Abstract
Objectives
In prior studies of febrile seizures (FS), prolonged FS are defined, absent empirical evidence, as lasting 10 or 15 minutes or more. We assessed the distribution of FS duration in a cohort with first FS, and the association between FS duration and baseline characteristics of the children.
Methods
We calculated the observed cumulative probability, S(t), that a FS would last at least t minutes, S(t) = exp(−t/ τ) . Data were also fit using a model obtained as the sum of two exponential distributions [S(t) = αexp(−t/τ1)+(1-α)exp(−t/τ2)]. After assessing the best fit, the cut off defining long FS was determined. Logisitic regression was used to examine associations between long FS and baseline characteristics, behavior and development.
Results
In 158 children with a first FS, median duration was 4.0 minutes. Duration of FS was best fit by a two-component mixture exponential model. Using this model we identified one population that accounts for 82.3% of FS and has a mean duration of 3.8 minutes (short FS) and a second population that accounts for 17.7% of FS and has a mean duration of 39.8 minutes (long FS). Long FS were significantly associated with developmental delay (p=0.010) and delays and younger age at first FS (p=0.048).
Interpretation
Like the distribution of afebrile seizure duration in children, the distribution of first FS duration is best modeled by assuming two populations. Developmental delay and younger age are associated with prolonged FS. Our data lend further support to defining 10 minutes as the upper limit for a simple FS.
Keywords: Febrile Seizures, Epidemiology, Children
Febrile seizures (FS) are common, affecting 2-5% of children by 6 years of age 1 2 and are associated with an increased risk of subsequent epilepsy. Epilepsy develops in 2-4% of children with a history of FS, four times more frequently than in children without and the risk is as great as 57% in children with focal, prolonged and recurrent FS 3. In prior studies, prolonged FS have been defined in the absence of empirical evidence as those lasting either 10 minutes or more or as those lasting 15 minutes or more.1 2 4-11 In children with first afebrile seizures, seizure duration is best fit as the sum of two exponential distributions with seizures lasting a mean of 3.6 minutes occurring in 76% of children and those lasting a mean of 31 minutes occurring in 24% of children 12. Similar information does not exist for first FS.
Using similar methodology to that reported by Shinnar et al in afebrile seizures 12, we analyzed the distribution of FS duration in a cohort presenting with a first FS. Additionally, we determined whether the distribution of FS duration differs from what was previously reported for first afebrile seizures in children, and the association between febrile seizure duration and baseline characteristics of the children.
Methods
Study Subjects
In this prospective cohort study 13, we identified children, aged 6 months to 5 years, with first FS by daily screening of the The Morgan Stanley Children’s Hospital of New York-Presbyterian Pediatric Emergency Department log and by review of pediatric hospital discharges with an ICD-9 code of 780.3 between March 1999 and April 2004. We defined FS as seizures occurring among children with a rectal temperature of at least 101° Fahrenheit (38.3° Celsius), in the absence of a history of unprovoked seizures or concurrent central nervous system infection as defined in the NIH consensus conference14 . Children with prior neonatal seizure were included.
Upon identification, we contacted the child’s primary care physician to request permission for the study team to contact the child’s parents or guardians. After obtaining permission from the parent, the physician notified the study team and the family was contacted to explain the study and offer participation.
Measures and procedures
If initial screening confirmed the first FS, we obtained informed consent and the child received an MRI of the brain within one week of the FS 13. Within one month, parents were interviewed regarding the child’s medical history, demographics, family history of FS and epilepsy in first-degree relatives, and child’s behavior. Adaptive behavior was assessed by the Vineland Scales of Adaptive Behavior 15 for all children and the Child Behavior Checklist 16 was completed for children aged 24 months or older.
At baseline, children received a neurological examination, which was classified as abnormal if the following findings were identified; hypertonia, hypotonia, or microcephaly. Also at this time, children received developmental testing, including the Bayley Tests of Infant Development 17 for all children under age 3 years and the Developmental Indicators for the Assessment of Learning (DIAL-3) 18 for children aged 3 years and older.
Classification of FSs
Medical records and the detailed parent interview were separately examined to arrive at a summary classification, incorporating all information. Disagreements were resolved in consensus discussions. The children described in this manuscript serve as controls in the FEBSTAT study (Shinnar et al, 2008), a study of the consequences of febrile status epilepticus. For consistency of FS classification in FEBSTAT, first and recurrent FS among FEBSTAT ‘controls’ were reclassified by study epileptologists (SS, DN, JP) as described above. Features examined included duration, focality (including Todd’s paralysis) and number of febrile seizures during the febrile illness.
IRB
This study was approved by the IRB at Columbia University. Parents gave written informed consent.
Statistical Analysis
Using the Kaplan-Meier Method 19, we estimated the cumulative probability, S(t), that a patient would have a FS lasting at least t minutes.
One Population Assumption
We first assumed that our sample came from a single population, regardless of FS duration. We fit the data using the exponential distribution:
where τ is the mean FS duration.
Two Population Assumption
Graphing of the observed S(t) against time revealed that the rate of decline was not constant over time. A model developed by Shinnar and colleagues 12 to describe the distribution of afebrile seizures among 407 children was used to describe the two populations, using a two component mixture exponential model.
This model involves the sum of two exponential distributions representing two populations of patients, the first with a mean seizure duration of τ1 ,which accounts for the proportion, α, of the cases, and the second with a mean seizure duration of τ2 accounting for the remaining proportion, (1- α), of cases.
Two Population Assumption: Febrile vs. Afebrile Parameter
We used a one sample proportion test to determine whether the proportions obtained in our study for the two population assumption differed from that which was observed previously for children with a first afebrile seizure 12.
Correlation between First and Second FS Durations
Among patients who had a second FS, we used the Pearson correlation coefficient to estimate the linear relationship between the duration of the first and second FS. We tested whether the population parameter, ρ, was statistically significant at the 0.05 level of significance.
Outcomes Analysis
We examined whether children with long FS differ from those with short FS with regards to baseline characteristics. Chi-squared or Fisher Exact test was used to test differences in categorical variables and the T-test for continuous variables; Wilcoxon Rank Sum Test was used for continuous variables that are not normally distributed.
Maternal Characteristics
The following factors were compared in children with short and long FSs: duration of pregnancy, maternal smoking during pregnancy, and household income (<15K, 15K to 50K, and ≥50K).
Demographics of children with FS
We examined age at first FS, gender, ethnicity (Hispanic or Non-Hispanic), and physician diagnosis of developmental delay.
Developmental Milestones
Parents were asked about when their children had reached developmental milestones if the child was in or older than the normal limits for that milestone. Parents were not queried for a milestone if the child was too young to have completed these developmental tasks. Children were considered delayed for a milestone if they completed the task at an older age than the upper limit of normal. Milestones assessed included: rolling over (2-5 months), smiling (2-3 months), sitting without support (5-8 months), pulling him/herself to a standing position (6-10 months), standing without support (11-14 months), taking first steps (12-14 months), beginning to walk without any support (12-15 months), running fairly well (18-24 months), making the sounds “mama” or “dada” (6.5-11.5 months), saying single words with meaning (12-14 months), and beginning to drink from a cup (10-17 months). Children could have a delayed milestone and not have a diagnosis of developmental delay.
Developmental Testing
Children under 3 years of age were assessed with the Bayley tests of infant development for cognitive and motor domains. Those older than 3 years were assessed with the DIAL-3 and cognitive and motor scores were analyzed; language standardized scores and concepts standardized scores were averaged to produce the cognitive score. Parents completed the Vineland Adaptive Behaviour Scales, covering communication, daily living, socialization, and motor skills.
Behavioral assessment
Parents completed the CBCL at baseline and at one year of follow-up for children age 24 months and older. The CBCL internal, external, and total scores were analyzed.
All statistical analyses were conducted using SAS 9.120 and graphics created using STATA/SE 9.0 21.
Results
There were a total of 159 children with a first febrile seizure. However, in one child there was no consensus on the exact duration, although the seizure was classified as lasting <10 minutes. This child was excluded from the empirical analyses, but included in the outcomes analysis. Among the 158 children in our sample with complete data on exact seizure duration, we observed a median FS duration of 4.0 minutes.
One Population Assumption
Assuming that the sample came from one population, the mean FS duration, τ, was determined to be 10.20 minutes. Therefore, S(t) was calculated as follows (2):
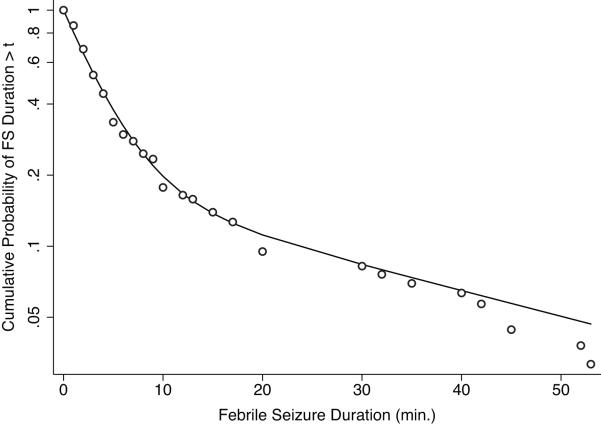
Figure 1 suggests that the one population assumption is not a good fit with a change of slope at about a FS duration of 10 minutes.
FIGURE 1.
Semilogarithmic plot of the observed cumulative probability (red dots), estimated cumulative probability assuming one population (green dashed line), and estimated cumulative probability assuming two populations (black line) of FS duration > t among those with an FS duration ≤ 60 minutes.
Two Population Assumption
Next we assumed that the sample came from two populations. Using this model the best fit for the data is represented by:
This two population model identifies one population that accounts for a proportion α = 82.3% of patients with a mean FS duration of 3.82 minutes, and a second population that accounts for 17.7% of patients with a mean FS duration of 39.82 minutes. The two population assumption yields a better fit of the data. The two distributions have some overlap so that for a given seizure, it is not always possible to say with certainty to which population it belongs. However, τ1 and τ2 are sufficiently far apart that in most cases it is clear to which population the seizure belonged. The cut-point between the two distributions in our data appeared to be at approximately 10 minutes, which also corresponds to a common lower limit for duration sufficiently prolonged to classify a febrile seizure as complex.22 In addition, the two population assumption yielded a better fit of the data in the children without developmental delay. In this group, the two population model identifies one population that accounts for a proportion α = 84.2% of patients with a mean FS duration of 3.79 minutes, and a second population that accounts for 15.8% of patients with a mean FS duration of 36.17 minutes.
Two Population Assumption: Febrile vs Afebrile Seizures
Among children with a first FS, 82.3% had short seizures. This was a higher proportion than the 76% previously reported in children with a first afebrile seizure, although the difference did not reach statistical significance (p=0.06).
Correlation between First FS Duration and Second FS Duration
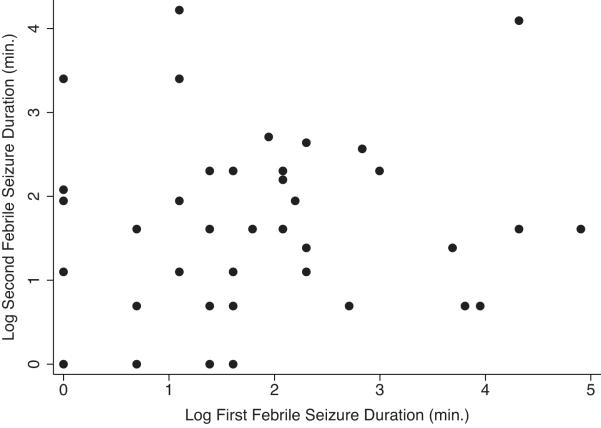
Forty eight (30.4%) of the children with first FS had a second FS. To meet the bivariate normality assumption necessary for the Pearson correlation, both the durations of the first febrile and second FSs were log-transformed (Fig 2). There was insufficient evidence to suggest a statistically significant linear relationship between the durations of the first and second FSs (r=0.12, p=0.40). Additional analysis comparing the risk for recurrent febrile seizures of long duration in children with long versus short first febrile seizure was consistent with these findings (RR=1.11; 95% CI=0.46-2.66).
FIGURE 2.
Scatter plot of Log Second FS Duration versus Log First FS Duration
Comparison of Outcomes between children with a short versus a long first FS
Maternal Characteristics
Children with short seizures did not differ from those with long seizures with respect to duration of pregnancy (p=0.75), maternal smoking during pregnancy (p=0.13), or household income (p=0.35, Table 1).
TABLE 1.
Comparison of children with short seizures and those with long seizures with respect to demographics and neuropsychological testing
| Duration ≤ 10 min. | Duration > 10 min. | p-value | ||
|---|---|---|---|---|
| Maternal Demographics | ||||
| Duration of pregnancy, weeks1 | 40 (38 - 40) | 40 (37 - 40) | 0.75 | |
| Smoked during pregnancy2 | ||||
| Yes | 11 (8.9%) | 5 (18.5%) | 0.13 | |
| No | 112 (91.1%) | 22 (81.5%) | ||
| Income, $3 | ||||
| < 15K | 50 (40.3%) | 14 (56.0%) | 0.35 | |
| 15K - 50K | 54 (43.6%) | 8 (32.0%) | ||
| ≥ 50K | 20 (16.1%) | 3 (12.0%) | ||
|
| ||||
| Patient Demographics | ||||
| Age at first FS, months | 19 (15 - 24) | 15 (12.5 - 20) | 0.048 | |
| Gender | ||||
| Female | 56 (42.8%) | 16 (57.1%) | 0.17 | |
| Male | 75 (57.2%) | 12 (42.9%) | ||
| Ethnicity | ||||
| Hispanic | 112 (85.5%) | 22 (78.6%) | 0.38 | |
| Non-Hispanic | 19 (14.5%) | 6 (21.4%) | ||
| Developmental delay4 | ||||
| Yes | 2 (1.6%) | 4 (14.3%) | 0.010 | |
| No | 127 (98.4%) | 24 (85.7%) | ||
| Neuropsychological Testing at Baseline | ||||
| Vineland standardized scores5 | ||||
| Communications | 95.7 ± 12.2 | 98.4 ± 12.9 | 0.29 | |
| Daily Living | 99.1 ± 12.8 | 100.9 ± 16.4 | 0.52 | |
| Socialization | 99.4 ± 12.7 | 101.9 ± 11.5 | 0.34 | |
| Motor Skills | 100.8 ± 13.1 | 98.8 ± 15.6 | 0.48 | |
| Bayley combined with DIAL-36 | ||||
| Cognitive | 89.6 ± 13.8 | 91.0 ± 17.2 | 0.64 | |
| Motor | 93.5 ± 13.7 | 87.5 ± 18.1 | 0.11 | |
Data are mean ± std. dev., median (IQR), or frequency (%). Tests of means were conducted using the T-test. Tests of medians were conducted using the Wilcoxon Rank Sum Test. Tests of proportions were conducted using the Chi-squared Test or Fisher’s Exact Test.
Missing information for 5 (3.8%) children with short seizures.
Missing information for 8 (6.1%) children with short seizures and 1 (3.6%) with a long seizure.
Missing information for 7 (5.3%) children with short seizures and 3 (10.7%) with long seizures.
Missing information for 2 (1.5%) children with short seizures.
Missing information for 3 (2.3%) children with short seizures.
Missing information for 12 (9.2%) children with short seizures and 4 (4.3%) with long seizures.
Demographics
Children with long FSs had a younger median age at first FS (15 months (IQR=12.5 – 20) vs 19 months (IQR=15 – 24), p=0.048) and were more likely to have developmental delay than children with short FSs (14.3% vs 1.6%, p=0.010). However, there was no difference with respect to gender (p=0.17), or ethnicity (p=0.38, Table 1) for children with short versus long FSs.
Developmental Milestones
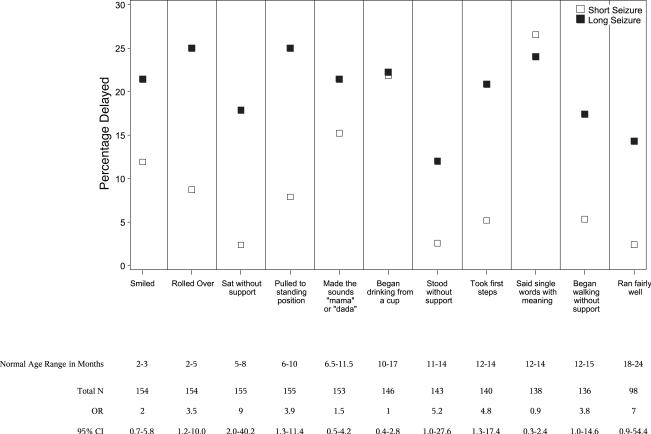
Children with long FSs were more likely to be delayed in rolling over, sitting without support, pulling him/herself to a standing position, and taking first steps, compared to children with shorter FSs (Fig 3). A higher proportion of children with long seizures were delayed in standing without support and walking without support compared to children with shorter FSs, however, this finding did not reach statistical significance. There was no difference with respect to other developmental milestones, including: smiling, running fairly well, making the sounds “mama” or “dada,” saying single words with meaning, and beginning to drink from a cup (Fig 4).
FIGURE 3.
Comparison of the percentage of children with delayed completion of developmental milestones between children with short seizures and those with long seizures
Developmental Testing at Baseline
At baseline, children with long FSs had lower motor scores than those with short FSs (87.5 ± 18.1 vs 93.5 ± 13.7, p=0.1), however this difference was not statistically significant. Similarly, FS duration was not associated with cognitive scores (p=0.64) nor with domains of adaptive behavior (communication (p=0.29), daily living (p=0.52), socialization (p=0.34), and motor skills; p=0.48, Table 1).
Discussion
Similar to analyses of children with a first unprovoked afebrile seizure,12 we found that seizure duration was best represented by a model that accounts for two populations of children, one with a short seizure duration and the other with a long one. Interestingly, the cut point between the two distributions appears to be at about 10 minutes, strengthening the evidence for making 10 minutes the dividing line for prolonged duration that should be classified as a complex FS. This definition has been previously used in the absence of empirical evidence 5-9 11 23 24.
Few studies of seizures evaluate seizure duration. The information used to arrive at a consensus in this study came from the ED record and from detailed parent interviews about the seizure episode. While most children experience a brief first febrile seizure followed by a short post-ictal period that resolves before reaching the ED, other children may have prolonged post-ictal periods. It is possible that some of these children are still experiencing non-motor seizures in the ED, and therefore have an even longer febrile seizure than we were able to assess. Such measurement error in the assessment of febrile seizure duration is unlikely to have biased our results.
The consequences febrile seizures are strongly influenced by seizure duration. Single brief febrile seizures are felt to be benign with no clinical consequences, and this is true in animal models as well.3 22 25-28 In contrast, prolonged febrile seizures are associated with an increased risk of developing epilepsy.3 4 Indeed, animal data indicate that the duration of febrile seizures is correlated with the severity and duration of subsequent unprovoked seizures as well as increased risk of cognitive dysfunction.28 29 Our findings that prolonged febrile seizures occur in a subgroup of children and appear to be a second population that is expected to be at increased risk for future epilepsy adds to the understanding that the brain may be differentially affected by prolonged febrile seizures.
Comparing children with first FS to those with a first unprovoked afebrile seizure,12 there are both striking similarities and differences. The sum of two exponentials model fits the data well in both cases, confirming that, in children with a first FS and those with a first unprovoked seizure, there are two populations, one of which is susceptible to prolonged seizures. The mean seizure duration in the children with first FS and those with first afebrile seizures was very similar (Short seizures: 3.87 minutes for first FS and 3.6 minutes for first unprovoked seizure; Long seizures: 39.33 minutes for first FS and 31 minutes for first unprovoked seizure). The difference between the FS and afebrile seizure population lies in the relative proportions of children in the short or long seizures duration groups. Among children with a first FS, 82.5% belonged to the population with short seizures versus 76% of those with a first unprovoked seizure, though the differences do not reach statistical significance (p=0.06).
The two component mixture exponential model applies to all first FS and to all first unprovoked seizures. However, the long seizure group may itself contain two populations as suggested by our prior work, examining FS with duration ≥ 30 minutes (febrile status epilepticus, FSE). In the FEBSTAT study, the distribution of FSE duration was best fit by a Weibull distribution with a shape parameter of 1.68 30, implying that the longer a seizure lasts, the less likely it is to stop on its own. However, there are very few children with FSs lasting > 60 minutes in a first FS cohort. Such extremely prolonged seizures are associated with long-term effects in animal data 25 26, and with MRI changes in children 31-33. Taken together, the data from the Columbia University study and from the FEBSTAT study suggest that while the two component mixture exponential model for first FS fits seizure durations up to about 60 minutes, at the tail of the distribution the exponential model assumption that the probability of a seizure stopping at time t is independent of how long it has already gone on, no longer holds. However, we were unable to examine this is a single cohort with febrile seizure. Nonetheless, the model holds for the vast majority of children with a first FS. Children with FSE represent only about 5% of all children with a first FS and those with duration >60 minutes are only 1-2% of cases. 2 11 13 34.
We failed to find a correlation between duration of the first FS and duration of the second FS, whereas a statistically significant correlation was observed between duration of first and second afebrile seizures in children 12. There are several reasons why the duration of first and second febrile seizure may be not be correlated. Whereas afebrile seizures are unprovoked, implying that the process leading to the clinical expression of a seizure and determining its duration is stable over time, febrile seizures arise from an interaction of the underlying insult caused by the febrile illness and a given child’s susceptibility. Importantly, this susceptibility to experience a febrile seizure changes over time since febrile seizures are an age related phenomenon and the susceptibility may differ at 12 months and 36 months. Not only may age related differences in febrile seizure susceptibility affect the duration of recurrences but features of the underlying illness may also contribute. These include functional interactions between interleukin-1α, γ-Aminobutyric acid and glutamate 35 that may depend upon the underlying illness provoking fever. Combined the interactions between mechanisms of fever and age-related susceptibility to seize with fever may reduce the consistency of febrile seizure duration between first and second febrile seizure. At odds with this is a prior study of first febrile seizure, which used different methodology to assess duration, but found that a prolonged first FS was associated with an increased risk of a second prolonged FS but not with an increased risk of having a second FS 23.
Children with developmental delay were more likely to have prolonged seizures in our first febrile seizure cohort, but the number of such children in any study of first febrile seizures is too small to permit further analysis. It is important to note that developmental delay is associated with prolonged febrile seizures in febrile status epilepticus. For example in their study of febrile status epilepticus,36 20% of the children had prior developmental delay, which was a risk factor for subsequent epilepsy and for recurrent febrile status. Thus, developmental delay appears to be a risk factor for prolonged febrile seizures.
We also observed a greater frequency of delays in reaching developmental milestones at baseline in children with long versus short FS, a difference restricted to motor milestones, and confirmed by developmental testing at baseline. Others have observed that children with prolonged FS are more likely to be neurologically abnormal 23 37 than children with single, short, non-focal FS. This may explain the increased risk for baseline motor delays we observed in children with long versus short FS. Their differences are most likely due to increased susceptibility to prolonged seizures among children with pre-existing neurological comorbidity who are over represented in studies of prolonged seizures of any etiology.38 39 Other studies of first FS have not conducted baseline developmental testing. Instead they have reported on cognition at 7 or 10 years of age. A British study assessed cognition at 10 years of age after excluding children with neurodevelopmental abnormality prior to the first FS 40. In the NCPP, the presence of neurodevelopmental abnormalities prior to the first FS predicted a lower IQ score at 7 years of age compared to siblings 41, but children without neurodevelopmental 14 abnormalities were indistinguishable from their siblings at age 7. In contrast, new animal data suggest that very prolonged febrile seizures can not only cause hippocampal injury and subsequent temporal lobe epilepsy but can also cause impaired learning and memory.29
Conclusion
The distribution of first febrile seizure duration is best modeled by assuming two underlying populations, a short and a long duration group. These two populations differ in development and in other factors. FSE may not fit the same duration model. Our data are remarkably similar to the distribution of seizure duration in children with a first unprovoked seizure. Our data lend further support to defining 10 minutes as the upper limit for a simple febrile seizure.
Acknowledgments
Acknowledgement statement: Supported by NS 43209 from NINDS (PI: S. Shinnar) and HD 36867 from NICHD (PI: DC Hesdorffer). The authors wish to acknowledge the FEBSTAT Study Team. Montefiore Medical Center, Bronx, NY: Shlomo Shinnar, MD, PhD; Christine O’Dell RN, MSN; Solomon L. Moshe, MD; David Masur, PhD; Erica Weiss, MA; Jacqueline Bello, MD; James Hannigan RT; Maryana Sigalova, MA; Jennifer Ayala, BA; Patricia Clements; Ann Mancini. Children’s Memorial Hospital, Chicago, IL: Douglas Nordli, MD; Leon Epstein, MD; Andrew Kim, MD; Aaliyah Hamidullah, BS; Julie Rinaldi, PhD, Victoria Vinarsky BASarah Ahlm, MA LCSW. Virginai Commonwealth University, Richmond, VA: John Pellocak, MD; Kathryn O’Hara, RN; Syndi Seinfeld, DO; James Culbert, PhD, Tanya Brazemore, R-EEGT; Jean Snow RT-R. International Epilepsy Consortium: Joyce Galloway; John Pellock, MD; Juan Lu, MD, PhD. Duke University Medical Center, Durham, NC: Darrell Lewis, MD; William Gallentine, DO; Elizabeth Rende, RN MSN CPNP; James MacFall, PhD; James Provenzale, MD; Jean Smith, MS; Melanie Bonner, PhD; Yuan Xu, BS; Allen Song, PhD; James Voyvodic, PhD. Eastern Virginia Medical School, Norfolk, VA: L. Matthew Frank, MD; Terrie Conklin, RN; Virginia Van de Water, PhD; Joanne Andy, RT; Susan Grasso, MD; Diane James, R-EEG T; David Kushner, MD; Susan Landers, RT. Columbia University, New York, NY: Dale Hesdorffer, PhD, Veronica J. Hinton, PhD; Emilia Bagiella, PhD; Emma Benn, MPH; Christine Roman, MPH; Stephen Chan, MD. Safety Monitor: Tracy Glauser
Footnotes
Presented in part at the Annual Meeting of the American Epilepsy Society in 2007.
The authors have no conflict of interest.
References
- 1.Van den Berg BJ, Yerushalmy J. Studies on convulsive disorders in young children. V. Excess of early fetal deaths among pregnancies preceding the birth of children with febrile or nonfebrile convulsions. J Pediatr. 1974;84(6):837–40. doi: 10.1016/s0022-3476(74)80759-6. [DOI] [PubMed] [Google Scholar]
- 2.Verity CM, Butler NR, Golding J. Febrile convulsions in a national cohort followed up from birth. II--Medical history and intellectual ability at 5 years of age. Br Med J (Clin Res Ed) 1985;290(6478):1311–5. doi: 10.1136/bmj.290.6478.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annegers JF, Hauser WA, Elveback LR, Kurland LT. The risk of epilepsy following febrile convulsions. Neurology. 1979;29(3):297–303. doi: 10.1212/wnl.29.3.297. [DOI] [PubMed] [Google Scholar]
- 4.Berg AT, Shinnar S. Unprovoked seizures in children with febrile seizures: short-term outcome. Neurology. 1996;47(2):562–8. doi: 10.1212/wnl.47.2.562. [DOI] [PubMed] [Google Scholar]
- 5.Deng CT, Zulkifli HI, Azizi BH. Febrile seizures in Malaysian children: epidemiology and clinical features. Med J Malaysia. 1994;49(4):341–7. [PubMed] [Google Scholar]
- 6.Doerfer J, Wasser S. An epidemiologic study of febrile seizures and epilepsy in children. Epilepsy Res. 1987;1(2):149–51. doi: 10.1016/0920-1211(87)90023-4. [DOI] [PubMed] [Google Scholar]
- 7.Forsgren L, Sidenvall R, Blomquist HK, Heijbel J. A prospective incidence study of febrile convulsions. Acta Paediatr Scand. 1990;79(5):550–7. doi: 10.1111/j.1651-2227.1990.tb11510.x. [DOI] [PubMed] [Google Scholar]
- 8.Iloeje SO. Febrile convulsions in a rural and an urban population. East Afr Med J. 1991;68(1):43–51. [PubMed] [Google Scholar]
- 9.Livingston S, Pauli LL, Pruce I. Febrile seizures. Arch Neurol. 1979;36(3):180. doi: 10.1001/archneur.1979.00500390098018. [DOI] [PubMed] [Google Scholar]
- 10.Nelson KB, Ellenberg JH. Prognosis in children with febrile seizures. Pediatrics. 1978;61(5):720–7. [PubMed] [Google Scholar]
- 11.Nelson KB, Ellenberg JH. Prenatal and perinatal antecedents of febrile seizures. Ann Neurol. 1990;27(2):127–31. doi: 10.1002/ana.410270206. [DOI] [PubMed] [Google Scholar]
- 12.Shinnar S, Berg AT, Moshe SL, Shinnar R. How long do new-onset seizures in children last? Ann Neurol. 2001;49(5):659–64. [PubMed] [Google Scholar]
- 13.Hesdorffer DC, Chan S, Tian H, Hauser W Allen, Dayan P, Leary LD, et al. Are MRI-detected brain abnormalities associated with febrile seizure type? Epilepsia. 2008;49(5):765–71. doi: 10.1111/j.1528-1167.2007.01459.x. [DOI] [PubMed] [Google Scholar]
- 14.NIH . Consensus development conference summary. NIH; Bethesda: 1980. [Google Scholar]
- 15.Sparrow SS, Cicchetti DV. Diagnostic uses of the Vineland Adaptive Behavior Scales. J Pediatr Psychol. 1985;10(2):215–25. doi: 10.1093/jpepsy/10.2.215. [DOI] [PubMed] [Google Scholar]
- 16.Achenbach TM. The Child Behavior Profile: I. Boys aged 6--11. J Consult Clin Psychol. 1978;46(3):478–88. doi: 10.1037//0022-006x.46.3.478. [DOI] [PubMed] [Google Scholar]
- 17.Bayley N. Bayley Scales of Infant Development. 2nd Edition Harcourt Brace and Company; San Antonio: 1993. [Google Scholar]
- 18.Mardell-Czudnowski CGD. A new test for assessing preschool motor development -DIAL-3. APAQ. 17(1):78–94. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- 20.9.1 version. SAS; Cary, NC: program] [Google Scholar]
- 21.College Station, TX: program] [Google Scholar]
- 22.Health NIo NIH Consensus Development summary. Febrile seizures: long-term management of children with fever-associated seizures. South Med J. 1980;73(11):1526. [PubMed] [Google Scholar]
- 23.Berg AT, Shinnar S. Complex febrile seizures. Epilepsia. 1996;37(2):126–33. doi: 10.1111/j.1528-1157.1996.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 24.Verity CM, Butler NR, Golding J. Febrile convulsions in a national cohort followed up from birth. I--Prevalence and recurrence in the first five years of life. Br Med J (Clin Res Ed) 1985;290(6478):1307–10. doi: 10.1136/bmj.290.6478.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brewster A, Bender RA, Chen Y, Dube C, Eghbal-Ahmadi M, Baram TZ. Developmental febrile seizures modulate hippocampal gene expression of hyperpolarization-activated channels in an isoform- and cell-specific manner. J Neurosci. 2002;22(11):4591–9. doi: 10.1523/JNEUROSCI.22-11-04591.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dube C, Richichi C, Bender RA, Chung G, Litt B, Baram TZ. Temporal lobe epilepsy after experimental prolonged febrile seizures: prospective analysis. Brain. 2006;129(Pt 4):911–22. doi: 10.1093/brain/awl018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pediatrics AAo Practice parameter: longterm treatment of the child with simple febrile seizures. Pediatrics. 1999;103:3. doi: 10.1542/peds.103.6.e86. [DOI] [PubMed] [Google Scholar]
- 28.Dube CM, Ravizza T, Hamamura M, Zha Q, Keebaugh A, Fok K, et al. Epileptogenesis provoked by prolonged experimental febrile seizures: mechanisms and biomarkers. J Neurosci. 30(22):7484–94. doi: 10.1523/JNEUROSCI.0551-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dube CM, Zhou JL, Hamamura M, Zhao Q, Ring A, Abrahams J, et al. Cognitive dysfunction after experimental febrile seizures. Exp Neurol. 2009;215(1):167–77. doi: 10.1016/j.expneurol.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinnar S, Hesdorffer DC, Nordli DR, Jr., Pellock JM, O’Dell C, Lewis DV, et al. Phenomenology of prolonged febrile seizures: results of the FEBSTAT study. Neurology. 2008;71(3):170–6. doi: 10.1212/01.wnl.0000310774.01185.97. [DOI] [PubMed] [Google Scholar]
- 31.Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Hippocampal abnormalities after prolonged febrile convulsion: a longitudinal MRI study. Brain. 2003;126(Pt 11):2551–7. doi: 10.1093/brain/awg262. [DOI] [PubMed] [Google Scholar]
- 32.Scott RC, King MD, Gadian DG, Neville BG, Connelly A. Prolonged febrile seizures are associated with hippocampal vasogenic edema and developmental changes. Epilepsia. 2006;47(9):1493–8. doi: 10.1111/j.1528-1167.2006.00621.x. [DOI] [PubMed] [Google Scholar]
- 33.VanLandingham KE, Heinz ER, Cavazos JE, Lewis DV. Magnetic resonance imaging evidence of hippocampal injury after prolonged focal febrile convulsions. Ann Neurol. 1998;43(4):413–26. doi: 10.1002/ana.410430403. [DOI] [PubMed] [Google Scholar]
- 34.Berg AT, Shinnar S, Shapiro ED, Salomon ME, Crain EF, Hauser WA. Risk factors for a first febrile seizure: a matched case-control study. Epilepsia. 1995;36(4):334–41. doi: 10.1111/j.1528-1157.1995.tb01006.x. [DOI] [PubMed] [Google Scholar]
- 35.Gatti S, Vezzani A, Bartfai T. Mechanisms of fever and febrile seizures: Putative role of the interleukin-1 system. In: Baram TZ, Shinnar S, editors. Febrile Seizures. Academic Press; San Diego: 2002. pp. 169–188. [Google Scholar]
- 36.Maytal J, Shinnar S. Febrile status epilepticus. Pediatrics. 1990;86(4):611–6. [PubMed] [Google Scholar]
- 37.Nelson KB, Ellenberg JH. Predictors of epilepsy in children who have experienced febrile seizures. N Engl J Med. 1976;295(19):1029–33. doi: 10.1056/NEJM197611042951901. [DOI] [PubMed] [Google Scholar]
- 38.Maytal J, Shinnar S, Moshe SL, Alvarez LA. Low morbidity and mortality of status epilepticus in children. Pediatrics. 1989;83(3):323–31. [PubMed] [Google Scholar]
- 39.Shinnar S, Pellock JM, Moshe SL, Maytal J, O’Dell C, Driscoll SM, et al. In whom does status epilepticus occur: age-related differences in children. Epilepsia. 1997;38(8):907–14. doi: 10.1111/j.1528-1157.1997.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 40.Verity CM, Greenwood R, Golding J. Long-term intellectual and behavioral outcomes of children with febrile convulsions. N Engl J Med. 1998;338(24):1723–8. doi: 10.1056/NEJM199806113382403. [DOI] [PubMed] [Google Scholar]
- 41.Ellenberg JH, Nelson KB. Sample selection and the natural history of disease. Studies of febrile seizures. Jama. 1980;243(13):1337–40. [PubMed] [Google Scholar]