Abstract
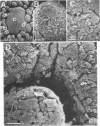
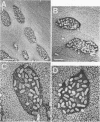
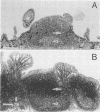
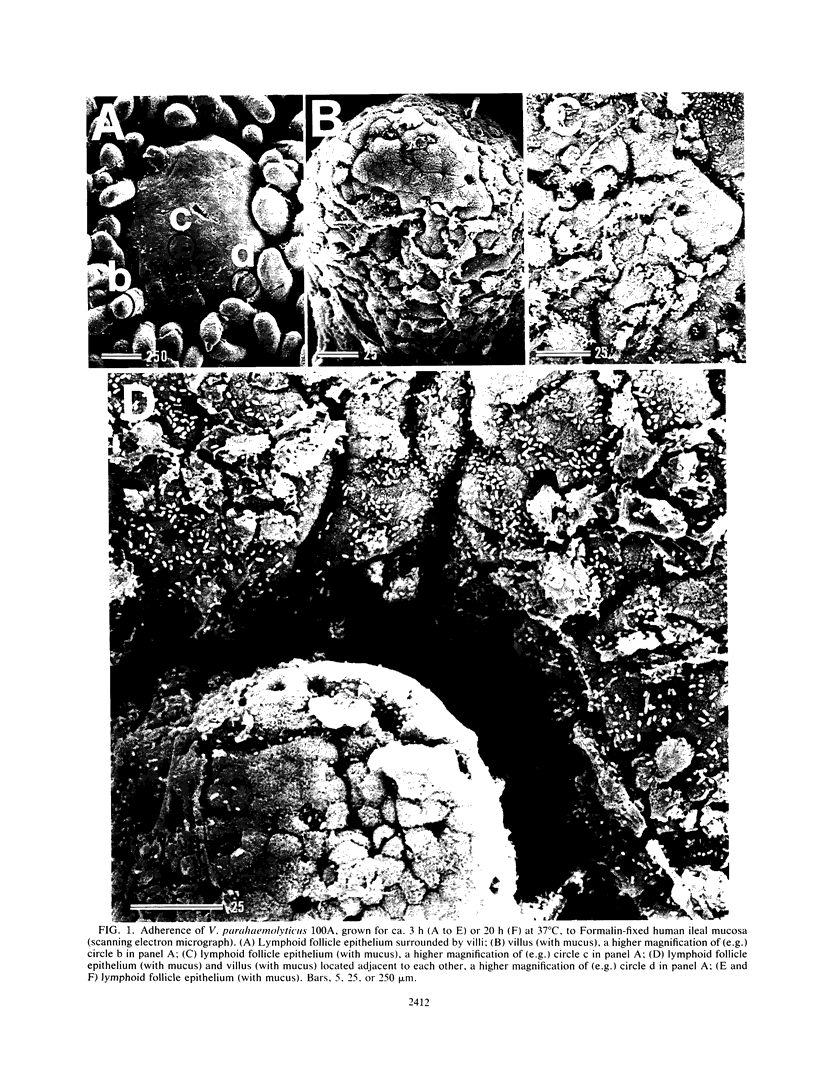
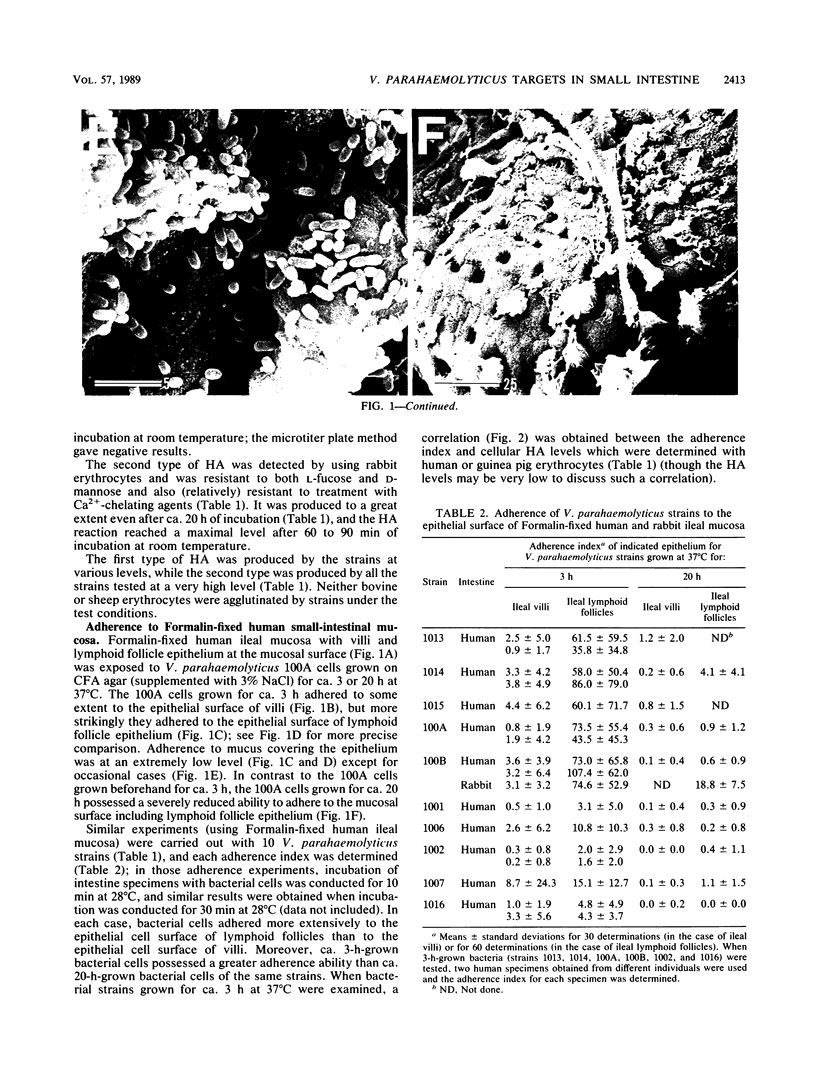
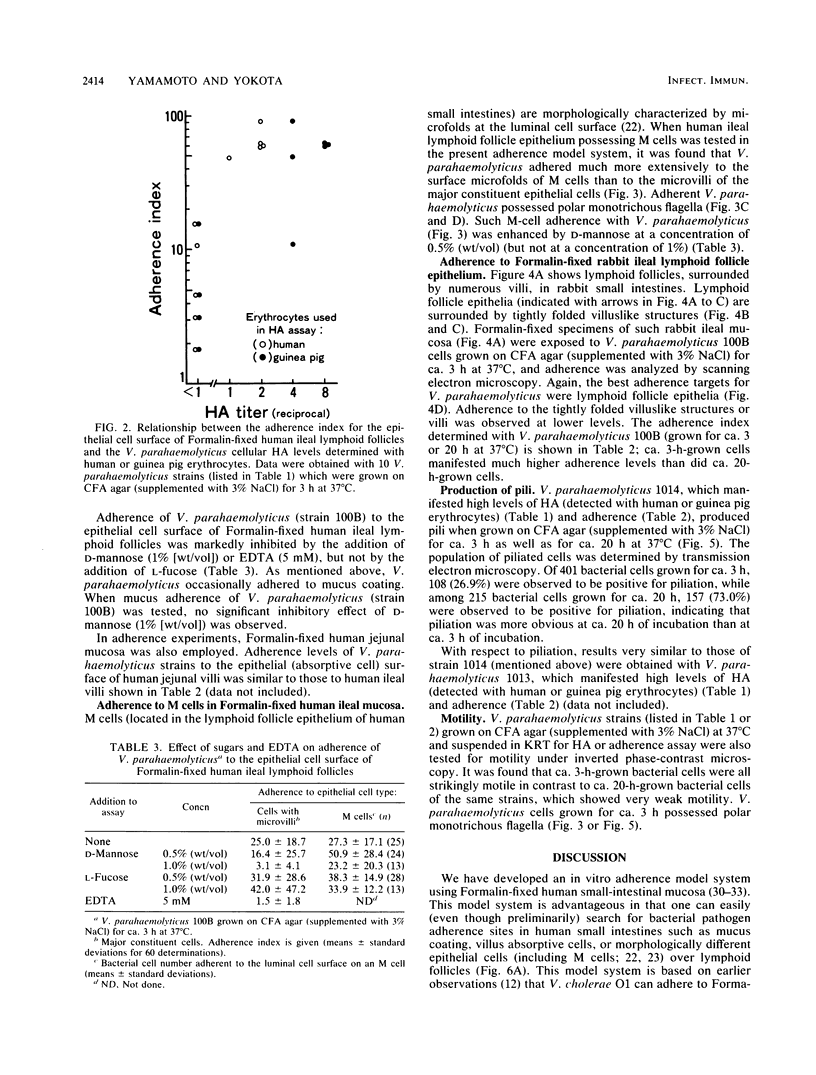
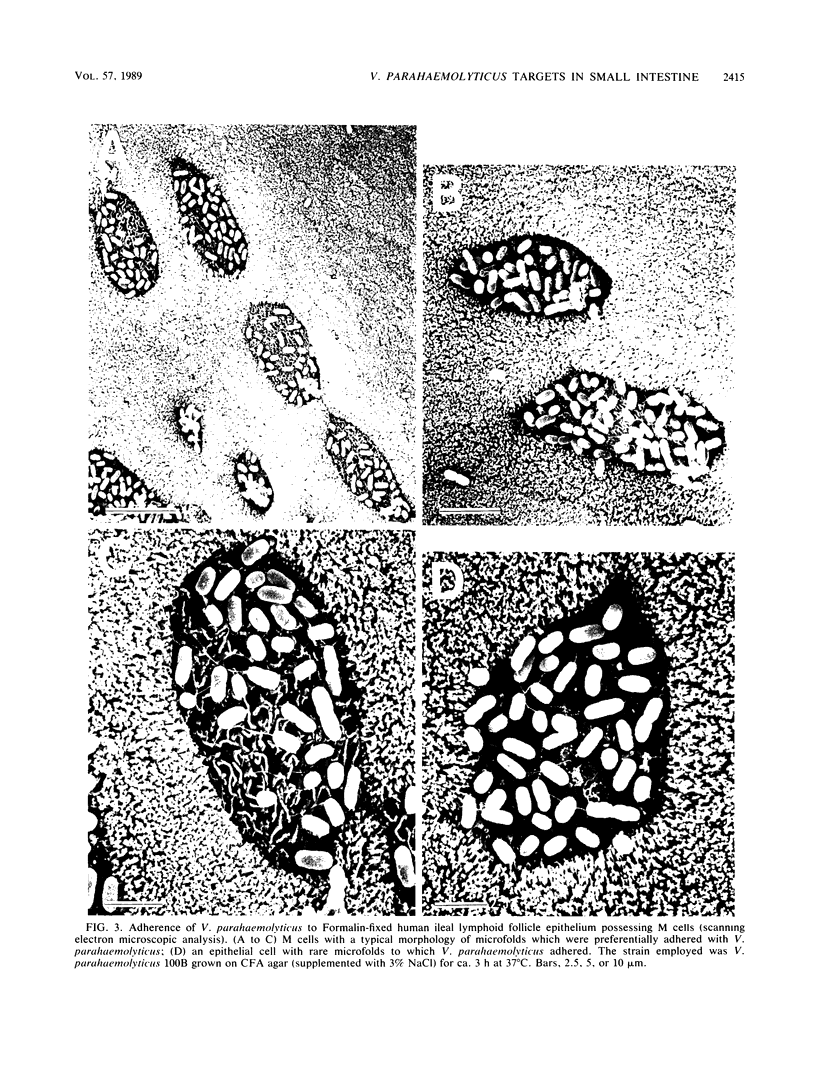
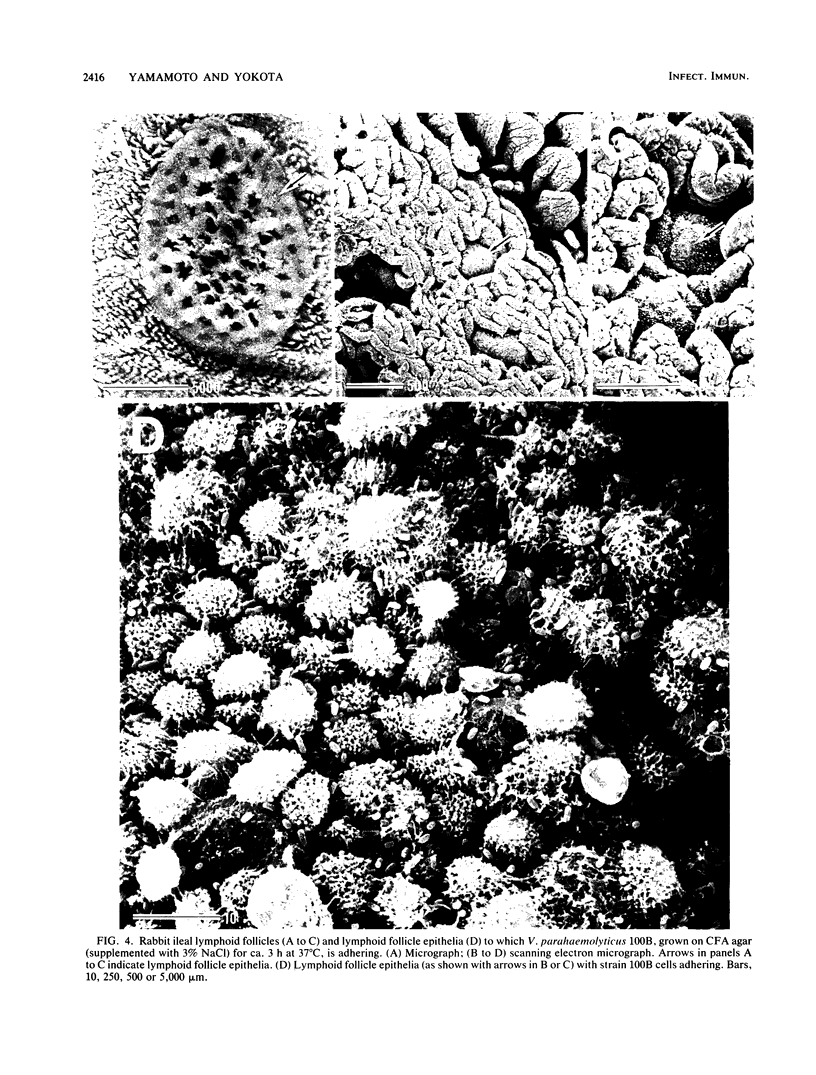
Formalin-fixed human small intestinal mucosa with mucus coating, villi, and lymphoid follicle epithelium at the mucosal surface was used to test the adherence sites of clinically isolated (Kanagawa phenomenon-positive) strains of Vibrio parahaemolyticus. V. parahaemolyticus strains grown on CFA agar (supplemented with 3% NaCl) for ca. 3 h at 37 degrees C possessed various levels of cell-associated hemagglutinins (HAs) which were detected with human or guinea pig erythrocytes. The observed adherence abilities of V. parahaemolyticus strains to human small intestinal mucosa correlated roughly with the HA levels of the strains. Under the test conditions, ileal lymphoid follicle epithelium (especially M cells) provided the best adherence target for V. parahaemolyticus. Adherence to villus absorptive cells or to mucus coating was observed at lower levels. In addition, all 3-h-grown V. parahaemolyticus strains tested produced high levels of HAs as detected with rabbit erythrocytes. The strains were all strikingly motile. In contrast, V. parahaemolyticus strains grown on CFA agar (supplemented with 3% NaCl) for ca. 20 h at 37 degrees C had much lower levels of HAs, adherence abilities, and motility. In contrast to the above observations, piliation of V. parahaemolyticus was more extensive at ca. 20 h of incubation at 37 degrees C than at ca. 3 h of incubation at 37 degrees C. The remarkable ability of V. parahaemolyticus to adhere to lymphoid follicle epithelium was also confirmed by using rabbit small intestinal mucosa.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Blake P. A., Weaver R. E., Hollis D. G. Diseases of humans (other than cholera) caused by vibrios. Annu Rev Microbiol. 1980;34:341–367. doi: 10.1146/annurev.mi.34.100180.002013. [DOI] [PubMed] [Google Scholar]
- Bolen J. L., Zamiska S. A., Greenough W. B., 3rd Clinical features in enteritis due to Vibrio parahemolyticus. Am J Med. 1974 Oct;57(4):638–641. doi: 10.1016/0002-9343(74)90017-5. [DOI] [PubMed] [Google Scholar]
- Boutin B. K., Townsend S. F., Scarpino P. V., Twedt R. M. Demonstration of invasiveness of Vibrio parahaemolyticus in adult rabbits by immunofluorescence. Appl Environ Microbiol. 1979 Mar;37(3):647–653. doi: 10.1128/aem.37.3.647-653.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calia F. M., Johnson D. E. Bacteremia in suckling rabbits after oral challenge with Vibrio parahaemolyticus. Infect Immun. 1975 Jun;11(6):1222–1225. doi: 10.1128/iai.11.6.1222-1225.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers M. M. In vitro adherence of Kanagawa-positive Vibrio parahaemolyticus to epithelial cells. J Infect Dis. 1977 Oct;136(4):588–592. doi: 10.1093/infdis/136.4.588. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Tjoa W. Hemagglutination of human group A erythrocytes by enterotoxigenic Escherichia coli isolated from adults with diarrhea: correlation with colonization factor. Infect Immun. 1977 Nov;18(2):330–337. doi: 10.1128/iai.18.2.330-337.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras S. P., Howard L. V. Adherence of Vibrio parahaemolyticus to human epithelial cell lines. Appl Environ Microbiol. 1980 Feb;39(2):369–371. doi: 10.1128/aem.39.2.369-371.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Boyce J. M., Aleem A. R., Wells J. G., Rahman A. S., Curlin G. T. Vibrio parahaemolyticus enterocolitis in Bangladesh: report of an outbreak. Am J Trop Med Hyg. 1978 Jan;27(1 Pt 1):106–112. doi: 10.4269/ajtmh.1978.27.106. [DOI] [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. W., Colwell R. R., Kaper J. B. Vibrio parahaemolyticus and related halophilic Vibrios. Crit Rev Microbiol. 1982;10(1):77–124. doi: 10.3109/10408418209113506. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Colwell R. R. Adsorption of Vibrio parahaemolyticus onto chitin and copepods. Appl Microbiol. 1975 Feb;29(2):269–274. doi: 10.1128/am.29.2.269-274.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T., Colwell R. R. Incidence of Vibrio parahaemolyticus in Chesapeake Bay. Appl Microbiol. 1975 Aug;30(2):251–257. doi: 10.1128/am.30.2.251-257.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett P. N., Daniel R. R. Attachment of a Vibrio parahaemolyticus strain to rabbit brush border membranes. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):245–250. doi: 10.1016/s0176-6724(88)80009-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y., Kato T., Obara Y., Akiyama S., Takizawa K., Yamai S. In vitro hemolytic characteristic of Vibrio parahaemolyticus: its close correlation with human pathogenicity. J Bacteriol. 1969 Nov;100(2):1147–1149. doi: 10.1128/jb.100.2.1147-1149.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasone N., Iwanaga M. Quantitative evaluation of colonizing ability of Vibrio cholerae O1. Microbiol Immunol. 1987;31(8):753–761. doi: 10.1111/j.1348-0421.1987.tb03137.x. [DOI] [PubMed] [Google Scholar]
- Nishibuchi M., Ishibashi M., Takeda Y., Kaper J. B. Detection of the thermostable direct hemolysin gene and related DNA sequences in Vibrio parahaemolyticus and other vibrio species by the DNA colony hybridization test. Infect Immun. 1985 Sep;49(3):481–486. doi: 10.1128/iai.49.3.481-486.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishibuchi M., Kaper J. B. Nucleotide sequence of the thermostable direct hemolysin gene of Vibrio parahaemolyticus. J Bacteriol. 1985 May;162(2):558–564. doi: 10.1128/jb.162.2.558-564.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. L., Jones A. L. Epithelial cell specialization within human Peyer's patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology. 1974 Feb;66(2):189–203. [PubMed] [Google Scholar]
- Owen R. L., Pierce N. F., Apple R. T., Cray W. C., Jr M cell transport of Vibrio cholerae from the intestinal lumen into Peyer's patches: a mechanism for antigen sampling and for microbial transepithelial migration. J Infect Dis. 1986 Jun;153(6):1108–1118. doi: 10.1093/infdis/153.6.1108. [DOI] [PubMed] [Google Scholar]
- Sakurai J., Matsuzaki A., Miwatani T. Purification and characterization of thermostable direct hemolysin of Vibrio parahaemolyticus. Infect Immun. 1973 Nov;8(5):775–780. doi: 10.1128/iai.8.5.775-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y. Thermostable direct hemolysin of Vibrio parahaemolyticus. Pharmacol Ther. 1982;19(1):123–146. doi: 10.1016/0163-7258(82)90044-4. [DOI] [PubMed] [Google Scholar]
- Taniguchi H., Ohta H., Ogawa M., Mizuguchi Y. Cloning and expression in Escherichia coli of Vibrio parahaemolyticus thermostable direct hemolysin and thermolabile hemolysin genes. J Bacteriol. 1985 May;162(2):510–515. doi: 10.1128/jb.162.2.510-515.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Kamano T., Uchimura M., Iwanaga M., Yokota T. Vibrio cholerae O1 adherence to villi and lymphoid follicle epithelium: in vitro model using formalin-treated human small intestine and correlation between adherence and cell-associated hemagglutinin levels. Infect Immun. 1988 Dec;56(12):3241–3250. doi: 10.1128/iai.56.12.3241-3250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Electron microscopic study of Vibrio cholerae O1 adherence to the mucus coat and villus surface in the human small intestine. Infect Immun. 1988 Oct;56(10):2753–2759. doi: 10.1128/iai.56.10.2753-2759.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Vibrio cholerae non-O1: production of cell-associated hemagglutinins and in vitro adherence to mucus coat and epithelial surfaces of the villi and lymphoid follicles of human small intestines treated with formalin. J Clin Microbiol. 1988 Oct;26(10):2018–2024. doi: 10.1128/jcm.26.10.2018-2024.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]