Abstract
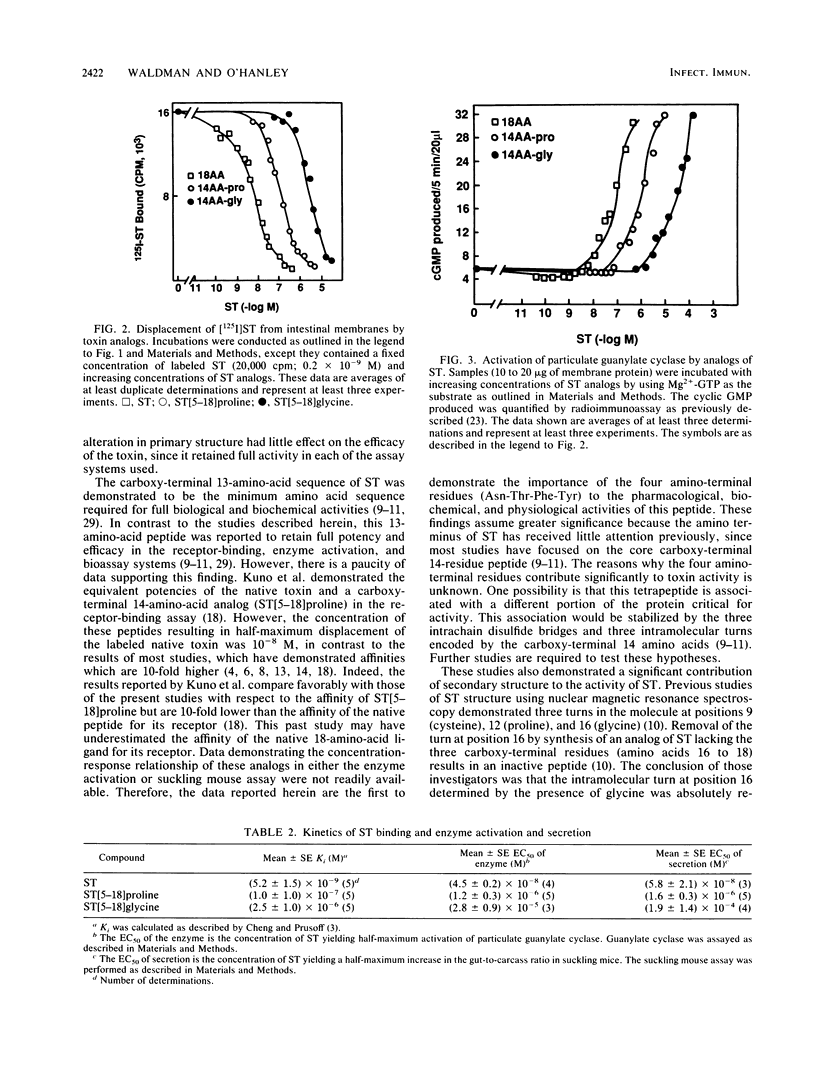
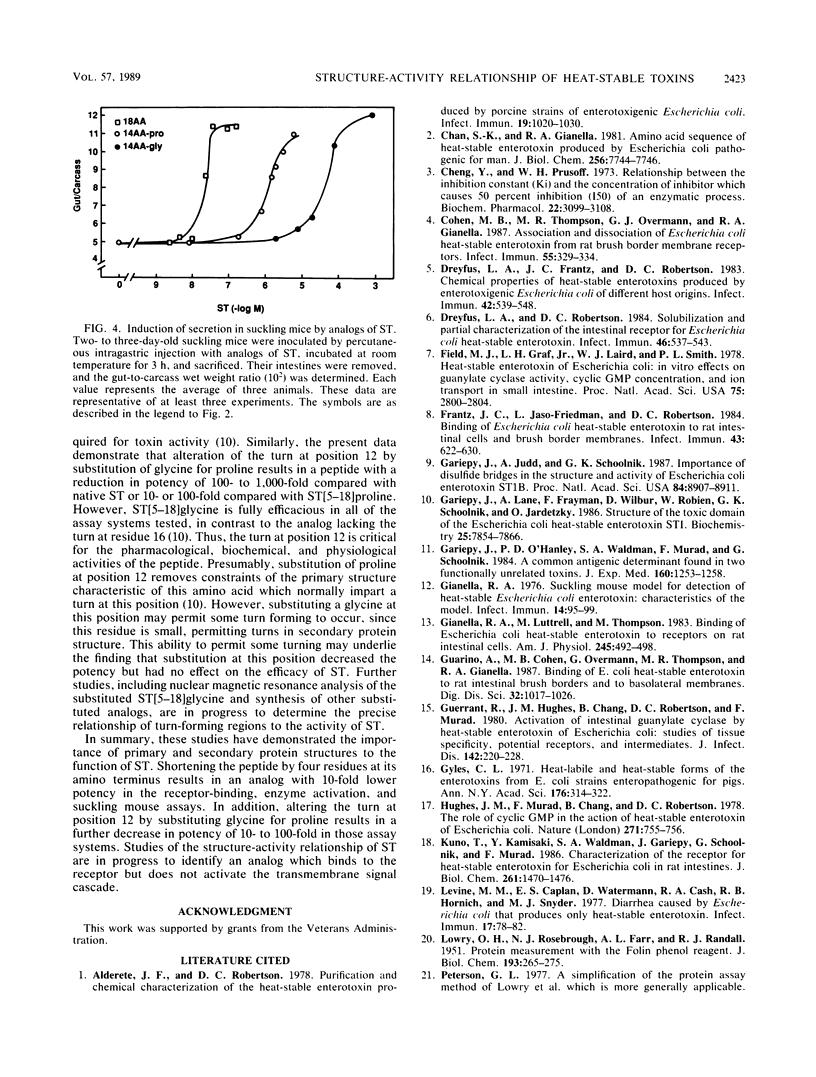
Analogs of Escherichia coli heat-stable enterotoxin (ST) differing in chain length or the presence of turn-forming residues were assessed for binding to receptors, activation of particulate guanylate cyclase, and stimulation of secretion in suckling mice. These analogs included the native 18-amino-acid peptide (ST), the 14-amino-acid carboxy terminus of this native peptide with a proline at position 12 (ST[5-18]proline), and the 14-amino-acid carboxy terminus in which the proline at position 12 was substituted with glycine (ST[5-18]glycine). Each analog bound to the receptor in a dose-dependent fashion, completely displacing [125I]ST in competitive binding assays. However, their potencies differed significantly: ST demonstrated the highest affinity (inhibition constant [Ki], 10(-9) M), followed by ST[5-18]proline (Ki, 10(-7) M) and ST[5-18]glycine (Ki, 10(-6) M). Similarly, these peptides maximally activated particulate guanylate cyclase and stimulated intestinal secretion in suckling mice. Their rank order of potency in these assays was similar to that described for receptor binding: ST greater than ST[5-18]proline greater than ST[5-18]glycine. These data demonstrate that the full peptide structure is not absolutely required for pharmacological, biochemical, or biological activity. However, the four amino-terminal residues contribute significantly to the potency of these peptides. In addition, the turn imposed by the proline residue at position 12 is not absolutely required for receptor occupancy or activation of the biochemical cascade that results in intestinal secretion. However, it significantly increases the potency of the toxin. These data illustrate the importance of primary and secondary structures to the biochemical, pharmacological, and physiological activities of the ST produced by E. coli.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Robertson D. C. Purification and chemical characterization of the heat-stable enterotoxin produced by porcine strains of enterotoxigenic Escherichia coli. Infect Immun. 1978 Mar;19(3):1021–1030. doi: 10.1128/iai.19.3.1021-1030.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S. K., Giannella R. A. Amino acid sequence of heat-stable enterotoxin produced by Escherichia coli pathogenic for man. J Biol Chem. 1981 Aug 10;256(15):7744–7746. [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cohen M. B., Thompson M. R., Overmann G. J., Giannella R. A. Association and dissociation of Escherichia coli heat-stable enterotoxin from rat brush border membrane receptors. Infect Immun. 1987 Feb;55(2):329–334. doi: 10.1128/iai.55.2.329-334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus L. A., Frantz J. C., Robertson D. C. Chemical properties of heat-stable enterotoxins produced by enterotoxigenic Escherichia coli of different host origins. Infect Immun. 1983 Nov;42(2):539–548. doi: 10.1128/iai.42.2.539-548.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus L. A., Robertson D. C. Solubilization and partial characterization of the intestinal receptor for Escherichia coli heat-stable enterotoxin. Infect Immun. 1984 Nov;46(2):537–543. doi: 10.1128/iai.46.2.537-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz J. C., Jaso-Friedman L., Robertson D. C. Binding of Escherichia coli heat-stable enterotoxin to rat intestinal cells and brush border membranes. Infect Immun. 1984 Feb;43(2):622–630. doi: 10.1128/iai.43.2.622-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy J., O'Hanley P., Waldman S. A., Murad F., Schoolnik G. K. A common antigenic determinant found in two functionally unrelated toxins. J Exp Med. 1984 Oct 1;160(4):1253–1258. doi: 10.1084/jem.160.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy J., Judd A. K., Schoolnik G. K. Importance of disulfide bridges in the structure and activity of Escherichia coli enterotoxin ST1b. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8907–8911. doi: 10.1073/pnas.84.24.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariépy J., Lane A., Frayman F., Wilbur D., Robien W., Schoolnik G. K., Jardetzky O. Structure of the toxic domain of the Escherichia coli heat-stable enterotoxin ST I. Biochemistry. 1986 Dec 2;25(24):7854–7866. doi: 10.1021/bi00372a011. [DOI] [PubMed] [Google Scholar]
- Giannella R. A. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect Immun. 1976 Jul;14(1):95–99. doi: 10.1128/iai.14.1.95-99.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A., Cohen M. B., Overmann G., Thompson M. R., Giannella R. A. Binding of E. coli heat-stable enterotoxin to rat intestinal brush borders and to basolateral membranes. Dig Dis Sci. 1987 Sep;32(9):1017–1026. doi: 10.1007/BF01297193. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Hughes J. M., Chang B., Robertson D. C., Murad F. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates. J Infect Dis. 1980 Aug;142(2):220–228. doi: 10.1093/infdis/142.2.220. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Kuno T., Kamisaki Y., Waldman S. A., Gariepy J., Schoolnik G., Murad F. Characterization of the receptor for heat-stable enterotoxin from Escherichia coli in rat intestine. J Biol Chem. 1986 Jan 25;261(3):1470–1476. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Levine M. M., Caplan E. S., Waterman D., Cash R. A., Hornick R. B., Snyder M. J. Diarrhea caused by Escherichia coli that produce only heat-stable enterotoxin. Infect Immun. 1977 Jul;17(1):78–82. doi: 10.1128/iai.17.1.78-82.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Staples S. J., Asher S. E., Giannella R. A. Purification and characterization of heat-stable enterotoxin produced by a strain of E. coli pathogenic for man. J Biol Chem. 1980 May 25;255(10):4716–4721. [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Thompson M. R., Giannella R. A. Revised amino acid sequence for a heat-stable enterotoxin produced by an Escherichia coli strain (18D) that is pathogenic for humans. Infect Immun. 1985 Mar;47(3):834–836. doi: 10.1128/iai.47.3.834-836.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman S. A., Kuno T., Kamisaki Y., Chang L. Y., Gariepy J., O'Hanley P., Schoolnik G., Murad F. Intestinal receptor for heat-stable enterotoxin of Escherichia coli is tightly coupled to a novel form of particulate guanylate cyclase. Infect Immun. 1986 Jan;51(1):320–326. doi: 10.1128/iai.51.1.320-326.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman S. A., Murad F. Cyclic GMP synthesis and function. Pharmacol Rev. 1987 Sep;39(3):163–196. [PubMed] [Google Scholar]
- Waldman S. A., O'Hanley P., Falkow S., Schoolnik G., Murad F. A simple, sensitive, and specific assay for the heat-stable enterotoxin of Escherichia coli. J Infect Dis. 1984 Jan;149(1):83–89. doi: 10.1093/infdis/149.1.83. [DOI] [PubMed] [Google Scholar]
- Yoshimura S., Ikemura H., Watanabe H., Aimoto S., Shimonishi Y., Hara S., Takeda T., Miwatani T., Takeda Y. Essential structure for full enterotoxigenic activity of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli. FEBS Lett. 1985 Feb 11;181(1):138–142. doi: 10.1016/0014-5793(85)81129-7. [DOI] [PubMed] [Google Scholar]



