Abstract
Objective
It has been hypothesized that slow wave activity, a well established measure of sleep homeostasis that increases after waking and decreases after sleep, may reflect changes in cortical synaptic strength. If so, the amplitude of sensory evoked responses should also vary as a function of time awake and asleep in a way that reflects sleep homeostasis.
Methods
Using 256-channel, high-density electroencephalography (EEG) in 12 subjects, auditory evoked potentials (AEP) and spontaneous waking data were collected during wakefulness before and after sleep.
Results
The amplitudes of the N1 and P2 waves of the AEP were reduced after a night of sleep. In addition, the decline in N1 amplitude correlated with low-frequency EEG power during non-rapid eye movement sleep and spontaneous wakefulness, both homeostatically regulated measures of sleep need.
Conclusion
The decline in AEP amplitude after a night of sleep may reflect a homeostatic reduction in synaptic strength.
Significance
These findings provide further evidence for a connection between synaptic plasticity and sleep homeostasis.
Keywords: Auditory evoked potentials, sleep homeostasis, synaptic plasticity, slow wave sleep, N1 and P2, electroencephalogram
Introduction
Sleep pressure increases with the duration of prior wakefulness and declines with sleep, a process known as sleep homeostasis. During non-rapid eye movement (NREM) sleep, the EEG is characterized by the occurrence of large amplitude slow waves. These events reflect near-synchronous slow oscillations (<1 Hz) in the membrane potential of cortical neurons, which alternate between a hyperpolarized down state, characterized by neuronal silence, and a depolarized up state, with elevated rates of neuronal firing (Steriade et al., 1993; Amzica and Steriade, 1998; Vyazovskiy et al., 2009). Slow wave activity (SWA, the NREM EEG power between 0.5 and 4.5 Hz) is a well established marker of sleep need, as it increases in proportion to the duration of prior wakefulness, declines during sleep, and is reduced by naps (Borbély, 1982; Werth et al., 1996; Rusterholz et al., 2010). In the waking EEG, low-frequency power (<9 Hz) also increases with time spent awake, suggesting that, during both sleep and wakefulness, low-frequency power might reflect sleep homeostasis. Previous studies have provided evidence for this relationship by establishing a correlation between the rise in low-frequency power during wakefulness and the subsequent increase in SWA during NREM sleep (Finelli et al., 2000; Vyazovskiy and Tobler, 2005). However, the mechanism responsible for the homeostatic regulation of low-frequency EEG power remains unknown.
Growing evidence suggests a link between sleep homeostasis and changes in synaptic strength (Massimini et al., 2009). For example, recent work in rats demonstrates that the slope of the local field potential (LFP) evoked by electrical stimulation, a classic measure of synaptic strength in vivo, is large before sleep, when sleep pressure is high, and low after sleep, when sleep pressure has dissipated. Importantly, the decline in SWA from early to late sleep predicted the decline in the slope of the evoked response, suggesting that sleep slow waves may be responsible for the pre- to postsleep reduction in synaptic strength (Vyazovskiy et al., 2008). The Human EEG, which mostly reflects currents generated by synchronously occurring postsynaptic potentials, also displays evidence of sleep-related plasticity. Indeed, paralleling the nightly reduction in SWA, the slope of individual sleep slow waves decreases, which reflects a decline in the synchrony of up-to-down and down-to-up state transitions (Riedner et al., 2007; Vyazovskiy et al., 2009). In addition, using a combination of transcranial magnetic stimulation (TMS) and high density EEG, theta burst TMS during wakefulness was found to potentiate cortically evoked responses and produce a local increase in SWA underlying the site of stimulation during subsequent sleep. Importantly, the larger the increase in evoked response amplitude, the larger was the increase in SWA (Huber et al., 2007). Furthermore, a single day of arm immobilization, a procedure presumably leading to local synaptic depression, produced a decline in somatosensory evoked potential amplitude and a local reduction in SWA over sensorimotor cortex contralateral to the immobilized arm (Huber et al., 2006). Together, these studies suggest that SWA, a well established measure of sleep homeostasis, may reflect alterations in synaptic strength occurring during wakefulness and drive synaptic renormalization during sleep (Tononi and Cirelli, 2006).
If synaptic renormalization does occur during sleep, then the amplitude of sensory evoked potentials might be reduced after a night of sleep. Auditory evoked potentials (AEPs) in the human EEG, an easily collected measure of auditory function, have been shown to reflect plastic changes occurring in auditory cortex (Pantev et al., 1999; Tremblay et al., 2001). For example, experienced musicians show larger amplitude AEPs compare to non-musicians in response to both instrumental and sine-wave tones, presumably reflecting a larger cortical representation (Baumann et al., 2008). More acute changes have also been found. Two minutes of high-frequency, repetitive, auditory stimulation is sufficient to produce a long-lasting increase (>1 hr) in AEP amplitude, an effect localized to auditory cortex (Clapp et al., 2005; Zaehle et al., 2007). Here we test the hypothesis that the amplitude of AEPs declines after a night of sleep. Moreover, we test whether the change in AEP amplitude correlates with known measures of sleep homeostasis, such as low-frequency power during sleep and wakefulness.
Methods
Experimental Design
Twelve healthy, medication-free, right-handed subjects (6 women; age 21.9 ± 0.5 years; mean ± s.e.m.) participated in the study. Data from 4 subjects (1 woman) was used from a previous study (Landsness, 2009). Subjects were screened prior to the study to assure normal hearing and no history of excessive daytime sleepiness, sleep complaints, or primary sleep disorders. Subjects reported a normal bedtime of 23:45 ± 14 minutes and wake time of 08:03 ± 8 minutes. The study was approved by the Institutional Review Board of the University of Wisconsin-Madison, and all subjects signed an Institutional Review Board-approved informed consent form.
As part of a larger study (Landsness et al., 2009), subjects spent an adaptation night and an experimental night (motor control) in the sleep laboratory. One week prior to, and for the entire duration of the study, subjects were instructed to refrain from alcohol, caffeine, and nicotine and to maintain regular sleep-wake schedules. Compliance was verified with self-reported sleep logs and wrist motor actigraphy (Actiwatch, Mini- Mitter, Inc., Bend, OR). On the experimental night, subjects arrived at the laboratory between 20:00 and 21:00, were outfitted with high-density EEG (256 electrode nets, Electrical Geodesics, Inc., Eugene, OR), sequentially performed a motor learning task, an auditory oddball task, and spontaneous waking recordings, then slept undisturbed in the laboratory (lights out: 23:40 ± 9 minutes). The next morning, after being woken up at 7 AM and given 30 minutes to combat sleep inertia, subjects again performed the auditory oddball task, spontaneous waking recordings, and then the motor learning task.
Auditory Oddball Task
AEPs were gathered using a standard oddball paradigm. Sinusoidal tones with 50 ms duration and frequencies of 800 Hz (standard) or 560 Hz (target) were generated using a personal computer running LabVIEW (National Instruments, Austin, TX) or E-Prime (Psychology Software Tools, Inc., Pittsburgh, PA). Tones were presented binaurally at an unchanging, comfortable volume through circumaural headphones or earbuds while subjects sat with eyes open, fixating on a point 90 cm in front of them. A total of 120 stimuli were presented with an inter-stimulus interval that varied randomly between 1900 and 3190 ms. Subjects were instructed to count the number of target tones presented to them, which occurred pseudo-randomly 20% of the time.
To ensure a high signal-to-noise ratio for averaging of the evoked response, analysis was restricted to the 96 standard tones. All EEG recordings during sleep and wakefulness were made using NetStation software (Electrical Geodesics, Eugene, OR) on DC amplifiers with 200 Hz analog low-pass filters. AEP recordings were sampled at 500 Hz, first-order high-pass filtered (Kaiser type FIR, 0.1 Hz), band-pass filtered (Kaiser type FIR, 1–15 Hz), and segmented (100 ms pre- to 500 ms post-tone). Artifact rejection was performed semi-automatically to exclude channels and segments with excessive muscle activity or eye movements. Bad channels were replaced using spherical spline interpolation. After artifact rejection, there was an average of 59.8 ± 11 (mean ± s.e.m) artifact-free segments in the presleep condition and 59.5 ± 9.7 (mean ± s.e.m) in the postsleep condition. All artifact-free segments were averaged. Finally, each channel was re-referenced to the average of the 185 channels directly overlying the scalp and baseline corrected by subtracting the average voltage during the 100 ms preceding the tone. Since the N1 is known to have a fronto-central negativity, we used a region of interest (ROI) approach to identify within 20 fronto-central channels the latency between 40 and 140 ms after the tone that showed the most negative sample. Within this ROI, the channel with the largest negative peak was identified and the latency of the peak was defined as the N1 latency for all 185 scalp channels. The P2 latency was defined in a similar fashion, but for the fronto-central channels showing the largest positive peak between 142 and 262 ms. Global mean field power (GMFP), the root mean square of the instantaneous voltage distribution across channels, was taken as a measure of global brain activation following presentation of the tone.
Spontaneous Waking Recordings
Spontaneous waking recordings were collected while subjects sat comfortably and fixated with their eyes open on a point in front of them for 2 minutes. Waking recordings were collected at 1000 Hz, first-order high-pass filtered (Kaiser type FIR, 0.6 Hz), low-pass filtered (Kaiser type FIR, 59 Hz), and average referenced to the 185 channels directly overlying the scalp. An automatic outlier detection tool based on amplitude threshold criteria was used to detect bad channels and confirmed by visual inspection. Rejected channels were then interpolated using spherical splines. Independent Component Analysis (ICA) as implemented in EEGLAB (Delorme and Makeig, 2004) was used to remove ocular, muscle, and electrocardiograph (ECG) artifacts. Only ICA components with specific activity patterns and component maps characteristic of artifactual activity (Makeig et al., 1997, Jung et al., 2000) were removed (see Supplementary Figure S1 for examples of artifactual components removed). Any remaining artifacts were excluded by visual inspection. After artifact rejection, an average of 79 ± 6 (mean ± s.e.m) seconds of data were used from the presleep condition and 73 ± 7 (mean ± s.e.m) seconds from the postsleep condition. Finally, power spectral analysis was performed on each channel in consecutive 4-second epochs (Welch’s averaged modified periodgram with a Hamming window, frequency resolution of 0.25 Hz). For every channel, the power of each frequency bin was expressed as a percent of the average power for that bin over all channels and conditions (pre- and postsleep).
Sleep Recordings and Scoring
All-night EEG recordings were sampled at 500 Hz, first-order high-pass filtered (Kaiser type FIR, 0.6 Hz), downsampled to 128 Hz (using an anti-aliasing, low-pass FIR filter with a Kaiser window), band-pass filtered (2-way least-squares FIR, 1–40 Hz), and average referenced to the 185 channels directly overlying the scalp. The 2-way least squares filter with a low frequency cutoff of 1 Hz was able to effectively filter any excessive sweat artifact recorded during sleep using the high-impedance, DC system while still maintaining the shape and amplitude characteristics of NREM slow waves. Similar to previous studies (Landsness et al., 2009), artifact rejection and power spectral analysis was performed semi-automatically on each channel in consecutive 6-second epochs (Welch’s averaged modified periodgram with a Hamming window, frequency resolution of 0.16 Hz). For every channel, the power of each frequency bin was expressed as a percent of the total power for all bins contained in an epoch. For correlation analysis, all channels and NREM sleep epochs were averaged together.
Sleep scoring was based on EEG channels F3, F4, C3, C4, O1, and O2, referenced to the contralateral mastoid channel A1 or A2, sub-mental electromyogram, electrooculogram, ECG, and pulse oximetry. Sleep was scored in 30 second epochs according to the American Academy of Sleep Medicine guidelines by an experienced sleep technician (Iber et al., 2007). All subjects had greater than 90% sleep efficiency and normal sleep architecture (Table 1).
Table 1.
Sleep measures for the experimental night. Left column, sleep measure description. Middle column, average value of each sleep measure over all subjects. Right column, standard error of the mean for each sleep measure over all subjects.
| Sleep Measures | Mean | s.e.m. |
|---|---|---|
| Sleep latency (min) | 9.9 | 1.9 |
| Wake after sleep onset (min) | 15.9 | 2.6 |
| Sleep efficiency (%) | 93.8 | 0.6 |
| Arousal Index (# per hr) | 10.8 | 1.7 |
| N1 sleep (min) | 25.9 | 4.1 |
| N2 sleep (min) | 221.1 | 10.7 |
| N3 sleep (min) | 68.8 | 8.2 |
| REM sleep (min) | 80.3 | 5.5 |
| Total sleep time (min) | 396.1 | 6.4 |
Statistics
As in previous studies (Nichols and Holmes, 2002; Huber et al., 2006), suprathreshold cluster tests (STCT) were used to correct for multiple comparisons and assess differences in: AEP amplitude after sleep by sample, GMFP amplitude after sleep by sample, the amplitude of N1 and P2 after sleep by channel, and correlations between AEP measures and topographic changes in spontaneous waking power (unless otherwise stated). For a cluster to be significant, it had to be larger than 95 percent of the clusters in the maximal cluster size distribution. 2-tailed paired t-tests were used to assess changes in N1 and P2 latency with sleep. The dependence between electrophysiological variables was assessed by linear regression. Statistical analysis was performed using MATLAB (The MathWorks, Inc., Natick, MA) and STATISTICA (StatSoft, Inc., Tulsa, OK).
Results
The amplitudes of N1 and P2 are reduced after sleep
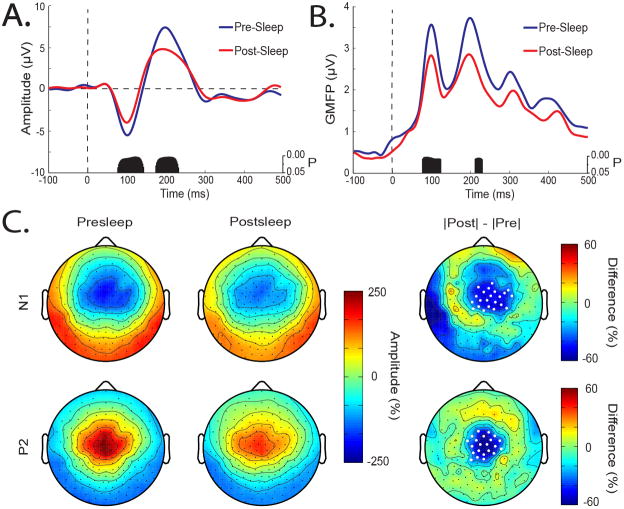
The amplitude of AEPs can be a sensitive measure of plastic changes occurring in auditory cortex (Clapp et al., 2005; Baumann et al., 2008). To test whether plastic processes might be occurring during sleep, we compared pre- and postsleep AEPs (Figure 1A). After averaging across trials, AEPs for each subject were taken from the channel showing the largest N1-P2 complex. Using this channel, a suprathreshold cluster test revealed a significant decline in N1 (78–146 ms) and P2 (176–234 ms) amplitude after sleep. Figure 1B displays GMFP, a measure of global brain activation, for the pre- and postsleep conditions. Similarly, a significant decline in N1 (80–124 ms) and P2 (214–230 ms) amplitude occurred after sleep. Figure 1C displays the AEP topographically using the instantaneous voltage distribution at the N1 and P2 latencies. As shown in Figure 1C, the N1 displayed the typical scalp pattern of fronto-central negativity with polarity reversal over the mastoids, while the P2 displayed central positivity with polarity reversal over the mastoids. After sleep, there was a significant reduction in the amplitude of both N1 and P2 (Figure 1C, right column, white dots mark significantly different channels). There were no significant differences between pre- and postsleep latency for N1 (presleep 105 ± 4 ms, postsleep 108 ± 4 ms, P=0.44, 2-tailed paired t-test) or P2 (presleep 198 ± 6 ms, postsleep 194 ± 8 ms, P=0.50, 2-tailed paired t-test). Taken together, these results indicate that after sleep the amplitude of the N1 and P2 waves of the AEP is attenuated over central scalp regions around their peak latencies.
Figure 1.
Decline in the amplitude of the N1 and P2 waves of the AEP after sleep. (A) Average AEP across subjects for presleep (blue) and postsleep (red) conditions. Tone presented at time 0. Black bars mark samples showing significant differences between pre- and postsleep conditions using a STCT. (B) Same as in A for average GMFP. (C) Instantaneous voltage distribution displayed topographically for N1 (top row) and P2 (bottom row) in the presleep (left column) and postsleep (middle column) conditions averaged across subjects. AEP amplitude was normalized by the average amplitude over both conditions and all channels. Values at each channel (dots) were color code and plotted at their corresponding position on the planer projection of the scalp surface. Right column, pre- to postsleep change in amplitude. Difference calculated as the absolute value of the presleep amplitude subtracted from the absolute value of the postsleep amplitude. White dots mark channels with significant pre- to postsleep differences using a STCT (Nichols and Holmes, 2001).
The power of low-frequency oscillations during NREM sleep predicts the decline in N1 amplitude after sleep
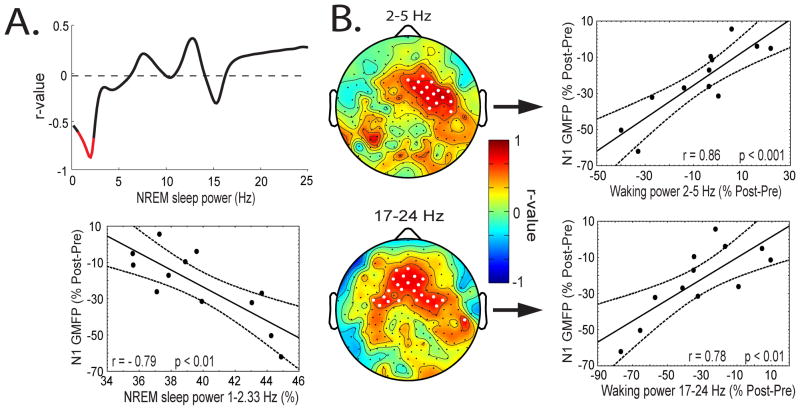
The overnight decline in AEP amplitude, as demonstrated above, suggests that some aspect of sleep may be responsible, though exactly which aspect remains unclear. Previous studies have pointed to specific sleep stages or total sleep time (Sforza and Haba-Rubio, 2006; Turcotte and Bastien, 2009). However, we found no significant relationships between the decline in N1 or P2 amplitude after sleep and total sleep time, sleep stage, or arousal index (Table 1 reports sleep measures for the experimental night). Recent findings have suggested that specific electrophysiological features of sleep, such as slow waves and spindles, may be responsible for these overnight changes (Huber et al., 2004; Vyazovskiy et al., 2007; Landsness et al., 2009). To test this, we correlated the decline in N1 amplitude with NREM sleep power by frequency bin, averaged over all channels. As shown in Figure 2A (top), the decline in N1 amplitude shows a significant negative correlation (marked in red) with the amount of low-frequency power (1–2.33 Hz, r = −0.79, P < 0.01, averaged over bins showing a significant correlation). No significant correlations were found between the decline in P2 amplitude and NREM sleep power. Thus, the more NREM sleep power in the frequency range of slow waves (1 – 2.33 Hz), the more the amplitude of the N1 decreased after sleep.
Figure 2.
Correlation between the decline in N1 GMFP amplitude after sleep and power during sleep and wakefulness. (A) Top, correlation between the decline in the N1 amplitude after sleep and power during NREM sleep by frequency bin, averaged across channels. Significant frequency bins are marked in red. Bottom, scatter plot showing the correlation between the decline in the N1 amplitude and NREM sleep power between 1 and 2.33 Hz. (B) Correlation between the decline in N1 amplitude after sleep and the pre- to postsleep change in spontaneous waking power between 2–5 Hz (top) and 17–24 Hz (bottom). Left, r-values plotted topographically. White dots mark channels showing significant correlations (top, STCT; bottom, uncorrected for multiple comparisons). Right, scatter plot, power average across channels showing a significant correlation. Pre- to postsleep differences calculated as postsleep values subtracted from presleep values. The decline in N1 GMFP amplitude was expressed as the percent difference from the mean (postsleep minus presleep). For normalization of waking power, see methods.
The decline in N1 amplitude correlates with the pre- to postsleep change in spontaneous waking power
Since spontaneous waking power in the EEG also changes as a function of the sleep-wake history (Cajochen et al., 2002), we correlated the pre- to postsleep decline in N1 amplitude with the pre- to postsleep change in spontaneous waking power at each channel for 1 Hz bins from 1 to 25 Hz. As shown in Figure 2B, two bands display significant correlations. The change in power between 2 and 5 Hz showed a significant positive correlation with the decline in N1 amplitude in a cluster of frontal channels (Figure 2B, top, STCT, white dots mark significantly different channels, r = 0.86, P < 0.001, power averaged over channels showing a significant correlation). The change in power between 17 and 24 Hz also showed a positive correlation with the decline in N1 amplitude after sleep also over frontal regions (Figure 2B, bottom, uncorrected for multiple comparisons, white dots mark significant channels, r = 0.78, P < 0.01, power averaged over channels showing a significant correlation). That is, the larger the decline in N1 amplitude after sleep, the larger was the decline in overnight waking power over frontal regions between 2 and 5 Hz and 17 and 24 Hz.
The power of low-frequency oscillations during NREM sleep predicts the pre- to postsleep change in spontaneous waking power
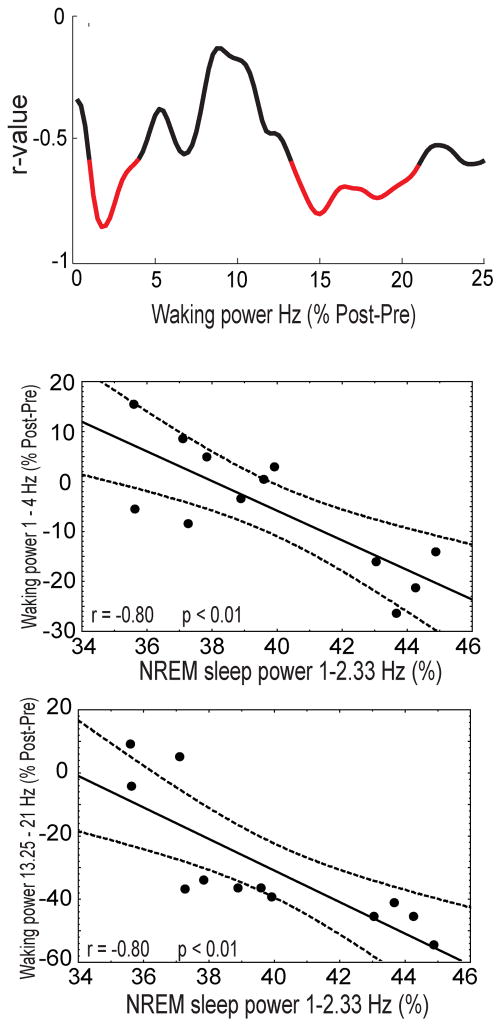
Because of previous reports (Finelli et al., 2000; Vyazovskiy and Tobler, 2005) and the ability of low-frequency oscillations during NREM sleep to predict the decline in N1 amplitude after sleep, we tested whether NREM sleep power (1–2.33 Hz) correlates with the change in spontaneous waking power. To do so, we correlated NREM sleep power (1–2.33 Hz) averaged over all channels with the change in waking power by frequency bin, averaged over all channels. As shown in Figure 3 (top), two main frequency bands show significant negative correlations. We found that the greater the power of low-frequency oscillations during NREM sleep, the larger the overnight decline in spontaneous waking power between 1 and 4 Hz (r = 0.80, P < 0.01, Figure 3, middle) and 13.25 and 21 Hz (r=0.80, P<0.01, Figure 3 bottom).
Figure 3.
Correlation between NREM sleep power and the pre- to postsleep change in spontaneous waking power. Top, correlation between NREM sleep power in the 1–2.33 Hz band and the change in spontaneous waking power after sleep by frequency bin averaged across channels (postsleep minus presleep). Significant bins are marked in red. Middle, scatter plot of NREM sleep power (1–2.33 Hz) and the percent change in spontaneous waking power (1–4 Hz). Bottom, scatter plot of NREM sleep power (1–2.33 Hz) and the percent change in spontaneous waking power (13.25–21 Hz).
Discussion
The present results provide evidence for a decline in the amplitude of the N1 and P2 waves of the human AEP after sleep. Since the EEG mostly reflects the summed action of currents generated by synchronously occurring postsynaptic potentials in cortex (Nunez and Srinivasan, 2006), the decline in AEP amplitude after sleep could reflect a reduction in the number, size, or synchrony of postsynaptic potentials evoked by auditory stimuli. Consistent with this interpretation, recent evidence shows that miniature excitatory postsynaptic currents are larger and more frequent after periods of wakefulness compared to after periods of sleep in cortical slices taken from rats and mice (Liu et al., 2010). In addition, the GluR1 subunit of α-amino-3-hydroxy- 5-methyl-4-isoxazolepropionic acid (AMPA) receptors, whose delivery to excitatory synapses is associated with an increase in synaptic strength, is enriched in synaptoneurosomes taken from the cortex of rats that have been mostly awake relative to those that have been mostly asleep (Vyazovskiy et al., 2007). Moreover, the mean firing rate of cortical neurons is high after periods of wakefulness and low after periods of sleep, indicating a generalized increase in the number of postsynaptic potentials impinging on cortical neurons after wakefulness and reduction after sleep (Vyazovskiy et al., 2009). Changes in the frequency and size of postsynaptic potentials may have an effect on the synchronization of cortical neurons. Indeed, during NREM sleep, the slope of individual sleep slow waves decreases, reflecting a decline in the synchrony of up-to-down and down-to-up state transitions (Riedner et al., 2007; Vyazovskiy et al., 2009).
Taken together, these studies indicate that synaptic strength may vary as a function of the sleep-wake cycle, as predicted by a recent hypothesis on sleep function, the synaptic homeostasis hypothesis. Specifically, the hypothesis predicts that plastic processes occurring during wakefulness lead to a net increase in synaptic strength in cortex, which is renormalized to baseline levels during sleep by slow waves (Tononi and Cirelli, 2006). In agreement with the hypothesis, the pre- to postsleep decline in N1 amplitude strongly correlated with NREM sleep power in the frequency range of slow waves, between 1 and 2.33 Hz. A similar result has been observed in rats, where the reduction in SWA from early to late sleep predicted the pre- to postsleep decline in evoked potential slope (Vyazovskiy et al., 2007). The fact that the correlation was restricted to 1 to 2.33 Hz of the SWA range (0.5 – 4.5 Hz) is likely due to two factors. First, a 1 Hz high-pass filter was used, effectively eliminating any correlation that may occur below 1 Hz. Secondly, compared to the upper-portion of the SWA band, the 1 to 2.33 Hz band is closer to the frequency of the neuronal slow oscillation that underlies sleep slow waves. Interestingly, although the pre- to postsleep decline in N1 amplitude correlated with low-frequency power during NREM sleep, the pre- to postleep decline in P2 amplitude did not. Why this should be the case is unclear, but several lines of evidence suggest that N1 and P2 are independent processes with different neuronal generators (Crowley and Colrain, 2004).
Studies measuring waking EEG power during sleep deprivation have found consistent increases in low- (<9 Hz) and higher-frequency (13 – 25) power (Aeschbach et al., 1999; Cajochen et al., 2002; Tinguely et al., 2006). Two studies have established a correlation between the rise in low-frequency power in wakefulness and the subsequent increase in SWA during NREM sleep in humans and rats (Finelli et al., 2000; Vyazovskiy and Tobler, 2005). In humans, the rise in low- and high-frequency waking power is largest over frontal areas, as is the subsequent increase in SWA during sleep, suggesting that frontal cortex is especially sensitive to sleep deprivation (Tinguely et al., 2006). In the present study, we found that the larger the decline in N1 amplitude after sleep, the larger was the decline in low- (2 –5 Hz) and high-frequency (17 – 25 Hz) waking power over frontal regions. Therefore, the amplitude of N1 and low- and high-frequency power during waking likely reflect the same process, sleep homeostasis. Consistent with this interpretation, we found that the power of low-frequency oscillations during NREM sleep also predicted the pre- to postsleep decline in low- and high-frequency waking power.
Although the decline in AEP amplitude with sleep and its correlation with established measures of sleep homeostasis suggests the decline is due to a reduction in synaptic strength, other explanations are possible. Changes in attention and arousal are known to affect AEP amplitude. Increased attentional effort is thought to increase N1 amplitude and decrease P2 amplitude by producing a broad negativity in the time window of N1 and P2 (Näätänen and Picton, 1987; Crowley and Colrain, 2004). However, we found no evidence for such a negativity, as both N1 and P2 amplitude declined. Sleep inertia, a period of reduced arousal after awakening from sleep, has been shown to decrease N1-P2 amplitude and increase N1 latency (Ferrara et al., 2002). However, our subjects were given suitable time to combat sleep inertia (Tassi and Muzet, 2000), and the latencies of N1 and P2 did not change after sleep. A reduction in sleep time may also lead to a reduction in the subjects’ level of arousal. In this study, subjects spent ~7 hours and 15 minutes in bed, which was less than their self-reported sleep time of 8 hours. However, as there was no correlation between the postsleep decline in AEP amplitude and total sleep time, sleep stage, or arousal index, it is unlikely that 45 minutes less sleep negatively impacted these results. Moreover, in a subset of subjects (N=7), there were no significant differences between pre- and postsleep performance on a objective measure of sleepiness, the psychomotor vigilance task (Dinges et al., 1997), using measures of average reaction time, number of lapses, and average reaction time for the slowest 10 percent of trials (data not shown). Therefore, it is unlikely the observed decline in AEP amplitude after sleep is due to difference in attention or arousal state. Another possibility is that differences in circadian time are responsible for the decline in AEP amplitude. Although this possibility cannot be excluded in the present study, the ability of low-frequency power during NREM sleep to predict the decline in N1 amplitude argues in favor of the homeostatic interpretation given above.
In summary, these findings suggest that the amplitude of the human AEP declines with sleep. The decline likely reflects a reduction in the number, size, or synchrony of postsynaptic potentials evoked by auditory stimuli, providing further evidence for a connection between synaptic plasticity and sleep homeostasis. In agreement with this interpretation, the overnight decline in N1 amplitude correlated with several measures of sleep homeostasis. More work is needed to investigate whether sensory evoked potentials in other modalities also show sleep-wake history dependent modulation and to separate the contribution of homeostatic and circadian processes.
Supplementary Material
Examples of artifactual components removed from spontaneous waking recordings using independent component analysis (ICA). For each column, upper-left panel is the component’s activity plotted topographically. Upper-right panel is the component’s activity in time. Lower panel is the component’s power spectrum. Left column, eye movements. Middle column, muscle activity. Right column, cardiac activity.
Acknowledgments
This research was funded by the National Institute of Mental Health (5T20MH077967 to GT and F30MH082601 to EL). The authors thank Jennifer Noe and Kate Sprecher for their logistical contributions, the research assistants for their role in data collection, and the polysomnographic technologists from Wisconsin Sleep for working with participants overnight and scoring the sleep recordings. Dr. Tononi has consulted for Sanofi-Aventis and Takeda, and he is currently the David P. White Chair in Sleep Medicine at the University of Wisconsin – Madison, endowed by Phillips Respironics. Dr. Tononi has also received unrelated research support from Phillips Respironics.
Footnotes
The other authors have indicated no financial conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aeschbach D, Matthews JR, Postolache TT, Jackson MA, Giesen HA, Wehr TA. Two circadian rhythms in the human electroencephalogram during wakefulness. Am J Physiol. 1999;227(6 Pt 2):R1771–9. doi: 10.1152/ajpregu.1999.277.6.R1771. [DOI] [PubMed] [Google Scholar]
- Amzica F, Steriade M. Electrophysiological correlates of sleep delta waves. Electroenceph Clin Neurophysiol. 1998;107(2):69–83. doi: 10.1016/s0013-4694(98)00051-0. [DOI] [PubMed] [Google Scholar]
- Baumann S, Meyer M, Jäncke L. Enhancement of Auditory-evoked Potentials in Musicians Reflects an Influence of Expertise but not Selective Attention. J Cogn Neurosci. 2008;20(12):2238–49. doi: 10.1162/jocn.2008.20157. [DOI] [PubMed] [Google Scholar]
- Borbély AA. A Two Process Model of Sleep Regulation. Hum Neurobiol. 1982;1(3):195–204. [PubMed] [Google Scholar]
- Cajochen C, Wyatt JK, Czeisler CA, Dijk DJ. Separation of Circadian and Wake Duration-Dependent Modulation of EEG Activation During Wakefulness. Neuroscience. 2002;114(4):1047–60. doi: 10.1016/s0306-4522(02)00209-9. [DOI] [PubMed] [Google Scholar]
- Clapp WC, Kirk IJ, Hamm JP, Shepherd D, Teyler TJ. Induction of LTP in the human auditory cortex by sensory stimulation. Eur J Neurosci. 2005;22(5):1135–40. doi: 10.1111/j.1460-9568.2005.04293.x. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: age, sleep and modality. Clin Neurophysiol. 2004;115(4):732–44. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Dinges DF, Pack F, Williams K, Gillen KA, Powell JW, Ott GE, et al. Cumulative sleepiness, mood disturbance, and psychomotor vigilance performance decrements during a week of sleep restricted to 4–5 hours per night. Sleep. 1997;20(4):267–277. [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ferrara M, Gennaro LDL, Ferlazzo F, Curcio G, Cristiani R, Bertini M. Topographical changes in N1-P2 amplitude upon awakening from recovery sleep after slow-wave sleep deprivation. Clin Neurophysiol. 2002;113(8):1183–90. doi: 10.1016/s1388-2457(02)00146-3. [DOI] [PubMed] [Google Scholar]
- Finelli LA, Baumann H, Borbély AA, Achermann P. Dual Electroencephalogram Markers of Human Sleep Homeostasis: Correlation Between Theta Activity in Waking and Slow-Wave Activity in Sleep. Neuroscience. 2000;101(3):523–9. doi: 10.1016/s0306-4522(00)00409-7. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Tononi G. Local sleep and learning. Nature. 2004;430(6995):78–81. doi: 10.1038/nature02663. [DOI] [PubMed] [Google Scholar]
- Huber R, Ghilardi MF, Massimini M, Ferrarelli F, Riedner BA, Peterson MJ, et al. Arm immobilization causes cortical plastic changes and locally decreases sleep slow wave activity. Nature Neurosci. 2006;9(9):1169–76. doi: 10.1038/nn1758. [DOI] [PubMed] [Google Scholar]
- Huber R, Esser SK, Ferrarelli F, Massimini M, Peterson MJ, Tononi G. TMS-Induced Cortical Potentiation during Wakefulness Locally Increases Slow Wave Activity during Sleep. Plos One. 2007;2(3):e276. doi: 10.1371/journal.pone.0000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iber C, Ancoli-Israel S, Chessonn A, Quan S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. 1. Westchester: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37(2):163–78. [PubMed] [Google Scholar]
- Landsness EC, Crupi D, Hulse BK, Peterson MJ, Huber R, Ansari H, et al. Sleep-Dependent Improvement in Visuomotor Learning: A Causal Role for Slow Waves. Sleep. 2009;32(10):1273–84. doi: 10.1093/sleep/32.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ZW, Faraguna U, Cirelli C, Tononi G, Gao XB. Direct Evidence for Wake-Related Increases and Sleep-Related Decreases in Synaptic Strength in Rodent Cortex. J Neurosci. 2010;30(25):8671–5. doi: 10.1523/JNEUROSCI.1409-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S, Jung TP, Bell AJ, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proc Natl Acad Sci U S A. 1997;94(20):10979–84. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimini M, Tononi G, Huber R. Slow waves, synaptic plasticity and information processing: insights from transcranial magnetic stimulation and high-density EEG experiments. Eur J Neurosci. 2009;29(9):1761–70. doi: 10.1111/j.1460-9568.2009.06720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Picton T. The N1 Wave of the Human Electric and Magnetic Response to Sound: A Review and an Analysis of the Component Structure. Psychophysiology. 1987;24(4):375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric Permutation Tests For Functional Neuroimaging: A Primer with Examples. Hum Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez PL, Srinivasan R. Electric Fields of the Brain: The Neurophysics of EEG. 2. New York: Oxford University Press; 2006. [Google Scholar]
- Pantev C, Wollbrink A, Roberts LE, Engelien A, Lütkenhöner B. Short-term plasticity of the human auditory cortex. Brain Res. 1999;842(1):192–9. doi: 10.1016/s0006-8993(99)01835-1. [DOI] [PubMed] [Google Scholar]
- Riedner BA, Vyazovskiy VV, Huber R, Massimini M, Esser S, Murphy M, et al. Sleep homeostasis and cortical synchronization: III. A high-density EEG study of sleep slow waves in humans. Sleep. 2007;30(12):1643–57. doi: 10.1093/sleep/30.12.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusterholz T, Dürr R, Achermann P. Inter-individual Differences in the Dynamics of Sleep Homeostasis. Sleep. 2010;33(4):491–8. doi: 10.1093/sleep/33.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sforza E, Haba-Rubio J. Event-Related Potentials in Patients With Insomnia and Sleep-Related Breathing Disorders: Evening-to-Morning Changes. Sleep. 2006;29(6):805–13. doi: 10.1093/sleep/29.6.805. [DOI] [PubMed] [Google Scholar]
- Steriade M, Nuñez A, Amzica F. A Novel Slow (< 1 Hz) Oscillation of Neocortical Neurons in vivo: Depolarizing and Hyperpolarizing Components. J Neurosci. 1993;13(8):3252–65. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4(4):341–53. doi: 10.1053/smrv.2000.0098. [DOI] [PubMed] [Google Scholar]
- Tinguely G, Finelli LA, Landolt HP, Borbély AA, Achermann P. Functional EEG topography in sleep and waking: State-dependent and state-independent features. Neuroimage. 2006;32(1):283–92. doi: 10.1016/j.neuroimage.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49–62. doi: 10.1016/j.smrv.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Tremblay K, Kraus N, McGee T, Ponton C, Otis B. Central Auditory Plasticity: Changes in the N1-P2 Complex after Speech-Sound Training. Ear Hear. 2001;22(2):79–90. doi: 10.1097/00003446-200104000-00001. [DOI] [PubMed] [Google Scholar]
- Turcotte I, Bastien CH. Is quality of sleep related to the N1 and P2 ERPs in chronic psychophysiological insomnia sufferers? Int J Psychophysiol. 2009;72(3):314–22. doi: 10.1016/j.ijpsycho.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Tobler I. Theta activity in the waking EEG is a marker of sleep propensity in the rat. Brain Res. 2005;1050(1–2):64–71. doi: 10.1016/j.brainres.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Cirelli C, Pfister-Genskow M, Faraguna U, Tononi G. Molecular and electrophysiological evidence for net synaptic potentiation in wake and depression in sleep. Nat Neurosci. 2008;11(2):200–8. doi: 10.1038/nn2035. [DOI] [PubMed] [Google Scholar]
- Vyazovskiy VV, Olcese U, Lazimy YM, Faraguna U, Esser SK, Williams JC, et al. Cortical Firing and Sleep Homeostasis. Neuron. 2009;63(6):865–78. doi: 10.1016/j.neuron.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth E, Dijk DJ, Achermann P, Borbély AA. Dynamics of the sleep EEG after an early evening nap: Experimental data and simulations. Am J Physiol Regul Integr Comp Physiol. 1996;271(3):R501–R10. doi: 10.1152/ajpregu.1996.271.3.R501. [DOI] [PubMed] [Google Scholar]
- Zaehle T, Clapp WC, Hamm JP, Meyer M, Kirk IJ. Induction of LTP-like changes in human auditory cortex by rapid auditory stimulation: An FMRI study. Restor Neurol Neurosci. 2007;25(3–4):251–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Examples of artifactual components removed from spontaneous waking recordings using independent component analysis (ICA). For each column, upper-left panel is the component’s activity plotted topographically. Upper-right panel is the component’s activity in time. Lower panel is the component’s power spectrum. Left column, eye movements. Middle column, muscle activity. Right column, cardiac activity.