Abstract
Background
The anterior cingulate cortex (ACC), orbito-frontal cortex (OFC) and basal ganglia have been implicated in pathological aggression. This study aimed at identifying neuroanatomical correlates of impulsive aggression in healthy children.
Methods
Data from 193 representative 6–18 year-old healthy children were obtained from the NIH MRI Study of Normal Brain Development after a blinded quality control (1). Cortical thickness and subcortical volumes were obtained with automated software. Aggression levels were measured with the Aggressive Behavior scale (AGG) of the Child Behavior Checklist (CBCL). AGG scores were regressed against cortical thickness and basal ganglia volumes using first and second-order linear models while controlling for age, gender, scanner site and total brain volume. ‘Gender by AGG’ interactions were analyzed.
Results
There were positive associations between bilateral striatal volumes and AGG scores (right: r=0.238, p=0.001; left: r=0.188, p=0.01). A significant association was found with right ACC and subgenual ACC cortical thickness in a second-order linear model (p<0.05, corrected). High AGG scores were associated with a relatively thin right ACC cortex. An ‘AGG by gender’ interaction trend was found in bilateral OFC and ACC associations with AGG scores.
Conclusion
This study shows the existence of relationships between impulsive aggression in healthy children and the structure of the striatum and right ACC. It also suggests the existence of gender specific patterns of association in OFC/ACC grey matter. These results may guide research on oppositional-defiant and conduct disorders.
Keywords: Aggression, Cortical Thickness, Anterior Cingulate Cortex, Orbito-Frontal Cortex, Striatum, Magnetic Resonance Imaging (MRI)
INTRODUCTION
All humans tend to experience periods of worry, sadness, happiness, and aggression, among others. All of these are brain based and the study of what brain regions are implicated in specific human behaviors is one of the great frontiers of modern neuroscience. The past decade has seen great improvement in the knowledge of structural and functional imaging correlates of a wide variety of childhood psychiatric disorders (2, 3). A primary methodological approach has been to compare and contrast so-called ‘normal controls’ to individuals with a specific disorder. This categorical approach to the study of human behavior and underlying brain biology belies empirical evidence suggesting that many human traits, including aggression, are continuously distributed (4, 5). Studying brain-behavior relations among healthy children exhibiting normal variation may provide critical insight on the neural substrate of human behavior and psychopathology. This is the aim of this particular study on childhood impulsive aggression.
Human aggression is a ubiquitous phenomenon with important social consequences. Although the underpinnings are clearly multifactorial, the neurobiology of impulsive aggression is gradually being better understood. Impulsive aggression can be defined as a lower threshold for activation of motoric aggressive responses to external stimuli without adequate reflection or regard for the aversive consequences of the behavior (6). In neurobiological terms, this is conceived as an inadequate “top-down” control of “bottom-up” drives from the limbic system. The anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) have been frequently implicated as important structures for the “top-down” regulation of aggressive impulses (6, 7). There is a well-documented clinical association between frontal lobe lesions and impulsive/aggressive behaviors (8–10). Functional PET studies have demonstrated reduced serotonin (a neurotransmitter associated with impulse control) transporter availability in the ACC of individuals with pathological impulsive aggression (11). In addition, the imagination of aggressive scenarios by healthy adult subjects was associated with decreased activity of the OFC on PET (12).
Although not as extensively studied as the ACC and OFC, there is evidence indicating that basal ganglia are also implicated in the regulation of aggressive behaviors. It is known from past animal studies that these structures participate in the motor component of aggression (13, 14). It is now recognized that basal ganglia are also involved in more complex behaviors through their extensive connections with the frontal lobes and limbic system (15, 16). Various studies illustrate the involvement of basal ganglia in aggressive behaviors. Glenn et al. (2010) have found increased striatal volume in psychopathic individuals (17). In a SPECT study of adolescents and adult psychiatric patients with a recent history of aggressive behavior, aggression was associated with increased left basal ganglia activity (18). In addition, there is clinical evidence linking basal ganglia pathologies, such as Huntington’s disease, Tourette’s disorder and Lesch-Nyhan syndrome on the one hand, and irritability/aggressive behaviors on the other (19–22). Further, catastrophic aggressive outbursts with rages in a patient with bilateral caudate infarcts have been reported (23).
The Aggressive Behavior syndrome (AGG) of the Child Behavior Checklist (CBCL) is a well-validated measure of aggressive tendencies in healthy children and those suffering from externalizing disorders (24–26). AGG primarily measures components of impulsive aggression. It has a high test-retest reliability (r =0.90, Cronbach’s alpha =0.94) and the stability is high, with Pearson correlations of 0.82 and 0.81 reported for 12- and 24-month intervals, respectively(27). It has been validated as an independent behavioral measurement in different cultures(28, 29). It tends to co-occur with the CBCL Rule Breaking scale that includes more instrumental forms of aggression; however factor analysis has revealed that these are separate syndromes with independent features (30–32). The genetic contribution to impulsive aggressive behaviors has been estimated to be above 50% and the genetics of the AGG scale in particular is between 52 and 71% (5, 33–36). AGG scores significantly predicts DSM-IV diagnosis of oppositional defiant disorder (ODD) and conduct disorder (CD) (24). In terms of gender specificity, it was previously found that teenage females tend to use more “indirect” or relational forms of aggression, as opposed to males who tend to express it in more direct ways (5, 37, 38).
Most neurobiological studies have focused on pathological aggression/violence (i.e. criminals, personality disorders, conduct disorder, etc.) and few studies have looked at correlates of aggressiveness in healthy children (2). Boes et al. (2008) identified decreased right anterior cingulate volume as a neuroanatomical correlate of aggression and defiance in boys from 7 to 17 years of age without psychopathology (39). In the current study, we assessed MRI neuroanatomical correlates of AGG scores in developmentally healthy children from 6 to 18 years of age using the NIH MRI Study of Normal Brain Development data (1). We hypothesize that, even in non-pathological subjects, there are anatomical variations in cortical thickness of the ACC(Brodmann Area (BA) 24, 25 and 32), the OFC(BA 11–12 and 47)(40, 41) and basal ganglia volume associated with CBCL AGG scores. The literature was insufficient to clearly predict the direction of the associations, although reduced grey matter at the cortical level was more frequently associated with aggressive behaviors in previous studies (39, 42, 43). We also hypothesize that the gender differences in aggression expression are linked to sex specific neuroanatomical correlates.
METHODS AND MATERIAL
Sampling and Recruitment
The NIH MRI Study of Normal Brain Development is a multi-site project providing a normative database to characterize healthy brain maturation in relationship to behavior (1). Subjects were recruited across the USA with a population-based sampling method seeking to minimize biases of selection (44). Based on available US Census 2000 data, a representative healthy sample of subjects was recruited at 6 pediatric study centers: Children's Hospital (Boston), Children's Hospital Medical Center (Cincinnati), University of Texas Houston Medical School (Houston), UCLA Neuropsychiatric Institute and Hospital (Los Angeles), Children's Hospital of Philadelphia (Philadelphia) and Washington University (St. Louis). Recruitment was monitored continuously in order to assure that the sample recruited across all pediatric centers was demographically representative on the basis of variables that included age, gender, race, ethnicity and socioeconomic status. Informed consent from parents and child assent were obtained for all subjects. The Objective 1 database used for this study included 431 children from 4 years and 6 months to 18 years and 3 months who underwent extensive cognitive, neuropsychological and behavioral testing, along with MRI brain imaging. Data from the first visit were used for this study. Given that the goal of this project was to study developmentally normal children, there were extensive exclusion criteria including current or past treatment for an axis 1 disorder, evidence of most axis I disorder on structured parent or child interview (DICA), substance use other than nicotine, family history of major axis 1 disorder, family history of inherited neurological disorder or mental retardation due to non-traumatic events, abnormality on neurological examination, gestational age at birth <37 weeks or >42 weeks, intra-uterine exposure to substances known or highly suspected to alter brain structure or function, etc. Structural MRI and clinical/behavioral data were consolidated and analyzed within a purpose-built database at the Data Coordinating Center of the Montreal Neurological Institute (MNI), McGill University.
Child Behavior Checklist (CBCL)
The CBCL is an age appropriate standardized questionnaire filled by parents. It is a validated assessment that is used as a screening tool by clinicians (26, 45). The CBCL/4–18 that was utilized in this study is divided into 8 subscales, including the AGG syndrome (46). The checklist provides raw values that can be converted to age adjusted t scores if needed. For AGG, t scores of less than 67 are considered normal, 67–70 borderline and over 70 to be in the clinical range. All children in this study had t scores of less than 70. Raw scores (0–11) were analyzed in this study because age was included as a control variable in the regression models.
MRI protocol
In order to collect data within the time limitations for this age range and allow automated morphometric analysis, 30–45 minutes of data acquisition was allocated, with 1 mm inplane resolution, 1–2 mm slice thickness, whole brain coverage and multiple contrasts (T1W, T2W and PDW) (1). A 3D T1-weighted spoiled gradient recalled (SPGR) echo sequence was selected. Inter-site reliability was monitored with both the American College of Radiology and living phantoms that were scanned at each site(1).
Automated Image Processing
Quality controlled (QC) native MR images were processed through the CIVET automated pipeline (version 1.1.9, 2006) (47). This pipeline includes the CLASP algorithm for generating cortical thickness measurements at 40,962 vertices per hemisphere (47–51). Cortical thickness is calculated as the distance between the 'outer CSF-grey matter' and 'grey matter-white matter' interfaces (48–50). Regional volumes of subcortical structures were obtained with the ANIMAL algorithm (51, 52). Subjects with missing values (either anatomical measures or AGG scores) and subjects over 18 year-old that had completed the Young Adult Self Report (YASR) instead of the CBCL were eliminated. A stringent visual quality control (blinded as to the AGG score of the subjects) of the native cortical thickness images and subcortical structures of each subject was implemented to ensure that there were no aberrations in values for a given subject (53). Only subjects that had successfully passed the QC for both cortical thickness (CIVET) and subcortical structures (ANIMAL) were kept for analysis, meaning that selected subjects had imaging data of the highest quality. This stringent process left a final sample of 193 subjects (105 females, 88 males).
Statistical Analysis: Cortical Thickness
Statistical analyses were implemented using SurfStat, a statistical toolbox created for MATLAB 7 (The MathWorks, Inc.) by Dr. Keith Worsley (http://wiki.bic.mni.mcgill.ca/index.php/SurfStat). Each subject's absolute native-space cortical thickness was linearly regressed against AGG score at each cortical point after accounting for the effects of age, total brain volume, gender and scanner (since different scanners were used at the six sites). Handedness and IQ were also considered as potential confounding variables. It has been shown in healthy children and in childhood disorders that grey matter thickness can follow quadratic patterns (gradual increase followed by a decrease)(3, 54). Therefore, a second-order regression model was also implemented to identify the best fitting model: Y = 1 + b1AGG + b2AGG^2 + b3Age + b4Gender + b5TotalBrainVolume + b6Scanner. ‘AGG by gender’ and ‘AGG by age’ interactions were analyzed (37, 39). To assess the specificity of findings to impulsive aggression, results were compared with other CBCL syndromes (Rule Breaking, Attention Problems, Anxious/Depressed, Internalizing).
A whole-brain correction, using random field-theory (RFT), was used to account for multiple comparisons (55). Based on the literature, the ACC (BA 24, 25 and 32) and OFC (BA 11–12 and 47) were identified as regions of interest (ROI). In these a priori identified ROI, the threshold to identify trends was set to a p-value of α=0.005, uncorrected. Despite these a priori hypotheses, all figures included in this study are whole brain associations and are not masked in any way.
Statistical Analysis: Basal Ganglia Volume
Volumetric analysis was done with SPSS Statistics 17.0 (SPSS Inc., Chicago). AGG scores were linearly regressed against the volume of the caudate, putamen and globus pallidus nuclei, controlling for age, gender, total brain volume and scanner. Given that the caudate and putamen nucleus are often considered as a single unit (striatum) due to their close histological and embryological relation, the analysis was repeated for both structures combined (56). A Bonferroni correction was applied to the usual α=0.05 to account for multiple comparisons. Taking into account the strong correlation between the size of basal ganglia among themselves (r=0.578), the adjusted signification threshold was established at α=0.0235.
RESULTS
Demographics
There were no differences between the initial NIH cohort (n=431) and the QC sample (n=193) used in the statistical analysis for gender, socioeconomic status, race and handedness (Table S1 in Supplement). Mean age was slightly older in the n=193 sample (11.8±0.25 vs. 10.4±0.20) because younger children tended to have lower image quality, resulting in a higher image processing failure rate. Mean AGG score (2.47±0.19) was also slightly lower, barely reaching significance. Mean age, race distribution, socioeconomic status, handedness and mean AGG scores were comparable between males and females (Table S2 in Supplement). There were no significant differences in the distribution of AGG scores, age and gender across the 6 scanner sites of the study (Table S3 in Supplement)
Cortical Thickness
No first-order linear association was found between AGG scores and cortical thickness when all subjects were pooled together. However, the second-order model and quadratic term regressions revealed an association between AGG and the right ACC thickness (figure 1, p≤0.05 RFT corrected, n=193). The association was located in the ventral part of the pregenual ACC (BA 24) and the subgenual ACC (BA 25) (scatterplots included in figure 2). This finding can be more concretely explained by a weak positive association in subjects with very low level of aggressiveness (AGG scores 0–2), while subjects with higher scores (AGG 3–11) had a strong linear negative association with the right ACC thickness (Figure S1 in Supplement). This finding was independent of gender and there were no ‘AGG by age’ or ‘gender by age’ interactions. Adding handedness, IQ or CBCL Anxious/Depressed score as control variables did not change the results. Other CBCL scales, including the Rule Breaking, showed either no, or different anatomical associations with cortical thickness.
Figure 1.
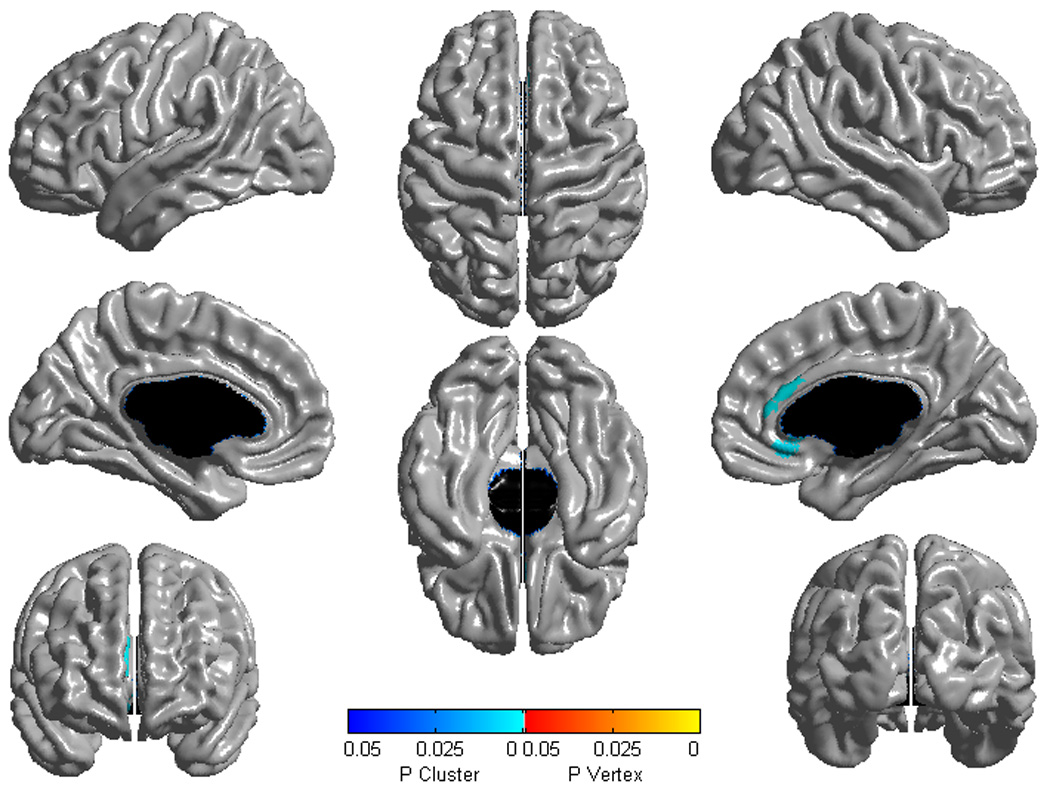
Brain areas where local cortical thickness is associated with CBCL Aggressive Behavior raw scores in a second-order (quadratic) model over the whole sample (n=193). Random field theory was used to correct for multiple comparisons over the whole cortical mantle.
Figure is shown at p≤0.05, RFT corrected. Blue areas are significant at the cluster level and red color corresponds to areas significant at the vertex level (none in this figure). Controlled for age, gender, scanner and total brain volume.
Figure 2.
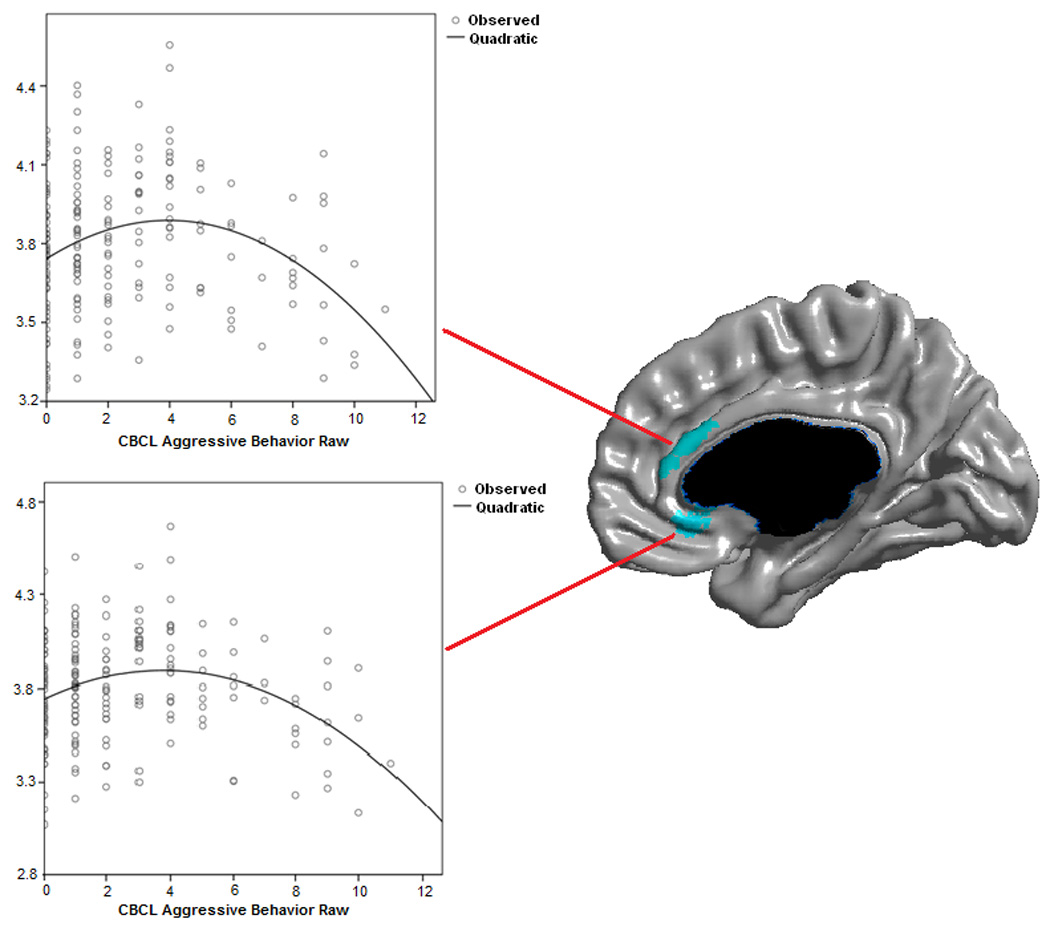
Scatterplots of CBCL Aggressive Behavior raw scores against local cortical thickness (mm).
Controlled for age, gender, scanner and total brain volume.
Figure 3 shows the map of the ‘AGG by gender’ interaction term against cortical thickness in a first-order linear model (females-males contrast, p≤0.005, n=193)( t map in Figure S2 in Supplement). Although there were no statistically significant associations, there were trends of differences in the right OFC (p<0.001), the left OFC (p<0.005) and bilateral ACC (p<0.005). In males (n=88), there were negative trends in bilateral OFC and a small part of the right ACC (Figure S3 in Supplement). In females (n=105), there was a positive trend of association between AGG and the subgenual ACC (Figure S4 in Supplement). In figure 3, associations outside of the ROIs in the left dorsolateral prefrontal cortex and the right anterior pole of the temporal lobe were not significant after RFT correction. There were no ‘AGG by gender’ interactions in a second-order model.
Figure 3.
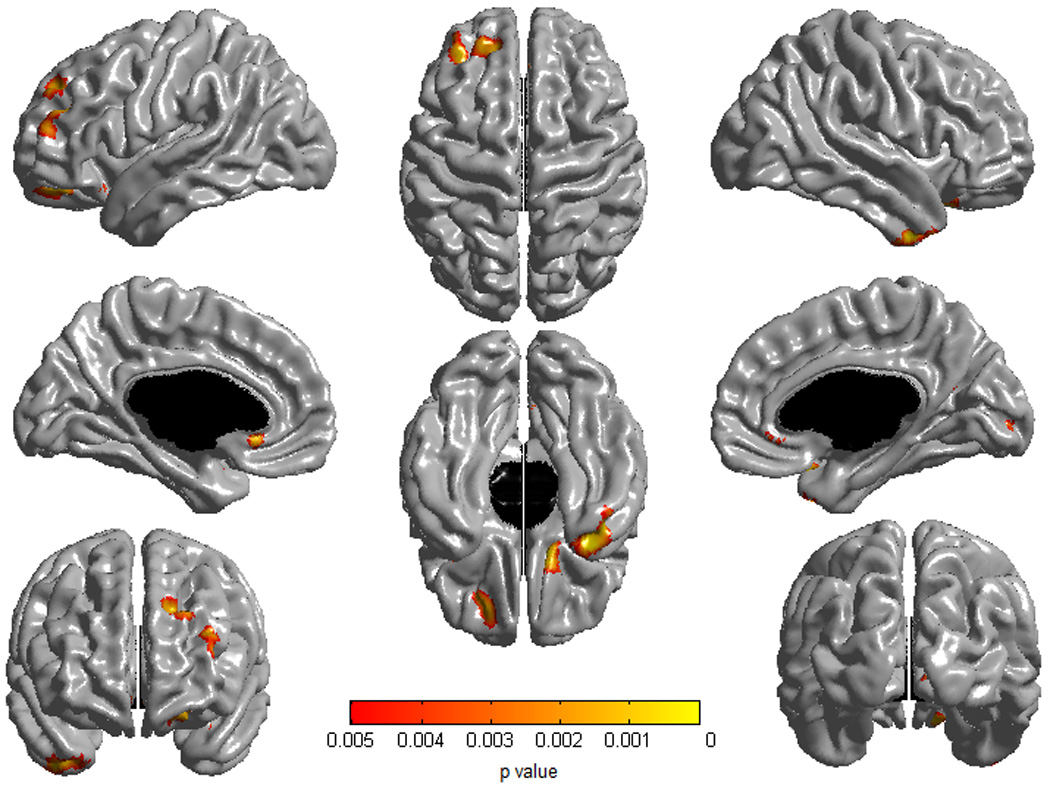
Brain areas where local cortical thickness is associated with the ‘CBCL Aggressive Behavior by gender’ interaction term (contrast females – males) over the whole sample (n=193). A first-order linear model was used. Threshold for trends was set at p=0.005 uncorrected for regions of interest (ACC/OFC).
Figure is shown at p≤0.005, uncorrected. This figure includes only one-sided associations because trends in the regions of interest were all in the same direction. Associations outside of the regions of interest were not significant after a random field theory correction. Controlled for age, gender, scanner and total brain volume.
Basal Ganglia Volumes
As shown in table 1 (n=193), AGG scores were positively associated with the volume of the right caudate (r=0.207, p=0.004), left caudate (r=0.191, p=0.008) and right putamen (r=0.193, p=0.008). There was a positive trend with the left putamen (r=0.134, p=0.066). There was no association with bilateral globus pallidus. The positive correlation between the volume of the right striatum and AGG was slightly greater than the association for either the putamen or caudate nucleus alone (right: r=0.238, p=0.001; left: r=0.188, p=0.01). Scatterplots are shown in figure 4. There were no significant ‘gender by basal ganglia’ or ‘age by basal ganglia’ interactions.
Table 1.
Pearson’s correlation coefficient between CBCL Aggressive Behavior raw scores and basal ganglia volumes in mm3 (n=193)
| Basal Ganglia |
Caudate right |
Caudate left |
Putamen right |
Putamen left |
Globus pallidus right |
Globus pallidus left |
Striatum right |
Striatum left |
|---|---|---|---|---|---|---|---|---|
|
Pearson’s Coefficient |
r=0.207 p=0.004 |
r=0.191 p=0.008 |
r=0.193 p=0.008 |
r=0.134 p=0.066 |
r=0.056 p=0.447 |
r=0.036 p=0.626 |
r=0.238 p=0.001 |
r=0.188 p=0.010 |
α=0.0235. Controlled for age, scanner, gender and total brain volume.
Figure 4.

Scatterplots of CBCL Aggressive Behavior raw scores against the left and right striatum volume (mm3)
Controlled for age, gender, scanner and total brain volume.
Comparison between Cortical Thickness and Basal Ganglia
Multiple regressions revealed that the left striatum explained 3.3% of the variance in AGG score, that the right striatum explained 5.7% of variance in AGG score and that the AGG score explained 6.2% of the variance in right ACC mean thickness. The right striatum explained, by itself, a statistically significant amount of variance despite controlling for the left striatum and ACC mean thickness while the left striatum association became non significant once the right striatum was taken into account. The association between the ACC thickness and AGG was significant even when controlling for striatal volumes.
DISCUSSION
As initially hypothesized, various neuroanatomical correlates of AGG were found in our large cohort of developmentally healthy children. At the subcortical level, the main findings were positive associations with striatal volume bilaterally. At the cortical level, thickness of the right ACC (BA 24–25) was observed to be associated with aggression in a second-order (quadratic linear) model. In a first-order model, the ‘AGG by gender’ interaction suggested trends of gender differences in bilateral OFC and bilateral ACC associations with AGG.
The ACC has strong connections to both the limbic system and higher prefrontal areas, and it has repeatedly been found to be involved in the regulation of emotions, especially its ventral subpart (57–62). Per example, multiple studies on borderline personality disorder, which is associated with emotional dysregulation and impulsivity, have found decreased volume in the ACC, both in adults and adolescents(63–66). The quadratic association between AGG scores and right ACC thickness found in this study further supports the crucial importance of this region in the regulation of aggressive impulses in the developing brain. Interestingly, decreased right ACC volume had been previously associated with aggression and defiance in healthy teenage males in a smaller study, but not in females (39). Our results were similar in both genders, possibly because of the larger sample size. The association in a quadratic model but not in a linear model could be explained by a gradually stronger negative association between right ACC thickness and aggression as children get closer to pathological levels. Another hypothesis would be that the CBCL could not discriminate as precisely small differences between children with very low levels of aggression (AGG 0–2), making it impossible to detect a small association at these levels.
Although it remains unclear as to why the association between ACC thickness and CBCL AGG was found only in the right hemisphere, previous studies on aggression have evinced similar asymmetrical findings (6, 18). Moreover, there is burgeoning evidence from imaging studies that the neural circuitry mediating response inhibition demonstrates a rightward lateralization (67). Prospective longitudinal studies, employing both structural and functional imaging modalities, are needed to address such possibilities.
The positive bilateral associations between striatal volume and the CBCL AGG suggest that these structures are implicated in the regulation of impulsive aggressive behaviors. In addition, given that the putamen and the head of the caudate fuse to form the ventral striatum (including the nucleus accumbens, a crucial component of the reward system) which has important connections with the limbic circuitry, they might also be associated with increased “bottom-up” aggressive impulses (56). In that regard, it is interesting to note that larger striatum has also been found in adult psychopathic individuals who often exhibit impulsive aggressions (17). This suggests that conduct disorder and antisocial personality disorder might be on a continuum with normal aggressive traits. The literature on the link between aggression and basal ganglia being less developed than for the ACC/OFC, it is possible that the role of striatum is related to impulsivity in general as opposed to aggression specifically. The absence of correlation between AGG and globus pallidus is not unexpected given that it had rarely been specifically associated with aggression in previous studies.
The trends of negative associations between aggressive behavior and OFC thickness in males support previous reports that implicated this region in the neurobiology of aggression (6, 7). Although results were not statistically significant, this research suggests that the OFC would also plays a role in normal aggressive traits in developing boys. Decreased “top-down” control could be linked to a different maturation of this brain region. Our findings interestingly parallel recent reports of structural studies in boys with conduct problems associated with aggression (42, 43). Both studies have identified grey matter volume of the OFC as a correlate of their clinical samples; however the direction of the association was inconsistent. Our findings support the results from Huebner et al. (2008) suggesting that decreased OFC grey matter is associated with increased aggressive tendencies (42). The results of Huebner at al. (2008), when interpreted in conjunction with the present study’s findings, suggest that normative aggressive behavior and more severe conduct problems may constitute a single underlying dimensional trait that is associated with distinct neurodevelopmental correlates—including difference in grey matter in the OFC in childhood.
In females, the absence of such trends in the OFC and the presence of a positive trend in the left subgenual ACC raise the issue of gender specific neuroanatomical correlates of aggression. Observed trends of differences might represent gender specific anatomical correlates of impulsive aggression, but might also be related to the AGG score not capturing as well relational forms of aggression that are more prevalent in females(37).
This cross-sectional study had several limitations that should be mentioned. First, although it is a clinically and scientifically well-validated tool, AGG is a single measure and remains a gross estimate of impulsive aggression. It is not aimed at measuring all aspects such as instrumental aggression. Further, although statistically significant, the associations were of small magnitude. This is expected given the complexity and heterogeneity of the measured phenomenon. The threshold used to identify trends in the ROI (p=0.005) is not the most stringent, but probably yielded valid findings given the strength of the a priori hypothesis, the small variation in the measured phenomenon (aggression in a very healthy cohort of children) and the intrinsic difficulties of measuring cortical thickness in the OFC because of its know susceptibility to field artifacts. In addition, it is important to point out that regions of associations were found primarily in the a priori defined ROI, increasing the confidence in the validity of the findings. Importantly, the right ACC’s quadratic association was significant after a stringent RFT correction and the positive striatal correlations were significant after a Bonferroni correction. This study has focused on regions thought to be implicated in the regulation of aggressive impulsions. It would have been interesting to study limbic structures such as the amygdala, however the automated measurement of these structures with ANIMAL in children has not yet been validated due to their subjective anatomical boundaries. Finally, less than half of all the initial sample subjects were used for analysis. These were subjects with the highest imaging quality and they remained representative of the American population in terms of demographics. Cortical thickness measurement being sensitive to very small movement artifact (frequent in children), this loss of subjects was compensated by a more precise and reliable measurement.
This being said, it is the largest study of neuroanatomical correlates of aggressive behaviors in typically developing children from a demographically representative sample. It is also one of the rare studies that looked at aggressive behavior at the trait level (39). Our findings were located in biologically plausible brain regions and mapped a fronto-striatal network of structures (striatum, right ACC, OFC) that has been repeatedly associated with regulation of impulses and affect in functional imaging studies (6, 7). Finally, the AGG scale has good correlation with clinical diagnoses of ODD and conduct CD (24). Psychiatric disorders being more and more conceptualized as a continuum from normal to pathological behaviors, these results suggest that the identified structures could play a role in the development of these pathologies.
In summary, several cortical and subcortical neuroanatomical correlates of AGG scores in healthy children were identified. These findings have significant implications for those who wish to study aggression in genetic, clinical and neuroimaging studies. Future interesting areas of research could include longitudinal investigation of regional developmental changes and their relationship to changes in AGG and functional imaging studies of the network formed by the right ACC, OFC and striatum and how they interact to regulate aggressive behaviors. A next step in this type of investigation would be to determine the genetic and epigenetic contributions to findings such as these. Finally, we believe that the right ACC and the striatum should be a focus of attention for future neurobiological studies of pediatric psychiatric diagnoses associated with aggression, mainly CD and ODD.
Supplementary Material
ACKNOWLEDGEMENT
This project has been funded in whole or in part with Federal funds from the National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Institute of Mental Health, and the National Institute of Neurological Disorders and Stroke (Contract #s N01-HD02-3343, N01-MH9-0002, and N01-NS-9-2314, -2315, -2316, -2317, -2319 and -2320).
Dr. Simon Ducharme receives financial support from the Canadian Institutes of Health Research with a Master’s Award: Frederick Banting and Charles Best Canada Graduate Scholarship. Dr. Sherif Karama was supported by the Fonds de Recherche en Santé du Québec.
We also acknowledge the important contribution and remarkable spirit of John Haselgrove, Ph.D. (deceased).
Brain Development Cooperative Group
Key personnel from the six pediatric study centers are as follows: Children’s Hospital Medical Center of Cincinnati, Principal Investigator William S. Ball, M.D., Investigators Anna Weber Byars, Ph.D., Mark Schapiro, M.D., Wendy Bommer, R.N., April Carr, B.S., April German, B.A., Scott Dunn, R.T.; Children’s Hospital Boston, Principal Investigator Michael J. Rivkin, M.D., Investigators Deborah Waber, Ph.D., Robert Mulkern, Ph.D., Sridhar Vajapeyam, Ph.D., Abigail Chiverton, B.A., Peter Davis, B.S., Julie Koo, B.S., Jacki Marmor, M.A., Christine Mrakotsky, Ph.D., M.A., Richard Robertson, M.D., Gloria McAnulty, Ph.D; University of Texas Health Science Center at Houston, Principal Investigators Michael E. Brandt, Ph.D., Jack M. Fletcher, Ph.D., Larry A. Kramer, M.D., Investigators Grace Yang, M.Ed., Cara McCormack, B.S., Kathleen M. Hebert, M.A., Hilda Volero, M.D.; Washington University in St. Louis, Principal Investigators Kelly Botteron, M.D., Robert C. McKinstry, M.D., Ph.D., Investigators William Warren, Tomoyuki Nishino, M.S., C. Robert Almli, Ph.D., Richard Todd, Ph.D., M.D., John Constantino, M.D.; University of California Los Angeles, Principal Investigator James T. McCracken, M.D., Investigators Jennifer Levitt, M.D., Jeffrey Alger, Ph.D., Joseph O’Neil, Ph.D., Arthur Toga, Ph.D., Robert Asarnow, Ph.D., David Fadale, B.A., Laura Heinichen, B.A., Cedric Ireland B.A.; Children’s Hospital of Philadelphia, Principal Investigators Dah-Jyuu Wang, Ph.D. and Edward Moss, Ph.D., Investigators Robert A. Zimmerman, M.D., and Research Staff Brooke Bintliff, B.S., Ruth Bradford, Janice Newman, M.B.A. The Principal Investigator of the data coordinating center at McGill University is Alan C. Evans, Ph.D., Investigators Rozalia Arnaoutelis, B.S., G. Bruce Pike, Ph.D., D. Louis Collins, Ph.D., Gabriel Leonard, Ph.D., Tomas Paus, M.D., Alex Zijdenbos, Ph.D., and Research Staff Samir Das, B.S., Vladimir Fonov, Ph.D., Luke Fu, B.S., Jonathan Harlap, Ilana Leppert, B.E., Denise Milovan, M.A., Dario Vins, B.C.,, and at Georgetown University, Thomas Zeffiro, M.D., Ph.D. and John Van Meter, Ph.D. Ph.D. Investigators at the Neurostatistics Laboratory, Harvard University/McLean Hospital, Nicholas Lange, Sc.D., and Michael P. Froimowitz, M.S., work with data coordinating center staff and all other team members on biostatistical study design and data analyses. The Principal Investigator of the Clinical Coordinating Center at Washington University is Kelly Botteron, M.D., Investigators C. Robert Almli Ph.D., Cheryl Rainey, B.S., Stan Henderson M.S., Tomoyuki Nishino, M.S., William Warren, Jennifer L. Edwards M.SW., Diane Dubois R.N., Karla Smith, Tish Singer and Aaron A. Wilber, M.S. The Principal Investigator of the Diffusion Tensor Processing Center at the National Institutes of Health is Carlo Pierpaoli, MD, Ph.D., Investigators Peter J. Basser, Ph.D., Lin-Ching Chang, Sc.D., Chen Guan Koay, Ph.D. and Lindsay Walker, M.S. The Principal Collaborators at the National Institutes of Health are Lisa Freund, Ph.D. (NICHD), Judith Rumsey, Ph.D. (NIMH), Lauren Baskir, Ph.D. (NIMH), Laurence Stanford, PhD. (NIDA), Karen Sirocco, Ph.D. (NIDA) and from NINDS, Katrina Gwinn-Hardy, M.D., and Giovanna Spinella, M.D. The Principal Investigator of the Spectroscopy Processing Center at the University of California Los Angeles is James T. McCracken, M.D., Investigators Jeffry R. Alger, Ph.D., Jennifer Levitt, M.D., Joseph O'Neill, Ph.D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest related to this article. Special thanks to the NIH contracting officers for their support.
REFERENCES
- 1.Evans A Group BDC. The NIH MRI study of normal brain development. NeuroImage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 2.Kruesi M, Casanova M, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Research: Neuroimaging. 2004;132:1–11. doi: 10.1016/j.pscychresns.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch J, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. PNAS. 2007;104(49):19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hudziak J, van Beijsterveldt C, Bartels M, Rietveld M, Rettew D, Derks E, et al. Individual differences in aggression: genetic analyses by age, gender, and informant in 3-, 7-, and 10-year-old Dutch twins. Behav Genet. 2003;33(5):575–589. doi: 10.1023/a:1025782918793. [DOI] [PubMed] [Google Scholar]
- 5.Ligthart L, Bartels M, Hoekstra R, Hudziak J, Boomsma D. Genetic contributions to subtypes of aggression. Twin Research and Human Genetics. 2005;8(5):483–491. doi: 10.1375/183242705774310169. [DOI] [PubMed] [Google Scholar]
- 6.Siever L. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterzer P, Stadler C. Neuroimaging of aggressive and violent behaviour in children and adolescents. Behav Neurosci. 2009;3:1–8. doi: 10.3389/neuro.08.035.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grafman J, Schwab K, Warden D, Pridgen A, Brown H, Salazar A. Frontal lobe injuries, violence and aggression. Neurology. 1996:46. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- 9.Damasio H, Grabowski T, Frank R, Galaburda A, Damasio A. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264(5162):1102–1105. doi: 10.1126/science.8178168. [DOI] [PubMed] [Google Scholar]
- 10.Brower M, Price B. Neuropsychiatry of frontal lobe dysfunction in violent and criminal behaviour: a critical review. J Neurol Neurosurg Psychiatry. 2001;71:720–726. doi: 10.1136/jnnp.71.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frankle W, Lombardo I, New A, Goodman M, Talbot P, Huang Y, et al. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a position emission study with [11C]McN 5652. Am J Psychiatry. 2005;162(5):915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- 12.Pietrini P, Guazzelli M, Basso G, Jaffe K, Grafman J. Neural correlates of imaginal aggressive behavior assessed by position emission tomography in healthy subjects. Am J Psychiatry. 2000;157(11):1772–1781. doi: 10.1176/appi.ajp.157.11.1772. [DOI] [PubMed] [Google Scholar]
- 13.Kolb B. Studies on the caudate-putamen and the dorsomedial thalamic nucleus of the rat: implication for mammalian frontal-lobe functions. Physiology & Behavior. 1977;18:237–244. doi: 10.1016/0031-9384(77)90128-7. [DOI] [PubMed] [Google Scholar]
- 14.de Almeida R, Benini Q, Betat J, Hipolide D, Miczek K, Svensson A. Heightened aggression after chronic flunitrazepam in male rates: potential links to cortical and caudate-putamen-binding sites. Psychopharmacology. 2008;197:309–318. doi: 10.1007/s00213-007-1031-5. [DOI] [PubMed] [Google Scholar]
- 15.Chow T, Cummins J. Frontal-subcortical circuits. In: Miller B, Cummings J, editors. The human frontal lobe lobes-functions and disorders. New York, NY: Guilford Press; 1999. p. 3. [Google Scholar]
- 16.Krishnan K. Brain imaging correlates. J Clin Psychiatry. 1999;60 suppl 15:50–54. [PubMed] [Google Scholar]
- 17.Glenn A, Raine A, Yaralian P, Yang Y. Increased volume of striatum in psychopathic individuals. Biol Psychiatry. 2010;67:52–58. doi: 10.1016/j.biopsych.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amen D, Stubblefield M, Carmichael B, Thisted R. Brain SPECT findings and aggressiveness. Annals of Clinical Psychiatry. 1996;8(3):129–137. doi: 10.3109/10401239609147750. [DOI] [PubMed] [Google Scholar]
- 19.Nyhan W. Behavior in the Lesch-Nyhan syndrome. Journal of Autism and Childhood Schizophrenia. 1976;6(3):235–252. doi: 10.1007/BF01543464. [DOI] [PubMed] [Google Scholar]
- 20.Stephens R, Sandor P. Aggressive behaviour in children with Tourette syndrome and comorbid attention-deficit hyperactivity disorder and obsessive-compulsive disorder. Can J Psychiatry. 1999;44:1036–1042. doi: 10.1177/070674379904401010. [DOI] [PubMed] [Google Scholar]
- 21.Visser J, Bär P, Jinnah H. Lesch-Nyhan disease and the basal ganglia. Brain Research Reviews. 2000;32:449–475. doi: 10.1016/s0165-0173(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblatt A, Leroi I. Neuropsychiatry of Huntington's disease and other basal ganglia disorders. Psychosomatics. 2000;41:24–30. doi: 10.1016/S0033-3182(00)71170-4. [DOI] [PubMed] [Google Scholar]
- 23.Petty R, Bonner D, Mouratoglou V, Silverman M. Acute frontal lobe syndrome and dyscontrol associated with bilateral caudate nucleus infarctions. British Journal of Psychiatry. 1996;168:237–240. doi: 10.1192/bjp.168.2.237. [DOI] [PubMed] [Google Scholar]
- 24.Hudziak J, Copeland W, Stanger C, Wadsworth M. Screening for DSM-IV externalizing disorders with the Child Behavior Checklist: a receiver-operating characteristic analysis. Journal of Child Psychology and Psychiatry. 2004;45(7):1299–1307. doi: 10.1111/j.1469-7610.2004.00314.x. [DOI] [PubMed] [Google Scholar]
- 25.Achenbach T. Manual for the Child Behavior Checklist/4-18. Burlington: University of Vermont; 1991. [Google Scholar]
- 26.Achenbach T, Ruffle T. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies Pediatrics in Review. 2000;21(1):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- 27.Achenbach T, Rescorla L. Manual for the ASEBA school-age forms & profiles. Burlington: University of Vermont: Research Center for Children, Youth & Families; 2001. [Google Scholar]
- 28.Crijnen A, Achenbach T, Verlhuist F. Problems reported by parents of children in multiple cultures: The Child Behavior Checklist syndrome constructs. Am J Psychiatry. 1999;156:569–574. doi: 10.1176/ajp.156.4.569. [DOI] [PubMed] [Google Scholar]
- 29.Heubeck B. Cross-cultural generalizability of CBCL syndromes across three continents: from the USA and Holland to Australia. Journal of Abnormal Child Psychology. 2000;28(5):439–450. doi: 10.1023/a:1005131605891. [DOI] [PubMed] [Google Scholar]
- 30.Stanger C, Achenbach T, Verhulst F. Accelerated longitudinal comparisons of aggressive versus delinquent syndromes. Developmental Psychopathology. 1997;9:43–58. doi: 10.1017/s0954579497001053. [DOI] [PubMed] [Google Scholar]
- 31.Hopwood C, Burt A, Markowitz J, Yen S, Shea M, Sanislow C, et al. The construct validity of rule-breaking and aggression in an adult clinical sample. Journal of Psychiatric Research. 2009;43(8):803–808. doi: 10.1016/j.jpsychires.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartels M, Hudziak J, van den Ord E, van Beijsterveldt C, Rietveld M, Boomsma D. Co-occurence of aggressive behavior and rule-breaking behavior at age 12: multi-rater analyses. Behav Genet. 2003;33(5):607–621. doi: 10.1023/a:1025787019702. [DOI] [PubMed] [Google Scholar]
- 33.Hudziak J, van Beijsterveldt C, Bartels M, Rietveld M, Rettew D, Derks E, et al. Individual differences in aggression: genetic analyses by age, gender, and informant in 3-, 7-, and 10-year-old Dutch twins. Behavior Genetics. 2003;33(5):575–589. doi: 10.1023/a:1025782918793. [DOI] [PubMed] [Google Scholar]
- 34.Hudziak J, Derks E, Althoff R, Copeland W, Boomsma D. The genetic and environmental contributions to oppositional defiant behavior: A multi-informant twin study. J Am Acad Child Adolesc Psychiatry. 2005;44:907–914. doi: 10.1097/01.chi.0000169011.73912.27. [DOI] [PubMed] [Google Scholar]
- 35.Craig I, Halton K. Genetics of human aggressive behaviour. Hum Genet. 2009;126:101–113. doi: 10.1007/s00439-009-0695-9. [DOI] [PubMed] [Google Scholar]
- 36.Rhee S, Waldman I. Genetics and environmental influences on antisocial behavior: a meta-analysis of twin and adoption studies. Psychol Bull. 2002;128:490–529. [PubMed] [Google Scholar]
- 37.Lagerspetz K, Björkqvist K, Peltonen T. Is indirect aggression typical of females? Gender differences in aggressiveness in 11- to 12-year-old children. Aggressive Behavior. 1988;14(6):403–414. [Google Scholar]
- 38.Moffitt T. The new look of behavioral genetics in developmental psychopathology: Gene-environment interplay in antisocial behaviors. Psychological Bulletin. 2005;131:533–554. doi: 10.1037/0033-2909.131.4.533. [DOI] [PubMed] [Google Scholar]
- 39.Boes A, Tranel D, Anderson S, Nopoulos P. Right anterior cingulate: a neuroanatomical correlate of aggression and defiance in boys. Behavioral Neuroscience. 2008;122(3):677–684. doi: 10.1037/0735-7044.122.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elliott R, Dolan R, Frith C. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10(3):308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- 41.Petrides M, Pandya D. Comparative architechtonics analysis of the human and macaque frontal cortex. In: Boller F, Grafman J, editors. Handbook of neuropsychology. Amsterdam: Elsevier Science BV; 1994. pp. 17–58. [Google Scholar]
- 42.Huebner T, Vloet T, Marx I, Konrad K, Fink G, Herpetz S, et al. Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47(5):540–547. doi: 10.1097/CHI.0b013e3181676545. [DOI] [PubMed] [Google Scholar]
- 43.De Brito SA, Mechelli A, Wilke M, Laurens KR, Jones AP, Barker GJ, et al. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132(Pt 4):843–852. doi: 10.1093/brain/awp011. [DOI] [PubMed] [Google Scholar]
- 44.Waber D, De Moor C, Forbes P, Almli C, Botteron K, Leonard G, et al. The NIH MRI study of normal brain development: Performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. Journal of the International Neuropsychological Society. 2007;13(5):729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- 45.Sadock B, Sadock V, Ruiz P. Kaplan & Sadocks's comprehensive textbook of psychiatry. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]
- 46.Achenbach TM. Manual for the Child behavior Checklist/4-18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 47.Ad-Dab'bagh Y, Lyttelton O, Muehlboeck J, Lepage C, Einarson D, Mok K, editors. The CIVET image-processing environment: A fully automated comprehensive pipeline for anatomical neuroimaging research. Proceedings of the 12th annual meeting of the organization for human brain mapping; Florence, Italy: 2006. [Google Scholar]
- 48.Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. NeuroImage. 2007;34(4):1535–1544. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Singh V, MacDonald D, Lee J, Kim S, Evans A. Automated 3D extraction and evaluation of the outer cortical surface using a Laplacian map and partial volume effect classification. NeuroImage. 2005;27:210–221. doi: 10.1016/j.neuroimage.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 50.MacDonald D, Kabani N, Avis D, Evans A. Automated 3D extraction of inner and outer surfaces of cerebral cortex from MRI. NeuroImage. 2000;13:340–356. doi: 10.1006/nimg.1999.0534. [DOI] [PubMed] [Google Scholar]
- 51.Collins D, Holmes C, Peters T, Evans A. Automatic 3D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- 52.Collins DL, Evans AC. Animal: Validation and Applications of Nonlinear Registration-Based Segmentation. International Journal of Pattern Recognition and Artificial Intelligence. 1997;11(8):1271–1294. [Google Scholar]
- 53.Karama S, Ad-Dab'bagh Y, Haier R, Deary I, Lyttelton O, Lepage C, et al. Positive association between cognitive ability and cortical thickness in a representative US sample of healthy 6 to 18 year-olds. Intelligence. 2009;37(2):145–155. doi: 10.1016/j.intell.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giedd J, Blumenthal J, Jeffries N, Castellanos F, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 55.Worsley K, Taylor J, Tomaiuolo F, Lerch J. Unified univariate and multivariate random field theory. Neuroimage. 2004;23 Suppl 1:189–195. doi: 10.1016/j.neuroimage.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 56.Blumenfeld H. Neuroanatomy through clinical cases. Sunderland, MA: Sinauer Associates, Inc.; 2002. [Google Scholar]
- 57.Mayberg H, Liotti M, Brannan S, McGinnis S, Mahurin R, Jerabek P, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 58.Allman J, Hakeem A, Erwin J, Nimchinsky E, Hof P. The evolution of an interface between emotion and cognition. Annals New York Academy of Sciences. 2001;935:107–117. [PubMed] [Google Scholar]
- 59.Bush G, Luu P, Posner M. Cognitive and emotional influences in anterior cingulate cortex. Trends Cognitive Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 60.Drevets W. Prefrontal cortical-amygdalar metabolism in major depression. Ann NY Acad Sci. 1999;877:614–637. doi: 10.1111/j.1749-6632.1999.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 61.Ongür D, Drevets W, Price J. Glial reduction in the subgenual prefrontal cortex in mood disorders. PNAS. 1998;95(22):13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vogt B, Pandya D. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J Comp Neurol. 1987;262:271–289. doi: 10.1002/cne.902620208. [DOI] [PubMed] [Google Scholar]
- 63.Hazlett E, New A, Newmark R, Haznedar M, Lo J, Speiser L, et al. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biol Psychiatry. 2005;58:614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 64.Whittle S, Chanen A, Fornito A, McGorry P, Pantelis C, Yücel M. Anterior cingulate volume in adolescents with first-presentation borderline personality disorder. Psychiatry Research: Neuroimaging. 2009;172:155–160. doi: 10.1016/j.pscychresns.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Tebartz van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, et al. Frontolimbic brain abnormalities in patients with borderline personality disorder: a volumetric magnetic resonance imaging study. Biol Psychiatry. 2003;54(2):163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- 66.Goodman M, Hazlett E, Avedon J, Siever D, Chu K, New A. Anterior cingulate volume reduction in adolescents with borderline personality disorder and co-morbid depression. Journal of Psychiatric Research. 2010 doi: 10.1016/j.jpsychires.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 67.Robbins TW. Shifting and stopping: fronto-striatal substrates, neurochemical modulation and clinical implications. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):917–932. doi: 10.1098/rstb.2007.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


