Abstract
Resistance to heavy metals is important for the survival of bacteria in contaminated environments. In this study, we show that the unicellular cyanobacterial species Synechococcus sp. IU 625 adapts to growth in the presence of mercuric chloride, recovering from pigmentation and morphological defects. Cells accumulate mercury within two hours of growth and by three days, the total mercury concentration is significantly reduced, with all remaining mercury associated with the cells. This suggests that Synechococcus sp. IU 625 can convert mercury to a volatile form.
Keywords: Cyanobacterium Synechococcus sp. IU 625, Mercury
Introduction
Synechococcus sp. IU 625 (S. IU 625) is a cyanobacterium also called Anacystis nidulans. It is a non-motile, unicellular, rod-shaped microorganism which is similar to Gram-negative bacteria in cell wall structure, cell division and the ability to harbor plasmids (Lau and Doolittle, 1979). Owing to the ubiquitous nature of cyanobacterial species and their adaptability to various ecological environments, they serve as excellent models for the investigation of various biological processes, including membrane transport and physiological changes in photosynthesis due to changes in environmental conditions (Sauer et al., 2001; Zdrou and Tromballa, 1981). They also serve as good indicators of environmental pollution, especially heavy metal contamination. Studies have found that heavy metals such as barium, aluminum, lead, cadmium, and mercury are toxic to S. IU 625 (Lee et al., 1991, 1992a, 1992b; Lee and Lustigman, 1996). However, even essential metals such as Mn2+, Cu2+, Zn2+ and Ni2+ inhibit the growth of S. IU 625 when present at high concentrations (Lee et al., 1993, 1994; Lee and Lustigman, 1996). Furthermore, the toxicity of selenium and mercury combinations have been evaluated (Lee et al., 2002).
Mercury (Hg) has no known biological function (Weiss-Magasic et al., 1997) and is the most lethal heavy metal on the Environmental Protection Agency Target Analyte List (TAL). In humans, mercury poisoning can cause diseases such as Minamata Disease/Hunter Russell syndrome (which results in reduced senses and speech impairment) or Pink Disease (painful limbs) (Davidson et al., 2004). The severity of symptoms depends on the elemental form of mercury, dosage, and duration of exposure. Mercury is highly toxic because of its high affinity for thiols, which play a vital role in protein function (Bruins et al., 2000; Kozak and Forsberg, 1979). It also non-specifically binds to DNA, inducing single-strand breaks (Williams et al., 1987), and affects oxidative phosphorylation and membrane permeability (Brown et al., 1983). Mercury exhibits high toxicity to both light and dark photosynthetic reactions in phototrophic organisms (Lu et al., 2000).
Water, soil, and rocks can contain mercury. Oxidized forms of mercury, such as Hg2+, can be reduced to Hg0, which is volatile at room temperature, or methylated to form methylmercury (Barkay et al., 2003), which is subject to bioaccumulation in the food chain (Davidson et al., 2004). Aquatic microorganisms often methylate mercury, ultimately accumulating in fish at the top of the food chain, providing the primary route for human organomercury exposure (Davidson et al., 2004). Bioaccumulation of mercury in microalgae occurs in two stages: short-term physical absorption at the cell surface and long-term uptake involving intracellular accumulation (Inthorn et al., 2002).
Because of the toxicity and ubiquity of metals in the environment, microorganisms have developed multiple ways of managing both essential and non-essential metals. The levels of these metals must be carefully regulated to ensure sufficient supply while avoiding toxicity – a process often referred to as metal homeostasis. Microorganisms can manage excess metals by simultaneously effluxing metal ions and restricting uptake (Brown et al., 1983; Brocklehurst et al., 1999). Some microorganisms can make metals less toxic by sequestering or enzymatically reducing the metal to a less toxic form. The latter is the case in most mercury resistant microorganisms (Brown et al., 1983). Since bacteria are likely to encounter toxic Hg2+ concentrations in their natural environments, mercury resistance determinants encoded by mer genes can be found among a number of species of bacteria (Nies, 1999; Silver, 1998), including close relatives of S. IU 625, such as S. elongatus. Our previous work showed that mercury inhibits the growth of S. IU 625 (Lee et al., 1992a) and suggested that S. IU 625 removes mercury from culture supernatants (Lee et al., 2002). In this study we expand on our previous findings, reporting that S. IU 625 recovers from mercury-induced pigmentation and morphological defects and describe the temporal dynamics of the reduction of mercury concentration in cell supernatants and the association of mercury with S. IU 625 cells.
Materials and methods
Culture and maintenance of Synechococcus sp. IU 625
S. IU 625 stock cultures were obtained from the American Type Culture Collection, Manassas, VA (ATCC No. 27344) rather than from Dr. Roy McGowan, New York (as in our previous publications). Five ml of cells were inoculated aseptically in 95 ml of sterilized Mauro's Modified Medium (3M medium) in 250 ml Erlenmeyer flasks. The medium was adjusted to a pH of 7.9 using 1M NaOH (Kratz and Myers, 1955). The final concentration of the cells in the culture was ~1.0 × 107 cells/ml. The cultures were grown at a constant temperature of 30°C in a Gyromax™ 747R incubator shaker (Amerex Instruments, Inc., Lafayette, CA, USA) with constant fluorescent light and continuous agitation at 100 rpm.
Preparation of mercuric chloride
A stock solution of 1% (104 mg/L) mercuric chloride (Sigma-Aldrich, Co., St. Louis, MO, USA) was prepared with triple deionized water in a sterile container.
Growth of Synechococcus sp. IU 625 cultures with mercuric chloride
Five ml of exponentially growing S. IU 625 cells were inoculated into 250 Erlenmeyer flasks containing 95 ml of sterilized 3M medium at a concentration of approximately 1.0 × 107 cells/ml. Each flask contained a concentration of 0, 0.1, 0.5, or 1.0 mg/L HgCl2, respectively. Cell growth was monitored in two ways: 1.) by direct cell count using a hemacytometer; and 2.) a turbidity study using a Spectronic Genesys 20 spectrophotometer at OD750nm (Thermo Scientific, Inc., Madison, WI, USA). These experiments were performed in triplicate.
Microscopic observation
Both short-term and long-term studies were carried out. The short-term study contained the time points of 0.5, 1, 2, 4, 6, 8, 12, 24 and 48 hours and the long-term study contained the time points of 1, 4, 8, 11, 15, 19, 22, and 25 days. S. IU 625 cells were grown for 25 days as described above. One ml aliquots of cells were collected in microcentrifuge tubes at each time point. Immediately after collection, cells were centrifuged for 1 minute and supernatants were discarded. Cells were then fixed using a solution of 30 mM sodium phosphate buffer (pH 7.5) and 2.5% formaldehyde. Using differential interference contrast settings, cell morphology was observed with a Zeiss AxioVision microscope (with a Hamamatsu ORCA-ER digital camera) and DNA was detected via DAPI (4′6- diamidino-2-phenylindole) fluorescence. Fixed cells were incubated with 2 μg/ml DAPI for 10 minutes in the dark and aliquoted onto a 1% agarose pad.
Distribution of mercury during the growth of Synechococcus sp. IU 625
To determine the amount of mercury in the cells with respect to the growth medium, samples were collected at 2, 4, and 24 hours for the short-term study and at 1, 3, 8, and 17 days for the long-term study. The 25 ml portion of each sample was digested with potassium permanganate and persulfate at acidic conditions in a water bath at 95°C. The mercury in the sample was reduced to the elemental state and detected by the cold vapor technique in a closed system (USEPA, 1994). The samples were sent to Accredited Analytical Resources, LLC, (Carteret, NJ) for analysis. The analytical procedure associated with the Cold Vapor technique is derived from the EPA Method 245.1.
Results and discussion
Synechococcus sp. IU 625 cultures recover from a pigmentation defect caused by a high concentration of mercuric chloride
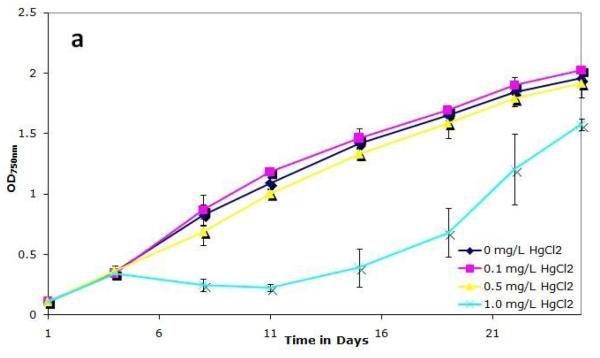
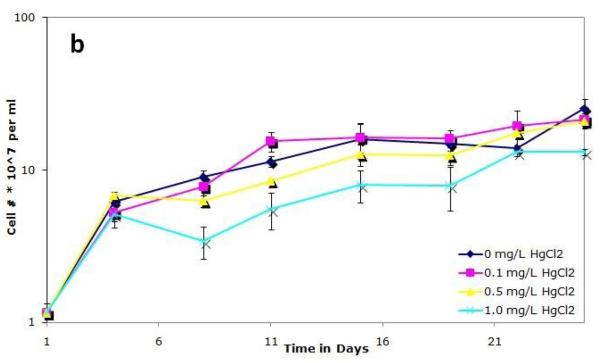
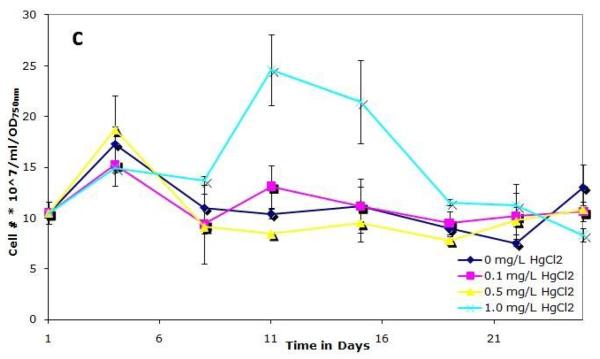
To examine how mercury affects the growth of S. IU 625, cultures were grown continuously in 3M broth with 0, 0.1, 0.5, or 1.0 mg/L of mercuric chloride for a twenty-six days and were monitored for growth and viability. Cultures were noticeably affected by 1 mg/L HgCl2, as evidenced by a change in color: they developed a pale green color in contrast to the normal dark green. A previous study reported that photoautotrophic growth is inhibited by HgCl2 (Pant, 2000) and that different species are susceptible to this effect. Figure 1a shows that the 0.1 and 0.5 mg/L HgCl2 cultures grow with similar kinetics to the control, whereas a decrease in optical density is observed with 1.0 mg/L mercuric chloride. The cell number per ml of the 0.1 and 0.5 mg/L cultures was similar to that of control cultures whereas a noticeable decrease in cell number per ml was apparent in 1.0 mg/L cultures (Fig. 1b). Figure 1c shows cell number per ml per OD, which is typically consistent in a given culture media during exponential growth. However, at a concentration of 1.0 mg/L mercuric chloride on days 11 and 15, a significantly higher cell number per ml is observed than expected for a given optical density (Fig. 1c). This can be attributed to a pigmentation defect. Normal cell numbers per ml per OD and pigmentation return to the 1.0 mg/L mercuric chloride culture after 19 days of incubation, suggesting that the cells are adapting, or developing resistance, to the presence of mercuric chloride.
Figure 1.
Growth of S. IU 625 in 100 ml of 3M media containing different concentrations of mercuric chloride (0-1.0 mg/L). Error bars represent standard deviation. (a) Turbidity study (OD750nm) of triplicate cultures at concentrations of 0, 0.1, 0.5 and 1.0 mg/L of HgCl2; (b) Direct count (cell number per ml) of triplicate cultures at concentrations of 0, 0.1, 0.5 and 1.0 mg/L of HgCl2; (c) Cell number per ml per OD of triplicate cultures at concentrations of 0, 0.1, 0.5 and 1.0 mg/L of HgCl2.
Synechococcus sp. IU 625 cultures recover from morphological changes caused by mercuric chloride
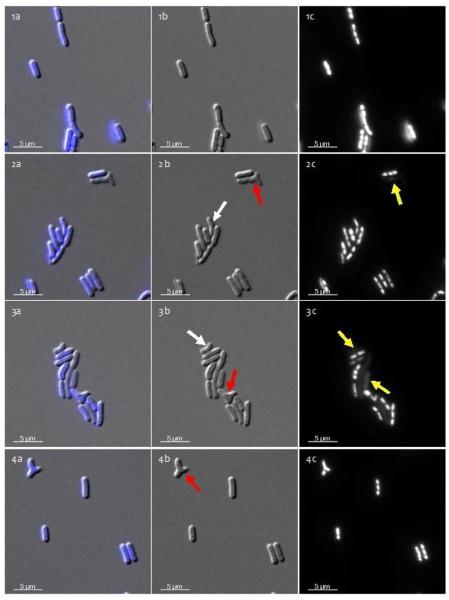
To determine if mercuric chloride affects cell morphology or chromosome segregation, samples were stained with DAPI and observed with a Zeiss AxioVision fluorescence microscope. Samples were observed from all time points. Twelve hour images shown in Figure 2 are representative of the other time points. No significant changes were observed in either cell length or the frequency of cells lacking DNA (i.e., those not staining with DAPI; see yellow arrows in Figure 2) between control and mercury-treated cultures, suggesting that HgCl2 does not inhibit chromosome segregation. However, morphological changes were observed in mercuric chloride-treated cultures (Fig. 2). The percentage of curved cells and cells with ectopic poles increased (data not shown). Cell curvature (white arrows) and ectopic pole (red arrows) formation suggest that mercuric chloride alters the bacterial cytoskeleton (Fig. 2, rows 2-4). In contrast to the morphological problems observed early in mercury-treated cultures, cell morphology returned to normal after 3 days, with morphology similar to that of the control at 12 hours (Fig. 2, row 1). These observations suggest that S. IU 625 is capable of adapting to mercury stress after damage initially occurs within the population.
Figure 2.
Microscopic observation of S. IU 625 with various HgCl2 concentration treatments at 12 hours. Column a: overlaid images; Column b: DIC images; Column c: DAPI-stained images. 1a-1c: 0.0 mg/L HgCl2 (control); 2a-2c: 0.1 mg/L HgCl2; 3a-3c: 0.5 mg/L HgCl2, and 4a-4c: 1.0 mg/L HgCl2. Yellow arrows indicate cells lacking DNA; white arrows indicate curved cells; red arrows indicate ectopic poles.
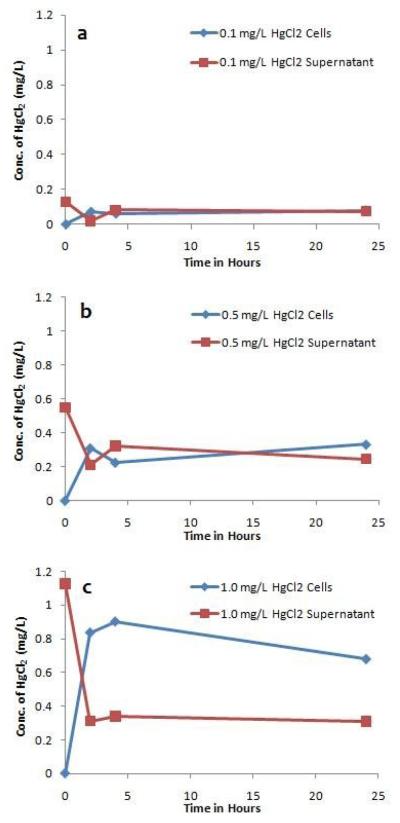
Mercury accumulates in Synechococcus sp. IU 625 within two hours of growth
In order to assess the ability of S. IU 625 to absorb mercury from its immediate environment, mercury concentrations were analyzed in HgCl2-treated culture supernatants and cell pellets (as well as uninoculated 3M broth). Figure 3 shows that significant levels of mercury accumulated in cells after 2 hours, indicating cellular permeability for mercury. At a concentration of 0.1 mg/L HgCl2, similar mercury concentrations (0.075 mg/L) were found in cells and the supernatant between 4 and 24 hours (Figure 3a). At 0.5 mg/L HgCl2, similar levels of mercury (~0.27 mg/L) remained in the supernatant and accumulated in the cells (Fig. 3b). At a concentration of 1.0 mg/L HgCl2, ~0.8 mg/L mercury accumulated in the cells between 2 and 24 hours, with a level of ~0.32 mg/L remaining in the media (Fig. 3c).
Figure 3.
The distribution profile of mercury during short-term (0 to 24 hours) growth of S. IU 625. Content of mercury within the cells and growth media at 0, 2, 4 and 24 hours of exposure to different concentrations of HgCl2. (a) 0.1 mg/L, (b) 0.5 mg/L and (c) 1.0 mg/L.
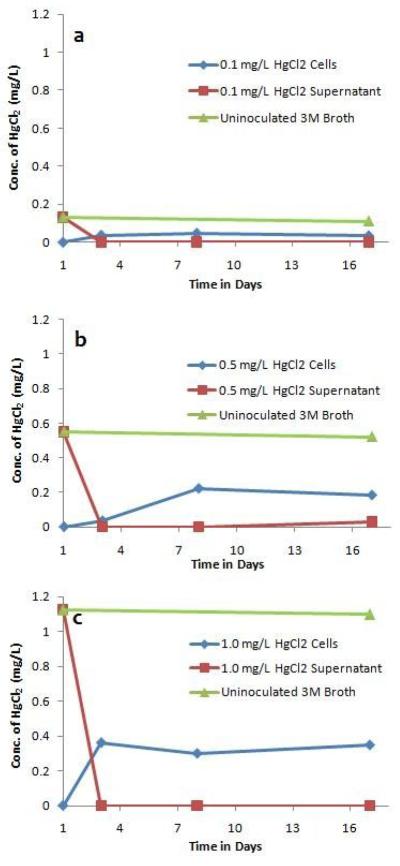
The total mercury concentration is reduced by three days, with all remaining mercury associated with Synechococcus sp. IU 625 cells
By three days, no detectable amounts of mercury remained in culture supernatants and a significant reduction in total mercury concentration was apparent, with all remaining mercury associated with S. IU 625 cells (Fig. 4). However, after 8 days, the concentration detected inside cells (for all three concentrations of mercuric chloride) was reduced to ~30% of the initial concentration of mercury added to the media. This is significant, because mercury levels remained relatively constant for 17 days in uninoculated 3M broth (Fig. 4, green line). The pH of S. IU 625 cultures grown in the absence (Lee et al., 1991) or presence (Lee et al., 2002) of mercuric chloride is within the range of pH 7-10. Mercuric chloride has been shown to be stable in solution up to a pH as high as 10.5 (Spence and Barton, 2003). Therefore, a change in culture pH is not predicted to affect mercuric chloride concentration. In many mercury-resistant bacteria, enzymes encoded by the mer operon can transport mercuric ions from the periplasm into the cytosol where mercuric reductase then reduces them to a volatile, elemental form. Elemental mercury may then dissipate through the membrane and be released into the environment (Barkay, 2003). S. IU 625 may use a similar process.
Figure 4.
The distribution profile of mercury during long-term (1 to 17 days) growth of S. IU 625. Content of mercury within the cells and growth media at 1, 3, 8 and 17 days of exposure to different concentrations of HgCl2. (a) 0.1 mg/L, (b) 0.5 mg/L and (c) 1.0 mg/L.
Concluding remarks
We found that S. IU 625 cultures recovered from pigmentation and morphological defects caused by mercuric chloride. Additionally, after 1.0 mg/L HgCl2 cultures reduced mercuric chloride concentration to ~0.34 mg/L (with all mercury associated with S. IU 625 cells) (Figure 4c), cell number per ml per OD initially increased before subsequently returning to normal (Figure 1c). This implicates HgCl2 with pigmentation and morphological defects and suggests acquired tolerance to 0.34 mg/L HgCl2. Cellular mercury accumulation did not compensate for the loss of extracellular mercury in the medium, suggesting that S. IU 625 detoxifies mercuric ions into their elemental form allowing them to be released as vapor. Further experimentation is required to identify mer gene homologues in S. IU 625 and expression levels must be quantitated at varying HgCl2 concentrations in order to begin to elucidate the mechanism of resistance. Additional insight may be gained by knocking out potential efflux pumps to examine their role in short-term resistance.
Acknowledgements
This work was supported by Seton Hall University Biological Sciences Research Fund and Start-Up Funds to TC, NIH Grant GM084860 to SRM and Montclair State University Faculty Scholarship Program to LHL. We thank Math Cuajungco and Andrea Hernandez for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors' contributions
TC, SRM, and LHL designed and planned the experiments. JT, WP, JRY, and CO performed the experiments and analyzed the results. TC, SRM, JT, JRY and LHL analyzed results and wrote the manuscript.
References
- Barkay T, Miller SM, Summers AO. Bacterial mercury resistance from atoms to ecosystems. FEMS Microbiol Rev. 2003;27:355–84. doi: 10.1016/S0168-6445(03)00046-9. [DOI] [PubMed] [Google Scholar]
- Brocklehurst KR, Hobman JL, Lawley B, Blank L, Marshall SJ, Brown NL, Morby AP. ZntR is a Zn(II)-responsive MerR-like transcriptional regulator of zntA in Escherichia coli. Mol Microbiol. 1999;31:893–902. doi: 10.1046/j.1365-2958.1999.01229.x. [DOI] [PubMed] [Google Scholar]
- Brown NL, Ford SJ, Pridmore RD, Fritzinger DC. Nucleotide sequence of a gene from the Pseudomonas transposon Tn501 encoding mercuric reductase. Biochemistry. 1983;22:4089–95. doi: 10.1021/bi00286a015. [DOI] [PubMed] [Google Scholar]
- Bruins MR, Kapil S, Oehme FW. Microbial resistance to metals in the environment. Ecotoxicol Environ Saf. 2000;45:198–207. doi: 10.1006/eesa.1999.1860. [DOI] [PubMed] [Google Scholar]
- Davidson PW, Myers GJ, Weiss B. Mercury exposure and child development outcomes. Pediatrics. 2004;113:1023–9. [PubMed] [Google Scholar]
- Inthorn D, Sidtitoon N, Silapanuntakul S, Incharoensakdi A. Sorption of mercury, cadmium and lead by microalgae. Science Asia. 2002;28:253–61. [Google Scholar]
- Kozak S, Forsberg CW. Transformation of mercuric chloride and methylmercury by the rumen microflora. Appl Environ Microbiol. 1979;38:626–36. doi: 10.1128/aem.38.4.626-636.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratz WA, Myers J. Photosynthesis and respiration of three blue-green algae. Plant Physiol. 1955;30:275–80. doi: 10.1104/pp.30.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau RH, Doolittle WF. Covalently closed circular DNAs in closely related unicellular cyanobacteria. J Bacteriol. 1979;137:648–52. doi: 10.1128/jb.137.1.648-652.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Lustigman B, Schwinge V, Chiu IY, Hsu S. Effect of mercury and cadmium on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1992a;49:272–8. doi: 10.1007/BF00191766. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B. Effect of barium and nickel on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1996;56:985–92. doi: 10.1007/s001289900142. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Chu IY, Hsu S. Effect of lead and cobalt on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1992b;48:230–6. doi: 10.1007/BF00194376. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Chu IY, Jou HL. Effect of aluminum and pH on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1991;46:720–6. doi: 10.1007/BF01689958. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Dandorf D. Effect of manganese and zinc on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1994;53:158–65. doi: 10.1007/BF00205154. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman B, Maccari J. Effect of copper on the growth of Anacystis nidulans. Bull Environ Contam Toxicol. 1993;50:600–7. doi: 10.1007/BF00191252. [DOI] [PubMed] [Google Scholar]
- Lee LH, Lustigman BK, Murray SR. Combined effect of mercuric chloride and selenium dioxide on the growth of the cyanobacteria, Anacystis nidulans. Bull Environ Contam Toxicol. 2002;69:900–7. doi: 10.1007/s00128-002-0144-0. [DOI] [PubMed] [Google Scholar]
- Lu CM, Chau CW, Zhang JH. Acute toxicity of excess mercury on the photosynthetic performance of cyanobacterium, S. platensis--assessment by chlorophyll fluorescence analysis. Chemosphere. 2000;41:191–6. doi: 10.1016/s0045-6535(99)00411-7. [DOI] [PubMed] [Google Scholar]
- Nies DH. Microbial heavy-metal resistance. Appl Microbiol Biotechnol. 1999;51:730–50. doi: 10.1007/s002530051457. [DOI] [PubMed] [Google Scholar]
- Pant A. Toxicity and uptake of mercurials in a cyanobacterium. The Environmentalist. 2000;20:295–300. [Google Scholar]
- Sauer J, Schreiber U, Schmid R, Volker U, Forchhammer K. Nitrogen starvation-induced chlorosis in Synechococcus PCC 7942. Low-level photosynthesis as a mechanism of long-term survival. Plant Physiol. 2001;126:233–43. doi: 10.1104/pp.126.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S. Genes for all metals--a bacterial view of the periodic table. The 1996 Thom Award Lecture. J Ind Microbiol Biotechnol. 1998;20:1–12. doi: 10.1038/sj.jim.2900483. [DOI] [PubMed] [Google Scholar]
- Spence R, Barton J. Stabilization of mercury in high pH tank sludges; WM'03 Conference; Tucson, AZ. 2003. [Google Scholar]
- USEPA (EPA 600-R-94-111).Methods for the determination of metals in environmental samples, Supplement I, Mercury in water by CVAA. 1994:245.1. [Google Scholar]
- Weiss-Magasic C, Lustigman B, Lee LH. Effect of mercury on the growth of Chlamydomonas reinhardtii. Bull Environ Contam Toxicol. 1997;59:828–33. doi: 10.1007/s001289900556. [DOI] [PubMed] [Google Scholar]
- Williams MV, Winters T, Waddell KS. In vivo effects of mercury (II) on deoxyuridine triphosphate nucleotidohydrolase, DNA polymerase (alpha, beta), and uracil-DNA glycosylase activities in cultured human cells: relationship to DNA damage, DNA repair, and cytotoxicity. Mol Pharmacol. 1987;31:200–7. [PMC free article] [PubMed] [Google Scholar]
- Zdrou I, Tromballa HW. Active transport of chloride by Anacystis nidulans. Arch Microbiol. 1981;129:325–30. [Google Scholar]