Abstract
Dysfunctional homeostasis of transition metals is believed to play a role in the pathogenesis of Alzheimer’s disease (AD). Although questioned by some, brain copper, zinc, and particularly iron overload are widely accepted features of AD which have led to the hypothesis that oxidative stress generated from aberrant homeostasis of these transition metals might be a pathogenic mechanism behind AD. This meta-analysis compiled and critically assessed available quantitative data on brain iron, zinc and copper levels in AD patients compared to aged controls. The results were very heterogeneous. A series of heavily cited articles from one laboratory reported a large increase in iron in AD neocortex compared to age-matched controls (p<0.0001) while seven laboratories failed to reproduce these findings reporting no significant difference between the groups (p=0.76). A more than three-fold citation bias was found to favor outlier studies reporting increases in iron and this bias was particularly prominent among narrative review articles. Additionally, while zinc was not significantly changed in the neocortex (p=0.29), copper was significantly depleted in AD (p=0.0003). In light of these findings, it will be important to re-evaluate the hypothesis that transition metal overload accounts for oxidative injury noted in AD.
Keywords: transition metals, meta-analysis, citation bias, publication bias, chelation
1. INTRODUCTION
The distribution and homeostasis of transition metals in Alzheimer’s disease (AD) brain and their potential role in the etiology of neurodegeneration has been debated for six decades or more. Goodman presented one of the earliest arguments for a role of iron in AD in 1953 with a detailed pathological/histological description of a series of post-mortem AD cases. He reported increased Prussian/Turnbull’s blue reactivity indicating abnormally high levels of tissue iron in a few of these patients. From these findings, he hypothesized that a defect in iron management may underlie late-onset cognitive loss in these cases. Hallgren and Sourander in 1958 and 1960 published the first quantitative analyses of brain iron in Alzheimer’s disease patients using colorimetric techniques. This failed to demonstrate a significant increase in tissue levels of non-heme iron in AD brain. However, like Goodman, they noted that many of the specimens showed increased reactivity to histological iron stains. In the intervening decades, brain iron overload gradually became widely accepted as a feature of AD (Benzi and Morreti 1995, Cuajungco et al 2000, Gerlach et al 1994, Schipper et al 1999, Smith et al 1997). Additionally, the observation that iron, zinc and copper were concentrated in beta-amyloid plaques led to the hypothesis that oxidative stress generated from aberrant homeostasis of these transition metals and pathologic metal-protein interactions might be mechanisms behind the aggregation and toxicity of senile plaques, ultimately leading to (or contributing to) the neurodegeneration associated with AD (Lovell et al 1998, Smith et al 1997, Markesbery 1999). In vitro studies demonstrated that iron, zinc and copper at near-physiologic conditions each bind beta-amyloid and are capable of precipitating it into aggregates (Bush et al 1994). The discovery that chelating these metals from plaques reduced plaque toxicity and increased amyloid solubility in vitro further supported the metals hypothesis and became a basis for the therapeutic use of chelators in neurodegenerative conditions (Cherny et al 1999, Rottkamp et al 2001, Schubert and Chevion 1995). Interest in manipulating brain levels of transition metals has risen, resulting in the development of many pharmacologic chelators and a number of clinical trials (Crapper McLachlan et al 1994, Lannfeld et al 2008, Liu et al 2010, Squitti et al 2002).
In the year 2000, one of the most-cited review articles on the subject of metals in AD declared that —a consensus has emerged in the literature that copper, zinc and iron are elevated in the AD-affected neocortex (Bush 2000). The purpose of this study is to evaluate if that consensus is accurate. The issue remains that most available data on brain transition metal concentrations is qualitative, and quantitative studies have generally lacked adequate power to determine whether changes are significant. Additionally, several of the most prominently cited papers in the field have studied tissue that has been fixed, which has been shown to compromise the integrity of data on transition metals (Jellinger et al 1990, Lovell et al 1998, Schrag et al 2010a). This has made it increasingly important to compile and critically assess available quantitative data on brain iron, zinc and copper levels in AD patients compared to aged controls. Meta-analysis and systematic review are routine components of clinical research, but are less often applied in the basic sciences. These tools, when applied to a thorough bibliography, help to systematically compare and evaluate literature, identify flawed studies and draw conclusions with maximal power.
2. METHODS
2.1 Literature search
Literature search was conducted by the first author. Appropriate articles were assembled by systematic queries of NCBI (PubMed), ISI Web of Science, OVID and GoogleScholar databases on the 8th of January 2010. Additionally, we reviewed the citation lists from each article retrieved for the meta-analysis and from relevant review articles. The indexes of certain journals were manually reviewed, including Journal of Radioanalytical and Nuclear Chemistry, Trace Elements in Medicine, Trace Elements and Electrolytes, and Microelement. Articles published in any year up to the date of search and in any language were included, as long as they were indexed in the databases described. Quantitative analytical techniques were included in the analysis; semi-quantitative approaches were excluded. Acceptable quantitative techniques included atomic absorption spectroscopy (INAA), inductively coupled plasma mass spectrometry (ICP-MS) or atomic emission spectroscopy (AA), particle-induced x-ray emission (PIXE), and neutron activation analysis (INAA). These methods have been shown to consistently produce equivalent results (Jervis et al 1985, Stedman and Spyrou 1997, Zhang et al 1997). Search terms therefore included a technique keyword (such as —atomic absorption or —neutron activation analysis ) and —Alzheimer’s disease. General search of high-yield keyword combinations, such as —iron, —Alzheimer’s and —human brain were also conducted. Abstracts were reviewed to collect only reports which compared human Alzheimer’s disease brain to aged control brain for total iron, zinc and/or copper levels in any brain region. Finally, when possible we contacted an author from each study to request access to any unpublished data and to clarify methodological details. We were made aware of two unpublished datasets; these could not be recovered for inclusion in this meta-analysis, but were described as finding no significant differences between groups.
2.2 Exclusion criteria
Exclusion criteria were non-quantitative analysis (including normalizing element concentration to protein concentration), tissue fixation (for any duration of time), the absence of neuropathological diagnosis and inappropriate control tissue (all cases were required to be over age 55). Iron has been shown to increase in the brain with age in neurologically normal subjects; however, it reaches a relatively steady state by about age 55, which is why this was chosen as a cut-off (Hallgren and Sourander 1958, Markesbery 1984). Neuropathologic diagnosis was considered necessary because the clinical diagnosis of AD is only about 61–84% specific for AD (Brunnstrom and Englund 2009, Gay et al 2008). Tissue fixation has been shown to alter, sometimes dramatically, the concentrations of brain metals, either through leaching (iron is reduced on average by 40% in formalin fixed brain, zinc by as much as 75%), or by concentrating elements through tissue dehydration or by deposition of metal contaminants which may be present in formalin (particularly for copper) (Schrag et al 2010a). Finally, normalization of metal levels to protein concentration was considered unreliable because one study found that much lower concentrations of protein were isolated from AD tissue compared to normal brain (Loeffler et al 1995).
2.3 Publication and citation pattern analysis
Publication bias was assessed by funnel plot; a trim-and-fill analysis utilizing the Lo estimator was applied (Taylor and Tweedie 2000). Additionally, to assess whether positive studies were typically published in higher impact journal, the 5-year mean impact factor was collected as recorded in Journal Citation Reports (ISI) for the journals publishing articles in the meta-analysis. Citation bias was evaluated by determining the number of citations each article had received according to ISI Web of Science. Data was evaluated both as total number of citations and citations/year. Partial years were accounted in the annual citation rate to the nearest month. Citation bias in narrative review articles describing iron levels in Alzheimer’s disease was also evaluated; review articles were collected by broadly screening PubMed with keywords —iron and —Alzheimer’s and manually filtering for articles which specifically make a claim regarding the level of tissue iron in AD. This search yielded 115 review articles published since 1990. Finally, citation maps were created to visually analyze the citation patterns of these narrative review articles. The specific objective of this analysis was to determine how the primary literature was used to build the argument that tissue iron is increased in Alzheimer’s disease. Therefore, the maps indicate not simply which articles are cited, but rather which articles are cited in the context of a specific claim regarding the concentration of iron in AD brain.
2.4 Data analysis
The studies which were included reported metals concentrations as either micromolar concentration or micrograms of metal per milligram of tissue. Tissue samples were either dessicated or native (referred to as dry weight or wet weight) -- wet weight was chosen as the standard measure for this study because it is the physiologically relevant mass. Because dry weight to wet weight conversion ratios have been extensively published for essentially all brain regions in both Alzheimer’s disease and control brain, all dry weight measures were converted to wet weight measures. This conversion affected only two studies and the conversion ratios are included in supp. Table 4 (Andrasi et al 2000, Deibel et al 1996). Data from individual studies were collected as means, standard deviations, and numbers of brains in each group. Effect size was calculated by Hedge’s g (with a small N bias correction) in a random effects model. Results were presented by brain region as weighted mean concentrations, and effect sizes. Studies were weighted in the analysis by inverse variance. Heterogeneity was assessed by Q-test with alpha = 0.05. The metal levels reported for the neocortex were nearly equivalent region-to-region, which enabled analysis of these regions jointly as well as individually. The pooled neocortical dataset included frontal lobe, temporal lobe, parietal lobe and hippocampal measurements. Normalcy of distribution was assessed with Lilliefors test for the pooled neocortical data (Lilliefors 1967). Figures were constructed using an Excel-based software add-on, MIX 1.7 (Bax et al 2007).
3. RESULTS
Thirty-two studies were identified in the primary screen; twenty studies remained after the application of objective exclusion criteria. All studies evaluated for inclusion in the meta-analysis were reported in the annotated references with explanations of the rationale for inclusion or exclusion. In general, clinical data and demographic information were limited to age, sex and neuropathological diagnosis at death; comorbid diseases were generally not described. For this reason, evaluation of potential confounders was limited. Additionally, only one of the studies reported the use of blinding in any part of the study (Ward and Mason 1987). The neuropathologic diagnoses in all cases were limited to parenchymal Alzheimer’s disease pathology -- vascular amyloid deposition and Lewy body pathologies were not described.
The main statistical measure we chose to describe the effect of AD on brain iron was Hedge’s G, hereafter called simply —effect size. This statistical tool describes the difference between two groups (here the metal concentration in control brain vs. AD brain) as a proportion of the pooled standard deviation of the two groups. An effect size of 1 indicates the experimental group is one standard deviation higher than the control group. Because of the methodological differences and heterogeneity between the studies, a random effects model was chosen for the analysis. For datasets which were not significantly heterogenous, the random effects model yielded comparable results to the fixed effects model and analysis in a fixed effects model would not alter the conclusions of the study. Detailed region by region data for each metal has been assembled and is present in supplementary tables 1,2 and 3.
3.1 Iron in Alzheimer’s disease neocortex
When neocortical brain regions were analyzed together, the effect of Alzheimer’s disease on brain iron was small, and did not reach significance (Fig 1; effect size = 0.23, 95%CI −0.07-0.53, p=0.13). However, this analysis was complicated by significant heterogeneity, Q=26.0 (p=0.017). Heterogeneity in the iron dataset appeared to derive primarily from data published by the University of Kentucky (U of K) (Fig 2). The results from studies published from the U of K (4 studies) were strikingly different from all other studies (11 studies from 7 independent laboratories) – the combined effect size reported by U of K studies alone was 0.67 (95%CI 0.35–1.00, p<0.0001) while the combined effect size reported by all other studies was −0.05 (95%CI −0.34-0.25, p=0.76) (Fig. 2). With the exclusion of U of K studies, heterogeneity was reduced to non-significant levels as assessed by the Q test. We found no obvious quality measures between the studies which would account for this heterogeneity. While brain iron concentration increases with aging and differences could therefore be attributed differences in age between control and AD groups, this was well-controlled in study selection and control patients were over 55 years of age in all the studies (Hallgren and Sourander 1958). The possibility that different analytical techniques could contribute to heterogeneous findings was also considered; however, the techniques employed in these studies have been shown to produce compatible results and five additional studies which did not originate at U of K also utilized INAA-based measurements (the technique employed in the U of K studies) without detecting significant changes (Andrasi et al 2000, Panayi et al 2002, Plantin et al 1987, Squitti et al 2006, Ward and Mason 1987). This suggests the artifact was not dependent on the analytical technique employed (Fig 2). Moreover, no differences in the methodology of tissue preparation between these studies would be expected to produce the discrepant results observed here.
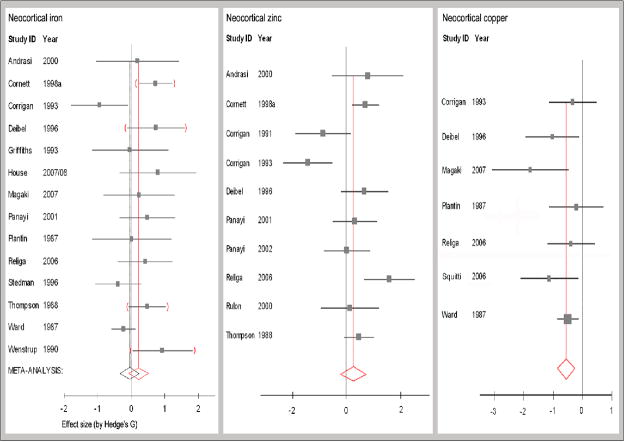
Figure 1. Meta-data of studies reporting the effect of Alzheimer’s disease on neocortical iron, zinc and copper concentrations.
Data from hippocampus, frontal, temporal and parietal lobes were combined to assess neocortical metal levels. Studies from the University of Kentucky (U of K) are indicated by red parentheses for iron data. The mean effect size indicated by red vertical lines includes data reported by U of K (p=0.13). The black vertical line indicates the meta-effect size for iron when this data source is excluded. There is no significant increase in neocortical iron in Alzheimer’s brain: effect size = −0.05, 95%CI −0.34-0.25; n= 206 control, 251 AD (p=0.76). There is a trend toward an increase in neocortical zinc, although the dataset is significantly heterogenous: effect size = 0.26, 95% CI −0.22-0.75; n= 166 control, 118 AD (p=0.29). Copper levels are significantly depleted in Alzheimer’s disease neocortex: effect size = −0.59, 95% CI −0.87- −0.31; n= 123 controls, 115 AD (p<0.0003). This correlates to a reduction of about 0.4 μg Cu/g tissue, or a 13.8% reduction.
Figure 2. Laboratory of origin and not analytical technique appears to be the source of heterogeneity in measurements of neocortical iron in Alzheimer’s disease.
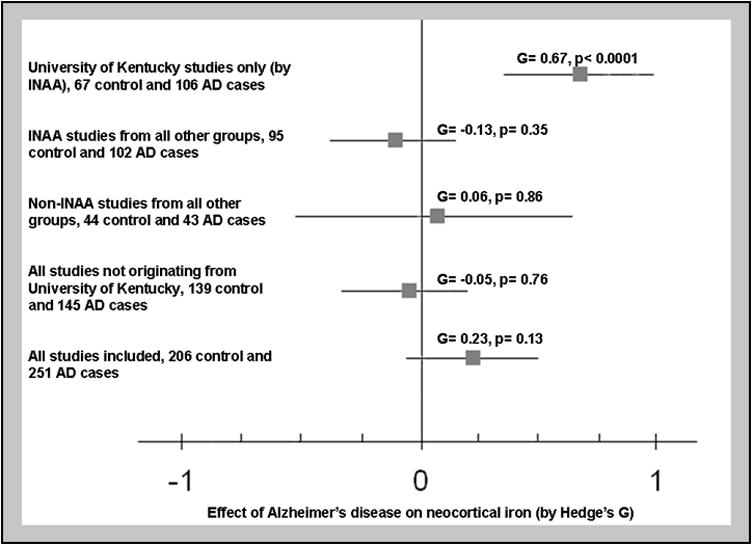
Subgroups analysis found that the University of Kentucky results indicate a significant positive effect size. Data from other groups using INAA for analysis found no significant change and groups using non-INAA techniques also found no significant change. The results reported by the University of Kentucky are different from the results obtained from groups whether they used INAA for analysis (p<0.0001) or non-INAA techniques (p<0.0001).
Region by region analysis (after the exclusion of the outlier data source) revealed significantly increased brain iron in Alzheimer’s disease only in the putamen (supp. Table 1). Putamen iron levels were increased by 21.4% (effect size 1.14, 95% CI 0.50–1.78). While only one study apart from those from the U of K measured iron levels in the amygdala (and it had a very small number of samples), the increase in iron concentration reported by the U of K was higher than that reported by the independent study. The effect size for each neocortical region individually was non-significant (effect size hippocampus −0.32, 95%CI −0.76-0.11; frontal lobe −0.02, 95%CI −0.41 -0.37; temporal lobe 0.56, 95%CI −0.22-1.34; parietal lobe 0.35 95%CI −0.26-0.96).
3.2 Zinc and copper in Alzheimer’s disease neocortex
For neocortical regions analyzed in aggrefate, no significant change in zinc concentration was found, although significant heterogeneity was found independent of the laboratory of origin (Fig 1). There was a significant change in zinc concentration in the parietal lobe, but the other lobes did not appear to be affected (effect size parietal lobe 0.50, 95%CI 0.06–0.94; hippocampus −0.08, 95%CI −1.00-0.85; frontal lobe 0.35, 95%CI −0.24-0.94; temporal lobe 0.47, 95%CI −0.13-1.07). Several individual studies indicated an increase in zinc concentration in each neocortical lobe (supp Table 2), however, with significant heterogeneity between studies. There was insufficient data to adequately analyze the effect size of Alzheimer’s disease on zinc for deep grey matter regions, although results seemed to parallel the changes in iron (strongest increases were reported for the putamen, globus pallidus and caudate nucleus).
Copper levels were depleted in the AD group in most regions (supp. Table 3) and cumulatively neocortical copper was significantly reduced in AD (Fig.1; effect size = −0.55, 95%CI −0.85- −0.25, p=0.0003). One study (conducted by the U of K) which was excluded for tissue fixation, reported a dramatic increase in copper concentration (>400%) in AD amygdala compared to controls (Lovell et al 1998). However, a second report from the same laboratory reported copper was depleted in the amygdala with an effect size of −1.42, 95%CI −2.40- −0.44. The effect of Alzheimer’s disease on hippocampal copper was −0.54, 95%CI −0.91- −0.16 and reported effect sizes for other neocortical regions ranged from −0.39 to −2.78.
3.3 Assessment for publication and citation bias
A systematic evaluation for publication bias and citation bias was conducted and the effect of observed biases was modeled. For these analyses the relevant studies previously excluded for technical rationale were included as they certainly contributed to the evolution of scientific opinions. To assess for publication bias, a funnel plot was constructed. Briefly, a funnel plot depicts the inverse of the variance in each study compared to the reported effect size. Theoretically, the studies with greatest power should have the lowest degree of variance (lying high on the y-axis) and should lie closest to the true effect size; studies with lower power should have greater variance (lying lower on the y-axis) and should distribute symmetrically about the estimated true effect size producing a scatter plot which resembles an inverted funnel. Publication bias argues that studies with non-significant results are less likely to be published. Therefore, if publication bias is present, the distribution should be asymmetric with the side of the funnel nearest the non-significant effect size containing fewer data points (Egger et al 1997). The funnel plot from this data set was symmetric and, no evidence of publication bias was found using a trim-and-fill analysis. A more-subtle publication bias may result in studies with significant results being published in higher impact journals, however no significant difference in the long-term impact factor of journals publishing significant results versus those reporting no change in iron levels was found in this dataset (Fig 3).
Figure 3. No evidence of publication bias is present in the dataset.
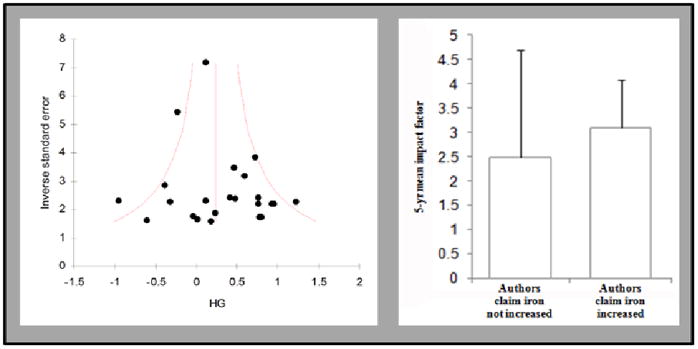
Funnel plot illustrates symmetric distribution of the studies and exclusion of the results from the University of Kentucky does not alter this analysis. The long-term impact factor of journals publishing studies which analyzed iron content of Alzheimer’s disease brain was not statistically different between those that argue that iron levels change and those that argue that it does not.
Citation bias was assessed by calculating both the total number of citations per paper and the annual rate of citation (Fig 4). While citation rate is a more-reliable estimate of citation bias, total number of citations offers an estimate of the total impact a study has had on subsequent studies, so we felt both measures were useful. Significant citation bias was found in the dataset favoring studies reporting positive effect size of Alzheimer’s disease on iron levels. The study by Lovell et al in 1998 received many times more citations than any other study and performed as an outlier – it was excluded from the calculation of citation bias. Studies reporting increased levels of iron were cited more than twice as many times (p<0.05) as those that found levels did not change. The rate of citation among these articles was also significantly greater (p<0.01). Citation bias was also assessed by whether the authors interpreted their results as demonstrating an alteration in iron levels because in several cases the authors’ interpretation differed from the results we obtained. Studies which the authors interpreted as showing an increase were cited more than three-times more than those concluded to be non-significant and the rates of citation were also more than three times higher (p<0.0001 for each). We modeled the effect of the citation bias by weighting the studies by the number of citations each had received (red arrow in Fig 5) and comparing that to the effect size observed when the studies were weighted by inverse variance both with and without exclusion criteria (the grey and green vertical arrows respectively in Fig 5). The quality measures employed had the opposite effect on the apparent effect size as the citation bias and this likely accounted for the widely held opinion that iron levels are increased in AD neocortex.
Figure 4. Significant citation bias is present favoring studies which found an increase in brain iron.
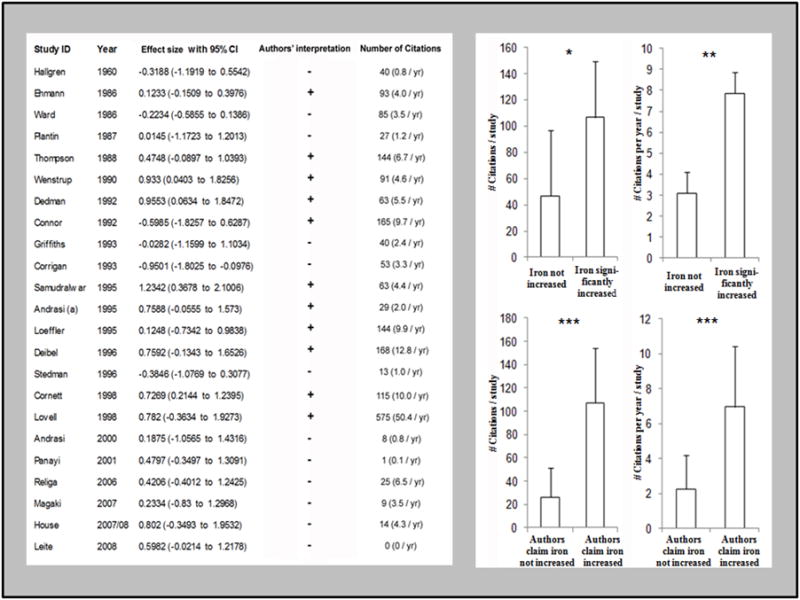
There was a significant increase in both the number (p<0.05) and frequency (p<0.01) of citations favoring studies which found a significant increase in brain iron in AD. A more than three-fold increase in the number and frequency of citation (p<0.0001 for each) was observed favoring articles which interpreted their data as supporting the hypothesis that iron is increased compared to those that do not. In some cases the authors’ interpretation appeared to differ from the data – in these cases the interpretation may have been based on data from a particular brain region or other non-quantitative findings or the data may have appeared significant prior to conversion to the standard measure (μg Fe/g wet weight tissue) which was necessary for inclusion in this study.
Figure 5. The divergent effects of quality measures and citation bias lead to different interpretations of the dataset.
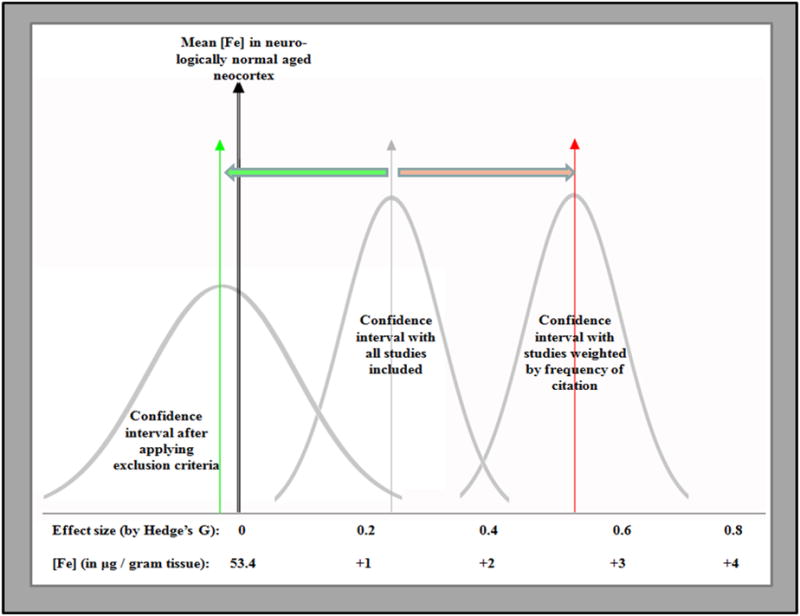
When all available studies were included in the analysis, a small positive effect size was observed. Introduction of quality-based exclusion criteria adjusted the effect size to −0.08 (green vertical arrow). Finally, when all available studies were included and weighted by the number of citations received (instead of conventional weighting by inverse variance), the apparent effect size was increased to 0.53 (red arrow). It should be noted that even this exaggerated effect size correlated to less than 3 g/g increase in brain iron (about 5% higher than control).
3.4 Citation mapping of narrative review literature
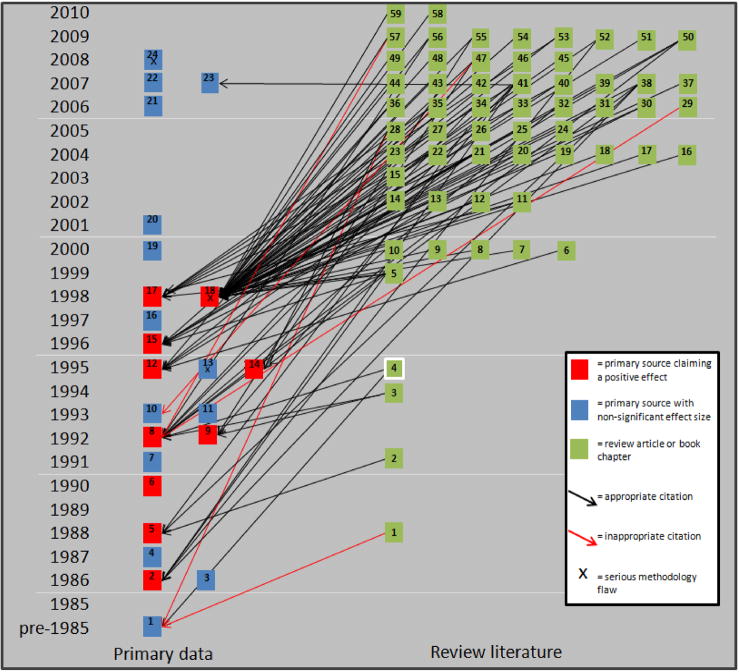
Assessing for a cumulative citation bias is useful, but does not describe how primary data is used in building scientific hypotheses. For a more-precise understanding, we manually analyzed a cross-section of review literature which described alterations in transition metal metabolism in AD. Relevant articles were found by querying PubMed and Science Citation Index for —iron or —metals and —Alzheimer’s and filtering for review articles. The articles retrieved were screened to determine whether or not they made a claim regarding brain iron levels in AD. Since 1990, 115 narrative review articles were found which make such a claim – all but one claim that iron is increased in AD. The primary studies referenced to support the claim are shown in Fig 6. Of the 115 relevant studies, 59 cited primary quantitative studies, 37 cited review articles and qualitative studies to support the claim, nine cited only review literature (Amit et al 2008, Brewer 2007, Qian and Wang 1998, Rogers et al 2008, Schipper 2004, Stankiewitz 2009, Takeda et al 2004, Youdim 2008, Zecca 2004), and eight cited no supporting literature at all (Atamna 2009, Connor and Lee 2006, Milton 2004, Perry et al 2003, Rouault and Cooperman 2006, Schipper 2000, Schipper et al 2009). Finally two studies inaccurately referenced iron measurements in either cerebrospinal fluid or Parkinsonian brain as their only source to defend the claim that iron is increased in Alzheimer’s disease (Maccioni et al 2009, Mandel and Silvia 2008). This sort of citation is shown in Fig 6 by red arrows indicating an inappropriate citation. The citation pattern of the 59 studies which cited primary/quantitative sources is shown in Fig 6. Since its publication in 1998, the article by Lovell et al has been the dominant source for the claim that iron is increased. This figure qualitatively demonstrates that a systematic citation bias is present in narrative review literature and that this bias is (at least qualitatively) considerably greater than the citation bias observed in the general literature. Additionally, it clarifies the degree to which brain iron overload as a feature of Alzheimer’s disease has become dogma. Less than 1% of the reviewed articles claimed iron was not increased, and eight articles cited no supporting literature for the claim.
Figure 6. Citation map illustrating citation bias in review literature describing the role of iron in Alzheimer’s disease.
Of 115 review articles reviewed, only 59 cited quantitative literature and only one (in white square) argued that iron levels were not altered in Alzheimer’s disease. Of the 94 citations shown in the map above, only 6 referenced negative literature and of those 3 misrepresented the findings of those studies to suggest they demonstrated an increase in brain iron.
4. DISCUSSION
While the bulk of the findings from this study described reports of iron levels in AD, several important findings relevant to zinc and copper were noteworthy. No significant change in bulk neocortical zinc levels was found, although a modest elevation in the parietal lobe was noted. The data on zinc levels in the neocortex was heterogeneous and no clear explanation for the heterogeneity could be deduced from the meta-data. However, some of the included studies indicated that their brain samples contained equal portions of white and grey matter, while others were more ambiguous; the study reporting the largest increase in zinc levels sampled from temporal lobe cortex (Religa et al 2006). Consistent with this pattern, we recently reported that zinc levels were unchanged in AD in temporal lobe white matter, but were significantly increased in the overlying cortical ribbon (Schrag et al 2010b). It is therefore possible that the heterogeneity of these results is due to differences in tissue sampling.
The available evidence suggests that copper is generally depleted in AD (although copper was noted to increase in the putamen by one study). A single discordant study originating from the U of K (excluded from this analysis) indicated a more than four-fold increased copper levels in AD (potentially a fixation artifact) -- this study is the most cited paper on the subject of copper in AD and appears to be the source for numerous articles reporting that copper levels are (several fold) increased in AD (Bush 2000, Cuajungco et al 2000, Filiz et al 2008, Lovell et al 1998, Rottkamp et al 2001). It is important to emphasize that the overwhelming number of studies report that copper is not increased in AD brain. Of note, a clinical trial of D-penicillamine, a copper chelator, was unable to produce any clinical improvement in the treated cohort of AD patients (in fact patients trended toward worse outcomes), although subjects experienced numerous toxicities resulting in one subject death and the early suspension of the trial (Squitti et al 2002).
Regarding iron, we conclude that Alzheimer’s disease does not appear to alter neocortical iron levels. Iron was modestly elevated in the AD putamen over controls, but no other brain region appeared to be affected. The increases in tissue iron in deep grey matter may be a significant component of Alzheimer’s disease pathology, but certainly do not account for the neocortical dysfunction observed in this disease. Moreover, AD is commonly comorbid with some degree of Lewy body disease which is strongly associated with increases in basal ganglia iron -- the findings in the putamen may be more reflective of this disease process (Chavhan et al 2009, Dexter et al 1991). Gradient echo T2* (GRE-T2*) and susceptibility weighted imaging (SWI) are iron-sensitive sequences that have been used to follow brain iron levels in AD patients. Several large studies of this technology have been conducted to evaluate the usefulness of following brain iron levels as a biomarker of AD. In these studies the putamen was the only region consistently found to contain increased levels of iron in AD (Ding et al 2009, Kirsch et al 2009, Zhu et al 2009). This is remarkably consistent with the results of this meta-analysis and validates the utility of this technology for noninvasively estimating tissue iron levels. However, increases in putamen iron are not specific to AD and therefore may not be particularly helpful in establishing a diagnosis. Few studies described the levels of iron in males versus females with AD, but the limited data available does not show any significant difference between sexes (Magaki et al 2007). Finally, none of the included studies specifically analyzed iron levels in brains from patients with early-onset and familial forms of AD.
Focal alterations in metals distribution have been suggested by several studies to be associated with the primary pathologies of AD. All three metals evaluated here are reported to accumulate within senile plaques, although other studies call into question the consistency of this observation. One study found 30% of plaques had no detectable iron in them while a few of the largest plaques had high concentrations of redox-active magnetite. Because of the inhomogeneous distribution of metals in the brain, it is important to cautiously interpret the finding that bulk levels of iron are unchanged – an underlying alteration in iron metabolism may still be present and if this were to result in an increased fraction of poorly liganded iron it is reasonable that it could account for the oxidative injuries observed in Alzheimer’s disease. Never-the-less, based upon these cumulative findings and because of the disproportionate impact of outlier data on the literature, we feel it will be important to re-evaluate brain metals-overload hypotheses particularly when considering additional clinical trials of metal chelating/modulating therapies. It is fundamentally important that the application of metal-targeted pharmacology restores not only normal metal levels, but also normal transition metal physiology.
Importantly, the data from this meta-analysis indicates that there is a wide-spread misconception in the scientific literature regarding the levels of several transition metals, most prominently iron, in AD brain. Less than 1% of the review articles analyzed in this study reported that iron levels were not increased. This family of studies spanning fifty years serves as a case-study in the development of a dogma and we tried to understand what factors contributed to the development of the distortion and what strategies might be reasonable to modify this risk. The misconception appears to have arisen as a result of significant citation bias (p<0.0001) in the absence of publication bias which amplified the contributions of one laboratory which were significantly different from all other published reports.
Systematic evidence describing citation bias is limited. The bulk of available evidence describing the phenomenon is focused on clinical studies where it is obviously of great importance – selective citation may misguide clinical and health-policy decision making which can immediately endanger patients. Citation bias has been reported in a wide range of topics, including literature describing smoking rate among schizophrenics (Chapman et al 2009), in clinical trials for various hepato-biliary diseases (Kjaergard et al 2002) and the effect of anti-inflammatory drugs on rheumatoid arthritis (Gotsche et al 1989) among others topics. Evidence for citation bias in basic science literature is more limited, although an elegant study recently demonstrated extensive bias in studies reporting the presence or absence of amyloid in muscle tissue in inclusion body myositis (Greenberg 2010). Citation bias is generally thought to favor studies reporting significant findings (what has sometimes been termed optimism bias), although there is evidence for mixed biases, with certain fields favoring over-citation of negative associations (Hutchison et al 1995, Nieminem et al 2007). Understanding how citation bias develops is important in developing strategies to control it. One reasonable hypothesis that has been presented is that studies reporting significant findings are likely to be published in higher impact factor journals and are therefore more visible which could account for higher citation rates. However, one of the earliest analyses of citation bias reported no significant correlation between citation rates of individual articles and journal impact factor (Seglen 1989). These findings are consistent with the results of our study – negative results were just as likely to be published in high impact factor journals. Another hypothesis suggests that a regional citation bias favors citation of articles originating in the United States or in English-speaking countries. Paris et al argued that Italian scientific contributions to the field of environmental science were systematically under-cited despite being published in journals with strong impact factors relative to the field (1998). We could not exclude some component of regional citation bias in our results. While the studies that received the greatest number of citations in our analysis originated in North America, those reporting —negative findings were cited at comparable rates regardless of the geographical origin of the publication – of course, this study is probably far too small to effectively identify such a bias. Finally, some evidence has previously suggested that narrative review articles were particularly prone to citation bias (Schmidt et al 2006). This pattern was strikingly evident in our analysis as well. Among other purposes, the narrative review is a venue to distill current research to frame scientific hypotheses. Unfortunately, the tacit assumption of unbiased literature sampling in the formulation of review literature has proved to be unreliable. This is probably not so conspiratorial as it is a human and technological reality which underscores the need for more rigorous systematic analysis of basic science data to provide the foundation for clinical trials in a given field.
Using meta-analysis and systemic review methodologies we have identified the wide-spread misconception in AD literature that iron, and to a lesser degree zinc and copper, levels are increased in AD brain. In addition to misleading research studies, this may also prove to be dangerous to AD patients. Therapies based on this largely unsupported dogma could result in unexpected toxicities and failure to be of therapeutic value. In light of our findings it will be important to re-evaluate the brain metal-overload hypothesis in AD and critically review related research and review articles.
Supplementary Material
RESEARCH HIGHLIGHTS.
Neocortical iron levels are not increased in Alzheimer’s disease compared to aged control subjects
Bulk neocortical zinc levels do not appear to be increased in Alzheimer’s disease compared to aged control subjects, although several studies indicate that zinc is selectively increased in grey matter in Alzheimer’s disease.
Copper levels are significantly decreased across multiple brain regions in Alzheimer’s disease. On average, copper is reduced by 13.8% in the neocortex (p=0.0003).
One data-source found that iron was significantly increased in Alzheimer’s disease neocortex and studies published by this group were cited more than 3 times as often as the studies from groups reporting non-significant changes in iron levels.
Citation bias is particularly prominent in narrative review articles describing iron metabolism in Alzheimer’s disease.
Amplification of outlier data by citation bias can influence scientific opinion.
Acknowledgments
We are grateful to Mark A. Smith who contributed greatly to this work. In light of his recent and unexpected death, we dedicate this work to his memory and his passion for dissolving dogma in his field. We also recognize the immense contribution of William Markesbery – our field has lost Titans this year.
This material was made available to Mark Lovell and Elizabeth Head at the Sanders Brown Center of Aging at the University of Kentucky prior to submission. We are grateful that they took the time to review this data and the graciousness with which they have treated us. This work was funded in part by the National Institutes of Health (AG20948). The authors are aware of no real or potential conflicts of interests related to this material.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew Schrag, Email: mschrag@llu.edu.
Claudius Mueller, Email: cmuelle1@gmu.edu.
Udochukwu Oyoyo, Email: uoyoyo@llu.edu.
Wolff M. Kirsch, Email: wkirsch@llu.edu.
6. Annotated references
- Andrasi E, Farkas E, Gawlik D, Rosick U, Bratter P. Brain iron and zinc contents of German patients with Alzheimer’s disease. Journal of Alzheimer’s Disease. 2000;2:17C–26. doi: 10.3233/jad-2000-2103. INCLUDED: Iron and zinc were measured by INAA for hippocampus, frontal, parietal, occipital lobes, thalamus, caudate putamen and globus pallidus. Dry weight values were reported without dry to wet weight ratios; standard conversion ratios were applied. Results are from Eotvos University. [DOI] [PubMed] [Google Scholar]
- Andrasi E, Farkas E, Scheibler H, Reffy A, Bezur L. Al, Zn, Cu, Mn and Fe levels in brain in Alzheimer’s disease. Archives of Gerontology and Geriatrics. 1995;21:89–97. doi: 10.1016/0167-4943(95)00643-y. EXCLUDED for formalin fixation: iron, zinc and copper were measured from ten brain regions by INAA and ICP-AES. [DOI] [PubMed] [Google Scholar]
- Collingwood J, Dobson J. In situ characterization and mapping of iron compounds in Alzheimer’s disease tissue. Journal of Alzheimer’s Disease. 2005;7:267–72. doi: 10.3233/jad-2005-7401. EXCLUDED tissue fixation and semi-quantitative analysis: iron was measured by x-ray fluorescence in the frontal cortex of a single Alzheimer’s case. [DOI] [PubMed] [Google Scholar]
- Connor J, Snyder B, Beard J, Fine R, Mufson E. Regional distribution of iron and iron-regulatory proteins in the brain in aging and Alzheimer’s disease. Journal of Neuroscience Research. 1992;31:327–335. doi: 10.1002/jnr.490310214. EXCLUDED for semi-quantitative methods (iron concentration normalized to protein concentration, not tissue weight): iron was measured by colorimetric assay in three brain regions. [DOI] [PubMed] [Google Scholar]
- Cornett C, Markesbery W, Ehmann W. Imbalances of trace elements related to oxidative damage in Alzheimer’s disease brain. Neurotoxicology. 1998a;19:339–45. INCLUDED: Iron and zinc were measured by INAA in hippocampus, amygdala, frontal, parietal and temporal lobes and cerebellum. Results are from the University of Kentucky. [PubMed] [Google Scholar]
- Cornett C, Ehmann W, Wekstein D, Markesbery W. Trace elements in Alzheimer’s disease pituitary glands. Biological Trace Element Research. 1998b;62:107–14. doi: 10.1007/BF02820026. INCLUDED: Iron and zinc were measured by INAA in pituitary. Results are from the University of Kentucky. [DOI] [PubMed] [Google Scholar]
- Corrigan F, Reynolds G, Ward N. Reductions of zinc and selenium in brain in Alzheimer’s disease. Trace Elements in Medicine. 1991;8:1–5. INCLUDED: Iron, zinc and copper were measured for putamen, zinc measurements for caudate, temporal and frontal lobes by INAA. Results are from the University of Surrey. [Google Scholar]
- Corrigan FM, Reynolds GP, Ward NI. Hippocampal tin, aluminum and zinc in Alzheimer’s disease. BioMetals. 1993;6:149–54. doi: 10.1007/BF00205853. INCLUDED: Iron, zinc and copper were measured by ICP-MS in hippocampus (dentate gyrus only). Results are from the University of Surrey. [DOI] [PubMed] [Google Scholar]
- Danscher G, Jensen KB, Frederickson CJ, Kemp K, Andreasen A, Juhl S, Stoltenberg M, Ravid R. Increased amount of zinc in the hippocampus and amygdala of Alzheimer’s diseased brains: a proton-induced x-ray emission spectroscopic analysis of cryostat sections from autopsy material. Journal of Neuroscience Methods. 1997;76:53–9. doi: 10.1016/s0165-0270(97)00079-4. EXCLUDED for semi-quantitative results: zinc was measured by PIXE in hippocampus and amygdala. Significant increases were reported in both regions. Results are from the University of Aarhus. [DOI] [PubMed] [Google Scholar]
- Dedman D, Treffry A, Candy JM, Taylor GAA, Morris CM, Bloxham CA, Perry RH, Edwardson JA, Harrison PM. Iron and aluminum in relation to brain ferritin in normal individuals and Alzheimer’s disease and chronic dialysis patients. Journal of Biochemistry. 1992;287:509–14. doi: 10.1042/bj2870509. EXCLUDED for semi-quantitative results: non-heme iron was measured by a colorimetric assay in the parietal lobe normalized to protein concentration. A significant increase in iron was reported. Results are the University of Sheffield. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel M, Ehmann W, Markesbery W. Copper, iron and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: possible relation to oxidative stress. Journal of Neurological Science. 1996;143:137–42. doi: 10.1016/s0022-510x(96)00203-1. INCLUDED: Iron, copper and zinc measurements by INAA in the hippocampus, amygdala, temporal and parietal lobes and cerebrellum. Dry weight values were reported w/o dry to wet weight ratios; standard conversion ratios were applied. Results are from the University of Kentucky. [DOI] [PubMed] [Google Scholar]
- Ehmann W, Markesbery W, Alauddin M, Hossain T, Brubaker E. Brain trace elements in Alzheimer’s disease. Neurotoxicology. 1986;1:197–206. EXCLUDED for absence of regional discrimination: iron and zinc measurements by INAA for bulk brain only. Results are from the University of Kentucky. [PubMed] [Google Scholar]
- Griffiths P, Crossman A. Distribution of iron in the basal ganglia and neocortex in postmortem tissue in Parkinson’s disease and Alzheimer’s disease. Dementia. 1993;4:61–5. doi: 10.1159/000107298. INCLUDED: Iron measurements by atomic absorption in frontal, parietal and temporal lobes, putamen, caudate, globus pallidus (lateral and medial separately) and substantia nigra. Results from University of Manchester. [DOI] [PubMed] [Google Scholar]
- House M, St Pierre TG, Kowdley KV, Montine T, Connor J, Beard J, Berger J, Siddaiah N, Shankland E, Jin LW. Correlation of proton transverse relaxation rates (R2) with iron concentrations in postmortem brain tissue from Alzheimer’s disease patients. Magnetic Resonance in Medicine. 2007;57:172–180. doi: 10.1002/mrm.21118. INCLUDED: see next entry. [DOI] [PubMed] [Google Scholar]
- House M, St Pierre T, McLean C. 1.4T study of proton magnetic relaxation rates, iron concentrations and plaque burden in Alzheimer’s disease and control postmortem brain tissue. Magnetic Resonance in Medicine. 2008;60:41–52. doi: 10.1002/mrm.21586. INCLUDED: Because the number of cases in these two studies are small, the data has been merged and cited in the analysis as House (2007/08). Iron measurements are reported from atomic absorption studies of the hippocampus, amygdala, caudate, globus pallidus, thalamus, cingulated gyrus, corpus callosum, frontal, temporal, parietal and occipital lobes. Dry to wet weight ratios are reported for each region. Results are from University of Western Australia. [DOI] [PubMed] [Google Scholar]
- 16.Leite R, Jacob-Filho W, Saiki M, Grinberg L, Ferretti R. Determination of trace elements in human brain tissue using neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry. 2008;278:581–4. EXCLUDED for lack of neuropathologic diagnosis of dementia cases: Iron and zinc were measured by INAA in frontal lobe and hippocampal tissue. [Google Scholar]
- Loeffler D, Connor JR, Juneau PL, Snyder BS, Kanaley L, Demaggio AJ, Nguyen H, Brickman CM, Lewitt PA. Transferrin and iron in normal, Alzheimer’s disease and Parkinson’s disease brain regions. Journal of Neurochemistry. 1995;65:710–6. doi: 10.1046/j.1471-4159.1995.65020710.x. EXCLUDED reported values were semi-quantitative (normalized to protein concentration). The amount of protein isolated per mass of tissue was also reported which was significantly different between groups: iron measurement were obtained from a colorimetric assay for caudate, putamen, substanti nigra, globus pallidus and frontal lobe. Significant increases were reported in the frontal cortex and globus pallidus, however, when the results were recalculated to normalize to tissue weight, no significant changes remained. Results are from Wayne State University. [DOI] [PubMed] [Google Scholar]
- Lovell M, Robertson J, Teesdale W, Campbell J, Markesbery W. Copper, iron and zinc in Alzheimer’s disease senile plaques. Journal of Neurological Science. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. EXCLUDED for tissue fixation: Iron, zinc and copper were measured in amygdala by microprobe-PIXE in the amygdala. Results are from University of Kentucky. [DOI] [PubMed] [Google Scholar]
- Magaki S, Raghavan R, Mueller C, Oberg KC, Vinters HV, Kirsch WM. Iron, copper and iron regulatory protein 2 in Alzheimer’s disease and related dementia. Neuroscience Letters. 2007;418:72–6. doi: 10.1016/j.neulet.2007.02.077. INCDLUDED: Iron and copper measurements by atomic absorption reported for hippocampus and frontal lobe. Results are from Loma Linda University. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayi A, Spyrou N, Part P. Differences in trace element concentrations between Alzheimer and —normal human brain tissue using instrumental neutron activation analysis. Journal of Radioanalytic and Nuclear Chemistry. 2001;249:437–41. INCLUDED: Iron and zinc were measured by INAA in frontal, parietal and temporal lobes. [Note: samples in this study were age-matched.] Results are from the University of Surrey. [Google Scholar]
- Panayi A, Spyrou N, Iverson B, White M, Part P. Determination of cadmium and zinc in Alzheimer’s brain tissue using inductively coupled plasma mass spectrometry. Journal of Neurological Science. 2002;195:1–10. doi: 10.1016/s0022-510x(01)00672-4. INCLUDED: Zinc measurements were measured by ICP-MS for hippocampus, thalamus, frontal, parietal and temporal lobes. Controls as young as 38 were included in the control group -- the mean age of controls was 66.8 years versus a mean of 80.0 years for the AD group. Because only zinc measurements were reported and zinc is less affected by ageing than iron, we decided to include this study. Results are from the University of Surrey. [DOI] [PubMed] [Google Scholar]
- Plantin L, Lying-Tunell U, Kristensson K. Trace elements in the human central nervous system studied with neutron activation analysis. Biological Trace Element Research. 1987;13:69–75. doi: 10.1007/BF02796622. INCLUDED: [DOI] [PubMed] [Google Scholar]
- Rao K, Rao R, Shanmugavelu P, Menon R. Trace elements in Alzheimer’s disease: a new hypothesis. Alzheimer’s Reports. 1999;2:241–6. EXCLUDED: copper, iron and zinc measured by ICP-AES for hippocampus and frontal cortex. Age of control subjects was not reported. The study reported a more than 1000 fold increase in iron and a 12 fold increase in zinc in the AD hippocampus – these results are likely inaccurate in the context of other reported values. [Google Scholar]
- Religa D, Strozyk D, Cherny RA, Volitakis I, Haroutunian V, Winblad B, Naslund J, Bush AI. Elevated cortical zinc in Alzheimer’s disease. Neurology. 2006;67:69–75. doi: 10.1212/01.wnl.0000223644.08653.b5. INCLUDED: Iron, zinc and copper were measured by ICP-MS in the temporal lobe. Results are from Harvard University. [DOI] [PubMed] [Google Scholar]
- Rulon L, Robertson JD, Lovell MA, Deibel MA, Ehmann WD, Markesbery WR. Serum zinc levels and Alzheimer’s disease. Biological Trace Element Research. 2000;75:79–85. doi: 10.1385/bter:75:1-3:79. INCLUDED: Zinc was measured by AA for the hippocampus, amygdala, temporal lobe and cerebellum. Results are from the University of Kentucky. [DOI] [PubMed] [Google Scholar]
- Samudralwar D, Diprete C, Ni B, Ehmann W, Markesbery W. Elemental imbalances in the olfactory pathway in Alzheimer’s disease. Journal of Neurological Science. 1995;130:139–45. doi: 10.1016/0022-510x(95)00018-w. INCLUDED: Iron and zinc were measured by INAA for the olfactory system and amygdala. Results are from the University of Kentucky. [DOI] [PubMed] [Google Scholar]
- Squitti R, Quattrochi C, Forno G, Antuono P, Wekstein DR, Capo CR, Salustri C, Rossini PM. Ceruloplasmin (2D PAGE) pattern and copper content in serum of Alzheimer’s disease patients. Biomark Insights. 2006;1:205–13. EXCLUDED: for semi-quantitative analysis. [PMC free article] [PubMed] [Google Scholar]
- Stedman J, Spyrou N. Elemental analysis of the frontal lobe of —normal brain tissue and that affected by Alzheimer’s disease. Journal of Radioanalytical and Nuclear Chemistry. 1997;217:163–6. INCLUDED: Iron and zinc were measured by INAA for the frontal lobe. Results are from the University of Surrey. [Google Scholar]
- Tandon L, Ni BF, Ding XX, Ehmann WD, Kasarskis EJ, Markesbery WR. RNAA for arsenic, cadmium, copper and molybdenum in CNS tissue from subjects with age-related neurodegenerative diseases. Journal of Radioanalytical and Nuclear Research. 1994;179:331–9. EXCLUDED for absence of regional discrimination: reported copper levels by RNAA in bulk brain samples only. Results are from the University of Kentucky. [Google Scholar]
- Thompson C, Markesbery W, Ehmann W, Mao Y, Vance D. Regional brain trace-element studies in Alzheimer’s disease. Neurotoxicology. 1988;9:1–8. INCLUDED: Iron and zinc measured by INAA for hippocampus, amygdala, and nucleus basalis of Meynert. Results are from the University of Kentucky. [PubMed] [Google Scholar]
- Ward N, Mason J. Neutron activation analysis techniques for identifying elemental status in Alzheimer’s disease. Journal of Radioanalytical and Nuclear Chemistry. 1987;113:515–526. INCLUDED: Iron and zinc were measured by INAA for hippocampus and bulk cortex. The study was blinded. NOTE: several subsequent papers (Religa 2006, Deibel 1996) questioned the reliability of this study suggesting that formalin-fixed tissue was used – this does not appear to be the case. The tissue was reportedly stored by refrigeration. The results obtained were consistent with those reported from other studies of non-fixed tissues and different from those that studies fixed tissues. This is the largest study in the analysis. [Google Scholar]
- Wenstrup D, Ehmann W, Markesbery W. Trace element imbalances in isolated subcellular fractions of Alzheimer’s disease brains. Brain Research. 1990;533:125–31. doi: 10.1016/0006-8993(90)91804-p. INCLUDED: Iron and zinc were measured by INAA for temporal lobe. Results are from the University of Kentucky. [DOI] [PubMed] [Google Scholar]
- Yoshimasu F, Yasui M, Yase Y, Iwata S, Gajdusek DC, Gibbs CJ, Chen KM. Studies on amyotrophic lateral sclerosis by neutron activation analysis—2. Comparative study of analytical results on Guam PD, Japanese ALS and Alzheimer’s disease cases. Folia Psychiatrica et Neurologica. 1980;34:75–82. doi: 10.1111/j.1440-1819.1980.tb01515.x. EXCLUDED for fixation: reported copper levels by neutron activation analysis. [DOI] [PubMed] [Google Scholar]
7. General references
- Amit T, Avramovich-Tirosh Y, Youdim M, Mandel S. Targeting multiple Alzheimer’s disease etiologies with multimodal neuroprotective and neurorestorative iron chelators. FASEB J. 2008;22:1296–305. doi: 10.1096/fj.07-8627rev. [DOI] [PubMed] [Google Scholar]
- Atamna H. Amino acids variation in amyloid-beta peptides, mitochondrial dysfunction and new therapies for Alzheimer’s disease. J Bioenerg Biomembr. 2009;41:457–64. doi: 10.1007/s10863-009-9246-2. [DOI] [PubMed] [Google Scholar]
- Bax L, Yu L, Ikeda N, Moons K. A systematic comparison of software dedicated to meta-analysis of causal studies. BMC Med Res Methodol. 2007;7:40. doi: 10.1186/1471-2288-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzi G, Moretti A. Are reactive oxygen species involved in Alzheimer’s disease. Neurobiol Aging. 1995;16:661–74. doi: 10.1016/0197-4580(95)00066-n. [DOI] [PubMed] [Google Scholar]
- Brewer G. Iron and copper toxicity in diseases of aging, particularly atherosclerosis and Alzheimer’s disease. Exp Biol Med. 2007;232l:323–35. [PubMed] [Google Scholar]
- Brunnstrom H, Englund E. Clinicopathological concordance in dementia diagnostics. Am J Geriatr Psych. 2009;17:664–70. doi: 10.1097/jgp.0b013e3181a6516e. [DOI] [PubMed] [Google Scholar]
- Bush AI, Pettingell WH, Multhaup G, Paradis MD, Vonsattel JP, Gusella JF, Beyreuther K, Masters CL, Tanzi RE. Rapid induction of Alzheimer Abeta amyloid formation by zinc. Science. 1994;265:1464–67. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- Bush A. Metals and neuroscience. Curr Opin Chem Biol. 2000;4:184–91. doi: 10.1016/s1367-5931(99)00073-3. [DOI] [PubMed] [Google Scholar]
- Chapman S, Ragg M, McGeechan K. Citation bias in reported smoking prevalence in people with schizophrenia. Australian and New Zealand Journal of Psychiatry. 2009;43:277–82. doi: 10.1080/00048670802653372. [DOI] [PubMed] [Google Scholar]
- Chavhan G, Babyn P, Thomas B, Schroff M, Haacke M. Principles, techniques and applications of T2*-based MR imaging and its special applications. Radiographics. 2009;29:1433–49. doi: 10.1148/rg.295095034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny RA, Legg JT, McLean CA, Fairlie DP, Huang X, Atwood CS, Beyreuther K, Tanzi RE, Masters CL, Bush AI. Aqueous dissolution of Alzheimer’s disease Abeta amyloid deposits by biometal depletion. J Biol Chem. 1999;274:23223–8. doi: 10.1074/jbc.274.33.23223. [DOI] [PubMed] [Google Scholar]
- Connor J, Lee S. HFE mutations and Alzheimer’s disease. J Alz Dis. 2006;10:267–76. doi: 10.3233/jad-2006-102-311. [DOI] [PubMed] [Google Scholar]
- Crapper McLachlan D, Kruck T, Kalow W, Andrews DF, Dalton AJ, Bell MY, Smith WL. Intramusclar desferrioxamine in patients with Alzheimer’s disease. Lancet. 1991;337:1304–8. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- Cuajungco MP, Faget KY, Huang X, Tanzi RE, Bush A. Metal chelation as a potential therapy for Alzheimer’s disease. Ann N YAcad Sci. 2000;920:292–304. doi: 10.1111/j.1749-6632.2000.tb06938.x. [DOI] [PubMed] [Google Scholar]
- Dexter D, Carayon A, Javoyagid F, Agid Y, Wells FR, Daniel SE, Jenner P, Marsden CD. Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain. 1991;114:1953–75. doi: 10.1093/brain/114.4.1953. [DOI] [PubMed] [Google Scholar]
- Ding B, Chen KM, Ling HW, Sun F, Li X, Wan T, Chai WM, Zhang H, Zhan Y, Guan YJ. Correlation of iron in the hippocampus with MMSE in patients with Alzheimer’s disease. J Mag Res Imaging. 2009;29:793–8. doi: 10.1002/jmri.21730. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. A non-parametric trim and fill method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected b y a simple, graphical test. BMJ. 1997;315:629. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiz G, Price KA, Caragounis A, Du T, Crouch PJ, White AR. The role of metals in modulating metalloprotease activity in AD brain. Eur Biophys J. 2008;37:315–21. doi: 10.1007/s00249-007-0244-1. [DOI] [PubMed] [Google Scholar]
- Gay B, Taylor K, Hohl U, Tolnay M, Staehelin H. The validity of clinical diagnoses of dementia in a group of consecutively autopsied memory clinic patients. J Nutr Health Aging. 2008;12:132–7. doi: 10.1007/BF02982566. [DOI] [PubMed] [Google Scholar]
- Gerlach M, Ben-Shachar D, Reiderer P, Youdim M. Altered brain metabolism of iron as a cause of neurodegenerative diseases. J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- Goodman L. Alzheimer’s disease; a clinico-pathologic analysis of 23 cases with a theory on pathogenesis. J Nerv Ment Dis. 1953;118:97–130. [PubMed] [Google Scholar]
- Gotzsche PC. Methodology and overt and hidden bias in reports of 196 double-blind trials of nonsteroidal anti-inflammatory drugs in rheumatoid arthritis. Controlled Clinical Trials. 1989;10:31–56. doi: 10.1016/0197-2456(89)90017-2. [DOI] [PubMed] [Google Scholar]
- Greenberg SA. How citation distortions create unfounded authority: analysis of a citation network. BMJ. 2010:339. doi: 10.1136/bmj.b2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The effect of age on the non-haemin iron in the human brain. J Neurochem. 1958;3:41–51. doi: 10.1111/j.1471-4159.1958.tb12607.x. [DOI] [PubMed] [Google Scholar]
- Hallgren B, Sourander P. The non-haemin iron in the cerebral cortex of Alzheimer’s disease. J Neurochem. 1960;5:307–10. doi: 10.1111/j.1471-4159.1960.tb13369.x. [DOI] [PubMed] [Google Scholar]
- Hamilton R. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10:378–84. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison B, Oxman A, Lloyd S. Comprehensiveness and bias in reporting clinical trials. Canadian Family Physician. 1995;41:1356. [PMC free article] [PubMed] [Google Scholar]
- Jellinger K, Paulus W, Grundke-Iqbal I, Peiderer P, Youdim M. Brain iron and ferritin in Parkinson’s and Alzheimer’s diseases. J Neural Transm Park Dis Dement Sect. 1990;2:327–40. doi: 10.1007/BF02252926. [DOI] [PubMed] [Google Scholar]
- Jervis RE, Landsberger S, Aufreiter S, Vanloon JC, Lecomte R, Monaro S. Trace elements in wet atmospheric deposition: application and comparison of PIXE, INAA and graphite furnace AAS techniques. Int J Environ Anal Chem. 1985;15:89–106. [Google Scholar]
- Kjaergard L, Gluud C. Citation bias of hepato-biliary randomized clinical trials. J Clin Epidemiology. 2002;55:407–10. doi: 10.1016/s0895-4356(01)00513-3. [DOI] [PubMed] [Google Scholar]
- Kirsch W, McAuley G, Holshouser B, Petersen F, Ayaz M, Vinters HV, Dickson C, Haacke EM, Britt W, Larsen J, Kim I, Mueller C, Schrag M, Kido D. Serial susceptibility weighted MRI measures brain iron and microbleeds in dementia. J Alz Dis. 2009;17:599–609. doi: 10.3233/JAD-2009-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S, Bush AI, Murdoch R, Wilson J, Ritchie CW. Safety, efficacy and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease; a phase IIa, double blind randomized placebo controlled trial. Lancet Neurol. 2008;7:779–86. doi: 10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- Lilliefors H. On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J Am Stat Assoc. 1967;62:399–402. [Google Scholar]
- Liu G, Men P, Perry G, Smith MA. Nanoparticle and iron chelators as a potential novel Alzheimer therapy. Methods Mol Biol. 2010;610:123–44. doi: 10.1007/978-1-60327-029-8_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni R, Rojo L, Fernandez J, Kuljis R. The role of neuroimmunomodulation in Alzheimer’s disease. Ann NY Acad Sci. 2009;1153:240–6. doi: 10.1111/j.1749-6632.2008.03972.x. [DOI] [PubMed] [Google Scholar]
- Mandel, Silvia A. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: special reference to epigallocatechin gallate. J Alz Dis. 2008;15:211. doi: 10.3233/jad-2008-15207. [DOI] [PubMed] [Google Scholar]
- Markesbery W. Brain trace elements concentrations in aging. Neurobiol Aging. 1984;5:19–28. doi: 10.1016/0197-4580(84)90081-2. [DOI] [PubMed] [Google Scholar]
- Markesbery W. The role of oxidative stress in Alzheimer’s disease. Arch Neurol. 1999;56:1449–52. doi: 10.1001/archneur.56.12.1449. [DOI] [PubMed] [Google Scholar]
- Milton N. Role of hydrogen peroxide in the aetiology of Alzheimer’s disease: implications for treatment. Drugs and Aging. 2004;21:81–100. doi: 10.2165/00002512-200421020-00002. [DOI] [PubMed] [Google Scholar]
- Nieminem P, Rucker G, Miettunen J, Carpenter J, Schumacher M. Statistically significant papers in psychiatry were cited more often than others. J Clin Epidemiology. 2007;60:939–46. doi: 10.1016/j.jclinepi.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Paris G, DeLeo G, Menozzi P, Gatto M. Region-based citation bias in science. Nature. 1998;396:210. doi: 10.1038/24249. [DOI] [PubMed] [Google Scholar]
- Perry G, Taddeo M, Petersen R, Castellani R, Harris PL, Siedlack SL, Cash AD, Liu Q, Nunomura A, Atwood CS, Smoth MA. Adventiously bound redox active iron and copper are at the center f oxidative damage in Alzheimer’s disease. Biometals. 2003;16:77–81. doi: 10.1023/a:1020731021276. [DOI] [PubMed] [Google Scholar]
- Qian Z, Wang Q. Expression of iron transport proteins and excessive iron accumulation in the brain in neurodegenerative disorders. Brain Research Reviews. 1998;27:257–67. doi: 10.1016/s0165-0173(98)00012-5. [DOI] [PubMed] [Google Scholar]
- Rogers J, Bush A, Cho H, Smith D, Thomson A, Friedlich A, Lahiri DK, Leedman P, Huang X, Cahill CC. Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer’ disease. Biochem Soc Trans. 2008;36:1282–7. doi: 10.1042/BST0361282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottkamp CA, Raina AK, Zhu X, Gaier E, Bush AI, Atwood CS, Chevion M, Perry G, Smith MA. Redox-active iron mediates amyloid-beta toxicity. Free Radic Biol Med. 2001;30:447–50. doi: 10.1016/s0891-5849(00)00494-9. [DOI] [PubMed] [Google Scholar]
- Rouault T, Cooperman S. Brain iron metabolism. Semin Pediatr Neurol. 2006;13:142–8. doi: 10.1016/j.spen.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Schipper H. Glial HO-1 expressions, iron deposition and oxidative stress in neurodegenerative disease. Neurotox Res. 1999;1:57–70. doi: 10.1007/BF03033339. [DOI] [PubMed] [Google Scholar]
- Schipper H. Heme-oxygenase-1: role in brain aging and neurodegeneration. Exp Gerontol. 2000;35:821–30. doi: 10.1016/s0531-5565(00)00148-0. [DOI] [PubMed] [Google Scholar]
- Schipper H. Brain iron deposition and the free radical-mitochondrial theory of ageing. Ageing Res Rev. 2004;3:265–301. doi: 10.1016/j.arr.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Schipper H, Gupta A, Szarek W. Suppresion of glial HO-1 activity as a potential neurotherapeutic intervention in AD. Curr Alz Res. 2009;6:424–30. doi: 10.2174/156720509789207985. [DOI] [PubMed] [Google Scholar]
- Schmidt LM, Gotzsche PC. Of mites and men: reference bias in narrative review articles – A systematic review. J Fam Practice. 2006;54:334–338. [PubMed] [Google Scholar]
- Schrag M, Dickson A, Jiffry A, Kirsch D, Vinters HV, Kirsch W. The effect of formalin fixation on the levels of brain transition metals in archived samples. Biometals. 2010a;23:1123–7. doi: 10.1007/s10534-010-9359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag M, Crofton A, Zabel M, Jiffry A, Kirsch D, Dickson A, Mao XW, Vinters HV, Domaille DW, Chang CJ, Kirsch WM. Effect of cerebral amyloid angiopathy on brain iron, copper and zinc in Alzheimer’s disease. J Alz Dis. 2010b doi: 10.3233/JAD-2010-101503. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D, Chevion M. The role of iron in beta-amyloid toxicity. Biochem Biophys Res Commun. 1995;216:702–7. doi: 10.1006/bbrc.1995.2678. [DOI] [PubMed] [Google Scholar]
- Seglen PO. From bad to worse: evaluation by Journal Impact. Trends Biochem Sci. 1989;14:326. doi: 10.1016/0968-0004(89)90163-1. [DOI] [PubMed] [Google Scholar]
- Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer’s disease is a source of redox-generated free radicals. Proc Nat Acad Sci. 1997;94:9866–8. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squitti R, Rossini PM, Cassetta E, Moffa F, Psqualetti P, Cortesi M, Colloca A, Rossi L, Finazzi-Agro A. D-penicillamine reduces serum oxidatitive stress in Alzheimer’s disease patients. Eur J Clin Inv. 2002;32:51–9. doi: 10.1046/j.1365-2362.2002.00933.x. [DOI] [PubMed] [Google Scholar]
- Stankiewicz J. Role of iron in neurotoxicity: a cause for concern in the elderly? Curr Op Clin Nutr Met Care. 2009;12:22. doi: 10.1097/MCO.0b013e32831ba07c. [DOI] [PubMed] [Google Scholar]
- Takeda A, Itoyama Y, Kimpara T, Zhu X, Avila J, Dwyer BE, Perry G, Smith MA. Heme catabolism and heme oxygenase in neurodegenerative disease. Antiox Redox Sig. 2004;6:888–94. doi: 10.1089/ars.2004.6.888. [DOI] [PubMed] [Google Scholar]
- Youdim M. Brain iron deficiency and excess; cognitive impairment and neurodegeneration with involvement of striatum and hippocampus. Neurotox Res. 2008;14:45. doi: 10.1007/BF03033574. [DOI] [PubMed] [Google Scholar]
- Zecca L. Iron, brain ageing and neurodegenerative disorders. Nature Reviews Neuroscience. 2004;5:863. doi: 10.1038/nrn1537. [DOI] [PubMed] [Google Scholar]
- Zheng J, Goessler W, Geiszinger A, Kosmus W, Chen B, Zhuang G, Xu K, Sui G. Multi-element determination in earthworms with instrumental neutron activation analysis and inductively coupled plasma mass spectrometry: a comparison. J Radioanal Nucl Chem. 1997;223:149–55. [Google Scholar]
- Zhu W, Zhong W, Wang W, Zhan C, Wang C, Qi J, Wang J, Lei T. Quantitative MR phase corrected imaging to investigate increased brain iron deposition in patients with Alzheimer’s disease. Radiology. 2009;253:497–504. doi: 10.1148/radiol.2532082324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.