Abstract
Objective
Recent research demonstrated that intermittent claudication patients have increased gait variability prior to the onset of claudication. However, it is unknown if these patients experience additional gait adaptations after the onset of claudication. Thus, we sought to determine how gait variability is affected by claudication in an effort to contribute to improved clinical management.
Methods
Twenty-six intermittent claudication patients and 20 controls walked on a treadmill at self-selected speed; intermittent claudication patients were tested before (pain free) and after (pain) the onset of claudication. Variability of the ankle, knee, and hip joint angles was assessed using the largest Lyapunov exponent, standard deviation and coefficient of variation. Dependent t-tests were used to compare the pain free and pain conditions. Independent t-tests were used to compare intermittent claudication patients and controls.
Findings
Pain free and pain conditions were not significantly different for any of the parameters evaluated except the ankle. Compared to controls, patients had significantly greater values for the largest Lyapunov exponent in both conditions for all joints.
Interpretation
Gait variability was essentially the same before and after the onset of claudication at the knee and the hip, and was increased in both conditions compared to controls. This indicates altered cooperation between components of the locomotor system of intermittent claudication patients, likely due to the associated myopathy since differences were present even before the onset of claudication. This research helps provide essential biomechanical knowledge of intermittent claudication that contributes to improved clinical management.
Keywords: Joint kinematics, peripheral arterial disease, Lyapunov exponent, locomotion, walking
Introduction
Peripheral arterial disease (PAD) is a disorder manifested from systemic atherosclerosis, characterized by blockages of the arteries supplying one (unilateral disease) or both (bilateral disease) of the legs. PAD affects up to twelve million people in the United States (Menard et al. 2004). Intermittent claudication (IC), defined as walking induced gait dysfunction and muscle pain (claudication pain) that is relieved by rest, is the most common manifestation of PAD. IC patients are less mobile, which leads to functional limitations and poor health outcomes such as impaired balance and increased risk of falls.
Gait in IC patients with a history of falls has not been previously investigated, but patients with IC tend to be older and to fall (Gardner, Montgomery 2001, American Heart Association, 2007). Biomechanical analysis has shown that gait variability is altered in the elderly and can actually be a predictor of falling (Maki 1997, Hausdorff 2007). Furthermore, recent work from our laboratory (Myers et al. 2009) using both linear (standard deviation, coefficient of variation) and nonlinear (largest Lyapunov exponent) tools of measuring gait variability has demonstrated that IC patients have increased amount and altered structure of gait variability at baseline ambulation prior to the onset of claudication pain as compared to healthy controls. These findings indicate significant deterioration in the locomotor system of IC patients, resulting in increased noise and instability of gait. Furthermore, it has been proposed that there is an “optimal” level of variability in gait patterns that is associated with a healthy state (Stergiou, Harbourne & Cavanaugh 2006). Deviation from the optimal level is linked with malfunction and disease, and would be considered altered or abnormal. Our previous results (Myers et al. 2009) are consistent with this idea. However, it is unknown if any additional adaptations, in terms of gait variability, are made when IC patients experience claudication pain.
The purpose of the current study was to determine how gait variability in IC patients is affected by claudication pain. Gait variability was determined by evaluating the joint kinematic variability of the lower extremities in IC patients before (pain free condition) and after (pain condition) the onset of claudication pain. The patients with IC were compared with healthy controls matched for age, height, body mass, and gender. We hypothesized that as patients experienced pain due to lack of blood flow in the claudicating limbs, gait variability would be significantly altered compared to baseline ambulation and healthy controls.
Methods
Participants
Twenty-six symptomatic IC patients (age: 64.8 (9.4) yr, body mass: 81.5 (15.7) kg, body height: 1.71 (0.05) m) diagnosed with moderate arterial occlusive disease and bilateral claudication were recruited from the vascular surgery clinics of the Veterans Affairs Medical Center of Nebraska and Western Iowa and the University of Nebraska Medical Center (Table 1). In addition, 20 height, mass, gender, and age matched healthy controls (age: 64.3 (13.2) yr, body mass: 81.6 (24.3) kg, body height: 1.72 (0.08) m) recruited from the community volunteered to participate. Informed consent was obtained from all subjects prior to data collection according to the guidelines of the respective medical centers’ Institutional Review Boards. Patients and controls were screened and evaluated by two board certified vascular surgeons. Patient evaluation included detailed history, and physical exam. Those IC patients with ambulation limiting cardiac, pulmonary, neuromuscular or musculoskeletal disease or those who experienced pain or discomfort during walking for any reason other than claudication (i.e. arthritis, low back pain, peripheral neuropathy) were excluded.
Table 1.
Baseline characteristics of Intermittent Claudication (IC) patients and control subjects. Continuous data are presented as mean (standard deviation).
| Control (N=20) | IC (N= 26) | P values | |
|---|---|---|---|
| Clinical characteristics | |||
| Gender (Male/Female) | 14/6 | 24/2 | 0.069 |
| Age (years) | 64.3 (13.2) | 64.7 (9.4) | 0.889 |
| Body mass (kg) | 81.6 (24.3) | 81.5 (15.7) | 0.977 |
| Body height (m) | 1.72 (0.08) | 1.71 (0.05) | 0.457 |
| Body Mass Index | 27.20 (6.72) | 27.85 (5.17) | 0.713 |
| Disease duration (years) | 0 | 1.90 (1.25) | NA |
| Ankle Brachial Index | |||
| Right limb | 1.2 (0.11) | 0.59 (0.19) | <0.001 |
| Left limb | 1.1 (0.09) | 0.59 (0.24) | <0.001 |
| Smokers (%) | 0 | 46.15 | <0.001 |
| Hypertension (%) | 10.00 | 76.92 | <0.001 |
| Diabetes mellitus (%) | 5.00 | 19.23 | 0.162 |
| Hyperlipidemia (%) | 5.00 | 69.23 | <0.001 |
| Calf pain during Pain condition (%) | 0 | 84.6 | <0.001 |
| Treadmill speed (km/hr)a | 3.70 (0.88) | 2.44 (0.54) | <0.001 |
NA, Not applicable
Treadmill speed was self-selected.
Control subjects had an ankle-brachial index ≥ 1.0 and no subjective or objective ambulatory dysfunction. Controls were screened in a similar fashion as IC patients and were excluded for the same ambulation limiting co-morbidities or if pain was experienced during walking. The gait of all recruited participants was tested in the biomechanics laboratory at the University of Nebraska at Omaha.
Experimental Procedure and Data Collection
Prior to data collection, reflective markers were placed at specific anatomical locations of each subject's lower limbs utilizing the models used by Vaughan et al. (Vaughan, Davis & O'Connor 1999) and Nigg et al.(Nigg, Cole & Nachbauer 1993). After markers were placed, subjects were allowed to get accustomed to the treadmill by starting to walk at 0.45 m/sec and being able to increase or decrease the speed until a comfortable speed was found; this speed was then identified as the self-selected speed. The patient was then allowed to rest to insure absence of claudication pain before data collection began. Three dimensional kinematics were acquired at 60 Hz using EVART software (Motion Analysis Corp., Santa Rosa CA) while subjects walked on a treadmill at their self-selected speed. Self-selected speed is the most comfortable and natural walking speed. For the pain free condition, patients walked on the treadmill for three minutes or until the onset of claudication pain, whichever came first. All kinematic measurements for the pain free condition were taken prior to the onset of claudication symptoms. Next, the treadmill was inclined to 10% grade and the subjects walked until the onset of claudication pain. Once the patient had pain, the treadmill was lowered to the 0% grade position and the patients walked on the level treadmill with claudication pain as long as tolerable for up to three minutes. Controls completed only the pain free condition. For safety purposes, blood pressure was monitored before and after the treadmill test.
Data Analysis
Data was exported and processed in custom software using Matlab (Mathworks Inc., MA). This software was used to calculate the relative joint angle time series for the ankle, knee and hip flexion/extension. A trial with a minimum of 30 footfalls was considered adequate for nonlinear and linear analysis (Abarbanel 1996, Keenan, Stergiou 2002, Sprott, Rowlands 1992). All joint angle time series were graphed and the number of data points required to reach 30 strides was counted. All data were cropped to the minimum data points required for 30 strides, insuring each time series included at least 30 gait cycles. All subjects were able to complete 30 strides for both the pain free and the pain condition. To insure the subjects had a significant level of claudication pain, the last 30 strides taken by each subject were analyzed for the pain condition. The data was analyzed unfiltered to obtain a more accurate representation of the variability within the locomotor system (Sprott, Rowlands 1992).
Gait Variability analysis
From each time series, range of motion was calculated for every gait cycle for the ankle, knee and hip angles. Means were then calculated for each variable and for each subject, as well as standard deviations and coefficients of variation. The calculation of these parameters was performed in Matlab (Mathworks Inc., MA). This analysis supplemented the nonlinear analysis of gait variability presented below and provided answers regarding the amount of variability present in the joint movement patterns over time.
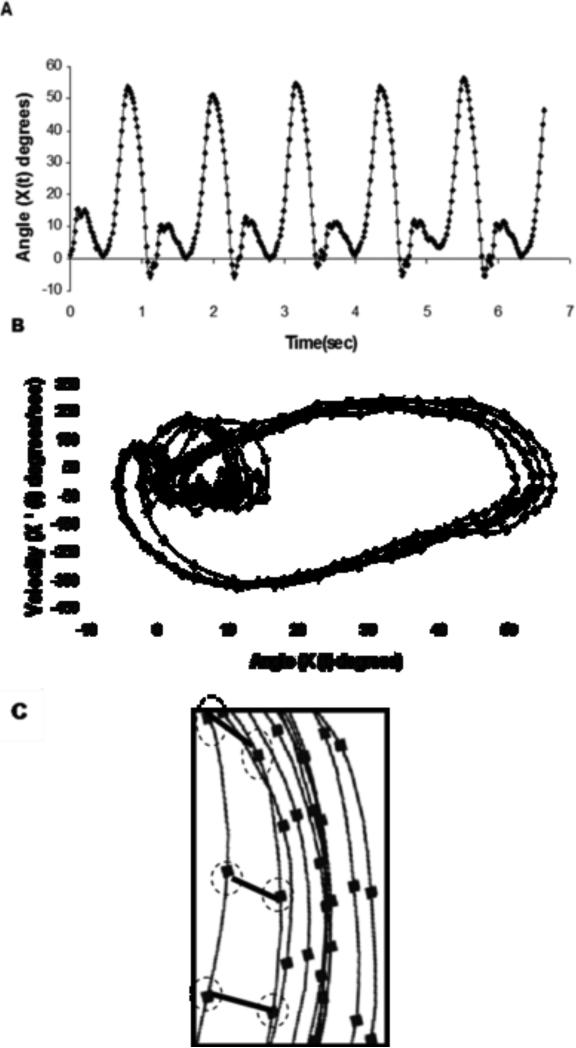
The nonlinear analysis included the calculation of the largest Lyapunov exponent which quantifies the mean rate of divergence of neighbored state-space trajectories and estimates the structure of variability in the system (Figure 1). The largest Lyapunov exponent was calculated for all joint angle series from the pain free and pain conditions of the IC patients and for the controls. Further description of the actual calculation of this measure is provided in Myers et al(Myers et al. 2009). The largest Lyapunov exponent from a stable system with little to no divergence will be zero (e.g. sine wave). Alternatively, the largest Lyapunov exponent for an unstable system that has a high amount of divergence will be positive with a greater value (above 0.5 when calculated using the Chaos Data Analyzer)(Abarbanel 1996, Buzzi et al. 2003, Stergiou et al. 2004). The Chaos Data Analyzer (professional version, American Institute of Physics)(Sprott, Rowlands 1992) was used to numerically calculate the largest Lyapunov exponent for each joint angle time series for each subject.
Figure 1.
A graphical representation of the state space of knee joint angle time series and the calculation of the Largest Lyapunov exponent. (A) An original knee flexion-extension time series from a control subject. (B) A two-dimensional state space created by the position and velocity time series from the same knee angle. (C) A section of the state space where the divergence of neighboring trajectories is outlined. In our study the state space was reconstructed using 10 embedding dimensions. The number of embedding dimensions from our data was calculated according to procedures outlined at Stergiou et al(24). The divergence from one cycle to the next is outlined for several points. The Largest Lyapunov exponent is calculated as the slope of the average logarithmic divergence of the neighboring trajectories.(24)
One of the assumptions made when calculating the largest Lyapunov exponent is that the source of the variation in a given time series is deterministic in nature (Buzzi et al. 2003, Stergiou et al. 2004, Miller, Stergiou & Kurz 2006). A deterministic time series is one that has an ordered pattern (each point in the series is related to its preceding points). Therefore, to ensure our time series met this assumption, we used the method of surrogation (Rapp 1994). Surrogation compares the original time series data set to an equivalent random data set with similar structure. Essentially, surrogation removes the deterministic characteristics from the actual joint angle data set by shuffling the data to produce a random series with the same mean, variance and power spectra as the original data. Significant differences in largest Lyapunov exponent values between the original and surrogate counterparts reveal that the variations in the original time series are not randomly derived, but they are deterministic in nature (Stergiou et al. 2004). Therefore, surrogated data sets were created for each original joint angle time series analyzed. This procedure was performed in Matlab (Mathworks Inc, MA) using the pseudoperiodic surrogation algorithm (Stergiou et al. 2004, Miller, Stergiou & Kurz 2006). The pseudoperiodic algorithm is used to determine if there is additional determinism in the fluctuations present in a time series that have inherent periodicity (e.g. gait cycles).
Statistical Analysis
The largest Lyapunov exponent values were calculated for both the surrogated and original joint angle time series data and compared using a dependent t-test (alpha=0.05). Significant differences between data sets indicate that the variations present in the original data set are not random, but they are deterministic in nature. Means for the standard deviation and the coefficient of variation of the range of motion and the largest Lyapunov exponent values were calculated for the ankle, knee and hip joints for both conditions of the IC patients and for the control group. Cohen's d was calculated to assess effect sizes of the results. Independent t-tests were used to compare the group means between the two groups. Dependent t-tests were used to compare gait variability measures in the pain free condition with those in the pain condition. Statistical comparisons were performed using SPSS (SPSS Inc, Chicago, IL). The level of significance was set at 0.05.
Results
There were no significant differences between IC patient and control group means for age (p=0.889), height (p=0.457), body mass (p=0.977) and body mass index (BMI; p=0.713) which confirms the two groups were appropriately matched (Table 1). Conversely there were significant differences in the clinical characteristics between the two groups, which was expected (Table 1). Nearly all IC patients, 84.6 percent, experienced calf pain during the pain condition.
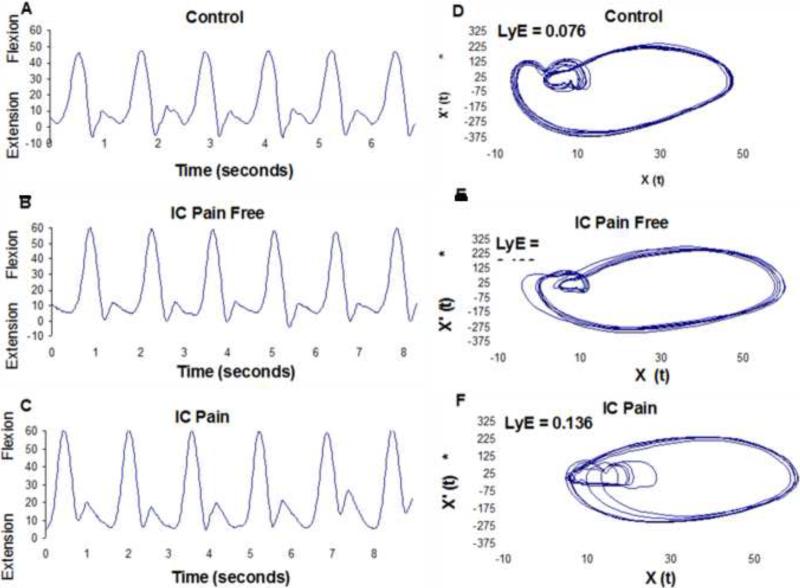
Nonlinear analysis results indicated a significant difference in the largest Lyapunov exponent values between the pain free and pain conditions of the IC patients at the ankle joint. The pain condition had a reduced value for the largest Lyapunov exponent compared to the pain free condition. Effect sizes for the comparisons of the largest Lyapunov exponent values in the pain free and pain conditions ranged from 0.03 to 0.44. Compared to controls, IC patients had significantly greater values for the largest Lyapunov exponent for the ankle, knee and hip joints during both the pain free and pain conditions (Table 2). The effect sizes ranged from 0.71 to 1.22 for the comparisons between healthy controls and IC patients during both conditions. These findings demonstrated that the joint movement patterns for both conditions in IC patients as compared to controls were exhibiting more divergence in consecutive strides which indicates altered neuromuscular organization (Figure 2). However, such differences in divergence were only present at the ankle joint between the two conditions.
Table 2.
Group means for the largest Lyapunov exponent of the original time series (LyE) and the surrogate time series (LyE-S) for patients with Intermittent Claudication (IC) in the Pain Free and Pain conditions and control groups. Data are reported as Mean (SD).
| Groupa | Ankle | Knee | Hip |
|---|---|---|---|
| LyE | |||
| Control (n=20) | 0.0761 (0.02) | 0.0713 ± 0.02 | 0.0734 (0.02) |
| IC Pain Free (n=26) | 0.0987 (0.02)a | 0.0952 ± 0.02a | 0.0940 (0.02)a |
| IC Pain (n=26) | 0.0900 (0.02)b,c | 0.0900 ± 0.02b | 0.0946 (0.02)b |
| LyE-S | |||
| Control | 0.0893 (0.02)d | 0.0829 (0.02)d | 0.0764 (0.01) |
| IC Pain Free | 0.1071 (0.02)d | 0.1011 (0.02) | 0.0907 (0.01) |
| IC Pain | 0.0970 (0.02) | 0.1005 (0.02) | 0.0911 (0 .02) |
Indicates significant difference (P < 0.05) between IC Pain Free and control groups.
Indicates significant difference (P<0.05) between IC Pain and control groups.
Indicates significant difference (P<0.05) between IC Pain Free and IC Pain conditions
Indicates significant differences between the original time series and their surrogate counterparts.
Figure 2.
A graphical comparison of knee joint angle variability between a (A) Control subject, (B) Intermittent Claudication (IC) patient in the pain free condition, and (C) IC patient in the pain condition. Graphs A, B, and C are the time series and graphs D, E, and F are two-dimensional state spaces created by plotting the position (X(t)) versus the velocity (X’ (t)) from the corresponding signals. The largest Lyapunov exponent (LyE) value of each time series is also given. It is clear that there is little or no difference between variability in the pain free and pain conditions of the IC patient. Additionally, it is apparent that PAD patients have more divergence and the healthy control graph traces nearly the same cycle with each stride.
No significant differences were found for the standard deviation and the coefficient of variation values for the hip or the knee between the two conditions. Significant differences were found only at the ankle where the standard deviation and the coefficient of variation values were significantly lower during the pain condition as compared to the pain free condition. The IC patients also had significantly lower standard deviation values at the ankle as compared to controls during the pain condition. Overall, the IC patients had significantly increased standard deviation values for most parameters as compared to controls during both pain free and pain conditions. Additionally, the IC patients had significantly greater coefficient of variation values than controls for the ankle, knee, and hip during the pain free condition and for the knee and hip during the pain condition (Table 3). Effect sizes ranged from 0.04 to 1.11 for the comparisons between IC patients and healthy controls, and from 0.02 to 0.72 for the comparisons between the pain free and pain conditions. These findings demonstrated a generally decreased amount of variability in the ankle flexion-extension patterns of the IC patients during the pain condition as compared with the healthy controls and as compared with the pain free condition in IC patients. However, no such differences existed for the other two joints.
Table 3.
Group means for the standard deviation (SD) and coefficient of variation (CoV) of the original time series for patients with Intermittent Claudication (IC) in the Pain Free and Pain conditions and control groups. Data are reported as Mean (SD).
| Groupa | Ankle | Knee | Hip |
|---|---|---|---|
| SD | |||
| Control (n=20) | 2.55 (0.81) | 1.79 (0.67) | 1.45 (0.52) |
| IC Pain Free (n=26) | 2.72 (1.15) | 2.14 (0.70) | 1.89 (0.57)a |
| IC Pain | 2.03 (0.81)b,c | 2.12 (1.00) | 1.99 (0.63)b |
| CoV | |||
| Control | 8.07 (2.86) | 3.18 (1.18) | 3.92 ± (1.52) |
| IC Pain Free | 11.03 (4.83)a | 4.38 (1.59)a | 6.09 ± (2.31)a |
| IC Pain | 8.23 (4.17)c | 4.27 (2.13)b | 6.31 ± (2.91)b |
a
Indicates significant difference (P < 0.05) between IC Pain Free and control groups.
Indicates significant difference (P<0.05) between IC Pain and control groups.
d Indicates significant difference (P < 0.05) between IC Pain Free and IC Pain conditions.
As expected, the surrogation analysis demonstrated the control group had surrogate data series with significantly greater largest Lyapunov exponent values than the original data at the ankle and the knee (Table 2). In the IC group, the surrogated largest Lyapunov exponent values were significantly greater than the original data only for the ankle during the pain free condition. There were no significant differences between original and surrogated data series during the pain condition (Table 2).
Discussion
The purpose of this study was to determine how gait variability in IC patients is affected by claudication pain. Gait variability was evaluated by exploring the joint kinematic variability of the lower extremities in IC patients before (pain free condition) and after (pain condition) the onset of claudication pain. The patients with IC were also compared with healthy controls matched for age, height, body mass, and gender. We hypothesized that as patients experienced pain in the claudicating limbs, gait variability would be further altered compared to baseline ambulation and compared to healthy controls. We used the largest Lyapunov exponent, the standard deviation and the coefficient of variation of the flexion-extension time series of the lower extremity joints to measure gait variability. Our results showed that there are only a few differences in gait variability between symptomatic IC patients during baseline walking in the absence of claudication pain and while walking with claudication pain. However, gait variability of all lower extremity joints during both the pain free and pain conditions were significantly different when compared to gait in healthy controls.
The linear measures of standard deviation and coefficient of variation assessed amount of variability. Of eighteen total comparisons of linear variability, ten were significantly different (Table 3). Eight of these ten differences occurred between IC patients and healthy controls. This indicates that the amount of gait variability in the lower extremity joint movement patterns stays relatively constant between pain free and pain conditions, but is different between IC patients and healthy controls. Four of those ten significant differences were located at the ankle while walking with claudication pain. Interestingly, biomechanical studies investigating joint kinematics and kinetics have consistently identified differences between IC patients and healthy controls at the ankle joint (Koutakis et al. 2010a, Koutakis et al. 2010b, Crowther et al. 2007). Increases in the amount of variability at the ankle as compared to controls are possibly the result of weakness in the posterior calf muscles in IC patients, which has been suggested in previous studies(Koutakis et al. 2010b) and demonstrated through isometric strength tests (McDermott et al. 2007). Additionally, claudication pain commonly occurs in the calf muscles (Vogt, Wolfson & Kuller 1992), and patients may be compensating by adjusting the amount of gait variability at the ankle. In our study, the only differences found between pain free and pain conditions of IC patients occurred at the ankle joint.
The surrogation analysis found that the largest Lyapunov exponent values of the original data series were significantly different than their surrogate counterparts for the ankle and knee in the control group. When surrogation was applied to both the pain free and pain conditions of IC patients, only the ankle during the pain free condition showed significant differences from its surrogate counterpart. Based on our findings, the variability present in healthy controls is deterministic, but this is less the case with the IC patients, especially while walking with claudication pain. It is important for normal gait to have deterministic properties because that allows individuals to appropriately adjust as conditions change within the environment. Walkers must frequently respond to changing environmental conditions like inclines or varying surfaces. This degradation of the variability structure in the IC patients is further evidence of the effect of the disorder on the gait patterns of these patients. The degradation of the deterministic behavior of joint angle variability with disease was hypothesized by Buzzi et al.(Buzzi et al. 2003) and is in agreement with our previous study of IC patients (Myers et al. 2009). Limitations with hip angle calculations due to hip marker blockages from arm swinging may be partially responsible for lack of significant differences between original and surrogate data series at the hip in the controls (Myers et al. 2009).
Gait alterations during baseline walking are consistent with previous research which identified a gait handicap in IC patients before the onset of claudication pain (Myers et al. 2009, Crowther et al. 2007). Specifically, the increase in the largest Lyapunov exponent agrees with previous investigations (Myers et al. 2009). Also, changes to the amount and structure of variability are consistent with previous comparisons between healthy young and elderly(Buzzi et al. 2003), healthy elderly and elderly fallers (Maki 1997, Lockhart, Liu 2008), and healthy individuals and patients with Parkinson's and Huntington's disease (Hausdorff 2007). For example, Lockhart and Liu found a 20.0% increase in the largest Lyapunov exponent of fall-prone elderly as compared with healthy elderly (Lockhart, Liu 2008). In the current study, IC patients had an average increase of 30% in the largest Lyapunov exponent values as compared with healthy controls. Based on the greater increases in the largest Lyapunov exponent values, gait variability is altered more in IC patients than in individuals who are prone to falls. Clinically, these changes suggest that IC patients may have a greater risk of experiencing a fall than typical fall-prone elderly individuals and interventions should be considered to decrease fall risk in IC patients.
The relatively few differences between pain free and pain conditions coupled with multiple differences between IC patients and controls, strongly demonstrate the level of gait impairment that is present in IC patients prior to the onset of claudication pain. While it may be puzzling that pain does not induce further changes in gait variability, a recent study by Gardner et al. had similar results, with few differences in spatial and temporal gait characteristics before and after the onset of claudication pain (Gardner et al. 2010). Anterior cruciate ligament reconstruction patients have also demonstrated altered gait variability and a recent study of the neuromuscular response to fatigue in these patients may provide further insight into our results (Moraiti et al. 2009). Patras et al investigated changes of lower extremity electromyographic activation in the limbs of patients with anterior cruciate ligament reconstruction (Patras et al. 2009). Results of the study showed that the healthy limb increased muscle activation following fatigue, but the reconstructed (pathological) limb did not change electromyographical activity, indicating an impaired neuromuscular response in the pathological limb. IC patients already exhibit pathological gait variability in a pain free state and no differences in gait variability at the proximal joints after the onset of claudication pain likely represents an inability of patients to respond to the pain. However, the ankle displayed decreased variability values for all three parameters assessed. A potential explanation for this could be that IC patients attempt to minimize use of the calf muscles, where claudication pain typically occurs. Reducing movement or altering the structure of variability due to joint problems has been found in individuals with chronic ankle instability, who limited the amount of dorsiflexion during jogging (Drewes et al. 2009) , and in patients with knee osteoarthritis, who have reduced largest Lyapunov Exponent values (Yakhdani et al. 2010). Thus, it is plausible that IC patients attempt to reduce the demands on the ischemic muscles.
Functional outcomes measures such as the six minute walk test and quality of life questionnaires have continually shown that IC patients do not function as well as those without IC (Gardner, Montgomery 2001, McDermott et al. 2001, Gardner, Forrester & Smith 2001). Our results are consistent with previous studies that have suggested that muscle weakness, abnormal muscle metabolism and muscle denervation as caused by chronic muscle ischemia or the onset of claudication pain itself maybe the reason for gait impairments (Gardner, Forrester & Smith 2001). The results of the current study show gait is altered even before the onset of pain and suggest gait differences are not caused by the pain itself, but rather by changes in the musculoskeletal system that have been documented in recent studies (Pipinos et al. 2008). Specifically, a number of reports have documented a metabolic myopathy in the muscles of IC patients that appears to be secondary to defective mitochondrial bioenergetics and related oxidative damage to skeletal muscle structures and components (Pipinos et al. 2008). There is also evidence that the chronic ischemia experienced by IC patients results in electrodiagnostic abnormalities representative of axonal nerve loss (Weber, Ziegler 2002). Therefore, the impairments in gait variability prior to the onset of pain likely reflect a combination of myopathy and neuropathy in limbs with IC, which disrupt the pathways used by the muscular and nervous systems to generate and control movement.
A neuro-musculoskeletal system with optimal joint angle variability is able to make flexible modifications to stresses that are placed on the body. Considering this, the results of the study demonstrate that the locomotor system in patients with IC is degraded, and thus the ability to adapt to changes in the environment is decreased even if the patient does not feel pain. The fact that these changes to neuromuscular control occur before pain symptoms makes one question the status of the locomotor system in asymptomatic IC patients, who experience decreased blood flow but have no IC symptoms. A study of elderly women by McDermott et al. (McDermott et al. 2000) found that only 6.7% of study participants had been told by a doctor that they had PAD; however, 35% of these participants had objective evidence of PAD based on their abnormal ankle-brachial indices (<0.90). Therefore, it is possible that many elderly individuals have asymptomatic PAD and its accompanying deterioration of the locomotor system, but are unaware that they have the disease.
Conclusions
Claudicating patients have increased amount and altered structure of gait variability at the ankle, knee, and hip joints compared to controls during the pain free condition. The greater values observed in the nonlinear analysis in IC patients indicate increased randomness in their gait patterns and loss of motor control. Altered gait variability in IC patients is consistent with the pathophysiology of IC, which includes ischemic injury to the muscles and nerves of the lower extremities. Gait variability of IC patients is not different before and after the onset of claudication pain, except at the ankle. Decreased amount of variability at the ankle during claudication pain is likely an attempt to minimize use of the ischemic muscles where the pain is located. Lack of gait variability changes in response to claudication pain for the proximal joints demonstrates an inability of IC patients to compensate for the deficit ankle, further showing the severity of the gait handicap in IC. Collectively these results indicate decline of the overall health of the locomotor system, and an increased risk of functional problems in IC patients even if they are not symptomatic. Future work can help improve clinical management of IC by examining specific mechanisms that may be triggering gait problems in IC patients, such as the effect of reduced blood flow, the role of myopathic and neuropathic changes and whether gait improves following treatment.
Acknowledgments
This work was supported by funds received from the American Geriatrics Society's Hartford Foundation Dennis W. Jahnigen Award to [JMJ], the Nebraska Research Initiative to [NS], the American Society of Biomechanics, the American Alliance for Health, Physical Education, Recreation, and Dance Research Grant program, the NASA Nebraska Space Grant Fellowship, and the National Institute on Aging (Award Number F31AG032788) to [SM]. The study sponsors had no role in the study design, collection, analysis, or interpretation of the data, or in the writing of the manuscript or decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abarbanel HDI. Analysis of observed chaotic data. Springer-Verlag; New York: 1996. [Google Scholar]
- American Heart Association Heart Disease and Stoke Statistics. 2007 [Google Scholar]
- Buzzi UH, Stergiou N, Kurz MJ, Hageman PA, Heidel J. Nonlinear dynamics indicates aging affects variability during gait. Clinical biomechanics (Bristol, Avon) 2003;18(5):435–443. doi: 10.1016/s0268-0033(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Crowther RG, Spinks WL, Leicht AS, Quigley F, Golledge J. Relationship between temporal-spatial gait parameters, gait kinematics, walking performance, exercise capacity, and physical activity level in peripheral arterial disease. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2007;45(6):1172–1178. doi: 10.1016/j.jvs.2007.01.060. [DOI] [PubMed] [Google Scholar]
- Drewes LK, McKeon PO, Kerrigan DC, Hertel J. Dorsiflexion deficit during jogging with chronic ankle instability. Journal of science and medicine in sport / Sports Medicine Australia. 2009;12(6):685–687. doi: 10.1016/j.jsams.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Gardner AW, Forrester L, Smith GV. Altered gait profile in subjects with peripheral arterial disease. Vascular medicine (London, England) 2001;6(1):31–34. [PubMed] [Google Scholar]
- Gardner AW, Montgomery PS. Impaired balance and higher prevalence of falls in subjects with intermittent claudication. The journals of gerontology.Series A, Biological sciences and medical sciences. 2001;56(7):M454–8. doi: 10.1093/gerona/56.7.m454. [DOI] [PubMed] [Google Scholar]
- Gardner AW, Montgomery PS, Ritti-Dias RM, Forrester L. The effect of claudication pain on temporal and spatial gait measures during self-paced ambulation. Vascular medicine (London, England) 2010;15(1):21–26. doi: 10.1177/1358863X09106836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM. Gait dynamics, fractals and falls: finding meaning in the stride-to-stride fluctuations of human walking. Human movement science. 2007;26(4):555–589. doi: 10.1016/j.humov.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan S, Stergiou N. The reliability of the lyapunov exponent during treadmill walking. Proceedings of the 27th Annual Meeting of the American Society of Biomechanics. Toledo, OH, pp. in cd-rom., ..2002. [Google Scholar]
- Koutakis P, Johanning J, Myers S, Stergiou N, Longo M, Pipinos I. Abnormal joint powers before and after the onset of claudication symptoms. Journal of Vascular Surgery. 2010a;52(2):340–7. doi: 10.1016/j.jvs.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutakis P, Pipinos I, Myers S, Stergiou N, Lynch T, Johanning J. Joint torques and powers are reduced during ambulation for both limbs in patients wtih unilateral claudication. Journal of Vascular Surgery. 2010b;51(1):80–88. doi: 10.1016/j.jvs.2009.07.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart T, Liu J. Differentiating fall-prone and healthy adults using local dynamic stability. Ergonomics. 2008;51(12):1860–1872. doi: 10.1080/00140130802567079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. Journal of the American Geriatrics Society. 1997;45(3):313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- McDermott MM, Ferrucci L, Guralnik JM, Tian L, Green D, Liu K, Tan J, Liao Y, Pearce WH, Schneider JR, Ridker P, Rifai N, Hoff F, Criqui MH. Elevated levels of inflammation, d-dimer, and homocysteine are associated with adverse calf muscle characteristics and reduced calf strength in peripheral arterial disease. Journal of the American College of Cardiology. 2007;50(9):897–905. doi: 10.1016/j.jacc.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Fried L, Simonsick E, Ling S, Guralnik JM. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the women's health and aging study. Circulation. 2000;101(9):1007–1012. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- McDermott MM, Ohlmiller SM, Liu K, Guralnik JM, Martin GJ, Pearce WH, Greenland P. Gait alterations associated with walking impairment in people with peripheral arterial disease with and without intermittent claudication. Journal of the American Geriatrics Society. 2001;49(6):747–754. doi: 10.1046/j.1532-5415.2001.49151.x. [DOI] [PubMed] [Google Scholar]
- Menard JR, Smith HE, Riebe D, Braun CM, Blissmer B, Patterson RB. Long-term results of peripheral arterial disease rehabilitation. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2004;39(6):1186–1192. doi: 10.1016/j.jvs.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Stergiou N, Kurz MJ. An improved surrogate method for detecting the presence of chaos in gait. Journal of Biomechanics. 2006;39(15):2873–2876. doi: 10.1016/j.jbiomech.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Moraiti CO, Stergiou N, Ristanis S, Vasiliadis HS, Patras K, Lee C, Georgoulis AD. The effect of anterior cruciate ligament reconstruction on stride-to-stride variability. Arthroscopy : The Journal of Arthroscopic & Related Surgery : Official Publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2009;25(7):742–749. doi: 10.1016/j.arthro.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Myers SA, Johanning JM, Stergiou N, Celis RI, Robinson L, Pipinos II. Gait variability is altered in patients with peripheral arterial disease. Journal of vascular surgery : official publication, the Society for Vascular Surgery [and] International Society for Cardiovascular Surgery, North American Chapter. 2009;49(4):924–931.e1. doi: 10.1016/j.jvs.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Nigg BM, Cole GK, Nachbauer W. Effects of arch height of the foot on angular motion of the lower extremities in running. Journal of Biomechanics. 1993;26(8):909–916. doi: 10.1016/0021-9290(93)90053-h. [DOI] [PubMed] [Google Scholar]
- Patras K, Ziogas G, Ristanis S, Tsepis E, Stergiou N, Georgoulis AD. High intensity running results in an impaired neuromuscular response in ACL reconstructed individuals. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2009;17(8):977–984. doi: 10.1007/s00167-009-0822-0. [DOI] [PubMed] [Google Scholar]
- Pipinos II, Judge AR, Selsby JT, Zhu Z, Swanson SA, Nella AA, Dodd SL. The myopathy of peripheral arterial occlusive disease: Part 2. Oxidative stress, neuropathy, and shift in muscle fiber type. Vascular and endovascular surgery. 2008;42(2):101–112. doi: 10.1177/1538574408315995. [DOI] [PubMed] [Google Scholar]
- Rapp PE. A guide to dynamical analysis. Integrative physiological and behavioral science : the official journal of the Pavlovian Society. 1994;29(3):311–327. doi: 10.1007/BF02691335. [DOI] [PubMed] [Google Scholar]
- Sprott J, Rowlands G. Chaos data analyzer: The professional version. American Institute of Physics; New York: 1992. [Google Scholar]
- Stergiou N, Buzzi UH, Kurz MJ, Heidel J. Nonlinear tools in human movement. In: Stergiou N, editor. Innovative analysis of human movement. Human Kinetics; Champaign, IL: 2004. pp. 63–90. [Google Scholar]
- Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. Journal of neurologic physical therapy : JNPT. 2006;30(3):120–129. doi: 10.1097/01.npt.0000281949.48193.d9. [DOI] [PubMed] [Google Scholar]
- Vaughan C, Davis B, O'Connor J. Dynamics of human gait. Kiboho Publishers; Cape Town, South Africa: 1999. [Google Scholar]
- Vogt MT, Wolfson SK, Kuller LH. Lower extremity arterial disease and the aging process: a review. Journal of clinical epidemiology. 1992;45(5):529–542. doi: 10.1016/0895-4356(92)90102-s. [DOI] [PubMed] [Google Scholar]
- Weber F, Ziegler A. Axonal neuropathy in chronic peripheral arterial occlusive disease. Muscle & nerve. 2002;26(4):471–476. doi: 10.1002/mus.10235. [DOI] [PubMed] [Google Scholar]
- Yakhdani HR, Bafghi HA, Meijer OG, Bruijn SM, van den Dikkenberg N, Stibbe AB, van Royen BJ, van Dieen JH. Stability and variability of knee kinematics during gait in knee osteoarthritis before and after replacement surgery. Clinical biomechanics (Bristol, Avon) 2010;25(3):230–236. doi: 10.1016/j.clinbiomech.2009.12.003. [DOI] [PubMed] [Google Scholar]