markers used in this study. For DAT1 we contrasted 9R/9R and 9R/10R with 10R/10R (we excluded those carrying the 11R allele). For 5-HTTLPR we compared s/s and s/l with l/l genotypes. We also determined an A/G SNP (rs25531) within the 5-HTTLPR repetitive element, the G-allele of which has been reported to render the l-allele transcriptionally less efficient (55). Genotyping for this was carried out at the Institute of Psychiatry and followed the protocol outlined in Wendland et al. (55), primers are available on request.
Procedure
The procedure for task administration is described in detail in Marco et al. (6). Families were required to withdraw ADHD medications for at least 48 hours before testing. The study had ethical approval from local site ethics committees.
Analysis
We tested whether the delay aversion and the IDIR data met normality assumptions. Delay aversion data were normally distributed. IDIR data were extremely skewed with the majority of cases (346; 54%) scoring zero. We therefore adopted different analytical approaches for the two outcomes. For both IDIR and delay aversion as the data were collected at different sites and within families, we used mixed-effects regression models to account for the three-level nested structure (e.g., controlling for intra-familial sibling relationships). Delay aversion was introduced as a continuous variable. In a first step, the specific hypotheses were tested using a mixed-effects regression model for normally distributed outcomes, with delay aversion as the outcome, a contrast of the s-allele carriers versus the other genotypes as predictors, and random intercepts at the levels of site and family. All models included ADHD status and its interaction with genotype. In a second step, models were adjusted for age, IQ and ODD. As there was one extreme outlier in the data, we tested the models also after setting the outlier to the 95th percentile of the distribution (Winsorization) to prevent it from heavily influencing the statistical parameters. For IDIR (given its non-normal distribution) the outcome was dichotomised to represent zero vs non-zero IDIR. Mixed-effects logistic regression models for binary outcomes were used to test for associations of genotype and ADHD status with IDIR, with random effects and a second analytical step as described for the delay aversion model. The mixed-effects regression models were done using the Stata v11.1 commands xtmixed and xtmelogit, respectively. These models were also applied to the A/G SNP supplementary analyses.
Results
Frequencies for common genotypes were as expected and in Hardy Weinberg Equilibrium: (DAT1: 9R/9R – N=27; 9R/10R – N=154; 10/10 – N=267. 5-HTTLPR: s/s – N=87; s/l – N= 233; l/l –N=139). DAT1 and 5-HTTLPR genotypes were not significantly associated (χ2=1.77; ps>.70). IDIR and delay aversion were uncorrelated (r=.07; p>.10). Table 1 reports IDIR and delay aversion for genotypes by ADHD status.
Table 1.
The relationship between impulsive drive for immediate reward (IDIR) and delay aversion and genotype as a function of ADHD status.
| DAT1 VNTR | 5-HTTLPR | |||||
|---|---|---|---|---|---|---|
| 9/9 | 9/10 | 10/10 | s/s | s/l | l/l | |
| IDIR | ||||||
| ADHD | 45.00 | 50.73 | 50.93 | 53.95 | 46.99 | 54.39 |
| none | 37.50 | 40.86 | 38.26 | 27.78 | 46.81 | 34.29 |
|
Delay Aversion |
||||||
| ADHD | 6.15 (20.41) |
12.16 (24.05) |
12.75 (24.98) |
7.87 (21.76) |
15.71 (27.50) |
11.40 (22.16) |
| none | 12.52 (21.47) |
8.53 (23.39) |
10.70 (24.62) |
11.42 (20.67) |
10.98 (23.94) |
2.52 (22.14) |
NB: IDIR - Impulsive Drive for Immediate Reward represents the proportion of individuals who chose the small-sooner reward on all trials in the post reward delay condition Delay aversion was based on the difference between the proportion of choices made for the smaller sooner reward in post-reward and no post reward delay condition. Higher scores indicate more small-sooner choices. Figures in parentheses are standard deviations.
Primary analysis
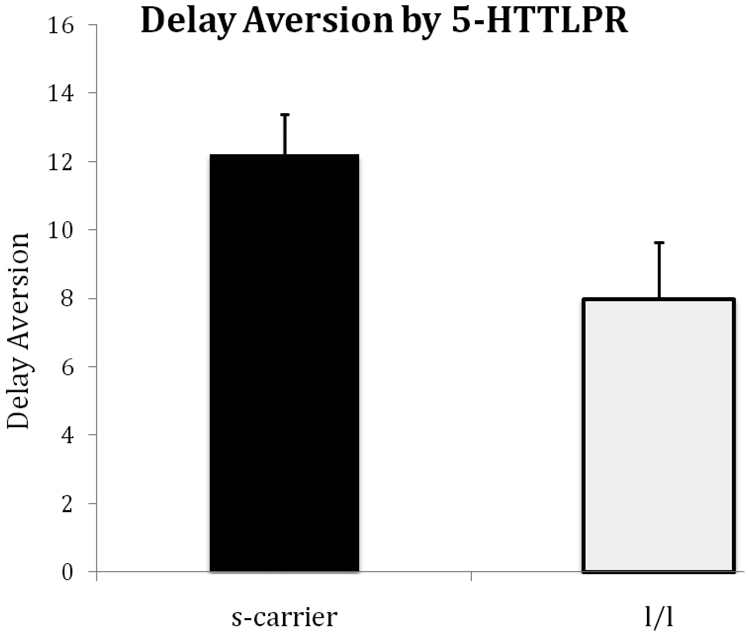
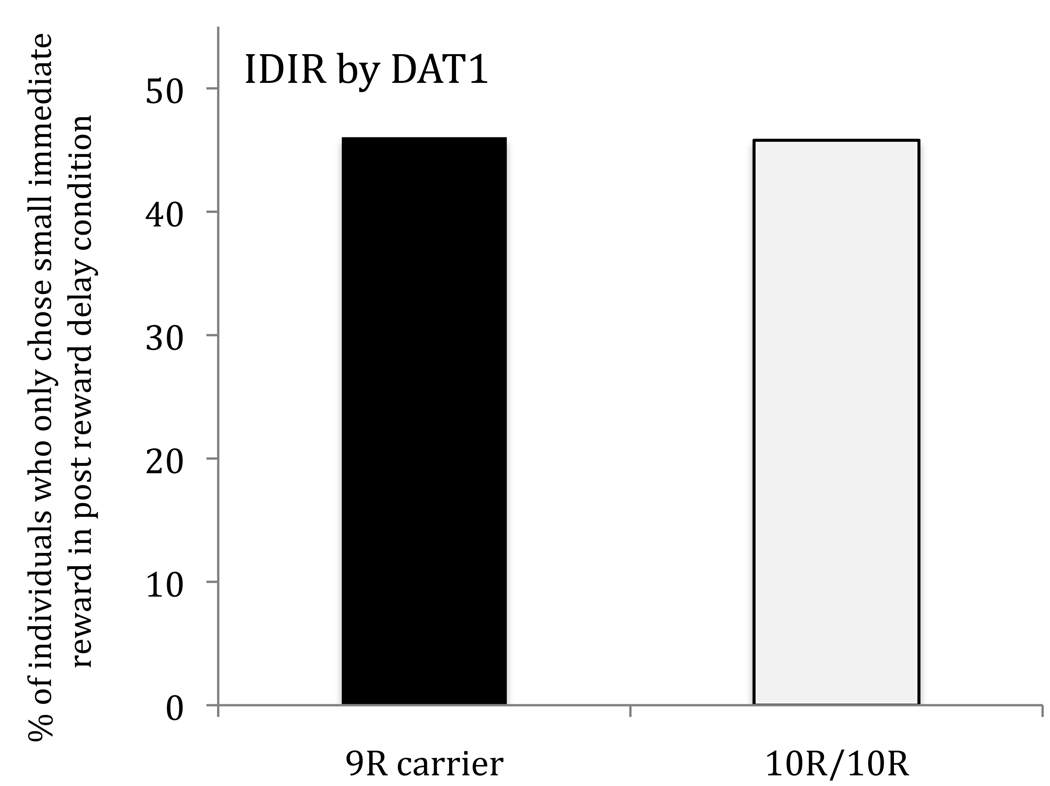
First we tested the predicted associations (Figure 1). IDIR did not vary by DAT1 genotype (10/10 v 9/9 and 9/10: χ2 (1)=<0.01;p=.99). As predicted, 5-HTTLPR s-allele carriers were more delay averse than non-carriers (s/s & s/l v l/l: χ2 (1)=4.57;p=.03). This effect was slightly stronger when analyses were conducted according to transcriptional activity status (.e. including SNP rs25531) - (“low activity” allele carriers being more delay averse than the “high/high” genotype (χ2(1)=5.37;p=.02; for delay aversion and IDIR by transcriptional genotype see supplementary information). There was a main effect of ADHD status on delay aversion (χ2 (1)=5.93;p=.01; as originally found in Marco et al.(6)) but no interaction between genotype and ADHD (χ2 (1)=2.77;p=.10; transcriptional activity groups - χ2(1)=0.64; p =.42). This pattern of significance did not change when IQ, ODD and age were added as covariates (effect of 5-HTTLPR s/s & s/l v l/l: χ2(1) = 6.30;p = .01; effect of ADHD status: χ2(1) = 8.03; p = .005 ; Interaction between ADHD status and genotype: χ2(1) = 3.02; p = .08), nor when outliers were Winsorized at the 95th percentile (value = −15).
Figure 1.
The mean level of MIDA delay aversion as a function of 5-HTTLPR status for the combined s/s and s/l genotype groups compared with the l/l group. Note: Delay aversion is calculated as the difference in percentage choices of the small-sooner option under no post reward and post reward delay conditions. Higher scores mean more delay aversion.
Exploratory analyses
Despite the lack of significant interaction, visual inspection of delay aversion means suggested a rather different pattern in probands and unaffected siblings by 5-HTTLPR genotype. To investigate this we conducted a set of exploratory post hoc analyses. These suggested that for the unaffected siblings there was a strong effect of the s-allele (χ2 (1)=7.29;p=.01) with s/s and s/l having similar levels of delay aversion and both different from the l/l carriers, while for ADHD cases the effect was carried largely by the s/l genotype with heterozygotes being more delay averse than the homozygotes (χ2 (1)=5.68;p=.02). Transcriptional activity status analysis gave the same pattern of results. For the unaffected siblings the “low/high” group being significantly more delay averse than the “high/high” group (χ2 (1)=6.26;p=.04). For the affected siblings, the comparison of “low/high” with the “high/high” group missed statistical significance (χ2 (1)=4.90;p=.09). These reduced levels of significance were likely related to the reduced number of participants for whom the rs25531 A/G SNP was available. Although not hypothesized, we also explored the associations between 5-HTTLPR and IDIR, and DAT1 and delay aversion. There were no significant effects (5-HTTLPR and IDIR, traditional grouping: χ2 (1)=0.65;p=.72; according to transcription activity: χ2 (1)=0.76;p=.38 ; DAT1 VNTR and delay aversion: χ2(1)=0.94;p=.63).
Discussion
The current results extend our understanding of different elements of impulsive choice, their genetic underpinnings and by extension their putative neurobiological basis. By providing evidence for differential genetic associations the results further validate the distinction between IDIR and delay aversion in models of impulsive choice (1). Using a hypothesis testing approach we predicted that IDIR (as measured by percentage of choices for the small-sooner reward in the post reward condition) would be associated with DAT1. This was based on the notion that IDIR is the result of altered signaling of delayed rewards modulated by dopamine function, which is affected by functional polymorphisms in the DAT1 gene. The result was negative and so the findings were at odds with the previous studies linking DAT1 genotype to impulsive choice, delayed responding (18), delay discounting and trait impulsivity (56). However bearing in mind the nature of the current sample it may be that effects of DAT1 on impulsive choice are sample specific and in particular may not underpin impulsive choice specifically in ADHD. The 10R allele may confer risk for ADHD only in combination with additional DNA variants in the DAT1 gene. Thus, we had found that a specific haplotype of the DAT1 gene is associated with combined-type ADHD (57), replicating a previous report from a different sample (58); additional DAT1 genetic variants from the 5′ region of the gene have also been reported to be associated with ADHD (59). In general, it has been difficult to identify robust and consistent associations between specific dopamine genotypes, including DAT1 and putative neuropsychological endophenotypes (60). The current result therefore adds to this rather fragmented picture, although it is not possible, of course, to rule out the effects of variations in dopamine genes, other than DAT1, involved in dopamine neurotransmission on IDIR.
Our second hypothesis was that delay aversion (the additional effect of linking small-sooner reward choices to delay reduction) would be associated with 5-HTTLPR genotype. This was based on the view that delay aversion was a specific case of a more general avoidant response to aversive events and therefore would be mediated by similar neurobiological mechanisms linked to serotonin function (34). As predicted, 5-HTTLPR genotype was associated with delay aversion with s-allele carriers more delay averse than non-carriers. This finding should be interpreted in relation to a more general link between 5-HTTLPR and impulsive choice seen in tryptophan depletion studies suggesting serotonin status affects waiting behavior and delay-related choice in other populations (26, 34, 61). However, it presents the first study to extend this to the effects of 5-HTTLPR genotype on impulsive choice behavior on laboratory tasks. It also represents one of the first studies implicating this genotype in ADHD neuropsychology.
Although not ideally placed to explore the moderation of these effects by ADHD status given the familial relations between affected and unaffected cases we conducted separate exploratory analyses for these groups. This confirmed the observation of a rather different pattern of results for the two groups and an unexpected heterosis effect in the ADHD group (the s/l group being the most delay averse). This raises the possibility that 5-HTTLPR genotype effects on impulsive behaviour may be dependent on disorder status or more generally on participant characteristics. This possibility has not been investigated systematically as most studies of 5-HTTLPR s-allele effects on amygdala reactivity have typically been in samples of healthy volunteers with no history of affective or other psychiatric disorders.
Although most studies have not specifically tested for it, a number of studies have found group-specific evidence of molecular heterosis at the 5HTT gene. Heterozygote subjects have shown lower [I125]beta-citalopram serotonin transporter binding in cocaine users (62), increased white-matter lesions among depressed patients (63), higher cognitive function in elderly adults (64) and lower availability of central 5HTT (16). In a recent study, Malmberg et al. (65) found associations between disruptive behaviour disorder and s/l genotype. Explanations for these effects include; (i) an inverted U-shaped response curve in which either too little or too much gene expression is deleterious; (ii) an independent third factor causing a hidden stratification of the sample such that both the two homozygote genotype (s/s and l/l) are independently associated with the highest phenotype score relative to the heterozygote (e.g., s/l); (iii) greater fitness in heterozygotes because they show a broader range of gene expression than both homozygotes (for a review see [(66)]). Clearly, although intriguing, our finding showing a disorder specific heterosis effect in families with ADHD children needs to be confirmed in other large independent samples with non-related controls.
The current results may take us further in understanding heterogeneity in ADHD. Previous studies (6) found that only a sub-set of ADHD children show impulsive responding on the MIDA. This may therefore be a marker of a sub-type of ADHD in which 5-HTTLPR polymorphisms play a particularly important role in the pathogenesis of the condition. This may explain the inconsistency in results relating to the association between ADHD and this genotype. The expectation is that effects would be larger for 5-HTTLPR genotypes in a refined delay averse sample of ADHD children. If this were the case it may be possible to isolate a sub-group whose ADHD is mediated by delay averse and might respond to serotonergic drugs (39) as a component of their treatment on the one hand or delay training on the other (25). The results of Zepf et al. (67) demonstrating that ADHD children with comorbid anxious-depression and/or aggression were sensitive to tryptophan depletion, highlights the possibility that a delay averse sub-group might be more likely to have these comorbidities.
The current study had many strengths. These included the large sample and the use of an experimental paradigm to dissect different elements of impulsive choice; however, there were a number of limitations. First, the skewed distribution of the IDIR measure and the need to dichotomize it for the analysis rather than use it as a continuous measure might have reduced its sensitivity compared with the delay aversion measure, the negative finding therefore needs to be interpreted with caution, although the effects were very far from significant. Second, the study did not include direct measures or manipulations of serotonin or dopamine levels which would have helped resolve issues around the functional significance of the different allelic combinations. Third, there were insufficient affected girls in this subset of the IMAGE sample to provide power to include gender as a factor in the analysis. Finally, the current sample with genotypic information did not include unrelated controls - this means that it remains uncertain how specific the role of these genotypes might be to ADHD because of the familial link and associated genetic overlap between probands and their unaffected sibs. Future studies should include biologically unrelated controls and groups of patients with other disorders to examine this issue.
Supplementary Material
Figure 2.
IDIR levels as a function of DAT1 VNTR genotype with the combined 9/9 and 9/10 groups compared to the 10/10 group. Note: IDIR=Impulsive Drive for Immediate Reward represents the proportion of individuals who chose the small-sooner reward on all trials in the post reward delay condition.
Acknowledgements
The IMAGE project is a multi-site, international effort supported by NIH grants R01MH081803 and R01MH62873 to S.V. Faraone, and, in London, by UK Medical Research Council grant G03001896 to J. Kuntsi. Site Principal Investigators for the genetics analysis were Philip Asherson, Tobias Banaschewski, Jan Buitelaar, Richard P. Ebstein, Michael Gill, Ana Miranda, Fernando Mulas, Robert D. Oades, Herbert Roeyers, Aribert Rothenberger, Joseph Sergeant, Edmund Sonuga-Barke, and Hans-Christoph Steinhausen. Principal investigators for the neuropsychological arm of the study were Tobias Banaschewski, Michael Gill, Jonna Kuntsi, Iris Manor, Ana Miranda, Fernando Mulas, Robert D. Oades, Herbert Royers, and Hans-Christoph Steinhausen. Chief investigators at each site were Penny Andreou, Rafaela Marco, Henrik Uebel, Hanna Christiansen, U. Mueller, Isabel Gabriels, and Shera Medad. We are very grateful to all the families and children that took part in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
E. Sonuga-Barke is a member of an Advisory Board to Shire, Flynn Pharma, UCB Pharma and Astra Zeneca; has received research support from Janssen Cilag, Shire and Qbtech; conference support from Shire; is on speaker board for Shire and UCB Pharma; and has been a consultant for UCB Pharma and Shire.
A. Miranda is advisor to Eli Lilly.
R. D. Oades has received support for investigator-initiated studies from UCB GmbH.
T. Banaschewski served in an advisory or consultancy role for Desitin, Lilly, Medice, Novartis, Pfizer, Shire, UCB and Viforpharma. He received conference attendance support or received speaker’s fee by Lilly, Janssen McNeil, Medice, Novartis, UCB. He received unrestricted grants for organizing a CME conference by Lilly, Janssen McNeil, Medice, Novartis, Shire, UCB. He is/has been involved in clinical trials conducted by Lilly, Shire and Novartis. The present work is unrelated to the above grants and relationships.
H. Uebel received conference attendance support or was paid for public speaking by Lilly, Janssen-Cilag, Novartis and Medice.
J. Kuntsi has received a speaker’s fee from Eli Lilly that has been used for educational and research activities.
J. Buitelaar has been in the past 3 years a consultant to / member of Advisory Board of / and/or speaker for Janssen Cilag BV, Eli Lilly, Bristol-Myer Squibb, Organon/Shering Plough, UCB, Shire, Medice, Servier, Bioprojet, Pfizer, and Servier.
H. Roeyers is a member of an Advisory Board to Shire and has received research funding and conference attendance support from Shire and Eli Lilly.
A. Rothenberger is on the Advisory Board and Speakers’ Bureau of Medice, Novartis, Shire and Eli Lilly; has received educational grants from Shire and Medice; and has received research support from Shire and Schwabe.
J. Sergeant is a member of an Advisory Board to Lilly and Shire; has received research funding from Lilly; and speaker’s fees from Lilly, Janssen-Cilag, Novartis and Shire.
H. C. Steinhausen has served as an advisor and speaker to Janssen-Cilag, Eli Lilly, Novartis, Shire, Medice and UCB.
P. Asherson has served as a consultant and on advisory boards for Eli Lilly, Shire, Janssen Cilag and Flynn Pharma. He received a research grant from Shire and an educational grant from Janssen-Cilag
In the past year, S. Faraone has received consulting fees and has been on Advisory Boards for Shire Development and has received research support from Pfizer, Shire and the National Institutes of Health. In previous years, Dr. Faraone has received consulting fees or has been on Advisory Boards or has participated in continuing medical education programs sponsored by: Shire, McNeil, Janssen, Novartis, Pfizer and Eli Lilly. In previous years he has received research support from Eli Lilly, Shire, Pfizer and the National Institutes of Health. Dr. Faraone receive royalties from a book published by Guilford Press: Straight Talk About Your Child’s Mental Health.
All other authors reported no biomedical financial interests or potential conflicts of interest.
References
- 1.Sonuga-Barke EJS, Sergeant JA, Nigg J, Willcutt E. Executive Dysfunction and Delay Aversion in Attention Deficit Hyperactivity Disorder: Nosologic and Diagnostic Implications. Child and Adolescent Psychiatric Clinics of North America. 2008;17:367–384. doi: 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Willcutt EG, Sonuga-Barke EJS, Nigg JT, Sergeant JA. Recent developments in neuropsychological models of childhood psychiatric disorders. In: Banaschewski T, Rhode LA, editors. Biological Child Psychiatry. Recent Trends and Developments. Advances in Biological Psychiatry. Basel: Karger; 2008. [Google Scholar]
- 3.Scheres A, Dijkstra M, Ainslie E, Balkan J, Reynolds B, Sonuga-Barke E, et al. Temporal and probabilistic discounting of rewards in children and adolescents: effects of age and ADHD symptoms. Neuropsychologia. 2006;44:2092–2103. doi: 10.1016/j.neuropsychologia.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Bidwell LC, Erik GW, John CD, Bruce FP. Testing for Neuropsychological Endophenotypes in Siblings Discordant for Attention-Deficit/Hyperactivity Disorder. Biological psychiatry. 2007;62:991–998. doi: 10.1016/j.biopsych.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonuga-Barke E. Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biological Psychiatry. 2005;57:1231–1238. doi: 10.1016/j.biopsych.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Müller U, et al. Delay and reward choice in ADHD: An experimental test of the role of delay aversion. Neuropsychology. 2009;23:367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- 7.Johansen E, Killeen P, Russell V, Tripp G, Wickens J, Tannock R, et al. Origins of altered reinforcement effects in ADHD. Behavioral and Brain Functions. 2009;5:7. doi: 10.1186/1744-9081-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. The Behavioral and brain sciences. 2005:28. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- 9.Tripp G, Wickens JR. Research Review: Dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. Journal of Child Psychology and Psychiatry. 2008;49:691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 10.Floresco SB, Tse MTL, Ghods-Sharifi S. Dopaminergic and Glutamatergic Regulation of Effort-and Delay-Based Decision Making. Neuropsychopharmacology. 2007;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- 11.Pietras C, Cherek D, Lane S, Tcheremissine O, Steinberg J. Effects of methylphenidate on impulsive choice in adult humans. Psychopharmacology. 2003;170:390–398. doi: 10.1007/s00213-003-1547-2. [DOI] [PubMed] [Google Scholar]
- 12.Shiels K, Hawk LW, Jr, Reynolds B, Mazzullo R, Rhodes J, Pelham WE, Jr, et al. The Effects of Methylphenidate on Discounting of Delayed Rewards in ADHD. Experimental and Clinical Psychopharmacology. 2009;17:291–301. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nature Neuroscience. 2008;11:966–973. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- 14.Hahn T, Dresler T, Ehlis A-C, Plichta MM, Heinzel S, Polak T, et al. Neural response to reward anticipation is modulated by Gray's impulsivity. NeuroImage. 2009;46:1148–1153. doi: 10.1016/j.neuroimage.2009.03.038. [DOI] [PubMed] [Google Scholar]
- 15.Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, et al. Neural Hyporesponsiveness and Hyperresponsiveness During Immediate and Delayed Reward Processing in Adult Attention-Deficit/Hyperactivity Disorder. Biological Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 16.van Dyck CH, Malison RT, Jacobsen LK, Seibyl JP, Staley JK, Laruelle M, et al. Increased Dopamine Transporter Availability Associated with the 9-Repeat Allele of the SLC6A3 Gene. Journal of Nuclear Medicine. 2005;46:745–751. [PubMed] [Google Scholar]
- 17.Dreher J-C, Kohn P, Kolachana B, Weinberger DR, Berman KF. Variation in dopamine genes influences responsivity of the human reward system. Proceedings of the National Academy of Sciences. 2009;106:617–622. doi: 10.1073/pnas.0805517106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14:60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gizer I, Ficks C, Waldman I. Candidate gene studies of ADHD: a meta-analytic review. Human Genetics. 2009;126:51–90. doi: 10.1007/s00439-009-0694-x. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Sham PC, Owen MJ, He L. Meta-analysis shows significant association between dopamine system genes and attention deficit hyperactivity disorder (ADHD) Human Molecular Genetics. 2006;15:2276–2284. doi: 10.1093/hmg/ddl152. [DOI] [PubMed] [Google Scholar]
- 21.Hariri AR, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Sciences. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Harmer CJ. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology. 2008;55:1023–1028. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neuroscience & Biobehavioral Reviews. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 24.Sonuga-Barke E, Wiersema J, van der Meere J, Roeyers H. Context-dependent Dynamic Processes in Attention Deficit/Hyperactivity Disorder: Differentiating Common and Unique Effects of State Regulation Deficits and Delay Aversion. Neuropsychology Review. 2010;20:86–102. doi: 10.1007/s11065-009-9115-0. [DOI] [PubMed] [Google Scholar]
- 25.Sonuga-Barke EJS, Houwer JD, Ruiter KD, Ajzenstzen M, Holland S. AD/HD and the capture of attention by briefly exposed delay-related cues: evidence from a conditioning paradigm. Journal of Child Psychology and Psychiatry. 2004;45:274–283. doi: 10.1111/j.1469-7610.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 26.Eagle DM, Lehmann O, Theobald DEH, Pena Y, Zakaria R, Ghosh R, et al. Serotonin Depletion Impairs Waiting but not Stop-Signal Reaction Time in Rats: Implications for Theories of the Role of 5-HT in Behavioral Inhibition. Neuropsychopharmacology. 2008;34:1311–1321. doi: 10.1038/npp.2008.202. [DOI] [PubMed] [Google Scholar]
- 27.Novkovic VH, Rudan V, Pivac N, Nedic G, Muck-Seler D. Platelet Serotonin Concentration in Children with Attention-Deficit/Hyperactivity Disorder. Neuropsychobiology. 2009;59:17–22. doi: 10.1159/000202825. [DOI] [PubMed] [Google Scholar]
- 28.Schweighofer N, Bertin M, Shishida K, Okamoto Y, Tanaka SC, Yamawaki S, et al. Low-serotonin levels increase delayed reward discounting in humans. Journal of Neuroscience. 2008;28:4528–4532. doi: 10.1523/JNEUROSCI.4982-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winstanley CA, Theobald DEH, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: Therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- 30.Wolff MC, Leander JD. Selective serotonin reuptake inhibitors decrease impulsive behavior as measured by an adjusting delay procedure in the pigeon. Neuropsychopharmacology. 2002;27:421–429. doi: 10.1016/S0893-133X(02)00307-X. [DOI] [PubMed] [Google Scholar]
- 31.Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 32.Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 33.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 34.Munafo Serotonin Transporter (5-HTTLPR) Genotype and Amygdala Activation: A Meta-Analysis (vol 63, pg 852, 2008) Biological Psychiatry. 2009;66 doi: 10.1016/j.biopsych.2007.08.016. 302-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- 36.Scherk H, Gruber O, Menzel P, Schneider-Axmann T, Kemmer C, Usher J, et al. 5-HTTLPR genotype influences amygdala volume. European Archives of Psychiatry and Clinical Neuroscience. 2009;259:212–217. doi: 10.1007/s00406-008-0853-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osinsky R, Reuter M, Kupper Y, Schmitz A, Kozyra E, Alexander N, et al. Variation in the serotonin transporter gene modulates selective attention to threat. Emotion. 2008;8:584–588. doi: 10.1037/a0012826. [DOI] [PubMed] [Google Scholar]
- 38.Aluja A, Garcia LF, Blanch A, De Lorenzo D, Fibla J. Impulsive-disinhibited personality and serotonin transporter gene polymorphisms: Association study in an inmate's sample. Journal of Psychiatric Research. 2009;43:906–914. doi: 10.1016/j.jpsychires.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Oades RD. Dopamine-serotonin interactions in attention-deficit hyperactivity disorder (ADHD) Progress in Brain Research. 2008;172:543–565. doi: 10.1016/S0079-6123(08)00926-6. [DOI] [PubMed] [Google Scholar]
- 40.Oades RD, Lasky-Su J, Christiansen H, Faraone SV, Sonuga-Barke EJS, Banaschewski T, et al. The influence of serotonin-and other genes on impulsive behavioral aggression and cognitive impulsivity in children with attention-deficit/hyperactivity disorder (ADHD): Findings from a family-based association test (FBAT) analysis. Behavioral and Brain Functions. 2008;4 doi: 10.1186/1744-9081-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ribases M, Ramos-Quiroga JA, Hervas A, Bosch R, Bielsa A, Gastaminza X, et al. Exploration of 19 serotoninergic candidate genes in adults and children with attention-deficit//hyperactivity disorder identifies association for 5HT2A, DDC and MAOB. Molecular Psychiatry. 2007;14:71–85. doi: 10.1038/sj.mp.4002100. [DOI] [PubMed] [Google Scholar]
- 42.Landaas ET, Johansson S, Jacobsen KK, Ribases M, Bosch R, Sanchez-Mora C, et al. An international multicenter association study of the serotonin transporter gene in persistent ADHD. Genes Brain Behav. 2010;9:449–458. doi: 10.1111/j.1601-183X.2010.00567.x. [DOI] [PubMed] [Google Scholar]
- 43.Brookes K, Xu X, Chen W, Zhou K, Neale B, Lowe N, et al. The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Molecular Psychiatry. 2006;11:934–953. doi: 10.1038/sj.mp.4001869. [DOI] [PubMed] [Google Scholar]
- 44.Conners CK, Sitarenios G, Parker JDA, Epstein JN. The Revised Conners' Parent Rating Scale (CPRS-R): Factor Structure, Reliability, and Criterion Validity. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/a:1022602400621. [DOI] [PubMed] [Google Scholar]
- 45.Conners CK, Sitarenios G, Parker JDA, Epstein JN. Revision and Restandardization of the Conners Teacher Rating Scale (CTRS-R): Factor Structure, Reliability, and Criterion Validity. Journal of Abnormal Child Psychology. 1998;26:279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- 46.Chen W, Taylor E. Parental account of children´s symptoms (PACS), ADHD phenotypes and its application to molecular genetic studies. In: Oades RD, editor. Attention-deficit/hyperactivity disorder and the hyperkinetic syndrome. Current ideas and ways forward. New York: Nova Science Publishers; 2006. pp. 3–20. [Google Scholar]
- 47.Goodman R. The Strengths and Difficulties Questionnaire: a research note. Journal of Child Psychology and Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen W, Zhou K, Sham P, Franke B, Kuntsi J, Campbell D, et al. DSM-IV combined type ADHD shows familial association with sibling trait scores: a sampling strategy for QTL linkage. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1450–1460. doi: 10.1002/ajmg.b.30672. [DOI] [PubMed] [Google Scholar]
- 49.Wechsler D. Wechsler Intelligence Scale for Children. London: The Psychological Corporation; 1991. [Google Scholar]
- 50.Wechsler D. Wechsler Intelligence Scale for Adults. London: The Psychological Corporation; 1997. [Google Scholar]
- 51.Sattler JM. Assessment of children: WISC-III and WPPSI-R supplement. San Diego: CA: 1992. [Google Scholar]
- 52.Kuntsi J, Rogers H, Swinard G, Borger N, van der Meere J, Rijsdijk F, et al. Reaction time, inhibition, working memory and 'delay aversion' performance: genetic influences and their interpretation. Psychol Med. 2006;36:1613–1624. doi: 10.1017/S0033291706008580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, et al. Reaction time performance in ADHD: improvement under fast-incentive condition and familial effects. Psychological Medicine. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uebel H, Albrecht B, Asherson P, Borger NA, Butler L, Chen W, et al. Performance variability, impulsivity errors and the impact of incentives as gender-independent endophenotypes for ADHD. Journal of Child Psychology and Psychiatry. 2010;51:210–218. doi: 10.1111/j.1469-7610.2009.02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 56.Paloyelis Y, Asherson P, Mehta MA, Faraone SV, Kuntsi J. DAT1 and COMT effects on delay discounting and trait impulsivity in male adolescents with attention deficit/hyperactivity disorder and healthy controls. Neuropsychopharmacology. 2010;35:2414–2426. doi: 10.1038/npp.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Asherson P, Brookes K, Franke B, Chen W, Gill M, Ebstein RP, et al. Confirmation that a specific haplotype of the dopamine transporter gene is associated with combined-type ADHD. American Journal of Psychiatry. 2007;164:674–677. doi: 10.1176/ajp.2007.164.4.674. [DOI] [PubMed] [Google Scholar]
- 58.Brookes K-J, Mill J, Guindalini C, Curran S, Xu X, Knight J, et al. A Common Haplotype of the Dopamine Transporter Gene Associated With Attention-Deficit/Hyperactivity Disorder and Interacting With Maternal Use of Alcohol During Pregnancy. Archives of General Psychiatry. 2006;63:74–81. doi: 10.1001/archpsyc.63.1.74. [DOI] [PubMed] [Google Scholar]
- 59.Brookes KJ, Xu X, Anney R, Franke B, Zhou K, Chen W, et al. Association of ADHD with genetic variants in the 5'-region of the dopamine transporter gene: evidence for allelic heterogeneity. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:1519–1523. doi: 10.1002/ajmg.b.30782. [DOI] [PubMed] [Google Scholar]
- 60.Kebir O, Tabbane K, Sengupta S, Joober R. Candidate genes and neuropsychological phenotypes in children with ADHD: review of association studies. Journal of Psychiatry and Neuroscience. 2009;34:88–101. [PMC free article] [PubMed] [Google Scholar]
- 61.Clark L, Roiser J, Cools R, Rubinsztein D, Sahakian B, Robbins T. Stop signal response inhibition is not modulated by tryptophan depletion or the serotonin transporter polymorphism in healthy volunteers: implications for the 5-HT theory of impulsivity. Psychopharmacology. 2005;182:570–578. doi: 10.1007/s00213-005-0104-6. [DOI] [PubMed] [Google Scholar]
- 62.Little KY, McLaughlin DP, Zhang L, Livermore CS, Dalack GW, McFinton PR, et al. Cocaine, ethanol, and genotype effects on human midbrain serotonin transporter binding sites and mRNA levels. American Journal of Psychiatry. 1998;155:207–213. doi: 10.1176/ajp.155.2.207. [DOI] [PubMed] [Google Scholar]
- 63.Steffens DC, Taylor WD, McQuoid DR, Krishnan KRR. Short/long heterozygotes at 5HTTLPR and white matter lesions in geriatric depression. International Journal of Geriatric Psychiatry. 2008;23:244–248. doi: 10.1002/gps.1869. [DOI] [PubMed] [Google Scholar]
- 64.Fiedorowicz JG, Moser DJ, Hynes SM, Beglinger LJ, Schultz SK, Ellingrod VL. LA allelic heterozygosity of the 5HTTLPR polymorphism is associated with higher cognitive function and lower interpersonal sensitivity. Psychiatric Genetics. 2007;17:3–4. doi: 10.1097/YPG.0b013e328010f498. 10.1097/YPG.1090b1013e328010f328498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malmberg K, Wargelius HL, Lichtenstein P, Oreland L, Larsson JO. ADHD and Disruptive behavior scores - associations with MAO-A and 5-HTT genes and with platelet MAO-B activity in adolescents. BMC Psychiatry. 2008;8 doi: 10.1186/1471-244X-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Comings DE, MacMurray JP. Molecular heterosis: A review. Molecular Genetics and Metabolism. 2000;71:19–31. doi: 10.1006/mgme.2000.3015. [DOI] [PubMed] [Google Scholar]
- 67.Zepf FD, Wockel L, Poustka F, Holtmann M. Diminished 5-HT functioning in CBCL pediatric bipolar disorder-profiled ADHD patients versus normal ADHD: susceptibility to rapid tryptophan depletion influences reaction time performance. Hum Psychopharmacol. 2008;23:291–299. doi: 10.1002/hup.934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.