Abstract
Transient receptor potential channels, of the vanilloid subtype (TRPV), act as sensory mediators, being activated by endogenous ligands, heat, mechanical and osmotic stress. Within the vasculature, TRPV channels are expressed in smooth muscle cells, endothelial cells, as well as in peri-vascular nerves. Their varied distribution and polymodal activation properties make them ideally suited to a role in modulating vascular function, perceiving and responding to local environmental changes. In endothelial cells, TRPV1 is activated by endocannabinoids, TRPV3 by dietary agonists, and TRPV4 by shear stress, epoxyeicosatrienoic acids (EETs), and downstream of Gq-coupled receptor activation. Upon activation, these channels contribute to vasodilation via nitric oxide (NO), prostacyclin (PGI2), and intermediate/small conductance potassium channel (IKCa/SKCa) dependent pathways. In smooth muscle, TRPV4 is activated by endothelial derived EETs, leading to large conductance potassium channel (BKCa) activation and smooth muscle hyperpolarization. Conversely, smooth muscle TRPV2 channels contribute to global calcium entry and may aid constriction. TRPV1 and TRPV4 are expressed in sensory nerves and can cause vasodilation through CGRP and substance P release as well as mediating vascular function via the baroreceptor reflex (TRPV1) or via increasing sympathetic outflow during osmotic stress (TRPV4). Thus, TRPV channels play important roles in the regulation of normal and pathological cellular function in the vasculature.
Keywords: TRPV channels, smooth muscle, endothelium, neurovascular
Introduction
The Transient Receptor Potential (TRP) superfamily is diverse, encoded for by 28 TRP channel genes present in the mammalian genome and grouped into six subfamilies based on DNA and protein sequence homology: TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPA (ankyrin), TRPP (polycystin), and TRPML (mucoliptin). TRP proteins form cation channels that can be gated by multiple stimuli, which include temperature, light, pressure, chemical ligands and mechanical and osmotic stress. All cells express multiple TRP channel proteins which often act as cellular sensors, regulating cellular function by responding to changes in extracellular stimuli. The focus of this review will be on the role of the TRPV subfamily in the vasculature. The TRPV subfamily can be further subdivided into 6 isoforms - TRPV1-6. However, at present only the TRPV isoforms 1–4 have clear roles in vascular function. This review will therefore start with a brief introduction to the structure, distribution, and functional roles of TRPV channels followed by a summary of our current knowledge of TRPV channels in vascular endothelium, arterial myocytes, and perivascular neurons and their roles in the regulation of vascular tone. More detailed information concerning the biophysical properties, channel domains, membrane topology, pharmacology and functional roles of TRP channels can be found in several excellent reviews covering these topics (Clapham, 2003, Nilius et al., 2007, Venkatachalam and Montell, 2007, Appendino et al., 2008, Klausen et al., 2009, Vriens et al., 2009, Vriens et al., 2007, Flockerzi et al., 2007, Di and Malik, 2010, Earley, 2010).
TRPV channels - General
Structure
It is generally accepted that TRP channel proteins have seven hydrophobic domains, six of which span the membrane (S1–S6), whilst the seventh hydrophobic domain, together with the carboxy- and N- termini reside intracellularly (Adamian and Liang, 2006, Clapham et al., 2001, Vannier et al., 1998). TRPV subunits can form both hetero- and homotetramers, (Cheng et al., 2007, Hellwig et al., 2005, Hoenderop et al., 2003, Chang et al., 2004, Stewart et al., 2010, Kottgen et al., 2008, Rutter et al., 2005, Kedei et al., 2001) although it is unknown what complexes are present within the vasculature. The pore region is formed between S5 and S6 and is responsible for TRPV channel cation selectivity and the greater calcium selectivity of TRPV5 and TRPV6 (Chung et al., 2008, Dodier et al., 2007, Garcia-Martinez et al., 2000, Voets et al., 2003, Voets et al., 2002). The pore region may also modulate temperature activation (Grandl et al., 2008, Grandl et al., 2010, Yang et al., 2010b). The N-terminus of TRPV channels contains six ankyrin repeats, collectively referred to as the ankyrin repeat domain (Jin et al., 2006, Lishko et al., 2007, McCleverty et al., 2006, Phelps et al., 2008). This domain allows TRPV channels to adequately traffic to the membrane (Chang et al., 2004, Erler et al., 2004), is required for tetramerization (Arniges et al., 2006, Erler et al., 2004) and modulatory protein-protein interactions (Al-Ansary et al., 2010, Lishko et al., 2007, Phelps et al., 2007, Stokes et al., 2005). The intracellular C-terminus is also involved in tetramerization (Garcia-Sanz et al., 2004), membrane trafficking (Becker et al., 2008), regulation (Liu et al., 2004) and temperature sensitivity (Brauchi et al., 2006, Liu et al., 2004). The C-terminus also contains a conserved hydrophophic domain, referred to as the TRP domain, and contains the consensus sequence; WKFQR, where W is conserved between all members of the TRP family. TRPV members also have a Q at position 4 and positive residues at positions 2 and 5 (Latorre et al., 2009). The TRP domain has been associated with PIP2 modulation (Rohacs, 2009, Rohacs et al., 2005), as well as tetramization (Chang et al., 2004, Garcia-Sanz et al., 2004) and lidocaine (Leffler et al., 2008), capsaicin and temperature sensitivity of TRPV1 (Garcia-Sanz et al., 2007).
Proposed TRPV channel functions
Thermosensation
TRPV1-4 channels are thermosensitive, but have different temperature activation profiles. Most evidence suggests that TRPV1 is activated by temperatures in the noxious range (>43 °C) with an activation threshold of 40 °C (Caterina et al., 1997, Tominaga et al., 1998). Heat shifts the voltage dependence of activation of TRPV1 expressed in HEK 293 cells, to more negative membrane potentials (V1/2 was approx. +70 mV at 25 °C compared to −50 mV at 42 °C), (Voets et al., 2004) and also sensitizes the channel by reducing the threshold for activation for repeated stimuli (Caterina et al., 1997). In vivo, TRPV1 is expressed in small/medium dorsal root ganglion (DRG) and trigeminal neurons. However, although TRPV1 ablation has illustrated that TRPV1 is important in thermal nociception (Caterina et al., 2000, Mishra and Hoon, 2010, Christoph et al., 2008), responses to noxious heat are not completely prevented after TRPV1 suppression (Caterina et al., 2000, Woodbury et al., 2004, Hiura, 2009), even after simultaneous TRPV1 and TRPV2 knockdown (Woodbury et al., 2004). The channel also does not seem to be important in thermoregulation (Iida et al., 2005, Mishra and Hoon, 2010, Romanovsky et al., 2009) but may be important in thermal hyperalgesia during inflammation or after tissue injury (Caterina et al., 2000, Davis et al., 2000, Vilceanu et al., 2010)
Cloned TRPV2 channels are activated by temperatures greater than 52 °C and are sensitized by repeated heat stimulation due to a reduction in the threshold for activation (from ~50 °C to 40 °C). As well, TRPV2 is expressed in medium and large diameter Aδ DRG neurons, which like TRPV2, are activated at temperatures greater that 52 °C (Caterina et al., 1999). It therefore seems likely then that TRPV2 channels in DRGs are involved in noxious heat sensation (Caterina et al., 2000), although responses to noxious heat (52 °C) still occurred in cultured C-fibers from TRPV1−/− mice following TRPV2 suppression (Woodbury et al., 2004).
TRPV3 has a lower temperature threshold (39 °C) than TRPV1 and TRPV2. Above this threshold, the channel displays steep temperature dependence up to the maximum temperature investigated (50 °C),(Smith et al., 2002). Repeated heat stimulation sensitizes the channel and is most effectively activated by rapid heating, producing a Q10 of 21.6 (for the steepest part of the Arrhenius curve) (Xu, 2002). In vivo, TRPV3 may not play a role in thermoregulation as TRPV3−/− mice have core body temperatures similar to wild type mice (Moqrich et al., 2005). However, its functionality in keratinocytes (Moqrich et al., 2005, Chung et al., 2003) and importance in environmental temperature sensing of warm and noxious heat (Moqrich et al., 2005) suggest that TRPV3 acts as a peripheral thermo sensor of noxious and non-noxious temperatures.
When TRPV4 is expressed in heterologous mammalian expression systems, an increase in temperature within the physiological range causes channel activation, increasing whole cell currents and/or calcium transients (Gao et al., 2003, Guler, 2002, Vriens et al., 2004, Wegierski et al., 2009, Watanabe, 2002). TRPV4 has a threshold temperature similar to TRPV3, ~25 °C, with a Q10 of 19.1 at temperatures greater than 24 °C. Unlike TRPV3, the channel desensitizes at temperatures outside the physiological range (>40 °C) (Watanabe, 2002b) and in response to repetitive heat stimulation (Chung et al., 2004, Watanabe, 2002b). Despite this desensitization, the channel still responds to changes in temperature, even during prolonged heat at 42 °C (Guler, 2002). However, when TRPV4 is expressed in a non-mammalian cell like yeast, the channel does not respond to heat (41 °C) (Loukin et al., 2009). Heat was also incapable of activating currents in inside-out patches of TRPV4 expressing HEK 293 cells (Watanabe, 2002b). Thus, In mammalian expression systems, as suggested by several recent studies, activation of TRPV4 by heat may occur downstream of various intracellular signaling cascades or require other molecular interactions. Specifically, TRPV4 activity is increased by TRPP2 (Kottgen et al., 2008), dependent on the ankyrin repeats in the N-terminus (Watanabe, 2002b), dependent on tyrosine residues Y555 (Vriens et al., 2004) and Y110 (Wegierski et al., 2009), and inhibited by PACSIN-3, a protein associated with endocytosis and trafficking (D’hoedt et al., 2008).
Although TRPV4 is found in the anterior hypothalamus, an area of the brain involved in thermoregulation (Guler, 2002), some evidence suggests that TRPV4 is not actually involved in thermal regulation. Core body temperatures of wild type and TRPV4 −/− mice, at different room temperatures, were similar (Liedtke and Friedman, 2003, Lee et al., 2005). Like TRPV3, TRPV4 is important in keratinocytes (Chung et al., 2003, Chung et al., 2004) and peripheral thermosensation in mice (Lee et al., 2005), however, TRPV4 does not seem to play a role in noxious heat sensation (Todaka et al., 2004, Suzuki et al., 2003a). Warmer temperatures can sensitize the channel to activation by other stimuli, such as hypotonic stress (HTS), 4α-PDD, shear stress and PMA (Gao et al., 2003, Liedtke et al., 2000, Liedtke et al., 2003), which may also be important for channel regulation in vivo.
Osmoreception
TRPV isoforms demonstrate osmosensitivity and may play important roles as osmoreceptors. In isolated urothelial cells, a functional role for TRPV1 in osmotic sensation has been observed. Hypotonic solution (HTS) causes the release of ATP, which is blocked by capsazepine and absent in TRPV1−/− mice (Birder et al., 2002). However, TRPV1 mRNA can not be detected in mouse urothelium (Everaerts et al., 2010), rendering this observation somewhat inconclusive. TRPV1 is also expressed in areas of the brain involved in osmoregulation such as the organum vasculosum of the lamina terminalis (OVLT) and supraoptic nucleus (SON). Neurons from the OVLT and the SON displayed increased firing rates after treatment with a hypertonic solution which did not occur in neurons from TRPV1−/− mice (Ciura and Bourque, 2006, Naeini et al., 2006).
In TRPV2 expressing CHO cells, HTS increased currents in cells in the cell-attached configuration. In mouse aortic myocytes, HTS-induced increases in whole cell currents and cell calcium were reduced after cells had been treated with antisense targeted against TRPV2 (Muraki et al., 2003).
A large body of evidence indicates that TRPV4 can be modulated by osmotic stimuli in multiple cells types. When TRPV4 is expressed in heterologous expression systems, HTS can increase whole cell currents (Nilius et al., 2001) and induce calcium influx which is reversed upon the return to isotonic solution (Liedtke et al., 2000, Strotmann et al., 2000). Responses to osmotic stimuli in TRPV4 containing cells, are sensitive to PLA2 and cytochrome P450 epoxygenase inhibition (Vriens et al., 2004, Loukin et al., 2010), although HTS responses still occur in yeast which lack endogenous EETs, a product of cytochrome P450 activity (Loukin et al., 2009). HTS-TRPV4 responses are also sensitive to PLC inhibition (Fernandes et al., 2008, Garcia-Elias et al., 2008), sensitized by IP3 receptor binding (Garcia-Elias et al., 2008), heat (Grant et al., 2007, Liedtke et al., 2000, Gao et al., 2003), TRPP2 (Kottgen et al., 2008), PAR2 activation (Grant et al., 2007), and inhibited by PACSIN-3 (D’hoedt et al., 2008). Phosphorylation of Y253 may be important in HTS activation (Xu 2003) although this is debatable (Vriens et al., 2004). Phosphorylation of Y110 by PKC/PKA also occurs in response to HTS (Wegierski et al., 2009). In vivo, the channel is associated with a central role in sensing peripheral changes in osmolarity (Liedtke and Friedman, 2003), HTS mediated nociception after sensitization by PGE2 (Alessandri-Haber 2003) and cell volume regulation (Becker et al 2008).
Mechanoreception
Mechanical stimulation, a condition that results in a physical perturbation of the membrane is usually achieved by membrane stretch, pressure or shear stress. The mechanosensitivity of TRP channels has been recently reviewed (Yin and Kuebler, 2010) and within the TRPV subfamily, mechanosensitivity has been mainly attributed to TRPV1, TRPV2 and TRPV4.
The majority of evidence implicating TRPV1 in mechanosensation has come from the use of TRPV1−/− mice. In excised bladders from TRPV1−/− mice, stretch-induced ATP release was less compared to controls (Birder et al., 2002). Intraluminal pressure in mouse jejunum increases the discharge of wide dynamic range afferent nerves, which is reduced in TRPV1 −/− mice (Rong et al., 2004). Intra-pelvic pressure in rats increases afferent renal nerve activity, and this response is reduced by capsazepine (Feng et al., 2008). However, in mutants of C.elgans that do not produce avoidance responses to mechanical nose touch, expression of TRPV1 was not sufficient to restore mechanical behavior (Liedtke et al., 2003) and mechanosensation is unaltered in TRPV1−/− mice (Caterina et al., 2000, Christoph et al., 2008, Mishra and Hoon, 2010). Nevertheless, TRPV1 may be involved in mechanical hypersensitivity and allodynia following spinal nerve ligation (Christoph et al., 2008).
The only convincing evidence that TRPV2 is mechanosensitive is from TRPV2 expressing CHO cells, where membrane stretch applied using negative pressure via the patch pipette stimulated inward currents at −40 mV (Muraki et al., 2003).
In TRPV4 expressing cells, whole cell currents were increased by cell inflation (Suzuki et al., 2003a), and cell suction (Loukin et al., 2010). Shear stress can also evoke responses in TRPV4 containing cells (Mendoza et al., Fernandes et al., 2008, Wegierski et al., 2009, Kottgen et al., 2008) that are reduced following TRPV4 suppression (Kottgen et al., 2008, Mendoza et al., 2010). In mutant C. elegans, TRPV4 expression was required to restore nose touch avoidance behaviors (Liedtke et al., 2003). Mechanical responses mediated by TRPV4 are greater at physiological temperatures (Gao et al., 2003, Liedtke et al., 2003), dependent on Y110 (Wegierski et al., 2009), enhanced following IP3 stimulation in cells co-expressing the IP3 receptor (Fernandes et al., 2008), yet do not require PLA2 or cytochrome P450 epoxygenase signaling (Loukin et al., 2010). Physiologically, mechanical activation of the channel is important in endothelium-dependent vasodilations (Mendoza et al.), sensitivity to acoustic injury (Liedtke and Friedman, 2003), stretch induced endothelial cell remodeling (Thodeti et al., 2009) and peripheral mechanosensation and mechanical hyperalgesia (Grant et al., 2007, Liedtke and Friedman, 2003, Suzuki et al., 2003b).
TRPV channels and vasomotor function
TRPV expression in vascular tissues
TRPV channels are differentially expressed throughout vascular endothelium, smooth muscle and innervating peri-vascular nerves (Table 1). In endothelial cells, investigations surrounding TRPV4 have been most numerous (Hartmannsgruber et al., 2007, Kohler et al., 2006, Loot et al., 2008, Mendoza et al., 2010, Watanabe, 2002b, Willette et al., 2008, Zhang et al., 2009), collectively suggesting that is also the most dominant isoform in this cell type. There is only one report for TRPV3 in endothelial cells (Earley et al., 2010) and TRPV2 has only been found in cultured vascular endothelial cells (Fantozzi et al., 2003). There are some reports that suggest that TRPV1 is present in vascular endothelium, however of these, three studies were conducted using cultured endothelial cells (Fantozzi et al., 2003, Golech et al., 2004, Yang et al., 2010) and another solely used immunohistochemistry (Bratz et al., 2008) with an antibody that produces non-specific staining (Everaerts et al., 2009). The final observation was made using RT-PCR in whole, chronically denervated arteries, with and without endothelium and although the endothelium was not isolated and tested specifically, TRPV1 mRNA expression was greater in vessels where the endothelium was kept intact, suggesting that TRPV1 was present in the endothelium (Bratz et al., 2008). However, TRPV1 mRNA was not found in the endothelium of rat middle cerebral arteries (Marrelli et al., 2007). In vascular smooth muscle, TRPV2 and TRPV4 appear to be the most abundantly expressed (Inoue et al., 2006, Yang et al., 2006); however, interestingly, TRPV4 mRNA was not present in mouse mesenteric arteries (Mendoza et al., 2010). TRPV3 expression in smooth muscle was the weakest (Inoue et al., 2006b, Yang et al., 2006) and was absent in mouse aorta (Muraki et al., 2003). TRPV1 may be expressed in vascular smooth muscle as mRNA for TRPV1 was found in de-endothelialized rat aorta and intralobular arteriers, although contamination of the sample by perivascular nerves or the endothelium was not examined (Yang et al., 2006). Other studies that have illustrated TRPV1 expression in vascular smooth muscle have done so using solely immunohistochemistry (Bratz et al., 2008, Kark et al., 2008), or by using cultured and not native cells (Wang et al., 2008). One study has shown that TRPV1 is not present in rat cerebral arteries (Marrelli et al., 2007). Only TRPV1 and TRPV4 have been associated with peri-vascular innervation, although these observations were mainly based on immunohistochemistry (Benfenati et al., 2007, Gao and Wang, 2010, Scotland et al., 2004, Tanaka et al., 2008, Toth et al., 2005) and would have been more beneficial if they had been confirmed with PCR. Evidence for TRPV5 and TRPV6 expression is limited, but these TRPV isoforms were not expressed in rat mesenteric, cerebral and aortic smooth muscle (Inoue et al., 2006), rat intralobular pulmonary artery and aorta (Yang et al., 2006) or human pulmonary artery smooth muscle cells (Wang et al., 2008).
Table 1.
Expression of TRPV channels in peri-vascular nerves, vascular endothelial cells and smooth muscle cells.
| Channel | Cell Type | Analysis | Reference |
|---|---|---|---|
| Peri-vascular nerves | |||
| TRPV1 | Mouse mesenteric arteries | IHC | (Scotland et al., 2004) |
| Astrocytes surrounding brain capillaries and larger arteries | IHC | (Tóth et al., 2005) | |
| TRPV4 | Cultured rat cortical astroctyes | RT, W, IHCIP | (Benfenati et al., 2007) |
| Rat mesenteric arteries | IHC | (Gao and Wang, 2010) | |
| Smooth muscle | |||
| TRPV1 | Rat intralobular artery and aorta | RTd, W | (Yang et al., 2006) |
| Rat skeletal muscle arteriole | IHC | (Kark et al., 2008) | |
| Cultured human pulmonary artery smooth muscle cells | RT, W | (Wang et al., 2008) | |
| Swine coronary artery | IHC | (Bratz et al., 2008) | |
| TRPV2 | Rat intralobular artery and aorta | RTd | (Yang et al., 2006) |
| Rat aorta, mesenteric and cerebral arteries | RT | (Inoue et al., 2006) | |
| Mouse aorta, mesenteric, basilar artery | RTi, IHCIP | (Muraki et al., 2003) | |
| Cultured human pulmonary artery smooth muscle cells | RT | (Wang et al., 2008) | |
| TRPV3 | Rat intralobular artery and aorta | RTd | (Yang et al., 2006) |
| Cultured human pulmonary artery smooth muscle cells | RT | (Wang et al., 2008) | |
| TRPV4 | Rat intralobular artery and aorta | RTd, Wd | (Yang et al., 2006) |
| Rat aorta, mesenteric and cerebral arteries | RT | (Inoue et al., 2006) | |
| Cultured human pulmonary artery smooth muscle cells | RT | (Wang et al., 2008) | |
| Mouse aorta artery | RTi | (Muraki et al., 2003) | |
| Rat cerebral artery | RTAS | Earley et al 2005 | |
| Rat aortic freshly isolated and cultured myoctes | RTi, IHCi | (Tanaka et al., 2008) | |
| Rat middle cerebral artery | RTw, IHC | (Marrelli et al., 2007) | |
| Mouse aorta | IHC* | (Zhang et al., 2009) | |
| Endothelial cells | |||
| TRPV1 | Cultured cerebrovascular endothelial cells | RT, IHC | (Golech et al., 2004) |
| Denervated rat mesenteric arteries | RT^ | (Poblete et al., 2005) | |
| Swine coronary artery | IHC, RT | (Bratz et al., 2008) | |
| Cultured human pulmonary endothelial cell | (Fantozzi et al., 2003) | ||
| Cultured mouse aortic endothelial cells | RT*, W* | (Yang et al., 2010) | |
| TRPV2 | Cultured human pulmonary endothelial cell | RT | (Fantozzi et al., 2003) |
| TRPV3 | Rat cerebral arteries | RTw, IHC | (Earley et al., 2010) |
| TRPV4 | Rat mesenteric arteries | IHC | (Gao and Wang, 2010) |
| Cultured human pulmonary endothelial cell | RT | (Fantozzi et al., 2003) | |
| Rat carotid artery | RTi | (Kohler et al., 2006) | |
| Cultured human pulmonary endothelial cell | RT | (Fantozzi et al., 2003) | |
| Rat middle cerebral artery | RTi, IHC | (Marrelli et al., 2007) | |
| Mouse carotid artery | IHC* | (Hartmannsgruber et al., 2007) | |
| Mouse mesenteric | RTi, HC* | (Mendoza et al., 2010) | |
| Mouse aorta | IHC* | (Zhang et al., 2009) | |
RT; RT-PCR, W; western blot, IHC; immunohistochemisty,
knock-out control,
anti-sense oligonucleotides used as control,
immunizing peptide control,
denervated arteries, +/− endothelium,
whole artery,
deendothelialized,
isolated cells.
TRPV channels and endothelial cell function
TRPV expression has been found in various vascular endothelial cells (Table 1), however functional roles have only been demonstrated for TRPV1, TRPV3 and TRPV4, all of which result in vasoldilation. Upon stimulation, the endothelium produces many vasoactive substances, but the main vasodilators are NO, prostacyclin (PGI2) and Endothelium-Derived Hyperpolarising Factor (EDHF) (Vanhoutte et al., 2009). EDHF has many identities and can differ between vascular beds (Edwards et al., 2010, Feletou and Vanhoutte, 2009), but for the purpose of this review, K+ efflux from endothelial intermediate and small conductance potassium channels (IKCa and SKCa respectively) is an important EDHF mechanism that follows TRPV stimulation. The importance of IKCa/SKCa channel function has been highlighted in transgenic mice (Kohler and Ruth, 2010). As NO, PGI2 and EDHF mechanisms can be calcium dependent, it is assumed that calcium influx through TRPV channels sufficiently activates these pathways.
TRPV1
As TRPV1 is expressed on endothelial cells, smooth muscle cells and peri-vascular nerves (Table 1), it is hard to distinguish true endothelial TRPV1 mediated responses. Further, endocannabinoids, which may also activate TRPV1, are known to activate many other receptors (CB1, CB2 and TRPV4), again making results difficult to interpret. Despite this, there is some evidence that shows capsaicin, a selective TRPV1 agonist, and endocannabinoids can affect vascular function through endothelial TRPV1 channels.
In rat skeletal muscle arteries, perivascular innervation appears to be absent, making skeletal muscle arteries an ideal preparation to study TRPV1 in vascular function (Kark et al., 2008). In this preparation, capsaicin (10 nM) produced endothelium-dependent vasodilations that were inhibited by L-NAME, an inhibitor of endothelial nitric oxide synthase (eNOS). Endothelial TRPV1 activation is therefore capable of causing vasodilation via a NO-dependent pathway. In porcine coronary artery endothelial cells, capsaicin (100 μM) induced calcium influx and relaxed arterial ring segments in a dose-dependent fashion. Capsaicin-induced relaxations were absent in endothelial denuded preparations and inhibited by the TRPV1 antagonist, capsazepine. Inhibition of eNOS with L-NAME, large conductance potassium channels (BKCa) with iberiotoxin and potassium channels in general with TEA (10 mM) reduced capsaicin-induced relaxations, suggesting that both NO and potassium channel activation is responsible for capsaicin-induced relaxations. Capsaicin, at concentrations above 1 μM, still produced a small relaxation in the presence of the inhibitors. Whether these effects were non-specific or due to an endothelium independent mechanism was not discussed (Bratz et al., 2008). In rat mesenteric arteries, Hoi et al., (2007) have shown that the effects of VSN16, a synthetic, cannabinoid-like compound, caused endothelium-dependent relaxations that were reduced by capsazepine. Responses were not completely TRPV1 dependent, as total inhibition only occurred in the presence of both capsazepine and O-1918, an antagonist of the novel endothelial ‘abnormal-cannabidiol receptor’ (see (Offertaler et al., 2003)). Relaxations were also reduced by inhibitors of eNOS (L-NNA), IKCa/BKCa (charybdotoxin) and SKCa (apamin), but not by cyclooxygenase (COX) inhibition with indomethacin (Hoi et al., 2007). It is unknown whether VSN16 activates TRPV1 directly or downstream of the abnormal-cannabidiol receptor, but the study highlights that activation of endothelial TRPV1 is part of a VSN16-dependent vasodilatory pathway that is reliant on NOS and IKCa/SKCa activation. Work by Poblete et al., (2005) has also shown that TRPV1 activation can result in endothelial-dependent NO release. In a chronically denervated mesenteric vascular bed, capsaicin and anandamide perfusion through the lumen, led to endothelium-dependent NO production (measured from the perfusate) that was completely inhibited by capsazepine and L-NNA. A selective TRPV1 antagonist, SB366791 also reduced endothelium-dependent, capsaicin and anandamide-induced, NO release in non-denervated arteries, confirming that TRPV1 activation was partially responsible. Although the concentration of anandamide used (100 nM, to mimic physiological concentrations) was not high enough to elicit a vasodilation, the study illustrates that activation of the TRPV1 channel, either by anandamide or capsaicin, can lead to endothelial-dependent NO release (Poblete et al., 2005). As anandamide and its metabolites can activate TRPV4 (Watanabe et al., 2003), the effects of anandaminde on TRPV4 were ruled out by showing that 4α-PDD infusion did not cause significant NO production (Poblete et al., 2005). Perhaps the most convincing evidence for a functional role of TRPV1 in endothelial cells has been presented by Yang et al., (2010) who have shown that in cultured mouse aortic endothelial cells, capsaicin can increase intracellular calcium, increase the phosphorylation of eNOS and activate PKA. Capsaicin was also capable of producing endothelium-dependent vasodilation and NO production (measured using DAF-2DA fluorescence) in isolated mouse mesenteric arteries, both of which were inhibited by L-NAME and reduced in vessels from TRPV1−/− mice. This study is also the first to demonstrate that the blood pressure of spontaneously hypertensive (SHR) rats could be reduced by chronic dietary capsaicin treatment. The chronic capsaicin diet also seemed to improve endothelium function as mesenteric arteries form these animals and from mice that had also been chronically fed a capsaicin diet, responded more robustly to acetylcholine (ACh), a muscarinic receptor agonist, compared to normally fed controls. Improved ACh-mediated dilations did not occur in chronically capsaicin fed TRPV1−/−mice and were also inhibited by L-NAME. TRPV1, PKA and eNOS expression was also increased in tissue from chronically capsaicin fed animals. Although it can not be certain that endothelial TRPV1 is responsible for capsaicin-dependent reductions in blood pressure in vivo, as the channel is expressed throughout the body, the data from isolated cells and mesenteric arteries clearly show that TRPV1 is present and functional within the endothelium and that activation leads to calcium influx, NO production, PKA activation and dilation (Yang et al., 2010). TRPV1 channel activation may also lead to calcitonin gene-related peptide (CGRP) release, which has been demonstrated in HUVECs (Luo et al., 2008, Ye et al., 2007). Thermal activation of TRPV1 causes a capsazepine-sensitive increases CGRP expression in rat mesenteric and aortic endothelial cells (Ye et al., 2007). Whether endothelial-TRPV1 mediated CGRP release can regulate arterial tone is not known, but it can dilate vessels when released from sensory nerves (Scotland et al., 2004), see below. CGRP may help protect against cell death (Ye et al., 2007). One study has shown that in rat carotid endothelium there does not appear to be a role for TRPV1 in endothelium-dependent dilation as capsaicin failed to activate currents in isolated endothelial cell and dilate isolated vessels (Kohler et al., 2006). Collectively, these in vitro studies suggest that TRPV1 channels are present and functional in vascular endothelial cells and may modulate vascular function via the production of NO, CGRP and/or IKCa/SKCa channel activation in response to capsaicin, temperature or locally produced endocannabinoids.
It should be noted that TRPV1 may not be required for normal blood pressure regulation as there is no statistical difference between the baseline mean arterial pressures of TRPV1−/− and wild type mice (Pacher et al., 2004, Wang and Wang, 2009). Further, TRPV1 expression does not help to reduce deoxycorticosterone acetate-salt induced hypertension, as blood pressures before and after deoxycorticosterone acetate-salt treatment were the same in wild type and TRPV1−/− mice, albeit TRPV1 might protect against deoxycorticosterone acetate-salt induced renal damage (Wang and Wang, 2009). Despite these observations, a role for TRPV1 in in vivo vascular control can not be completely ruled out as cinnamaldehyde (320 μmol/kg, i.v.), a TRPA1 agonist, caused a greater pressor effect on mean arterial pressure in TRPV1−/− mice compared to controls, which suggests that TRPV1 activation may oppose some vasoconstrictor mechanisms (Pozsgai et al., 2010).
TRPV3
To date there is only one investigation into TRPV3 and the endothelial control of vascular function (Earley et al., 2010). Earley and colleagues provide convincing evidence for a role of endothelial TRPV3 channels in rat cerebral arteries. Carvacrol, an activator of TRPV3 (and TRPA1) produced sensitizing whole cell currents in isolated endothelial cells. Currents were insensitive to the TRPA1 inhibitor, HC-030031, implicating TRPV3 as the source. In pressurized cerebral arteries (70 mmHg), carvacrol caused endothelium-dependent dilations, smooth muscle hyperpolarization, and reduced arterial wall calcium. The sensitivity of the TRPV3-induced dilations to TRAM-34, a selective IKCa blocker (Wulff et al., 2000), as well as to apamin and barium, inhibitors of SKCa, and smooth muscle inwardly rectifying potassium channels (Kir) respectively, suggests that TRPV3 activation increases cell calcium, activating IKCa and SKCa. This results in K+ efflux, which activates smooth muscle Kir. Kir activation hyperpolarizes the smooth muscle cells and causes relaxation. There was no significant difference between carvacrol elicited dilations in the presence and absence of NOS and COX blockade, suggesting that NO and prostacyclin do not contribute to TRPV3 mediated dilations (Earley et al., 2010).
TRPV4
Endothelium-dependent TRPV4 channels regulate vascular function by stimulating NO, PGI2 and EDHF production. The polymodal nature of TRPV4 allows activation by many endogenous stimuli, namely, shear stress, heat, hypotonic environments, Gq signaling, and directly via EETs.
Heterologously expressed TRPV4 channels can be activated fairly selectively by 4αPDD (EC50; 0.2 μM mTRPV4) (Watanabe, 2002a), GSK1016790A (EC50; 18 nM mTRPV4) (Thorneloe et al., 2008), RN-1747 (EC50; 4 μM r/mTRPV4) (Vincent et al., 2009), arachidonic acid metabolites (EC50 of 5,6-EETs; 130 nM mTRPV4) (Watanabe et al., 2003) and bisandrographolides (EC50; 950 nM mTRPV4)(Smith et al., 2006). Regarding specificity, it should be noted that GSK1016790A and RN-1747 can also activate TRPV1 (Vincent et al., 2009, Willette et al., 2008) and 4αPDD can activate a non-specific current in non-transfected TRPV4 cells (Thorneloe et al., 2008). Pharmacological activation of TRPV4 leads to profound dilations in mouse mesenteric arteries (Kohler et al., 2006, Mendoza et al., Earley et al., 2009) mouse carotid artery (Loot et al., 2008), rat aortic rings (Willette et al., 2008) and rat carotid artery (Kohler et al., 2006). TRPV4 mediated dilations are absent in TRPV4 −/− mice (Mendoza et al., Earley et al., 2009, Hartmannsgruber et al., 2007). Activation of TRPV4 increases whole cell currents (Kohler et al., 2006, Hartmannsgruber et al., 2007) and calcium influx (Zhang et al., 2009, Mendoza et al., Watanabe, 2002b, Wu et al., 2009, Kohler et al., 2006).in isolated endothelial cells; these responses are blocked by ruthenium red.
NO, PGI2 and EDHF are vasodilatory pathways that are activated following TRPV4 stimulation (Earley et al., 2009, Kohler et al., 2006, Mendoza et al., 2010, Willette et al., 2008). In mouse mesenteric arteries, GSK1016790A -induced dilations were reduced from 67 % to 27 % following eNOS inhibition. The residual dilation was completely abolished upon the subsequent addition of high K+ containing solutions (Mendoza et al., 2010). GSK1016790A also failed to cause vasodilation of mouse aortic rings from eNOS −/− mice (Willette et al., 2008). In rat carotid artery, joint NOS and COX inhibition reduced 4α-PDD induced dilations from 70 to 10 %, but was surprisingly ineffective in the resistance artery, A. gracilis, where a robust 4α-PPD induced dilation still remained in the presence of L-NNA and indomethacin (Kohler et al., 2006). As well, perfusion of 4α-PDD through the rat mesenteric vasculature did not lead to NO production (Poblete et al., 2005). Earley et al., (2009) showed that 11, 12-EET induced dilations still remained after NOS and COX inhibition in mouse mesenteric arteries and required inhibition of endothelial IKCa and SKCa channels to reduce dilations by 50 % (Earley et al., 2009). The remaining 50 % of the response was due to TRPV4 mediated effects on the smooth muscle. TRPV4 responses to 4αPDD (and HTS) may also require PLA2 signaling as inhibitors of PLA2 and cytochrome P450 epoxygenase dramatically reduced 4αPDD-induced currents in TRPV4 expressing oocytes. It was speculated that threshold calcium entry through TRPV4 is needed to bind to and activate PLA2, which produces EETs to activate/augment TRPV4 activity (Loukin et al., 2010).
Endothelial cells are in constant contact with the shear forces induced by the circulating blood. Increases in shear stress, arising from increases in blood flow or blood viscosity, are sensed by the endothelium, which then modulates vascular tone accordingly, dilating vessels to reduce intra-luminal shear force. Endothelial TRPV4 channels are involved in shear stress induced vasodilation. In gracilis muscle arterioles, shear stress (an increase of 2 dyn/cm2) causes a ruthenium red sensitive dilation (Kohler et al., 2006). In the mouse carotid artery, an increase in the shear stress, from 3 to 7 dyn/cm2, induces a 40 % dilation which does not occur in TRPV4 −/− mice (Hartmannsgruber et al., 2007). Flow induces a 30 % dilation in mouse carotid arteries, that is much lower in TRPV4−/− mice (15.5 %) (Hartmannsgruber et al., 2007) and a 49 % dilation in mouse mesenteric arteries that is reduced to 24 % in TRPV4−/− vessels (Loot et al., 2008).
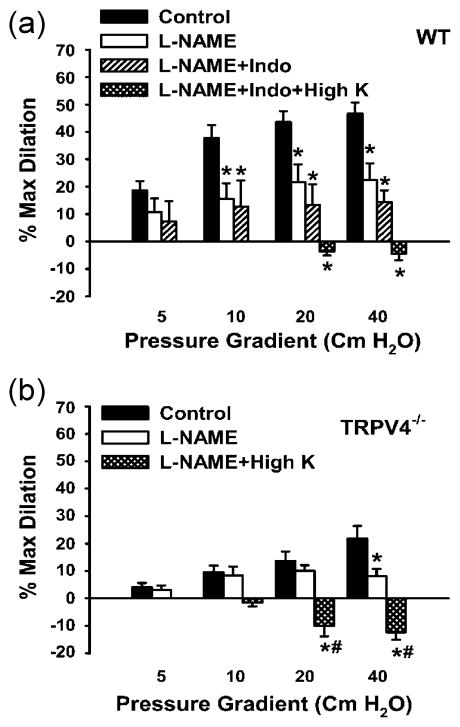
Shear stress-induced activation of TRPV4 dilates vessels via the subsequent production of NO, PGI2 and EDHF, however the relative contribution of each differs between vessel types: Shear stress induced dilations were completely dependent on NO in rat carotid artery. Inhibition of NOS with L-NNA reduced dilations to less than 5 % (Kohler et al., 2006). In gracilis muscle arterioles, shear stress (10 dyn/cm2) induced dilation was again, almost completely inhibited by NOS blockade (Kohler et al., 2006). Mendoza et al., (2010) showed that both NOS and high K+ solutions were required to block the shear stress mediated dilation in mouse mesenteric arteries, whereas COX inhibition had no effect (Figure 1). In the mouse carotid artery, inhibitors of IKCa and SKCa channels were required to inhibit the remaining shear stress induced vasodilation after NOS and COX inhibition (Hartmannsgruber et al., 2007). Loot et al., (2008) found that EDHF was the primary mechanism by which shear stress activation of TRPV4 caused dilation. In mouse carotid arteries, in the absence of NOS and COX inhibition, flow induced dilations were similar between WT and TRPV4−/− mice. After the inhibition of NOS and COX, the remaining dilation, presumably due to EDHF, was absent in TRPV4 −/− mice. Thus, shear stress leads to endothelium-dependent, TRPV4 mediated vasodilation, mainly via NO and EDHF production.
Figure 1.
Shear stress induced dilations are TRPV4 dependent and require NO and EDHF production. (a) In pressurized mouse mesenteric arteries, shear stress induced dilations were achieved by increasing luminal flow (by increasing pressure). L-NAME (100 μM) inhibited responses. Indomethacin (10 μM) produced no additional inhibition, but the addition of high K+ successfully prevented dilations. (b) In TRPV4−/− mice, shear stress induced dilations were less than wildtype (A) and also inhibited by L-NAME and high K+. (Data are from Mendoza et al., 2010. Am J Physiol Heart Circ Physiol. “Am Physiol Soc, with permission”).
Shear stress-TRPV4 mediated dilations appear to occur downstream of PLA2 and are not due to direct channel activation. Inhibition of PLA2, inhibited shear stress responses in gracilis muscle arterioles and in mouse carotid artery (Hartmannsgruber et al., 2007, Kohler et al., 2006) and were independent of PKC and tyrosine kinase (Kohler et al., 2006). Within the cell membrane, PLA2 converts membrane phospholipids to arachidonic acid, which in turn is metabolized by Cytochrome P450 epoxygenase activity to various epoxyeicosatrienoic acid products (5,6-EET, 8,9-EET, 11,12-EET). Vriens et al., (2004) showed that inhibition of PLA2 and cytochrome P450 epoxygenase prevents hypotonic-induced calcium responses in TRPV4 expressing HEK cells. In mouse carotid arteries, Loot et al., (2008) also showed that inhibition of CYP epoxygenase with MS-PPOH, reduced the EDHF mediated dilation that only occurred in wild type mice. No further dilation could be elicited by the sequential addition of ruthenium red; suggesting that CYP epoxygenase and TRPV4 are part of the same pathway for EDHF mediated dilation.
Activation of endothelial muscarinic receptors is an effective vasodilatory pathway resulting in NO, PGI2 and EDHF release. Downstream activation of TRPV4 and subsequent calcium influx mediates this response in some tissues. Although there was no difference between ACh mediated dilations of mouse carotid arteries from WT and TRPV4 −/− mice (Hartmannsgruber et al., 2007, Loot et al., 2008), and ruthenium red had no affect on ACh responses in gracilis muscle arterioles (Kohler et al., 2006), ACh-induced responses were partially inhibited by ruthenium red in mouse carotid artery (Kohler et al., 2006). Furthermore, ACh-mediated dilations were blunted in TRPV4 −/−mouse mesenteric arteries, reduced from an 88 % dilation in wild-type arteries to 49 % in TRPV4 −/− arteries. In TRPV4 −/− arteries the remainder of the dilation was inhibited by L-NAME implying that ACh-TRPV4 activation led to EDHF release in WT. The NO component of the ACh response, measured using DAF fluorescence, in carotid arteries was also less in the TRPV4−/− mice (Zhang et al., 2009). Earley et al., (2009) also concluded that EDHF was responsible for ACh-TRPV4 mediated dilations in mouse mesenteric arteries. In vessels incubated with L-NAME and indomethacin, ACh still induced endothelium-dependent dilation and smooth muscle hyperpolarization. This residual EDHF-mediated dilation was reduced by 75% in vessels from TRPV4 −/− mice (Earley et al., 2009). EDHF and NO components of a carbachol induced relaxation were both reduced in mouse mesenteric arteries from TRPV4−/− mice compared to WT (Saliez et al., 2008). Thus, muscarinic receptor activation leads to a downstream activation of TRPV4 that is responsible for the release of NO and EDHF.
Similar to shear-stress effects, muscarinic-dependent activation of TRPV4 may require PLA2. Marrelli et al., (2007) suggest that UTP mediated vasodilation is a result of activating TRPV4 via PLA2 signaling. UTP caused calcium influx in the endothelial cells of pressurized rat middle cerebral arteries, which was partially reduced by the individual application of ruthenium red and a PLA2 inhibitor. However, since the compounds were never added together, which would be necessary to assess whether the effect was additive, it is not clear whether they were part of the same mechanism.
It is becoming increasingly apparent that endogenous TRPV4 agonists exist, including various EETs and anandamide (Watanabe et al., 2003). Earley et al., (2009) showed that 11,12-EET dependent dilation and smooth muscle cell hyperpolarization, in mouse mesenteric arteries, is partially the result of endothelium-dependent TRPV4 activation. Responses were absent in vessels from TRPV4 −/− mice and blocked by inhibitors of TRPV4 and IKCa/SKCa channels (Earley et al., 2009). 5,6-EET increased ruthenium red sensitive currents in whole cell and inside out patches of mouse aorta endothelial cells (Watanabe et al., 2003). Arachidonic acid, 5,6-EET and 8,9-EET increased cell calcium in native mouse carotid artery endothelial cells (Vriens et al., 2005). Responses to 5,6-EET and 8,9-EET were completely TRPV4 dependent as there was no effect in cells from TRPV4 −/− mice, and the effect of arachidonic acid was reduced by ~50 % in TRPV4 −/− mice. As would be predicted, modulation of CYP2C9 also altered the response to arachidonic acid - CYP2C9 inhibition with sulfaphenazole reduced the response, whereas increased CYP2C expression led to enhanced arachidonic acid responses. Responses to arachidonic acid and EETs were also increased upon inhibition of EET degradation. Arachidonic acid also increased endothelial cell calcium and dilated pressurized MCAs from rats (Marrelli et al., 2007).
In accordance with the temperature and osmotic sensitivity of TRPV4, endothelial cells respond to changes in temperature and HTS. In native mouse carotid endothelial cells, HTS produced whole-cell currents that were reduced by ~70 % in TRPV4−/−animals (Hartmannsgruber et al., 2007). HTS also stimulated TRPV4-like whole-cell currents in freshly isolated endothelial cells from rat carotid artery which were inhibited by PLA2 inhibition (Kohler et al., 2006), which in mammalian cells (albeit, not in yeast) is indicative of TRPV4 ((Loukin et al., 2009), Loukin et al., 2010, Vriens et al., 2004),. HTS also increased intracellular calcium levels in isolated mouse aortic endothelial cells, a response that was much reduced in TRPV4 −/− mice (Vriens et al., 2005). TRPV4-like responses to heat have also been observed in native mouse aortic endothelial cells (Watanabe, 2002b, Vriens et al., 2005) which were reduced in TRPV4 −/− mice (Vriens et al., 2005). Kohler et al., (2006) showed that raising temperature to 37 °C increased cell calcium in rat carotid artery endothelial cells and attributed the phenomena, based on pharmacological experiments in the intact vessel, to TRPV4. Although in vivo temperature and tonicity are maintained relatively constant, TRPV4-mediated responses to changes in these parameters may be important during times of extreme heat or osmotic stress.
Evidence by Marrelli et al. (2007) shows that calcium influx in endothelial cells occurs mainly at the side of the endothelial cell closest to the smooth muscle, and not the luminal side. Only abluminally applied Mn2+, which would presumably be able to reach the side of the endothelial cell closest to the smooth muscle, could quench the Fura-2 signal in the endothelial cells of pressurized rat middle cerebral arteries. Although it can not be concluded definitively that TRPV4 was responsible, as effects of TRPV4 inhibitors on Mn2+ quench were not studied, the response was reduced by PLA2 inhibition. TRPV4 was also co-localized with caveolae, which are cholesterol rich domains that attract many signaling molecules and ion channels. In HUVECS, TRPV4 and Cav-1 (a structural protein of caveolae) co-immunoprecipitate (Saliez et al., 2008). The Cav-1 −/−mouse also showed reduced gap junction protein (connexins 37, 40 and 43) expression. As these proteins can form myoendothelial gap junctions, the data suggest that TRPV4 channels that are associated with Cav-1 are also located close to myoendothelial gap junctions. The localization of TRPV4 at the smooth muscle face of endothelial cells is in accordance with its role as a vasodilatory mediator. The close proximity to the smooth muscle would allow easy access of TRPV4-associated, endothelial derived vasodilators to the neighboring smooth muscle, either via the gap junctions or by diffusion.
Evidence summarized above indicates that production of endothelial-dependent vasodilators can be TRPV4-dependent. Therefore, it may be assumed that the channel is of substantial importance for normal vascular function in vivo. However, TRPV4−/−mice actually have similar resting blood pressures when compared to wild-type mice (Zhang et al., 2009, Earley et al., 2009) and a number of studies investigating gain of function mutations in humans do not report any cardiovascular complications (Landoure et al., 2010, Rock et al., 2008, Zimon et al., 2010). However the channel is functional since activation of TRPV4 in vivo, reduces blood pressure (Gao et al., 2009, Gao and Wang, 2010, Willette et al., 2008), possibly via NO and IKCa/SKCa-dependent pathways (Gao and Wang, 2010, Willette et al., 2008), as well as leading to circulatory collapse (Willette et al., 2008). Interestingly, L-NNA induced hypertension is greater in TRPV4−/−versus wild-type mice (Earley et al., 2009) and salt-induced hypertensive rats are more sensitive to ruthenium red treatment compared to normotensive controls (Gao et al., 2009). Thus, it seems plausible that TRPV4 function becomes necessary during times of excessive vasoconstricting stimuli, allowing the vasculature to respond appropriately during times of stress.
TRPV channels and vascular smooth muscle function
TRPV channels also play key roles in vascular smooth muscle cell function. Amongst the TRPV channels, TRPV2 and TRPV4 are the most likely candidates for vasomotor roles in the vascular smooth muscle cells per se.
TRPV2
TRPV2 channels, as indicated by measurement of message and protein, are present in rat (Inoue et al., 2006) and mouse (Muraki et al., 2003) mesenteric, cerebral, and aortic myocytes. Muraki et al., (2003) demonstrated that cell swelling following exposure to hypotonic bath solution activates a nonselective cation current and increases intracellular Ca2+ levels in mouse aortic myocytes. These responses are suppressed by ruthenium red, a TRPV channel inhibitor, and by treatment of isolated myocytes with TRPV2 antisense oligonucleotides. TRPV2-like currents, membrane depolarization, and increased intracellular [Ca2+] were induced by membrane stretch in CHO cells transfected with TRPV2. These findings are consistent with the general observation that TRPV2 channels are mechanosensitive (O’Neil and Heller, 2005). Muraki et al. (Muraki et al., 2003) speculate that TRPV2 channel activation and Ca2+ entry following myoctye stretch could contribute to the myogenic vasoconstrictor responses of resistance arteries. However, functional evidence of a vasoconstrictor role for TRPV2 channels in intact blood vessels has not been reported.
TRPV2 channels in mesenteric artery myocytes are activated by endogenous cannabinoids and may contribute to the vasodilator response evoked by these substances (Qin et al., 2008). Further, TRPV2 channels in vascular smooth muscle cells appear to be activated by growth factors and contribute to vascular smooth muscle migration (Stokes et al., 2005).
TRPV4
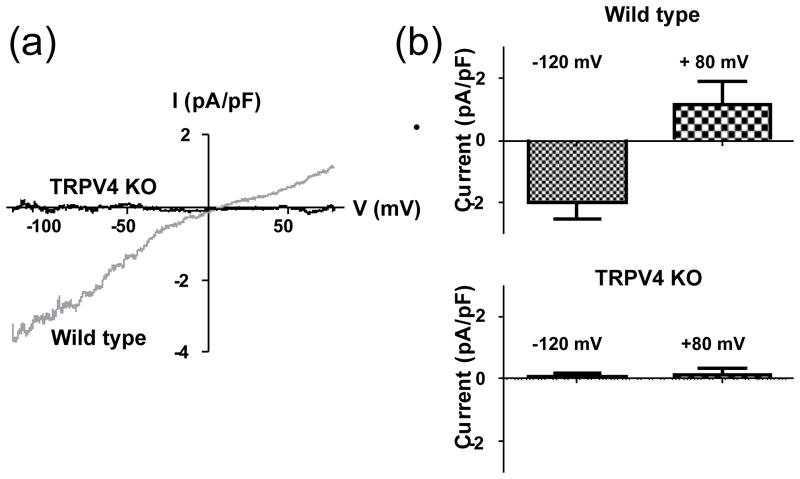
Recent studies support the hypothesis that TRPV4 channels located in vascular smooth muscle are important mediators of vasodilation induced by epoxyeicosatrienoic acids, which are one type of endothelium-derived hyperpolarizing factor (Campbell et al., 1996). TRPV4 is clearly present in smooth muscle cells in the mesenteric and cerebral vascular beds (Marrelli et al., 2007, Earley et al., 2005) and in aortic myocytes (Tanaka et al., 2008). Evidence for the functional role of TRPV4 in vascular smooth muscle comes from studies of cerebral (Earley et al., 2005) and mesenteric (Earley et al., 2009) resistance arteries. EETs activate TRPV4 channels in myocytes isolated from these arteries (Figure 2) (Earley et al., 2005, Earley et al., 2009). Subsequent Ca2+ influx through the EETs-activated TRPV4 channels (which have substantial permeability to Ca2+; (Voets et al., 2002)) induces the release of Ca2+ through ryanodine receptors located on the sarcoplasmic reticulum. These Ca2+ release events, termed Ca2+ sparks (Nelson et al., 1995), then activate nearby voltage-activated, BKCa channels in the sarcolemma and increase the frequency of macroscopic outward K+ currents (STOCs). The increased BKCa current activity causes smooth muscle hyperpolarization and vasodilation. These studies support the hypothesis that TRPV4 is part of a Ca2+ signaling complex that includes ryanodine receptors and BKCa channels and also suggest that the TRPV4 channel serves as a receptor molecule for EETs in vascular smooth muscle. TRPV4 activity in vascular myocytes also contributes to regulation of peripheral vascular resistance in vivo (Earley et al., 2009). This study demonstrated that elevations in blood pressure to a hypertensive challenge are exaggerated in TRPV4−/− mice, indicating that TRPV4 channels are involved in a negative feedback mechanism that limits the response to hypertensive stimuli. The proposal that vascular TRPV4 channels may play significant roles in blood pressure regulation is supported by a recent study demonstrating the substantial hypotensive responses to activators of TRPV4 in normotensive and salt-loaded, hypertensive rats (Gao et al., 2009).
Figure 2.
Ionic currents activated by 11–12 EET are absent In vascular smooth muscle cells from TRPV4 null mice. (a) Examples of whole-cell currents (difference currents) activated by 11,12 EET (3 μmol/L) in mesenteric arterial smooth muscle cells isolated from WT and TRPV4 KO mice. (b) Summary data for 11,12 EET-activated currents in WT (n=5) and TRPV4 KO (n=3) mouse mesenteric artery myocytes. *:P<0.05 vs. WT. (Data are from Earley et al., 2009. Am J Physiol Heart Circ Physiol. “Am Physiol Soc, with permission”)
TRPV channels and neurovascular control
TRPV1
TRPV1 channels are involved in neurally mediated vasodilation. Stimulation of perivascular sensory nerves dilates isolated mesenteric arteries and this response is mediated by CGRP (Han et al., 1990). TRPV1 channels in the perivascular nerves are involved in this response as evidenced by an absence of neurally-evoked CGRP release and dilation of mesenteric arteries from TRPV1 null mice. (Wang et al., 2006). Similarly, hindlimb cutaneous vasodilation in vivo following spinal cord stimulation is absent in rats treated with resiniferotoxin, which desensitizes TRPV1 receptors, suggesting that TRPV1 channels in sensory nerves are important mediators of neurogenic vasodilation (Wu et al., 2006). TRPV1 channels are also expressed in afferent neuronal baroreceptor pathways. Recent results indicate that baroreflex signaling is compromised when TRPV1 channels are inhibited (Sun et al., 2009). In this study resiniferotoxin eliminated TRPV1 immunoreactivity in barroreceptor signaling pathways. This apparent ablation of TRPV1 channels in the barroreceptor afferents was associated with substantial impairment of the normal barroreflex in response to increases in mean arterial pressure. These results suggest that TRPV1 channels are potential mechanoreceptors that sense changes in arterial pressure and play a key role in blood pressure homeostasis. A study by Scotland and colleagues (Scotland et al., 2004) suggests that TRPV1 channels in mesenteric perivascular sensory nerves contribute to a mechanism of myogenic tone that involves release of Substance P. Ablation of TRPV1 in vascular sensory fibers, block of 20-HETE production, or block of NK1 receptors inhibited myogenic tone. These findings lead the authors to propose that increased intravascular pressure enhances 20-HETE release from vascular smooth muscle cells which then activates sensory TRPV1 channels, triggering neuronal Ca2+ entry and release of Substance P, which then causes smooth muscle contraction. However, although the data in this study are provocative, they are inconsistent with numerous previous reports indicating no direct role of periarterial nerves in vascular myogenic behavior (Bayliss, 1902, Johansson, 1989).
TRPV4
As reviewed above, TRPV4 channels are widely distributed in the cardiovascular system, found both in endothelial cells and vascular smooth muscle cells. Like TRPV1, TRPV4 channels are also found in perivascular sensory nerves and may contribute to the hypotensive response to activation of TRPV4 channels in vivo (Gao et al., 2009, Gao and Wang, 2010). Similar to the situation involving TRPV1 channels in mesenteric periarterial sensory nerve fibers, activation of neuronal TRPV4 channels appears to be coupled to release of CGRP and subsequent mesenteric artery dilation.
TRPV4 channels may also contribute to increased sympathetic nervous activity and blood pressure associated with hyposmotic conditions in the hepatic portal circulation (McHugh et al., 2010). These studies indicate that TRPV4 channels play a role as a hepatic portal osmoreceptor. The authors found that increased blood pressure after water ingestion in wild type mice is absent in TRPV4 null mice. Administration of isosmotic saline did not elicit an increase in BP, suggesting osmolality as the stimulus. The authors propose the osmopressor response to water, and involvement of TRPV4 represent new factors to be considered in the physiology of blood pressure regulation.
Conclusion
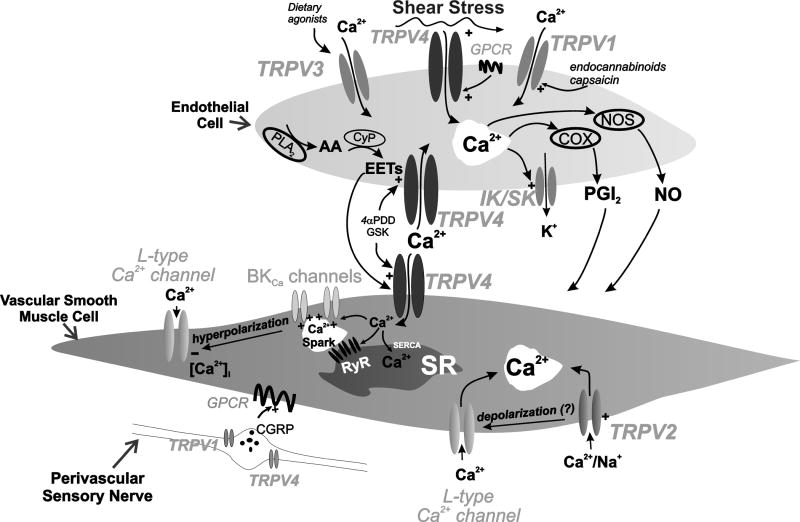
The available evidence strongly supports the proposal that TRPV channels modulate vascular endothelial and smooth muscle cell function, and perivascular neuronal activity (Figure 3). The presence of TRPV1, TRPV3 and TRPV4 in the vascular endothelium permits vasodilatory responses to a variety of stimuli including endocannabinoids (TRPV1), dietary agonists (TRPV3), shear stress, Gq-protein coupled receptor activation and EETs (TRPV4). Endothelial TRPV-mediated Ca2+-entry causes dilations by increasing NO, PGI2, or IKCa/SKCa channel activity. TRPV2 and TRPV4 are present in smooth muscle cells in numerous vascular beds. TRPV2 channels in aortic smooth muscle may be activated by mechanical stimulation, and Ca2+-entry through smooth muscle TRPV4 channels activates ryanodine receptors and BK channels, leading to vasodilation. Activation of TRPV1 or TRPV4 channels in perivascular sensory nerves induces release of CGRP which causes vasodilation. Although, as alterations in TRPV1 and TRPV4 expression do not affect resting blood pressure (Earley et al., 2009, Landoure et al., 2010, Pacher et al., 2004, Rock et al., 2008, Wang and Wang, 2009, Zhang et al., 2009, Zimon et al., 2010) these channels may not be required for day-today regulation of blood pressure, but may provide useful pathways to counteract vasoactive stimuli (Earley et al., 2009, Gao et al., 2009, Pozsgai et al., 2010). While disturbances in TRPV channel expression, structure, or activity in cells that encompass the vascular wall would be predicted to be associated with cardiovascular pathology, to date the only known TRPV channelopathies have been linked to bone disorders (Mizoguchi et al., 2008). Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss (Mizoguchi et al., 2008) and neurodegenerative skeletal muscle dyscrasias (Auer-Grumbach et al., 2010). Mutations in the N-terminal ankyrin domain of TRPV4 cause congenital and scapuloperoneal spinal muscular atrophy, and hereditary motor and sensory neuropathy 2C (Auer-Grumbach et al., 2010).
Figure 3.
Diversity of mechanisms by which vascular TRPV channels are regulated. In endothelial cells, TRPV2 can be activated by endocanabinoids and pharmacologically by capsaicin, TRPV3 by the dietary agonist, carvacrol, and TRPV4 by shear stress, GPCR activation, EETs and pharmacologically by GSK1016790A (GSK) and 4αPDD. Activation leads to calcium influx and the differential activation of NOS, COX and IKCa (IK)/SKCa (SK) channels to cause vasodilation. In the smooth muscle, activation of TRPV4 by endothelial derived EETs leads to calcium influx, stimulation of calcium sparks, subsequent activation of BKCa, K+ efflux and smooth muscle cell hyperpolarisation. TRPV2 channels in the smooth muscle mediate calcium entry and aid constriction. Activation of TRPV2 and TRPV4 in perivascular nerves leads to the release of CGRP (calcitonin-gene-related-peptide) and vasodilation. Abbreviations: AA (arachidonic acid), CyP (cytochrome P450 epoxygenase), PLA2 (phospholipase A2), EETs (epoxyeicosatrienoic acids), NO (nitric oxide), PGI2 (prostacyclin), eNOS (endothelial nitric oxide synthase), COX (cyclooxygenase), large/intermediate/small conductance potassium channel (BKCa/IKCa/SKCa respectively), GPCR (G-protein coupled receptor), SR (sarcoplasmic reticulum), RyR (ryanodine receptor), SERCA (sarco/endoplasmic reticulum Ca2+-ATPase).
Nevertheless, it seems likely that development of agents that selectively modulate vascular TRPV channel function will prove useful in treatment of vascular diseases, particularly those associated with elevated vasomotor tone such as hypertension and vasospasm.
Supplementary Material
Acknowledgments
This work was supported by NIH grant HL58231 and the Totman Medical Research Trust
Footnotes
Conflict of Interest
The authors state that there is no conflict of interest.
References
- Adamian L, Liang J. Prediction of transmembrane helix orientation in polytopic membrane proteins. BMC Structural Biology. 2006;6:13. doi: 10.1186/1472-6807-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ansary D, Bogeski I, Disteldorf BMJ, Becherer U, Niemeyer BA. ATP modulates Ca2+ uptake by TRPV6 and is counteracted by isoform-specific phosphorylation. FASEB J. 2010;24:425–435. doi: 10.1096/fj.09-141481. [DOI] [PubMed] [Google Scholar]
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain. 2005;118:70–79. doi: 10.1016/j.pain.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Appendino G, Minassi A, Pagani A, Ech-Chahad A. The Role of Natural Products in the Ligand Deorphanization of TRP Channels. Current Pharmaceutical Design. 2008;14:2–17. doi: 10.2174/138161208783330781. [DOI] [PubMed] [Google Scholar]
- Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, Valverde MA. Human TRPV4 Channel Splice Variants Revealed a Key Role of Ankyrin Domains in Multimerization and Trafficking. J Biol Chem. 2006;281:1580–1586. doi: 10.1074/jbc.M511456200. [DOI] [PubMed] [Google Scholar]
- Auer-Grumbach M, Olschewski A, Papic L, Kremer H, McEntagart ME, Uhrig S, Fischer C, Frohlich E, Balint Z, Tang B, Strohmaier H, Lochmuller H, Schlotter-Weigel B, Senderek J, Krebs A, Dick KJ, et al. Alterations in the ankyrin domain of TRPV4 cause congenital distal SMA, scapuloperoneal SMA and HMSN2C. Nat Genet. 2010;42:160–164. doi: 10.1038/ng.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss W. On the local reactions of the arterial wall to changes of internal pressure. J Physiol. 1902;28:220–231. doi: 10.1113/jphysiol.1902.sp000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Muller M, Leuner K, Jendrach M. The C-terminal domain of TRPV4 is essential for plasma membrane localization. Molecular Membrane Biology. 2008;25:139–151. doi: 10.1080/09687680701635237. [DOI] [PubMed] [Google Scholar]
- Benfenati V, Amiry-Moghaddam M, Caprini M, Mylonakou MN, Rapisarda C, Ottersen OP, Ferroni S. Expression and functional characterization of transient receptor potential vanilloid-related channel 4 (TRPV4) in rat cortical astrocytes. Neuroscience. 2007;148:876–892. doi: 10.1016/j.neuroscience.2007.06.039. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, de Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol. 2008;294:H2489–2496. doi: 10.1152/ajpheart.01191.2007. [DOI] [PubMed] [Google Scholar]
- Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A Hot-Sensing Cold Receptor: C-Terminal Domain Determines Thermosensation in Transient Receptor Potential Channels. J Neurosci. 2006;26:4835–4840. doi: 10.1523/JNEUROSCI.5080-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–23. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chang Q, Gyftogianni E, van de Graaf SFJ, Hoefs S, Weidema FA, Bindels RJM, Hoenderop JGJ. Molecular Determinants in TRPV5 Channel Assembly. Journal of Biological Chemistry. 2004;279:54304–54311. doi: 10.1074/jbc.M406222200. [DOI] [PubMed] [Google Scholar]
- Cheng W, Yang F, Takanishi CL, Zheng J. Thermosensitive TRPV Channel Subunits Coassemble into Heteromeric Channels with Intermediate Conductance and Gating Properties. The Journal of General Physiology. 2007;129:191–207. doi: 10.1085/jgp.200709731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoph T, Bahrenberg G, De Vry J, Englberger W, Erdmann VA, Frech M, Kogel B, Rohl T, Schiene K, Schroder W, Seibler J, Kurreck J. Investigation of TRPV1 loss-of-function phenotypes in transgenic shRNA expressing and knockout mice. Molecular and Cellular Neuroscience. 2008;37:579–589. doi: 10.1016/j.mcn.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Chung MK, Guler AD, Caterina MJ. TRPV1 shows dynamic ionic selectivity during agonist stimulation. Nat Neurosci. 2008;11:555–564. doi: 10.1038/nn.2102. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Caterina MJ. Warm Temperatures Activate TRPV4 in Mouse 308 Keratinocytes. Journal of Biological Chemistry. 2003;278:32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- Chung MK, Lee H, Mizuno A, Suzuki M, Caterina MJ. TRPV3 and TRPV4 Mediate Warmth-evoked Currents in Primary Mouse Keratinocytes. Journal of Biological Chemistry. 2004;279:21569–21575. doi: 10.1074/jbc.M401872200. [DOI] [PubMed] [Google Scholar]
- Ciura S, Bourque CW. Transient Receptor Potential Vanilloid 1 Is Required for Intrinsic Osmoreception in Organum Vasculosum Lamina Terminalis Neurons and for Normal Thirst Responses to Systemic Hyperosmolality. J Neurosci. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–24. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham D, Runnels L, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387 – 396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- D’hoedt D, Owsianik G, Prenen J, Cuajungco MP, Grimm C, Heller S, Voets T, Nilius B. Stimulus-specific Modulation of the Cation Channel TRPV4 by PACSIN 3. Journal of Biological Chemistry. 2008;283:6272–6280. doi: 10.1074/jbc.M706386200. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Di A, Malik AB. TRP channels and the control of vascular function. Current Opinion in Pharmacology. 2010;10:127–132. doi: 10.1016/j.coph.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Dodier Y, Dionne F, Raybaud A, Sauve R, Parent L. Topology of the selectivity filter of a TRPV channel: rapid accessibility of contiguous residues from the external medium. Am J Physiol Cell Physiol. 2007;293:C1962–1970. doi: 10.1152/ajpcell.00406.2007. [DOI] [PubMed] [Google Scholar]
- Earley S. Vanilloid and Melastatin Transient Receptor Potential Channels in Vascular Smooth Muscle. Microcirculation. 2010;17:237–249. doi: 10.1111/j.1549-8719.2010.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Gonzales AL, Garcia ZI. A Dietary Agonist of Transient Receptor Potential Cation Channel V3 Elicits Endothelium-Dependent Vasodilation. Molecular Pharmacology. 2010;77:612–620. doi: 10.1124/mol.109.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 Forms a Novel Ca2+ Signaling Complex With Ryanodine Receptors and BKCa Channels. Circ Res. 2005;97:1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- Earley S, Pauyo T, Drapp R, Tavares MJ, Liedtke W, Brayden JE. TRPV4-dependent dilation of peripheral resistance arteries influences arterial pressure. Am J Physiol Heart Circ Physiol. 2009;297:H1096–1102. doi: 10.1152/ajpheart.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Félétou M, Weston A. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Archiv European Journal of Physiology. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA. Ca2+-selective Transient Receptor Potential V Channel Architecture and Function Require a Specific Ankyrin Repeat. Journal of Biological Chemistry. 2004;279:34456–34463. doi: 10.1074/jbc.M404778200. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Sepúlveda M, Gevaert T, Roskams T, Nilius B, De Ridder D. Where is TRPV1 expressed in the bladder, do we see the real channel? Naunyn-Schmiedeberg’s Archives of Pharmacology. 2009;379:421–425. doi: 10.1007/s00210-008-0391-7. [DOI] [PubMed] [Google Scholar]
- Everaerts W, Vriens J, Owsianik G, Appendino G, Voets T, De Ridder D, Nilius B. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am J Physiol Renal Physiol. 2010;298:F692–701. doi: 10.1152/ajprenal.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JXJ. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1233–1245. doi: 10.1152/ajplung.00445.2002. [DOI] [PubMed] [Google Scholar]
- Félétou M, Vanhoutte P. EDHF: An update. Clinical Sciences. 2009:117. doi: 10.1042/CS20090096. [DOI] [PubMed] [Google Scholar]
- Feng NH, Lee HH, Shiang JC, Ma MC. Transient receptor potential vanilloid type 1 channels act as mechanoreceptors and cause substance P release and sensory activation in rat kidneys. Am J Physiol Renal Physiol. 2008;294:F316–325. doi: 10.1152/ajprenal.00308.2007. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Lorenzo IM, Andrade YN, Garcia-Elias A, Serra SA, Fernandez-Fernandez J, Valverde MA. IP3 sensitizes TRPV4 channel to the mechano-and osmotransducing messenger 5′-6′-epoxyeicosatrienoic acid. The Journal of Cell Biology. 2008;181:143–155. doi: 10.1083/jcb.200712058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V, Nilius B, Plant T, Strotmann R. TRPV4. In: STARKE K, BORN GVR, DUCKLES SP, EICHELBAUM M, GANTEN D, HOFMANN F, et al., editors. Transient Receptor Potential (TRP) Channels. Springer; Berlin Heidelberg: 2007. [Google Scholar]
- Gao Fa, Wang DHabc. Hypotension induced by activation of the transient receptor potential vanilloid 4 channels: role of Ca2+-activated K+ channels and sensory nerves. Journal of Hypertension. 2010;28:102–110. doi: 10.1097/HJH.0b013e328332b865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Sui D, Garavito RM, Worden RM, Wang DH. Salt Intake Augments Hypotensive Effects of Transient Receptor Potential Vanilloid 4: Functional Significance and Implication. Hypertension. 2009;53:228–235. doi: 10.1161/HYPERTENSIONAHA.108.117499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Wu L, O'Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem. 2003;278:27129–37. doi: 10.1074/jbc.M302517200. [DOI] [PubMed] [Google Scholar]
- Garcia-Elias A, Lorenzo IM, Vicente Rn, Valverde MA. IP3 Receptor Binds to and Sensitizes TRPV4 Channel to Osmotic Stimuli via a Calmodulin-binding Site. Journal of Biological Chemistry. 2008;283:31284–31288. doi: 10.1074/jbc.C800184200. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez C, Morenilla-Palao C, Planells-Cases R, Merino JM, Ferrer-Montiel A. Identification of an Aspartic Residue in the P-loop of the Vanilloid Receptor That Modulates Pore Properties. Journal of Biological Chemistry. 2000;275:32552–32558. doi: 10.1074/jbc.M002391200. [DOI] [PubMed] [Google Scholar]
- Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernandez-Ballester G, Ferrer-Montiel A. Identification of a Tetramerization Domain in the C Terminus of the Vanilloid Receptor. J Neurosci. 2004;24:5307–5314. doi: 10.1523/JNEUROSCI.0202-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Sanz N, Valente P, Gomis A, Fernandez-Carvajal A, Fernandez-Ballester G, Viana F, Belmonte C, Ferrer-Montiel A. A Role of the Transient Receptor Potential Domain of Vanilloid Receptor I in Channel Gating. J Neurosci. 2007;27:11641–11650. doi: 10.1523/JNEUROSCI.2457-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golech SA, McCarron RM, Chen Y, Bembry J, Lenz F, Mechoulam R, Shohami E, Spatz M. Human brain endothelium: coexpression and function of vanilloid and endocannabinoid receptors. Molecular Brain Research. 2004;132:87–92. doi: 10.1016/j.molbrainres.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Grandl J, Hu H, Bandell M, Bursulaya B, Schmidt M, Petrus M, Patapoutian A. Pore region of TRPV3 ion channel is specifically required for heat activation. Nat Neurosci. 2008;11:1007–1013. doi: 10.1038/nn.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, Altier C, Cenac N, Zamponi GW, Bautista-Cruz F, Lopez CB, Joseph EK, Levine JD, Liedtke W, Vanner S, Vergnolle N, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. The Journal of Physiology. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guler AD. Heat-evoked activation of the ion channel, TRPV4. J Neurosci. 2002;22:6408–6814. doi: 10.1523/JNEUROSCI.22-15-06408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SP, Naes L, Westfall TC. Inhibition of periarterial nerve stimulation-induced vasodilation of the mesenteric arterial bed by CGRP (8–37) and CGRP receptor desensitization. Biochemical and Biophysical Research Communications. 1990;168:786–791. doi: 10.1016/0006-291x(90)92390-l. [DOI] [PubMed] [Google Scholar]
- Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, Liedtke W, Hoyer J, Köhler R. Arterial Response to Shear Stress Critically Depends on Endothelial TRPV4 Expression. PLoS ONE. 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci. 2005;118:917–928. doi: 10.1242/jcs.01675. [DOI] [PubMed] [Google Scholar]
- Hiura A. Is thermal nociception only sensed by the capsaicin receptor, TRPV1? Anatomical Science International. 2009;84:122–128. doi: 10.1007/s12565-009-0048-8. [DOI] [PubMed] [Google Scholar]
- Hoenderop JGJ, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJM. Homo- and heterotetrameric architecture of the epithelial Ca2+ channels TRPV5 and TRPV6. EMBO J. 2003;22:776–785. doi: 10.1093/emboj/cdg080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoi PM, Visintin C, Okuyama M, Gardiner SM, Kaup SS, Bennett T, Baker D, Selwood DL, RHC Vascular pharmacology of a novel cannabinoid-like compound,3-(5-dimethylcarbamoyl-pent-1-enyl)-N-(2-hydroxy-1-methyl-ethyl)benzamide (VSN16) in the rat. Br J Pharmacol. 2007;152:751–764. doi: 10.1038/sj.bjp.0707470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida T, Shimizu I, Nealen ML, Campbell A, Caterina M. Attenuated fever response in mice lacking TRPV1. Neuroscience Letters. 2005;378:28–33. doi: 10.1016/j.neulet.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Inoue R, Jensen LJ, Shi J, Morita H, Nishida M, Honda A, Ito Y. Transient Receptor Potential Channels in Cardiovascular Function and Disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- Jin X, Touhey J, Gaudet R. Structure of the N-terminal Ankyrin Repeat Domain of the TRPV2 Ion Channel. Journal of Biological Chemistry. 2006;281:25006–25010. doi: 10.1074/jbc.C600153200. [DOI] [PubMed] [Google Scholar]
- Johansson B. Myogenic tone and reactivity: definitions based on muscle physiology. J Hypertens Suppl. 1989;7:S5–S8. [PubMed] [Google Scholar]
- Kark Ts, Bagi Z, Lizanecz Eb, Pasztor ET, Erdei Nr, Czikora Ãg, Papp Zn, Edes In, Porszasz Rb, Toth A. Tissue-Specific Regulation of Microvascular Diameter: Opposite Functional Roles of Neuronal and Smooth Muscle Located Vanilloid Receptor-1. Molecular Pharmacology. 2008;73:1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the Native Quaternary Structure of Vanilloid Receptor 1. Journal of Biological Chemistry. 2001;276:28613–28619. doi: 10.1074/jbc.M103272200. [DOI] [PubMed] [Google Scholar]
- Klausen TKr, Pagani A, Minassi A, Ech-Chahad A, Prenen J, Owsianik G, Hoffmann EK, Pedersen SF, Appendino G, Nilius B. Modulation of the Transient Receptor Potential Vanilloid Channel TRPV4 by α-Phorbol Esters: A Structure-Activity Study. Journal of Medicinal Chemistry. 2009;52:2933–2939. doi: 10.1021/jm9001007. [DOI] [PubMed] [Google Scholar]
- Kohler R, Ruth P. Endothelial dysfunction and blood pressure alterations in K<sup>+</sup>-channel transgenic mice. Pflugers Archiv European Journal of Physiology. 2010;459:969–976. doi: 10.1007/s00424-010-0819-z. [DOI] [PubMed] [Google Scholar]
- Köhler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a Functional Role of Endothelial Transient Receptor Potential V4 in Shear Stress-Induced Vasodilatation. Arterioscler Thromb Vasc Biol. 2006;26:1495–1502. doi: 10.1161/01.ATV.0000225698.36212.6a. [DOI] [PubMed] [Google Scholar]
- Kottgen M, Buchholz Br, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T, Nitschke R, Suzuki M, Kramer-Zucker A, Germino GG, Watnick T, Prenen J, et al. TRPP2 and TRPV4 form a polymodal sensory channel complex. The Journal of Cell Biology. 2008;182:437–447. doi: 10.1083/jcb.200805124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landoure G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, Shi Y, Taye AA, Kong L, Munns CH, Choo SS, Phelps CB, Paudel R, Houlden H, Ludlow CL, Caterina MJ, et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet. 2010;42:170–174. doi: 10.1038/ng.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latorre R, Zaelzer C, Brauchi S. Structure-functional intimacies of transient receptor potential channels. Quarterly Reviews of Biophysics. 2009;42:201–246. doi: 10.1017/S0033583509990072. [DOI] [PubMed] [Google Scholar]
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered Thermal Selection Behavior in Mice Lacking Transient Receptor Potential Vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler A, Fischer MJ, Rehner D, Kienel S, Kistner K, Sauer SK, Gavva NR, Reeh PW, Nau C. The vanilloid receptor TRPV1 is activated and sensitized by local anesthetics in rodent sensory neurons. The Journal of Clinical Investigation. 2008;118:763–776. doi: 10.1172/JCI32751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, AndrejSali, Hudspeth AJ, Friedman JM, Heller S. Vanilloid Receptor-Related Osmotically Activated Channel (VR-OAC), a Candidate Vertebrate Osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lishko PV, Procko E, Jin X, Phelps CB, Gaudet R. The Ankyrin Repeats of TRPV1 Bind Multiple Ligands and Modulate Channel Sensitivity. Neuron. 2007;54:905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- Liu B, Ma W, Ryu S, Qin F. Inhibitory modulation of distal C-terminal on protein kinase C-dependent phospho-regulation of rat TRPV1 receptors. The Journal of Physiology. 2004;560:627–638. doi: 10.1113/jphysiol.2004.069054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loot AE, Popp Rd, Fisslthaler B, Vriens J, Nilius B, Fleming I. Role of cytochrome P450-dependent transient receptor potential V4 activation in flow-induced vasodilatation. Cardiovascular Research. 2008;80:445–452. doi: 10.1093/cvr/cvn207. [DOI] [PubMed] [Google Scholar]
- Loukin SH, Su Z, Kung C. Hypotonic shocks activate rat TRPV4 in yeast in the absence of polyunsaturated fatty acids. FEBS Letters. 2009;583:754–758. doi: 10.1016/j.febslet.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and Brachyolmia-causing Mutant TRPV4 Channels Respond Directly to Stretch Force. Journal of Biological Chemistry. 2010;285:27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrelli SP, O’Neil RG, Brown RC, Bryan RM., Jr PLA2 and TRPV4 channels regulate endothelial calcium in cerebral arteries. Am J Physiol Heart Circ Physiol. 2007;292:H1390–1397. doi: 10.1152/ajpheart.01006.2006. [DOI] [PubMed] [Google Scholar]
- McCleverty CJ, Koesema E, Patapoutian A, Lesley SA, Kreusch A. Crystal structure of the human TRPV2 channel ankyrin repeat domain. Protein Science. 2006;15:2201–2206. doi: 10.1110/ps.062357206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh J, Keller NR, Appalsamy M, Thomas SA, Raj SR, Diedrich A, Biaggioni I, Jordan J, Robertson D. Portal osmopressor mechanism linked to transient receptor potential vanilloid 4 and blood pressure control. Hypertension. 2010;55:1438–43. doi: 10.1161/HYPERTENSIONAHA.110.151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza SA, Fang J, Gutterman DD, Wilcox DA, Bubolz AH, Li R, Suzuki M, Zhang DX. TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress. Am J Physiol Heart Circ Physiol. 2010;298:H466–476. doi: 10.1152/ajpheart.00854.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Hoon MA. Ablation of TrpV1 neurons reveals their selective role in thermal pain sensation. Molecular and Cellular Neuroscience. 2010;43:157–163. doi: 10.1016/j.mcn.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi F, Mizuno A, Hayata T, Nakashima K, Heller S, Ushida T, Sokabe M, Miyasaka N, Suzuki M, Ezura Y, Noda M. Transient receptor potential vanilloid 4 deficiency suppresses unloading-induced bone loss. J Cell Physiol. 2008;216:47–53. doi: 10.1002/jcp.21374. [DOI] [PubMed] [Google Scholar]
- Moqrich A, Hwang SW, Earley TJ, Petrus MJ, Murray AN, Spencer KSR, Andahazy M, Story GM, Patapoutian A. Impaired Thermosensation in Mice Lacking TRPV3, a Heat and Camphor Sensor in the Skin. Science. 2005;307:1468–1472. doi: 10.1126/science.1108609. [DOI] [PubMed] [Google Scholar]
- Muraki K, Iwata Y, Katanosaka Y, Ito T, Ohya S, Shigekawa M, Imaizumi Y. TRPV2 Is a Component of Osmotically Sensitive Cation Channels in Murine Aortic Myocytes. Circ Res. 2003;93:829–838. doi: 10.1161/01.RES.0000097263.10220.0C. [DOI] [PubMed] [Google Scholar]
- Naeini RS, Witty MF, Seguela P, Bourque CW. An N-terminal variant of Trpv1 channel is required for osmosensory transduction. Nat Neurosci. 2006;9:93–98. doi: 10.1038/nn1614. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995;270:633–7. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Nilius B, Prenen J, Wissenbach U, Bödding M, Droogmans G. Differential activation of the volume-sensitive cation channel TRP12 (OTRPC4) and volume-regulated anion currents in HEK-293 cells. Pflugers Archiv European Journal of Physiology. 2001;443:227–233. doi: 10.1007/s004240100676. [DOI] [PubMed] [Google Scholar]
- O’Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–203. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- Offertaler Ls, Mo FM, Bátkai Sn, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective Ligands and Cellular Effectors of a G Protein-Coupled Endothelial Cannabinoid Receptor. Molecular Pharmacology. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- Pacher P, Batkai S, Kunos G. Haemodynamic profile and responsiveness toanandamide of TRPV1 receptor knock-out mice. J Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps C, Procko E, Lishko P, Wang R, Gaudet R. Insights into the roles of conserved and divergent residues in the ankyrin repeats of TRPV channels. Channels. 2007;1:148–151. doi: 10.4161/chan.4716. [DOI] [PubMed] [Google Scholar]
- Poblete IsM, Orliac MaL, Briones R, Adler-Graschinsky E, Huidobro-Toro JP. Anandamide elicits an acute release of nitric oxide through endothelial TRPV1 receptor activation in the rat arterial mesenteric bed. The Journal of Physiology. 2005;568:539–551. doi: 10.1113/jphysiol.2005.094292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozsgai G, Bodkin JV, Graepel R, Bevan S, Andersson DA, Brain SD. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovascular Research. 2010;87:760–768. doi: 10.1093/cvr/cvq118. [DOI] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci. 2008;28:6231–8. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock MJ, Prenen J, Funari VA, Funari TL, Merriman B, Nelson SF, Lachman RS, Wilcox WR, Reyno S, Quadrelli R, Vaglio A, Owsianik G, Janssens A, Voets T, Ikegawa S, Nagai T, et al. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet. 2008;40:999–1003. doi: 10.1038/ng.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T. Phosphoinositide regulation of non-canonical transient receptor potential channels. Cell Calcium. 2009;45:554–565. doi: 10.1016/j.ceca.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohacs T, Lopes CMB, Michailidis I, Logothetis DE. PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 2005;8:626–634. doi: 10.1038/nn1451. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Almeida MC, Garami A, Steiner AA, Norman MH, Morrison SF, Nakamura K, Burmeister JJ, Nucci TB. The Transient Receptor Potential Vanilloid-1 Channel in Thermoregulation: A Thermosensor It Is Not. Pharmacological Reviews. 2009;61:228–261. doi: 10.1124/pr.109.001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. The Journal of Physiology. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter AR, Ma QP, Leveridge M, Bonnert TP. Heteromerization and colocalization of TrpV1 and TrpV2 in mammalian cell lines and rat dorsal root ganglia. Neuroreport. 2005;16:1735–1739. doi: 10.1097/01.wnr.0000185958.03841.0f. [DOI] [PubMed] [Google Scholar]
- Saliez J, Bouzin C, Rath G, Ghisdal P, Desjardins F, Rezzani R, Rodella LF, Vriens J, Nilius B, Feron O, Balligand JL, Dessy C. Role of Caveolar Compartmentation in Endothelium-Derived Hyperpolarizing Factor-Mediated Relaxation: Ca2+ Signals and Gap Junction Function Are Regulated by Caveolin in Endothelial Cells. Circulation. 2008;117:1065–1074. doi: 10.1161/CIRCULATIONAHA.107.731679. [DOI] [PubMed] [Google Scholar]
- Scotland RS, Chauhan S, Davis C, De Felipe C, Hunt S, Kabir J, Kotsonis P, Oh U, Ahluwalia A. Vanilloid Receptor TRPV1, Sensory C-Fibers, and Vascular Autoregulation: A Novel Mechanism Involved in Myogenic Constriction. Circ Res. 2004;95:1027–1034. doi: 10.1161/01.RES.0000148633.93110.24. [DOI] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE, Hayes PD, Reilly P, Facer P, Wright JE, Jerman JC, Walhin JP, Ooi L, Egerton J, Charles KJ, Smart D, Randall AD, Anand P, Davis JB. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature. 2002;418:186–190. doi: 10.1038/nature00894. [DOI] [PubMed] [Google Scholar]
- Smith PL, Maloney KN, Pothen RG, Clardy J, Clapham DE. Bisandrographolide from Andrographis paniculata Activates TRPV4 Channels. Journal of Biological Chemistry. 2006;281:29897–29904. doi: 10.1074/jbc.M605394200. [DOI] [PubMed] [Google Scholar]
- Stewart AP, Smith GD, Sandford RN, Edwardson JM. Atomic Force Microscopy Reveals the Alternating Subunit Arrangement of the TRPP2-TRPV4 Heterotetramer. Biophysical Journal. 2010;99:790–797. doi: 10.1016/j.bpj.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes AJ, Wakano C, Carmen KAd, Koblan-Huberson M, Turner H. Formation of a physiological complex between TRPV2 and RGA protein promotes cell surface expression of TRPV2. Journal of Cellular Biochemistry. 2005;94:669–683. doi: 10.1002/jcb.20331. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant T. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Sun H, Li DP, Chen SR, Hittelman WN, Pan HL. Sensing of blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J Pharmacol Exp Ther. 2009;331:851–9. doi: 10.1124/jpet.109.160473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation with mice lacking TRPV4. J Biol Chem. 2003a doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Watanabe Y, Oyama Y, Mizuno A, Kusano E, Hirao A, Ookawara S. Localization of mechanosensitive channel TRPV4 in mouse skin. Neuroscience Letters. 2003b;353:189–192. doi: 10.1016/j.neulet.2003.09.041. [DOI] [PubMed] [Google Scholar]
- Tanaka R, Muraki K, Ohya S, Yamamura H, Hatano N, Itoh Y, Imaizumi Y. TRPV4-Like Non-selective Cation Currents in Cultured Aortic Myocytes. Journal of Pharmacological Sciences. 2008;108:179–189. doi: 10.1254/jphs.08133fp. [DOI] [PubMed] [Google Scholar]
- Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. TRPV4 Channels Mediate Cyclic Strain-Induced Endothelial Cell Reorientation Through Integrin-to-Integrin Signaling. Circ Res. 2009;104:1123–1130. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, Chendrimada TP, Lashinger ESR, Gordon E, Evans L, Misajet BA, DeMarini DJ, Nation JH, Casillas LN, Marquis RW, Votta BJ, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-Dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1-piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a Novel and Potent Transient Receptor Potential Vanilloid 4 Channel Agonist Induces Urinary Bladder Contraction and Hyperactivity: Part I. Journal of Pharmacology and Experimental Therapeutics. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- Todaka H, Taniguchi J, Satoh J-i, Mizuno A, Suzuki M. Warm Temperature-sensitive Transient Receptor Potential Vanilloid 4 (TRPV4) Plays an Essential Role in Thermal Hyperalgesia. J Biol Chem. 2004;279:35133–35138. doi: 10.1074/jbc.M406260200. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tóth A, Boczán J, Kedei N, Lizanecz E, Bagi Z, Papp Z, Édes I, Csiba L, Blumberg PM. Expression and distribution of vanilloid receptor 1 (TRPV1) in the adult rat brain. Molecular Brain Research. 2005;135:162–168. doi: 10.1016/j.molbrainres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Shimokawa H, Tang EHC, Feletou M. Endothelial dysfunction and vascular disease. Acta Physiologica. 2009;196:193–222. doi: 10.1111/j.1748-1716.2009.01964.x. [DOI] [PubMed] [Google Scholar]
- Vannier B, Zhu X, Brown D, Birnbaumer L. The Membrane Topology of Human Transient Receptor Potential 3 as Inferred from Glycosylation-scanning Mutagenesis and Epitope Immunocytochemistry. J Biol Chem. 1998;273:8675–8679. doi: 10.1074/jbc.273.15.8675. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilceanu D, Honore P, Hogan QH, Stucky CL. Spinal Nerve Ligation in Mouse Upregulates TRPV1 Heat Function in Injured IB4-Positive Nociceptors. The Journal of Pain. 2010;11:588–599. doi: 10.1016/j.jpain.2009.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent F, Acevedo A, Nguyen MT, Dourado M, DeFalco J, Gustafson A, Spiro P, Emerling DE, Kelly MG, Duncton MAJ. Identification and characterization of novel TRPV4 modulators. Biochemical and Biophysical Research Communications. 2009;389:490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature. 2004;430:748–754. doi: 10.1038/nature02732. [DOI] [PubMed] [Google Scholar]
- Voets T, Janssens A, Prenen J, Droogmans G, Nilius B. Mg2+-dependent Gating and Strong Inward Rectification of the Cation Channel TRPV6. J Gen Physiol. 2003;121:245–260. doi: 10.1085/jgp.20028752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. Molecular Determinants of Permeation through the Cation Channel TRPV4. J Biol Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- Vriens J, Appendino G, Nilius B. Pharmacology of Vanilloid Transient Receptor Potential Cation Channels. Molecular Pharmacology. 2009;75:1262–1279. doi: 10.1124/mol.109.055624. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2 Permeable Cation Channel TRPV4 by Cytochrome P450 Epoxygenases in Vascular Endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Janssens A, Voets T, Nilius B. Determinants of 4alpha-Phorbol Sensitivity in Transmembrane Domains 3 and 4 of the Cation Channel TRPV4. Journal of Biological Chemistry. 2007;282:12796–12803. doi: 10.1074/jbc.M610485200. [DOI] [PubMed] [Google Scholar]
- Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. P Natl Acad Sci USA. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LH, Luo M, Wang Y, Galligan JJ, Wang DH. Impaired vasodilation in response to perivascular nerve stimulation in mesenteric arteries of TRPV1-null mutant mice. J Hypertens. 2006;24:2399–408. doi: 10.1097/01.hjh.0000251900.78051.56. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang DH. Aggravated renal inflammatory responses in TRPV1 gene knockout mice subjected to DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F1550–1559. doi: 10.1152/ajprenal.00012.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang J, Wang C, Liu J, Shi L, Xu M, Wang C. Functional Expression of Transient Receptor Potential Vanilloid-Related Channels in Chronically Hypoxic Human Pulmonary Arterial Smooth Muscle Cells. Journal of Membrane Biology. 2008;223:151–159. doi: 10.1007/s00232-008-9121-9. [DOI] [PubMed] [Google Scholar]
- Watanabe H. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002a;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002b;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Wegierski T, Lewandrowski U, Müller B, Sickmann A, Walz G. Tyrosine Phosphorylation Modulates the Activity of TRPV4 in Response to Defined Stimuli. J Biol Chem. 2009;284:2923–2933. doi: 10.1074/jbc.M805357200. [DOI] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue T-l, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, et al. Systemic Activation of the Transient Receptor Potential Vanilloid Subtype 4 Channel Causes Endothelial Failure and Circulatory Collapse: Part 2. J Pharm Exp Ther. 2008;326:443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Zwick M, Wang S, Lawson JJ, Caterina MJ, Koltzenburg M, Albers KM, Koerber HR, Davis BM. Nociceptors Lacking TRPV1 and TRPV2 Have Normal Heat Responses. J Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Komori N, Qin C, Farber JP, Linderoth B, Foreman RD. Sensory fibers containing vanilloid receptor-1 (VR-1) mediate spinal cord stimulation-induced vasodilation. Brain Res. 2006;1107:177–84. doi: 10.1016/j.brainres.2006.05.087. [DOI] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hānsel W, Grissmer S, Cahalan MD, Chandy KG. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature. 2002;418:181–186. doi: 10.1038/nature00882. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhao H, Tian W, Yoshida K, Roullet JB, Cohen DM. Regulation of a Transient Receptor Potential (TRP) Channel by Tyrosine Phosphorylation. SRC family kinase-dependent tyrosine phosphorylation of TRPV4 on TYR-253 mediates its response to hypotonic stress. J Biol Chem. 2003;278:11520–11527. doi: 10.1074/jbc.M211061200. [DOI] [PubMed] [Google Scholar]
- Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, He H, Zhao Z, Cao T, Yan Z, Liu D, Arendshorst WJ, Huang Y, Tepel M, Zhu Z. Activation of TRPV1 by Dietary Capsaicin Improves Endothelium-Dependent Vasorelaxation and Prevents Hypertension. Cell Metabolism. 2010;12:130–141. doi: 10.1016/j.cmet.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. P Natl Acad Sci USA. 2010;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1267–76. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- Ye F, Deng PY, Li D, Luo D, Li NS, Deng S, Deng HW, Li YJ. Involvement of endothelial cell-derived CGRP in heat stress-induced protection of endothelial function. Vascular Pharmacology. 2007;46:238–246. doi: 10.1016/j.vph.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Yin J, Kuebler W. Mechanotransduction by TRP Channels: General Concepts and Specific Role in the Vasculature. Cell Biochemistry and Biophysics. 2010;56:1–18. doi: 10.1007/s12013-009-9067-2. [DOI] [PubMed] [Google Scholar]
- Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Transient Receptor Potential Vanilloid Type 4-Deficient Mice Exhibit Impaired Endothelium-Dependent Relaxation Induced by Acetylcholine In Vitro and In Vivo. Hypertension. 2009;53:532–538. doi: 10.1161/HYPERTENSIONAHA.108.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimon M, Baets J, Auer-Grumbach M, Berciano J, Garcia A, Lopez-Laso E, Merlini L, Hilton-Jones D, McEntagart M, Crosby AH, Barisic N, Boltshauser E, Shaw CE, Landoure G, Ludlow CL, Gaudet R, et al. Dominant mutations in the cation channel gene transient receptor potential vanilloid 4 cause an unusual spectrum of neuropathies. Brain. 2010;133:1798–1809. doi: 10.1093/brain/awq109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.