Abstract
Peroxynitrite (PN, ONOO−) and its reactive oxygen precursor superoxide (SO, O2·−), are critically important in the development of pain of several etiologies including in the development of pain associated with chronic use of opiates such as morphine (also known as opiate-induced hyperalgesia and antinociceptive tolerance). This is now an emerging field in which considerable progress has been made in terms of understanding the relative contribution of SO, PN, and nitroxidative stress in pain signaling at the molecular and biochemical levels. Aggressive research in this area is poised to provide the pharmacological basis for development of novel non-narcotic analgesics that are based upon the unique ability to selectively eliminate SO and/or PN. As we have a better understanding of the role of SO and PN in pathophysiological settings, targeting PN may be a better therapeutic strategy than targeting SO. This is due to the fact that unlike PN, which has no currently known beneficial role, SO may play a significant role in learning and memory [1]. Thus, the best approach may be to spare SO while directly targeting its downstream product, PN. Over the last 15 years, our team has spearheaded research concerning the roles of SO/PN in pain and these results are currently leading to the development of solid therapeutic strategies in this important area.
Keywords: superoxide, peroxynitrite, pain, superoxide dismutase mimetics, peroxynitrite decomposition catalysts, rostral ventromedial medulla (RVM), central sensitization, glutamatergic neurotransmission, neuroimmune activation
Introduction
Pain that is refractory to the typical arsenal of analgesic drugs is a major health issue in the US [2]. While selective cyclooxygenase-2 (COX-2) inhibitors can be effective in certain types of chronic pain, their now well-document side-effects including increased risks of heart attack and stroke [3] limit their use. While opioid narcotics such as the mainstay morphine are the most effective treatments for acute and chronic severe pain, their clinical utility is nearly always hampered by the development of analgesic tolerance as well as painful hypersensitivity known now as morphine-induced hyperalgesia [4–6]. Typically the development of tolerance to morphine treatment necessitates escalating doses to achieve equivalent pain relief [7]. Unfortunately, with extended treatment, the onset of morphine-induced hypersensitivity counteracts the therapeutic impact of such dose increases [4–6]. Thus, in order to maintain the control of chronic severe pain in a growing number of older patients with a variety of complicating conditions, opioid treatment leads to debilitating side effects of oversedation, reduced physical activity, respiratory depression, constipation, and even potential for addiction [7]. Therefore, we have been keenly interested in developing new approaches that would maintain opiate efficacy during chronic dosing without engendering tolerance or unacceptable side effects. Considerable evidence implicates SO and PN [formed from the diffusion controlled reaction of SO with nitric oxide (NO)] in the development of chronic pain, the transition of acute to chronic pain, as well as opiate-induced hyperalgesia and antinociceptive tolerance. Thus, PN appears to be a key toxic mediator for target-based therapeutic strategies. To date, a number of approaches for the development of metal-based catalysts and non-metal scavenger systems [8–9] have been reported to effectively prevent the formation of PN through the dismutation of SO (superoxide dismutase mimetics, SODms) or to decompose PN once it is formed (PN-decomposition catalysts, PNDCs) [10–14]. The contributions of SO and PN to the development of peripheral and central sensitization associated with pain are also adding evidence that these species are novel targets for pain management (Figure 1). Importantly, numerous studies demonstrate that pharmacologic inhibition of SO and PN can prevent and reverse the characteristic pathologies associated with inflammatory pain, neuropathic pain, and morphine-induced hyperalgesia and tolerance.
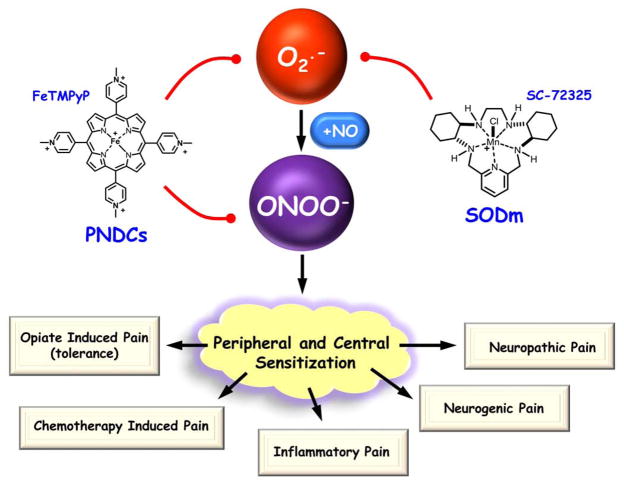
Fig. 1. Superoxide and peroxynitrite are targets for novel pain therapy.
Superoxide (O2·−) and peroxynitrite (ONOO−) are key mediators in the development of peripheral and central sensitization of the various pain etiologies. The use of superoxide-dismutase mimetics (SODm, i.e. SC-72325) and peroxynitrite decomposition catalysts (PNDCs, i.e. FeTMPyP) reduce nitroxidative stress and attenuate the development of peripheral and central sensitization; providing promising novel therapy for chronic pain management.
Alterations in glutamatergic neurotransmission and neuroinflammation as well as modulation of ion channels such as the transient receptor potential cation channel, subfamily V, member 1 (TRPV1) underlie the development of central sensitization (a putative pathophysiologic state underlying persistent and chronic pain) associated with acute and chronic inflammatory and non-inflammatory neuropathic pain. These alterations take place in the periphery, in the spinal cord, and at supraspinal sites such as in the RVM. A comprehensive analysis in the scientific domain as it pertains to pain and non-pain related areas reveals that each of these signaling pathways can be affected by SO and PN. To fully appreciate the roles of SO and PN in pain, this review will discuss their involvement in pain signaling. It is not our intention to discuss the chemistry of SODm or PNDCs since this will be covered in this special issue of FRBM. In addition, it is not our goal to discuss the relative contribution of NO in pain signaling as this topic has been covered for over a decade. More importantly, it has become apparent that therapeutic strategies targeting this reactive oxygen species have not yet yielded successful outcomes. We will briefly discuss potential pharmacologic alternatives that target PN and will suggest future directions in nitroxidative pain research based upon collective scientific outcomes in the field.
Brief overview of existing therapeutic strategies targeting superoxide and peroxynitrite
Improvement in our knowledge of the mechanisms underlying the transition of acute to chronic pain, the formation and exacerbation of neuropathic pain and the development of opioid-induced tolerance and hyperalgesia, would have a major impact in the treatment of pain in general. Over the last decade, our research efforts have established the key role of SO and PN in the development of pain of several etiologies. This research has provided the foundation for improving our mechanistic knowledge regarding the formation and maintenance of chronic pain states but has also illuminated a promising strategy for treatment of these conditions; namely the eradication of PN through prevention of formation or catalyzed decomposition. The most promising class of SO scavengers is comprised of compounds that are essentially functional mimetics of the SOD enzymes. To date, two classes have emerged as promising candidates for potential clinical agents and important pharmacological tools. These are iron (III) and manganese (III) complexes of the synthetic porphyrins (e.g. MnTE-2-PyP5+, FeTM-4-PyP5+), and functionalized manganese (II) polyazamacrocycles (e.g. SC-72325) [9, 15–16]. Both of these very distinct classes of metal complexes catalyze the disproportionation of SO to hydrogen peroxide and molecular oxygen. While most of the manganese (II) polyazamacrocycles have little reactivity toward PN, the metalloporphyrins highlighted above are potent PNDCs [9, 15–16]. Thus, most metalloporphyrins possess dual SODm and PN decomposing activities.
Roles of superoxide and peroxynitrite in pain
Superoxide and PN have emerged as powerful pronociceptive reactive oxygen and nitrogen species [17–18]. This realization was achieved by use of SODm such as SC-72325 or PNDCs including FeTM-4-PyP5+ and MnTE-2-PyP5+ as pharmacological tools to identify them and to dissect signaling pathways. Direct contribution of SO and PN was demonstrated by showing that intraplantar injection of SO or PN leads to the development of hyperalgesia [19–20]. Subsequent studies revealed that increased formation of SO/PN is critically important in the development of thermal hyperalgesia associated with acute and chronic inflammation [19–24], in response to spinal activation of the N-methyl-D-aspartate receptor (NMDAR) [25], in the development of orofacial pain [24] and in the development of opiate-induced hyperalgesia and antinociceptive tolerance [26–28]. An imbalance between oxidant/antioxidant activities has also been observed in other models of inflammatory nociception. For example, increased levels of hydrogen peroxide (the dismutated product of SO) [29] and decreased levels of SOD activity in the spinal trigeminal nucleus coincided with facial hyperalgesia induced by a formalin injection into the lip [30]. Superoxide is also increased in dorsal horn neurons during neuropathic pain induced by spinal nerve ligation [31] and neurogenic-induced hyperalgesia via capsaicin administration[32]. Importantly, the PNDCs evaluated to date, synergize with non-selective COX-1/COX-2 inhibitors, selective COX-2 inhibitors [20], and opiates (Salvemini, manuscript in preparation). This has an enormous added advantage, as it would allow for the possibility of increasing the efficacy of these drugs at much lower doses thereby reducing their well-documented side-effect profile [33–34]. These findings led us to put forth the hypothesis that targeting SO and PN should lead to development of novel analgesics for the management of pain [17–18]. Importantly, it should be noted that SO and PN have no role in acute and thus beneficial physiological nociception [19–20]. A role for nitroxidative stress (herein defined as stress induced in the presence of SO, PN and related species) was supported using a variety of non-selective agents such as phenyl N-tert-butylnitrone (PBN) and 4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl (TEMPOL) [15, 17, 35]. These agents showed efficacy in inflammatory [22] and neurogenic pain [32, 36–38], visceral pain [39], neuropathic pain [31, 37, 40–41], and chemotherapy-induced pain [42]. As we emphasized [9, 35, 43], non-selective agents such as TEMPOL or PBN cannot be used to delineate the contribution of a specific nitroxidative species (i.e. SO vs. PN), since these agents will remove many reactive oxygen and nitrogen species including but not limited to NO, SO, PN and hydroxyl radicals [9, 35, 43]. When using such non-selective probes (for example TEMPOL or PBN) it is important to discuss results in terms of “nitroxidative stress and nitroxidative species” rather than implicating a specific species such as SO or PN. This would lead to inappropriate implication of one species versus another in a particular setting- for instance results obtained with TEMPOL implicate nitroxidative species and not SO (SO would only be one component) and results should be discussed accordingly to avoid misinterpretations.
Enzymatic pathways leading to overt formation of superoxide and peroxynitrite in pain
Unraveling the enzymatic sources that produce SO and PN and understanding the signaling pathways engaged by these species in nociceptive processing is of paramount importance [17–18]. Inactivation of mitochondrial manganese superoxide dismutase (MnSOD), the enzyme that normally keeps SO under tight control, [29] is a central source for SO-derived PN in several diseases driven by overt production of PN [44]. Such enzymatic inactivation results from nitration of Tyr-34 by PN in a manganese-catalyzed process [45]. In a series of studies, our group revealed that spinal nitration and inactivation of MnSOD provides a critical “feed-forward” mechanism that allows for the accumulation of SO and PN during the development and maintenance of central sensitization [19, 25–28]. These findings were confirmed and subsequently extended by others [32, 38]. Thus, inactivation of mitochondrial MnSOD is a central site for the increased production of SO and PN in nociceptive signaling [17–18, 46]. Superoxide and PN can also be generated from the mitochondrial electron transport chain; to this end it was reported that production of mitochondrial SO by intrathecal injection of inhibitors of the electron transport complex (i.e. antimycin A or rotenone) in mice leads to mechanical hyperalgesia [47]. Another important SO-generating enzyme system is nicotinamide adenine dinucleotide phosphate (NADPH) oxidase [48] which was recently implicated in the development of central sensitization associated with inflammatory hyperalgesia [49] and peripheral nerve injury-induced neuropathic pain [50]. This SO-generating enzyme is dormant in resting cells and produces SO only upon activation. The principal regulation of NADPH oxidase is post-translational and depends on the assembly of several membrane-bound and cytosolic components to form an active enzyme complex [51]. In resting cells, the enzyme consists of two membrane-bound components, gp91phox and p22phox, and several cytosolic components, including p47phox, p40phox, p67phox, and rac1/2 [51]. Gp91phox is a flavocytochrome and the catalytic core of the enzyme. Upon activation, the cytosolic components translocate to the membrane and associate with membrane components to form an assembled, activated, and SO-producing enzyme complex [51]. Although this enzyme is best characterized in immune cells and leukocytes for its involvement in SO production, it is now known that various protein components of NADPH oxidase are expressed in neurons, astrocytes, and microglia [52–54]. Importantly, SO auto-augments its formation by up-regulating the expression of the Rac1 and gp91phox subunits of the holoenzyme and creates a self-perpetuating cascade [55–56]. Furthermore, protein kinase C (PKC), a kinase activated in peripheral [57–60] and central sensitization [61–65], regulates many of the NADPH oxidase subunits directly [66–67] or through the activation of extracellular signal-regulated kinases (ERK)1/2 or mitogen-activated protein kinase (MAPK) pathways [67–69]. Serine phosphorylation of the p47phox subunit by PKC α [67, 70–72], βII [66–67, 70], δ [67, 70, 73], ε [67], or ζ [70, 74] initiates the translocation of the cytosolic NADPH-oxidase regulatory complex and stimulates SO production. PKC-induced phosphorylation of other NADPH oxidase subunits (p67phox [75], p40phox [76], and gp91phox [77]) that induce or enhance SO production have also been described. PKC has also been demonstrated to be a key regulator in growth factor receptor-induced NOX1 expression [78], an isoform equivalent to catalytic gp91phox subunit. Therefore, post-translational nitration and inactivation of MnSOD and activation of NADPH oxidase may represent two pathways that operate in synchrony to maintain central sensitization (Figure 2). We have reported this to be the case in the development of morphine-induced hyperalgesia and antinociceptive tolerance [79].
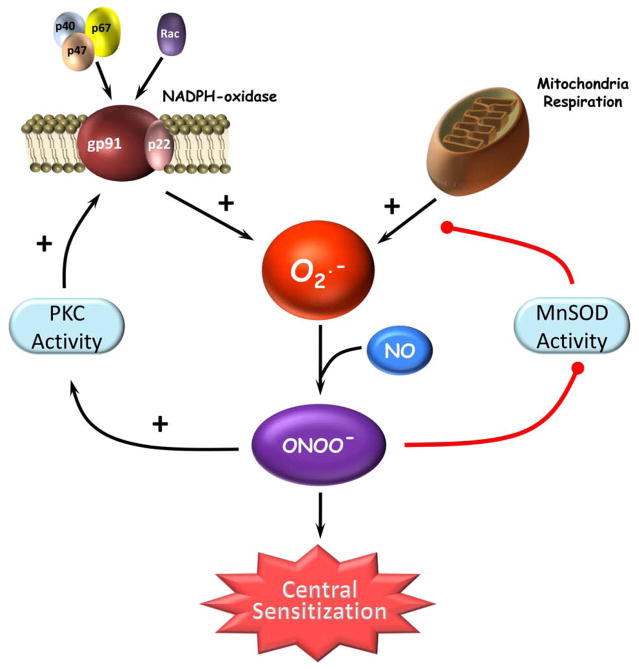
Fig. 2. Peroxynitrite-reinforced superoxide production in central sensitization: two feed forward mechanisms.
Two major sites of superoxide (O2·−) production, NADPH oxidase and mitochondrial respiration, are active in the development of central sensitization. Peroxynitrite (ONOO−) formed from NADPH oxidase- and mitochondrial-derived superoxide nitrates and inactivates the manganese SOD (MnSOD) enzyme preventing the removal of mitochondrial-derived superoxide. Peroxynitrite enhances protein kinase C (PKC) activity and, in turn, enhances translocation of NADPH oxidase regulatory subunits to the membrane to increase the NADPH oxidase-derived superoxide production. Combined, these two mechanisms amplify superoxide-derived peroxynitrite formation leading to the development of central sensitization.
Potential signaling pathways engaged by superoxide and peroxynitrite in nociceptive signaling
Modulation of protein kinases, alterations in glutamatergic neurotransmission, neuroinflammation, and modulation of ion channels such as TRPV1 underlie the development of central sensitization associated with acute and chronic inflammatory and non-inflammatory neuropathic pain. These alterations take place in the periphery, in the spinal cord and at supraspinal sites such as in the RVM. A comprehensive analysis in the scientific domain as it pertains to pain and non-pain related areas reveals that each of these signaling pathways can be affected by SO and PN. Additionally, nitroxidative species may be involved more subtly in central sensitization at least in part by sensitizing wide dynamic range neurons in the dorsal horn [36]. We will briefly review some of these signaling pathways.
Protein kinases, superoxide and peroxynitrite
Activity of protein kinases such as PKC, protein kinase A (PKA) and calcium/calmodulin-dependent protein kinase II (CaMKII) are central to many of the neuronal and glia signal transduction pathways leading to the development of peripheral and central sensitization. Activation of PKC, in particular, is necessary for the development of peripheral [57, 59–60, 80] and central sensitization [61–65] of several pain etiologies. Peripheral activation of PKC occurs within many of the G protein-coupled receptor signaling pathways that include protease- [81] and interleukin (IL)-6-induced [82] TRPV1 sensitization, prostaglandin (PG) E2-mediated sensory neuron sensitization [83–84], paclitaxel-induced hyperalgesia [85], calcitonin gene related peptide (CGRP) production in dorsal root ganglia through sensory neuron specific receptor (Mrg) stimulation [86], and TRPV4-induced mechanical hypersensitivity [87]. Activation of PKC in the central nervous system (CNS) occurs in neurons in response to peptide neurotransmitters [61, 88] and following glutamatergic receptor stimulation [89–92] that enhance synaptic plasticity. In astrocytes, PKC activation modulates various processes in central sensitization that include: cytokine/PGE2-mediated increases in inducible NOS (iNOS) [93], bradykinin-induced phospholipase A2 (PLA2) activation and COX2 production[94–95], IL-1β-induced reduction in gap junction formation [96], morphine-induced ERK activation [97], sphingosine 1-phosphate-induced nerve growth factor (NGF) expression [98], and glutamate-induced glial activation and subsequent reduction in the glutamate transporter (GT) GLT-1 surface expression [99–100]. The importance of PKA activity is demonstrated in tumor necrosis factor (TNF)-α [101] or PGE2-mediated [83–84, 102–103] sensitization of peripheral sensory neurons, TRPV1 [104–105] and TRPV4 [87] sensitization, paclitaxel-induced hyperalgesia [85], and phosphorylation and activation of NMDA receptors [106]. CAMKII activity facilitates the development of inflammatory- [107–109] and capsaicin-mediated hyperalgesia [110], morphine tolerance [111–113], and central sensitization [114].
The activation of protein kinases can be modulated, in part, by nitroxidative species. As described earlier, PKC is a recognized regulator of NADPH oxidase activity [89, 115] in a wide variety of pathologies and tissues. However, SO can activate PKC as demonstrated by increased autophosphorylation and activation of PKC by endogenous SO in hippocampal tissue [116] and by enhanced PKC-modulated persistent sodium currents [117] in the presence of SO, whereas removal of SO by antioxidants or SODm attenuates high frequency-stimulated PKC activation in neurons [116]. In addition to SO, PN facilitates PKC activation and translocation to the membrane [118] by direct nitration of PKCα, γ, and ε isoforms [118–120] or by stimulating proteolytic activation of PKC [121]. Ibi et al [49] demonstrated that accelerated PKCε translocation, possibly resulting from cysteine oxidation in the catalytic 1A site of PKCε [49] in response to NADPH oxidase-derived SO production in DRG neurons, was essential for the development of thermal and mechanical hyperalgesia. However, the effects of PN on PKC activity can be 1) dose-dependent as low PN concentrations enhance co-factor dependant PKC activity and higher PN concentrations irreversibly inhibit PKC activity through nitrotyrosine formation [120] and 2) cell-type specific as PN can be lethal in neurons [122], whereas PN-mediated PKC activity is cytoprotective for astrocytes [123–124], endothelial cells [125], and monocyte/macrophage through activation of cPLA2 and arachidonic acid formation [126].
In addition to PKC, nitroxidative species may play a role in PKA-mediated and CAMKII-mediated hyperalgesia, though the mechanisms are less well understood. Evidence for nitroxidative activation of PKA comes from studies where the antioxidant, PBN, reduced the levels of PKA-specific NMDA receptor phosphorylation and attenuated capsaicin-induced hyperalgesia [37] and where PN decomposition prevented PGE2-mediated thermal hyperalgesia and potential downstream PKA activity following intraplantar SO administration [20]. Although there is little evidence for direct nitroxidative activation of CAMKII, CAMKII is activated in the presence of mitochondrial SO-stimulated calcium influx following electrical stimulation [116] and PN-mediated p38 activation in PC12 cells requires, in part, the activation of CAMKII [127]. One possible nitroxidative-mediated CAMKII regulatory mechanism may occur through inactivation of the protein phosphatases responsible for downregulating CaMKII autophosphorylation and activity [128–129], since a reduction in SOD activity or increased SO reduces the protein phosphatase levels and dephosphorylation of CAMKII-activated cAMP response element-binding (CREB) [130] by calcineurin, respectively.
Alterations of glutamatergic neurotransmission by nitration
Dysfunction of the glutamatergic pathway is a key component of nociception [4, 131–134]. A key property of PN lies is its ability to post-translationally nitrate tyrosine and consequently modify protein function [12, 135–137]. Protein nitration is increasingly recognized as an important occurrence during cell signaling and regulation of protein activity [138]. The advent of proteomics and the development of immunological and analytical methodologies have revealed that tyrosine nitration is limited to specific proteins though the basis for this selectivity is not fully understood [12, 135–137]. Although there is no defined mechanism of removal of this modification, there is evidence that such a signal could be turned off by protein degradation or denitration by a “denitrase” [139]. Of the known proteins that are post-translationally modified by PN, the following are of particular significance: neuronal NMDARs [140–142], PKC [118–120], glia-derived GTs such as GLT-1 and GLAST [143–144] and glutamine synthetase (GS) [145–150]. These proteins are involved in ensuring optimal glutamatergic neurotransmission and thus optimal neuronal activation whereas dysregulation of their biological properties, such as would occur by nitration, will have critical consequences in events underlying central sensitization (findings summarized in Figure 3). [18, 46]
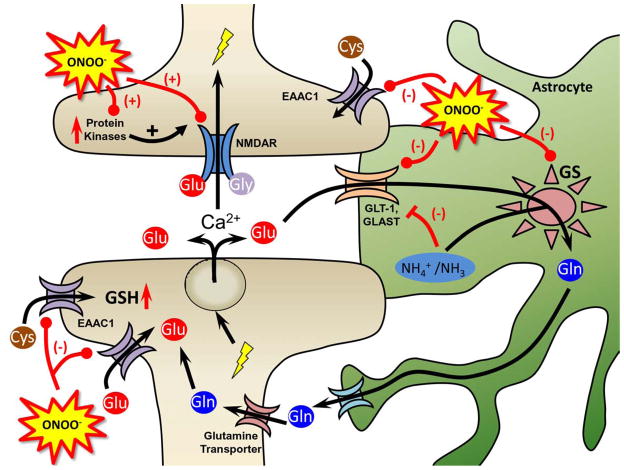
Fig. 3. The role of peroxynitrite in glutamatergic homeostasis and signaling.
Peroxynitrite (ONOO−) enhances glutamatergic signaling through nitration and activation of NMDARs and the protein kinases responsible for NMDAR activation. Peroxynitrite further enhances glutamatergic signaling by nitrating and inactivating the glutamate transporters (GLT-1, GLAST, and EAAC1) that remove glutamate (Glu) from the synapse and extrasynaptic regions and glutamine synthetase (GS) that converts glutamate, ammonia and ATP to glutamine, which is then taken back up by the neurons via the glutamine transporter. Nitration and inactivation of these enzymes results in toxic levels of glutamate and activation of neuroimmune responses. Inactivation of GS may also lead to increase ammonia levels that can inhibit glutamate transport. In addition to glutamate uptake, EAAC1 transports cysteine (Cys) into the neuron a process that is key in the biosynthesis of glutathione (GSH), a major cellular antioxidant. Compromised Cys transport by nitrated EAAC1 could lead to increased neuronal nitroxidative stress.
Glutamate (a primary endogenous ligand for the NMDAR) is a major neurotransmitter mediating the fast excitatory transmission at central synapses, and it plays critical roles in synaptic plasticity and development. It is now well established that glutamate and glutamate neurotransmission plays a critical role in the development of acute and chronic inflammatory pain (i.e. arthritis), neurogenic pain [104, 151], neuropathic pain [152–154] and in the development of opioid hyperalgesia/tolerance [155–156] associated, in part, with increased neuronal NMDA receptor activity in the spinal cord as reflected by increased phosphorylation of its NR1 subunit [152–153, 155–183]. The increased expression [184–186] and PKC-mediated phosphorylation [184, 187] of NR1, a NMDAR subunit essential for central sensitization, [188] increases the activation of NMDARs and thus neuronal activity [189]. For example, and in agreement with these findings, spinal administration of an antisense oligonucleotide to NR1 attenuates hyperalgesia/tolerance [190–191]. In addition, a free radical scavenger reduces spinal NR1 phosphorylation in neuropathic and inflammatory hyperalgesia, underscoring the contribution of nitroxidative species to NMDAR activity in central sensitization [37]. PN interacts with NMDARs leading to nitration of the tyrosine residues present on NR1 subunits [140–142]. This event is an irreversible reaction that leads to constant potentiation of the synaptic currents and calcium influx, overt activation of NMDARs and ultimately excitotoxicity [140–142]. In addition to its direct effects on NMDARs, PN nitration and activation of PKC provides a potential mechanism for enhancing NR1 phosphorylation. Collectively, these processes lead to neuronal activation which has been extensively studied following noxious stimulation by quantifying neurons expressing the proto-oncogene c-fos protein in regions of the dorsal horn of the spinal cord that are responsible for nociceptive signaling (i.e. laminae I, II, and V) and have been corroborated with electrophysiologic patterns of activity [192–198]. Importantly, c-fos expression increases in the superficial dorsal horn in morphine tolerant rats [199–200] and inhibition of NMDAR activation [201] reduces c-fos expression. Phosphorylation of neuronal NR1 increases neuronal excitation, as evidenced by increased c-fos expression in the spinal cord [189]. This phenomenon has been linked to the development of central sensitization associated with pain of several etiologies as well as in the development of morphine-induced hyperalgesia and antinociceptive tolerance [189, 202–204]. In support, administration of a NR1 antisense oligonucleotide reduces c-fos expression [191].
Extracellular glutamate concentration has to be kept low enough to terminate glutamate receptor activation and to protect neurons from glutamate excitotoxicity [205–207]. Glutamate is not metabolized by extracellular enzymes, but rather has to be removed from the synaptic cleft by cellular uptake: increased [glutamate] will affect both neuronal and glial cell function with dysregulation of this pathway impacting optimal neuronal activity and neurotransmission. The homeostasis of extracellular glutamate is tightly regulated by sodium-dependent high-affinity GTs in the plasma membranes of both neurons and glia [208–211]. At least five membrane GT subtypes have been cloned including GLAST (EAAT1), GLT-1 (EAAT2), EAAC1 (EAAT3), EAAT4, and EAAT5. Three GT subtypes isolated in the spinal cord [GLAST and GLT-1 associated with glial cells [212–213] (mainly in astrocytes but also found in microglia), and EAAC1 associated with neurons [214–219]] are considered essential to maintain low resting levels of glutamate (<1 μM) and to prevent over-stimulation of NMDARs [210, 220–223]. The glia-derived GLAST and GLT-1 concentrated in the superficial dorsal horn of the spinal cord are responsible for >90% of total glutamate transport [224]. If GLAST/GLT-1 function is compromised (i.e. reduced or eliminated) such as would occur when these GTs are nitrated, [glutamate] increases in the CSF contributing to rapid alterations in synaptic transmission [225–228]. Besides regulating synaptic levels of glutamate, these GTs play a crucial role in the uptake of cysteine, and thus contribute to the overall thiol redox state of cells normally regulated by intracellular levels of glutathione (GSH). GSH plays a critical role in protecting cells from oxidative stress as well as maintaining the thiol redox state. The depletion of GSH enhances oxidative stress leading to neuronal degeneration as shown in several studies [229–230]. Glutathione is a tripeptide composed of glutamate, cysteine and glycine residues with an unusual peptide bond between the α-amine of cysteine and the side chain carboxylate of glutamate. In neurons, cysteine is the rate-limiting substrate for GSH synthesis [231] and in neurons approximately 90% of total cysteine uptake is mediated by GTs and in particular EAAC1[232–234]. Thus, EAAC1 transports cysteine at a rate comparable to that of glutamate, with an affinity 10- to 20-fold higher than that of GLAST or GLT-1 [235]. Recent studies have shown that PN-mediated nitration of EAAC1 in neurons reduces the uptake capacity of cysteine leading to a depletion of intracellular GSH and neuronal cell death [236]. Integrating these findings, central sensitization could also develop due to excitotoxicity from increased synaptic concentrations of glutamate and a decrease in neuronal thiol redox state due to decreased intracellular levels of cysteine and thus GSH (Figure 3).
In contradistinction to the central role of GTs in regulating the homeostasis of extracellular glutamate and cysteine, GS another key enzyme in the regulation of glutamate neurotransmission in the CNS plays a pivotal role in its intracellular metabolic fate [237]. GS catalyzes the synthesis of glutamine from glutamate, and is responsible for the detoxification of ammonia in the brain.[237] GS is of critical importance in the CNS as although glutamate has a number of metabolic enzymes, the only known pathway for the synthesis of glutamine is via GS. GS is a major control point for nitrogen metabolism and since glutamine is the amino group donor in many important biosynthetic reactions, reducing its concentration in astrocytes could lead to defects in nitrogen homeostasis and hinder key biosynthetic pathways In the CNS, GS is located mainly in astrocytes and one of the primary roles of these cells is to protect neurons against excitotoxicity by taking up excess ammonia and glutamate, converting them into glutamine [237–238]. Indeed neurons depend on astrocytes for protection against glutamate toxicity [239] since it has been reported that glutamate is highly toxic to neurons in the absence of astrocytes [240]. Glutamine is then transported out of the astrocyte via glutamine transporter into neurons, where it serves as a precursor for the formation of glutamate and γ-aminobutyric acid (GABA) [241–242]. Nitration of GS (on Tyr 160) is intimately linked to inactivation of its biological function triggering loss of enzyme activity [148]. GS inactivation through nitration has been shown to occur after in vitro treatment with PN [147], in ammonia-intoxicated rat brain [149], endotoxin-treated rat liver [148], brains of epilepsia [150], hepatic encephalopathy [146] and as shown by our group pain of various etiologies [27, 243]. Nitration of GS will facilitate neuronal excitation [237, 244]. Inhibition of GS will also lead to [ammonia] with ensuing additional detrimental effects. One of the earliest events associated with increased [ammonia] is astrocyte swelling. This phenomenon could be very important in contributing to increased synaptic [glutamate] and overall excitotoxicity since astrocyte swelling has been linked to increased release of glutamate due to the opening of channels activated by swelling [245–246]. Other astrocytic activities such as neurotransmitter uptake, GS synthesis, blood-brain barrier transport and so forth can also be disrupted in response to astrocyte swelling [237]. Furthermore, astrocyte swelling leads to the formation of nitroxidative species and in turn, nitroxidative species trigger astrocyte swelling providing a self-amplifying signaling loop, which may enhance pathways leading to central sensitization [149, 247]. Furthermore, through feedback regulation, a decrease in the GS activity, as would occur when this enzyme is nitrated, reduces the activity of GTs [237] and in turn, reduced GT activity (following direct nitration or in response to reduced GS) can directly increase NMDAR activity [184, 226] underscoring the reciprocal feed-forward impact of post-translational nitration on these pathways. Increased levels of glutamate can be decreased by reducing the production of cytokines such as TNF-α and IL-6 that have been shown to inhibit glutamate uptake [248]. Since PN increases cytokine production (vide infra), it is likely that PN modulates glutamate homeostasis via the cytokine signaling pathway.
In summary, post-translational modifications of proteins involved in the tight regulation of glutamate homeostasis may provide a unifying link in signaling events underlying central sensitization.
Neuroinflammation, superoxide and peroxynitrite
Neuroinflammation following the activation of glial cells is important to the development and maintenance of central sensitization [249]. Numerous stimuli such as neurotransmitters and proinflammatory mediators can activate glial cells (e.g. astrocytes and microglia), which results in glial cell release of pro-inflammatory cytokines, excitatory amino acids, and nitroxidative species that contribute to central sensitization [250]. For example, the neuropeptide CGRP causes the release of proinflammatory cytokines from astrocytes (TNF-α and IL-1β) and microglia (IL-6) [251]. Additionally, prevention of neuroinflammation with minocycline blocks neuropathic hyperalgesia and allodynia [252]. Neuroinflammation may occur through Toll-like receptor-4 (TLR4) signaling in glial cells; this signaling contributes to both hyperalgesia and allodynia [253–257]. A series of studies by Watkins and colleagues has established that TLR4 is necessary for the actions of morphine including TLR4 activation for morphine metabolite (morphine-3-glucuonide)-induced hyperalgesia, allodynia, and neuroimmune activation [257–258]. Stimulation of TLR-4 can result in the production of the proinflammatory cytokines TNF-α and IL-1β that contribute to central sensitization through the downstream initiation of inhibitor of κBα (IκBα), c-Jun N-terminal kinase (JNK), and p38 pathways that promote proinflammatory cytokine transcription through nuclear factor κB (NFκB) and activator protein (AP-1) [253, 259]. It is well known that PN also activates the redox-sensitive transcription factor NFκB and several MAPKs, including p38 and ERK1/2, that regulate the production of many proinflammatory mediators and cytokines [131–133, 260–265]; to this end we have previously reported that spinal PN contributes to the development of central sensitization associated with inflammation and chronic administration of morphine via increased formation of glia-derived cytokines [17–19, 26–27, 79, 266]. Thus, systemic or intrathecal delivery of SODm blocks increased formation of cytokines in spinal cord and attenuates hyperalgesia [19, 25, 27]. Inhibition of cytokine formation also prevents the development of peripheral sensitization and hyperalgesia associated with inflammation [19, 26].
Superoxide, peroxynitrite and cyclooxygenase
One potential molecular pathway by which SO and/or PN may influence inflammatory events associated with the development of altered pain sensitivity is through modulation of COX enzymes [267–268]. As originally reported by our group [269] and subsequently extended by several other investigators [268, 270–271], the COX enzymes (constitutive COX-1 and inducible COX-2) are “receptor targets” for the multifaceted action of NO and as such are regulated in its presence. Although the mechanisms by which NO activates COX enzymes remain undefined, we now know that PN is involved in this activation through the oxidative inactivation and/or modification of key amino acids residues in the COX polypeptide backbone [272–273]. In addition to effects on COX-2 enzyme activity, PN (and NO) increase the production of PGs from macrophages by acting post-transcriptionally or translationally to increase COX-2 protein levels or to increase its mRNA stability, at least in part through SO and the p38 MAPK pathway [270–271, 274–277]. Furthermore, iNOS binds COX-2, and iNOS-derived NO increases the catalytic activity of COX-2 through S-nitrosylation in a macrophage cell line [278]. Other possibilities in this complex reaction biochemistry have been raised and discussed in detail [279]. We have reported that activation of COX-1 and activation/induction of COX-2 by SO and PN and subsequent increase in PGE2 contributes to the development of peripheral sensitization associated with inflammation [20].
Superoxide, peroxynitrite and TRPV1
The TRPV1 integrates multiple endogenous and exogenous pain stimuli and its activation results in intracellular sodium and calcium influx [280–283]. These receptors are found in the periphery (small and medium primary afferent neurons) and CNS within areas responsible for nociceptive signaling [284–286] and are essential to inflammatory thermal hyperalgesia [287–289]. Indeed, TRPV1 activation is also associated with enhanced glutamatergic signaling [290–294] and facilitation of long-term potentiation [295], which are important contributors to central sensitization. The unique ability of TRPV1 to modulate pain resulted in therapeutic targeting of this receptor for pain management [296–298].
Sensitization of TRPV1 during inflammatory pain depends upon numerous mechanisms. For example, phospholipase C activation (via growth factors, neurotransmitters, and inflammatory mediators), PGE2 (via cAMP activation of PKA), and prostacyclin all enhance TRPV1 sensitization [105, 298–299]. Phosphorylation of serine and/or threonine residues also results in TRPV1 sensitization. Sources of TRPV1 phosphorylation include PKA, PKC and CAMKII [105, 300–304]. The sensitization of TRPV1 may be regulated by nitroxidative species through the modulation of PKC and PKA (see above). Nitroxidative species are involved in both the downstream actions of TRPV1 activation and modulating TRPV1 expression and sensitization.
Numerous studies suggest that TRPV1 sensitization results in the production of nitroxidative species [305]. Production of reactive oxygen species can be TRPV1 dependent such as following TRPV1-mediated substance P release [306] and during reactive oxygen species-mediated afferent nerve fiber stimulation [307]. Direct activation of TRPV1 in vitro enhances the production of unstable hydroxyl radicals which is blocked with administration of a free radical scavenger and the TRPV1 antagonist, capsaizepine, in a joint inflammation model [308]. In addition, the NADPH oxidase-mediated production of reactive oxygen species in activated microglia is dependent upon TRPV1 receptor activity [309]. The NADPH/gp91phox production of SO also contributes to neurogenic vasodilation following TRPV1 activation and its mediated release of the neuropeptides substance P and CGRP [310]. Activation of TRPV1 in retinal explants was recently shown to enhance a biomarker of PN-mediated protein nitration, 3-nitrotyrosine [311]. Capsaicin administration and low pH solutions activate TRPV1 receptors and results in calcium influx with subsequent SO production in synoviocytes from arthritic rats [312]. This TRPV1-mediated calcium influx and nitroxidative species production is associated with reduced SOD levels and implicated in the mechanisms of cell death in numerous cell types [313–316]. Cell death via TRPV1 activation may result from the activities of nitroxidative species through p38 activation [317] and PN-mediated oxidative stress [316]. Recent evidence suggests that TRPV1 activation contributes to the maintenance of inflammation through the production of reactive oxygen species which act as signaling molecules to increase the expression of the TNF receptor in dorsal root ganglion neurons [318].
Conversely, nitroxidative species also modulate the activity, expression, and sensitivity of TRPV1 receptors. For example, SO stimulates TRPV1 activity in inflammatory states [307, 319] and this may occur through PKC phosphorylation of TRPV1 [320–321]. More specifically, peripheral SO-derived hydrogen peroxide requires TRPV1 activity to maintain thermal hyperalgesia [322]. Further, in vitro studies demonstrate that NADPH oxidase activity induces TRPV1 channel activity [323] and t-BOOH, a reactive oxygen species donor, increases TRPV1 protein expression [308]. Oxidative modification of TRPV1 cysteine residues (e.g. the formation of inter-cysteine disulfide bonds within its cytoplasmic termini) results in resistance to desensitization as well as sensitization of normal and reactivation of desensitized TRPV1 [324–325]. This direct oxidative modification and subsequent sensitization of TRPV1 may result in long-lasting pain signaling in nociceptive neurons [324].
Nitroxidative species may regulate TRPV1 expression through growth factors, transcription factors, and MAPK kinases. Nerve growth factor, a well described regulator of TRPV1 expression, acts through the tyrosine kinase receptor A (TrkA) and depends upon the Rac1/NADPH oxidase pathway-mediated activation of p38 MAPK to increase TRPV1 expression in PC12 and dorsal root ganglion cells [56, 326]. Puntambekar and colleagues [56] proposed that the generation of reactive oxygen species (e.g. SO) and their subsequent activity as signaling molecules causes a positive feedback regulation to induce TRPV1 expression which helps to maintain peripheral neuron integrity, inflammation, and pain perception.
Interestingly, TRPV1 receptors also contribute to the development of hyperalgesia and allodynia through activation by endogenous lipids, the oxidized linoleic acid metabolites (9- and 13-hydroxyoctadecadienoic acid), which act as TRPV1 agonists in the periphery and spinal cord [327–328]. Because of the well-documented nitroxidative stress that occurs in the periphery and spinal cord during inflammatory hyperalgesia [19, 25, 329–330], it is possible that SO and PN also regulate TRPV1 activity through oxidation of linoleic acid and the increased formation of oxidized metabolites.
Nitroxidative species in supraspinal descending facilitation of nociception
A recent wealth of evidence has demonstrated that supraspinal descending modulation of spinal nociception is essential to pathologic pain states [331–337]. The role of nitroxidative species in supraspinal descending facilitation of nociception during pathologic pain is unclear; however, there is increasing evidence that suggests their activities in the brain may be critical to pain of several etiologies. We reported that PN-mediated activity, as evidenced by expression of the nuclear enzyme poly (ADP-ribose) polymerase (PARP) and decreased MnSOD activity, occurs in the brain during hyperalgesia and is prevented with PNDCs (MnTnHex-2-PyP5+ and MnTE-2-PyP5+) [266]. Further, intracerebroventricular injections of free radical scavengers (i.e. PBN) attenuate inflammation and nerve injury-induced hypersensitivity to noxious and innocuous stimuli [36, 338]. More specifically, in the amygdala, facilitation of somatosensory and visceral nociception following type 1 metabotropic glutamate receptor activation is mediated by reactive oxygen species [339].
As previously described by our laboratory [46], one supraspinal nociceptive modulating center, the RVM, is a promising locus for nitroxidative species activities that substantially contribute to pain. The RVM facilitates nociception and drives central sensitization through well-described glutamatergic signaling pathways that depend upon the synthesis of the PN precursor NO [331]. During central sensitization, time-dependent cellular, biochemical, and molecular changes occur in the RVM that are comparable to those that occur in the spinal cord (e.g. enhanced glutamatergic signaling, NMDAR activation, neuroimmune activation with release of IL-1, IL-6, and TNF-α, and protein kinase activation) [335, 340–347]. As nitroxidative species are intimately involved with each of these changes in the spinal cord, it strongly suggests their potential role within the RVM. These findings have directed our current investigations and support our hypothesis that nitroxidative species activity within the RVM contributes to central sensitization [46].
Apoptosis, superoxide and peroxynitrite
Neuronal apoptosis is emerging as a significant contributor to the development of hyperalgesia and sensitization particularly in neuropathic pain and morphine-induced antinociceptive tolerance [157, 226, 348]. Nitroxidative species are potent inducers of apoptosis in neurodegenerative disease such as Alzheimer’s disease [349–350]. In pain, the role for nitroxidative stress-induced apoptosis is suggested by evidence that administration of a PNDC attenuates spinal apoptosis as marked by the reduction in spinal DNA damage and PARP activation in addition to preventing the development of morphine-induce hypersensitivity [27].
The specific mechanisms through which nitroxidative species initiate apoptosis in pain are poorly understood. However, PN administration leads to the nitration of mitochondrial proteins associated with the respiratory chain [351] and regulation of the mitochondrial permeability transition pore complex (MPTPC) [352]. Protein nitration inactivates mitochondrial respiratory chain proteins [351] that, in turn, alter the mitochondrial membrane potential and reduce adenosine triphosphate (ATP) production [353]. The alterations in mitochondrial membrane potential and cellular energy status from mitochondrial protein nitration lead to the release apoptogenic protein such as caspases [354–358] and apoptosis inducing factor [359–360] through the MPTPC. Additionally, PN can nitrate the adenosine nucleotide translocator [352] protein that regulates the MPTPC activity [361], thus providing additional mechanism of nitroxidative stress-induced apoptosis. Peroxynitrite also induces DNA strand breaks and activates the PARP enzyme [362–363]. High PARP activity in response to high concentrations of PN reduces nicotinamide adenine dinucleotide (NAD+) concentrations that, in turn, reduces glycolysis and electron transport [364]. Excessive PARP activation and reduction in ATP leads to necrosis [365–366], however, caspases released from by the MPTPC cleave PARP reducing the necrosis and favoring apoptosis [367].
Conclusions and future outlook
Pharmacologic investigations of SO and PN and nitroxidative stress are critical to advance the body of knowledge concerning the contribution of these species to pain. As we move forward, it will be of paramount importance for researchers to fully understand the dominant in vivo pharmacological activities and selectivities of compounds capable of attenuating nitroxidative stress. To sort out the mechanistic details accurately, researchers need better pharmacological tools than are currently available (e.g. compounds with documented selectivity toward either SO or PN but not both). Until these types of selective scavengers are developed, we need to use what we have appropriately. It is important to note that retrospective analysis in clinical trials with recombinant bovine Cu/Zn SOD (Orgotein®) [9, 368–369] reveals that in humans, removal of SO (and thus PN) may have some analgesic effects. Interestingly, the first clinical pilot studies with the native enzyme were done as early as 1970’s in rheumatoid arthritis (RA) and osteoarthritis (OA) with preliminary results demonstrating efficacy. Further studies showed that Orgotein® given by intraarticular injection, attenuated inflammation and pain of RA and OA [370–377] and led to a 60% decrease in the consumption of analgesics. Furthermore, Orgotein® was effective when given by intraarticular injection to patients with temporomandibular joint dysfunction and associated pain who had failed to respond to standard therapy [378] and reduced pain in patients with duodenal ulcer pain [379]. Other clinical settings in which SOD (whether recombinant or native bovine) was used included patients with Crohn’s disease and various forms of periarticular inflammation. Eighteen patients with Peyronie’s disease (exhibiting severe symptoms) who received Orgotein injected monthly into indurated areas of the penis, showed marked improvement, notably the loss of pain on erection [380–383]. Additional beneficial effects were seen in trigeminal pain, fibromyalgia and temporomandibular joint dysfunction [30, 384], chronic pancreatitis [385], and post-irradiation of breast cancer fibrosis [386]. Whether PN is the culprit or not remains to be proven, but it will be interesting and exciting to see whether levels of nitrated proteins correlate with pain in humans. We believe that continued research in this field will soon provide a valid pharmacological basis for developing PN-targeted therapeutic agents as novel non-narcotic analgesics in the management of pain and in particular chronic pain. The metalloporphyrin-based PN scavengers are exciting lead candidates in strategies that target PN alone or in synergistic combination with opioids or COX inhibitors. As metalloporphyrin systems have evolved in nature to be encased in protein (e.g. the cytochromes), small molecule porphyrin-based PNDCs will require peripheral synthetic modification to impart human pharmaceutical properties (i.e. membrane solubility, reduced charge, reduced non-target binding, reduced toxicity, optimal pharmacokinetics, etc). As the metal center in these systems is site for antioxidant action, the periphery of the porphyrin macrocycle is wide-open for synthetic manipulations to control in vivo performance without negative perturbation of the catalytic apparatus. In the field of catalytic antioxidants, structure activity studies regarding the in vivo biodistribution of metalloporphyrin PNDCs of varying functionality has been largely unexplored. We believe that through medicinal chemistry modification of the ligand periphery, true drug candidates with tuned selectivities can be engineered.
Acknowledgments
Supported by NIH/NIDA R01 DA024074 and NIH/NIAMS RC1 AR05823.
List of Abbreviations
- TEMPOL
4-hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl
- AP-1
Activator protein 1
- ATP
Adenosine triphosphate
- CREB
cAMP response element-binding
- CGRP
Calcitonin gene related peptide
- CaMKII
Calcium/calmodulin-dependent protein kinase II
- JNK
c-Jun N-terminal kinase
- cAMP
Cyclic adenosine monophosphate
- COX
Cyclooxygenase
- Cys
Cysteine
- NO
Nitric oxide
- EAAC
Excitatory amino acid channel
- EAAT
Excitatory amino acid transporter
- ERK
Extracellular signal-regulated kinases
- FeTM-4-PyP5+
Fe(III)tetrakis(1-methyl-4-pyridyl)porphyrin pentachlorideporphyrin
- GABA
γ-Aminobutyric acid
- Glu
Glutamate
- GT
Glutamate transporters
- GLAST
Glutamate-aspartate transporter
- GLT-1
Glutamate transporter 1
- Gln
Glutamine
- GS
Glutamine synthetase
- GlnT
Glutamine Transporter
- GSH
Glutathione
- Gly
Glycine
- IκBα
Inhibitor of κBα
- IL
Interleukin
- MnSOD
Manganese Superoxide Dismutase
- MPTPC
Mitochondrial permeability transition pore complex
- MAPK
Mitogen-activated protein kinase
- MnTE-2-PyP5+
Mn(III) 5,10,15,20-tetrakis(N-n-hexylpyridinium-2-yl)porphyrin
- NGF
Nerve growth factor
- NADPH
Nicotinamide adenine dinucleotide phosphate oxidase
- nNOS
Neuronal nitric oxide synthase
- NMDAR
N-methyl-D-aspartate receptor
- NFκB
Nuclear factor κB
- OA
Osteoarthritis
- PN
ONOO−, Peroxynitrite
- PNDCs
Peroxynitrite-decomposition catalysts
- PBN
Phenyl N-tert-butylnitrone
- PLA2
Phospholipase A2
- PARP
Poly (ADP-ribose) polymerase
- PG
Prostaglandin
- PKA
Protein kinase A
- PKC
Protein kinase C
- RVM
Rostral ventromedial medulla
- Mrg
Sensory neuron specific receptor
- SO
O2·−, Superoxide
- SODms
Superoxide dismutase mimetics
- TLR4
Toll-like receptor-4
- TRPV1
Transient receptor potential cation channel, subfamily V, member 1
- TNF
Tumor necrosis factor
- Tyr
Tyrosine
- TrkA
Tyrosine kinase receptor A
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Massaad CA, Klann E. Reactive Oxygen Species in the Regulation of Synaptic Plasticity and Memory. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renfrey S, Downton C, Featherstone J. The painful reality. Nat Rev Drug Discov. 2003;2:175–176. doi: 10.1038/nrd1038. [DOI] [PubMed] [Google Scholar]
- 3.Grosser T, Fries S, FitzGerald GA. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J Clin Invest. 2006;116:4–15. doi: 10.1172/JCI27291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 5.Arner S, Rawal N, Gustafsson LL. Clinical experience of long-term treatment with epidural and intrathecal opioids--a nationwide survey. Acta Anaesthesiol Scand. 1988;32:253–259. doi: 10.1111/j.1399-6576.1988.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 6.Ossipov MH, Lai J, King T, Vanderah TW, Malan TP, Jr, Hruby VJ, Porreca F. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- 7.Foley KM. Misconceptions and controversies regarding the use of opioids in cancer pain. Anticancer Drugs. 1995;6(Suppl 3):4–13. doi: 10.1097/00001813-199504003-00002. [DOI] [PubMed] [Google Scholar]
- 8.Salvemini D, Wang ZQ, Zweier JL, Samouilov A, Macarthur H, Misko TP, Currie MG, Cuzzocrea S, Sikorski JA, Riley DP. A nonpeptidyl mimic of superoxide dismutase with therapeutic activity in rats. Science. 1999;286:304–306. doi: 10.1126/science.286.5438.304. [DOI] [PubMed] [Google Scholar]
- 9.Salvemini D, Riley DP, Cuzzocrea S. SOD mimetics are coming of age. Nat Rev Drug Discov. 2002;1:367–374. doi: 10.1038/nrd796. [DOI] [PubMed] [Google Scholar]
- 10.Salvemini D, Wang ZQ, Stern MK, Currie MG, Misko TP. Peroxynitrite decomposition catalysts: therapeutics for peroxynitrite-mediated pathology. Proc Natl Acad Sci U S A. 1998;95:2659–2663. doi: 10.1073/pnas.95.5.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salvemini D, Jensen MP, Riley DP, Misko TP. Therapeutic manipulations of peroxynitrite. Drug News Perspect. 1998;11:204–214. [PubMed] [Google Scholar]
- 12.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 13.Batinic-Haberle I, Spasojevic I, Stevens RD, Bondurant B, Okado-Matsumoto A, Fridovich I, Vujaskovic Z, Dewhirst MW. New PEG-ylated Mn(III) porphyrins approaching catalytic activity of SOD enzyme. Dalton Trans. 2006:617–624. doi: 10.1039/b513761f. [DOI] [PubMed] [Google Scholar]
- 14.Batinic-Haberle I, Spasojevic I, Stevens RD, Hambright P, Neta P, Okado-Matsumoto A, Fridovich I. New class of potent catalysts of O2-dismutation. Mn(III) ortho-methoxyethylpyridyl- and di-ortho-methoxyethylimidazolylporphyrins. Dalton Trans. 2004:1696–1702. doi: 10.1039/b400818a. [DOI] [PubMed] [Google Scholar]
- 15.Batinic-Haberle I, Reboucas JS, Spasojevich I. Superoxide Dismutase Mimics: Chemistry, Pharmacology and Therapeutic Potential. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2009.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvemini D, Riley DP. Nonpeptidyl mimetics of superoxide dismutase in clinical therapies for diseases. Cell Mol Life Sci. 2000;57:1489–1492. doi: 10.1007/PL00000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salvemini D, Neumann W. Targeting peroxynitrite driven nitroxidative stress with synzymes: A novel therapeutic approach in chronic pain management. Life Sci. 2010;86:604–614. doi: 10.1016/j.lfs.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Salvemini D, Neumann WL. Peroxynitrite: a strategic linchpin of opioid analgesic tolerance. Trends Pharmacol Sci. 2009;30:194–202. doi: 10.1016/j.tips.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZQ, Porreca F, Cuzzocrea S, Galen K, Lightfoot R, Masini E, Muscoli C, Mollace V, Ndengele M, Ischiropoulos H, Salvemini D. A newly identified role for superoxide in inflammatory pain. J Pharmacol Exp Ther. 2004;309:869–878. doi: 10.1124/jpet.103.064154. [DOI] [PubMed] [Google Scholar]
- 20.Ndengele MM, Cuzzocrea S, Esposito E, Mazzon E, Di Paola R, Matuschak GM, Salvemini D. Cyclooxygenases 1 and 2 contribute to peroxynitrite-mediated inflammatory pain hypersensitivity. FASEB J. 2008;22:3154–3164. doi: 10.1096/fj.08-108159. [DOI] [PubMed] [Google Scholar]
- 21.Tang N, Ong WY, Yeo JF, Farooqui AA. Anti-allodynic effect of intracerebroventricularly administered antioxidant and free radical scavenger in a mouse model of orofacial pain. J Orofac Pain. 2009;23:167–173. [PubMed] [Google Scholar]
- 22.Khattab MM. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur J Pharmacol. 2006;548:167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Bezerra MM, Brain SD, Girao VC, Greenacre S, Keeble J, Rocha FA. Neutrophils-derived peroxynitrite contributes to acute hyperalgesia and cell influx in zymosan arthritis. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:265–273. doi: 10.1007/s00210-006-0123-9. [DOI] [PubMed] [Google Scholar]
- 24.Yeo JF, Ling SF, Tang N, Ong WY. Antinociceptive effect of CNS peroxynitrite scavenger in a mouse model of orofacial pain. Exp Brain Res. 2008;184:435–438. doi: 10.1007/s00221-007-1211-x. [DOI] [PubMed] [Google Scholar]
- 25.Muscoli C, Mollace V, Wheatley J, Masini E, Ndengele M, Wang ZQ, Salvemini D. Superoxide-mediated nitration of spinal manganese superoxide dismutase: a novel pathway in N-methyl-D-aspartate-mediated hyperalgesia. Pain. 2004;111:96–103. doi: 10.1016/j.pain.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Ndengele MM, Cuzzocrea S, Masini E, Vinci MC, Esposito E, Muscoli C, Petrusca DN, Mollace V, Mazzon E, Li D, Petrache I, Matuschak GM, Salvemini D. Spinal ceramide modulates the development of morphine antinociceptive tolerance via peroxynitrite-mediated nitroxidative stress and neuroimmune activation. J Pharmacol Exp Ther. 2009;329:64–75. doi: 10.1124/jpet.108.146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muscoli C, Cuzzocrea S, Ndengele MM, Mollace V, Porreca F, Fabrizi F, Esposito E, Masini E, Matuschak GM, Salvemini D. Therapeutic manipulation of peroxynitrite attenuates the development of opiate-induced antinociceptive tolerance in mice. J Clin Invest. 2007;117:3530–3539. doi: 10.1172/JCI32420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Batinic-Haberle I, Ndengele MM, Cuzzocrea S, Reboucas JS, Spasojevic I, Salvemini D. Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways. Free Radic Biol Med. 2009;46:212–219. doi: 10.1016/j.freeradbiomed.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- 30.Viggiano A, Monda M, Viggiano A, Viggiano D, Viggiano E, Chiefari M, Aurilio C, De Luca B. Trigeminal pain transmission requires reactive oxygen species production. Brain Res. 2005;1050:72–78. doi: 10.1016/j.brainres.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 31.Park ES, Gao X, Chung JM, Chung K. Levels of mitochondrial reactive oxygen species increase in rat neuropathic spinal dorsal horn neurons. Neurosci Lett. 2006;391:108–111. doi: 10.1016/j.neulet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz ES, Lee I, Chung K, Chung JM. Oxidative stress in the spinal cord is an important contributor in capsaicin-induced mechanical secondary hyperalgesia in mice. Pain. 2008;138:514–524. doi: 10.1016/j.pain.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salvemini D. Inhibitors of the ceramide metabolic pathway as adjuncts to opiates for pain. 20080241121. US patent publication. 2009
- 34.Salvemini D. Peroxynitrite and opiate antinociceptive tolerance: a painful reality. Arch Biochem Biophys. 2009;484:238–244. doi: 10.1016/j.abb.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang ZQ, Salvemini D. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee I, Kim HK, Kim JH, Chung K, Chung JM. The role of reactive oxygen species in capsaicin-induced mechanical hyperalgesia and in the activities of dorsal horn neurons. Pain. 2007;133:9–17. doi: 10.1016/j.pain.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao X, Kim HK, Chung JM, Chung K. Reactive oxygen species (ROS) are involved in enhancement of NMDA-receptor phosphorylation in animal models of pain. Pain. 2007;131:262–271. doi: 10.1016/j.pain.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz ES, Kim HY, Wang J, Lee I, Klann E, Chung JM, Chung K. Persistent pain is dependent on spinal mitochondrial antioxidant levels. J Neurosci. 2009;29:159–168. doi: 10.1523/JNEUROSCI.3792-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang J, Cochran V, Abdi S, Chung JM, Chung K, Kim HK. Phenyl N-t-butylnitrone, a reactive oxygen species scavenger, reduces zymosan-induced visceral pain in rats. Neurosci Lett. 2008;439:216–219. doi: 10.1016/j.neulet.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 40.Siniscalco D, Fuccio C, Giordano C, Ferraraccio F, Palazzo E, Luongo L, Rossi F, Roth KA, Maione S, de Novellis V. Role of reactive oxygen species and spinal cord apoptotic genes in the development of neuropathic pain. Pharmacol Res. 2007;55:158–166. doi: 10.1016/j.phrs.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 41.Tal M. A novel antioxidant alleviates heat hyperalgesia in rats with an experimental painful peripheral neuropathy. NeuroReport. 1996;7:1382–1384. doi: 10.1097/00001756-199605310-00010. [DOI] [PubMed] [Google Scholar]
- 42.Kim HK, Zhang YP, Gwak YS, Abdi S. Phenyl N-tert-butylnitrone, a free radical scavenger, reduces mechanical allodynia in chemotherapy-induced neuropathic pain in rats. Anesthesiology. 2010;112:432–439. doi: 10.1097/ALN.0b013e3181ca31bd. [DOI] [PubMed] [Google Scholar]
- 43.Salvemini D, Doyle TM, Cuzzocrea S. Superoxide, peroxynitrite and oxidative/nitrative stress in inflammation. Biochem Soc Trans. 2006;34:965–970. doi: 10.1042/BST0340965. [DOI] [PubMed] [Google Scholar]
- 44.Macmillan-Crow LA, Cruthirds DL. Invited review: manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–336. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 45.MacMillan-Crow LA, Thompson JA. Tyrosine modifications and inactivation of active site manganese superoxide dismutase mutant (Y34F) by peroxynitrite. Arch Biochem Biophys. 1999;366:82–88. doi: 10.1006/abbi.1999.1202. [DOI] [PubMed] [Google Scholar]
- 46.Little JW, Doyle T, Salvemini D. Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids. 2010 doi: 10.1007/s00726-010-0633-0. [DOI] [PubMed] [Google Scholar]
- 47.Kim HY, Chung JM, Chung K. Increased production of mitochondrial superoxide in the spinal cord induces pain behaviors in mice: the effect of mitochondrial electron transport complex inhibitors. Neurosci Lett. 2008;447:87–91. doi: 10.1016/j.neulet.2008.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nauseef WM. The NADPH-dependent oxidase of phagocytes. Proc Assoc Am Physicians. 1999;111:373–382. doi: 10.1111/paa.1999.111.5.373. [DOI] [PubMed] [Google Scholar]
- 49.Ibi M, Matsuno K, Shiba D, Katsuyama M, Iwata K, Kakehi T, Nakagawa T, Sango K, Shirai Y, Yokoyama T, Kaneko S, Saito N, Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J Neurosci. 2008;28:9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim D, You B, Jo EK, Han SK, Simon MI, Lee SJ. NADPH oxidase 2-derived reactive oxygen species in spinal cord microglia contribute to peripheral nerve injury-induced neuropathic pain. Proc Natl Acad Sci U S A. 2010;107:14851–14856. doi: 10.1073/pnas.1009926107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Babior BM, Lambeth JD, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- 52.Abramov AY, Canevari L, Duchen MR. Beta-amyloid peptides induce mitochondrial dysfunction and oxidative stress in astrocytes and death of neurons through activation of NADPH oxidase. J Neurosci. 2004;24:565–575. doi: 10.1523/JNEUROSCI.4042-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo JA, Erickson RW, Curnutte JT. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21:374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Tejada-Simon MV, Serrano F, Villasana LE, Kanterewicz BI, Wu GY, Quinn MT, Klann E. Synaptic localization of a functional NADPH oxidase in the mouse hippocampus. Mol Cell Neurosci. 2005;29:97–106. doi: 10.1016/j.mcn.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muzaffar S, Shukla N, Angelini GD, Jeremy JY. Superoxide auto-augments superoxide formation and upregulates gp91(phox) expression in porcine pulmonary artery endothelial cells: inhibition by iloprost. Eur J Pharmacol. 2006;538:108–114. doi: 10.1016/j.ejphar.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 56.Puntambekar P, Mukherjea D, Jajoo S, Ramkumar V. Essential role of Rac1/NADPH oxidase in nerve growth factor induction of TRPV1 expression. J Neurochem. 2005;95:1689–1703. doi: 10.1111/j.1471-4159.2005.03518.x. [DOI] [PubMed] [Google Scholar]
- 57.Fischer MJ, Reeh PW. Sensitization to heat through G-protein-coupled receptor pathways in the isolated sciatic mouse nerve. Eur J Neurosci. 2007;25:3570–3575. doi: 10.1111/j.1460-9568.2007.05582.x. [DOI] [PubMed] [Google Scholar]
- 58.Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009;4:e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sculptoreanu A, Aura Kullmann F, de Groat WC. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur J Neurosci. 2008;27:3171–3181. doi: 10.1111/j.1460-9568.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sluka KA, Rees H, Chen PS, Tsuruoka M, Willis WD. Capsaicin-induced sensitization of primate spinothalamic tract cells is prevented by a protein kinase C inhibitor. Brain Res. 1997;772:82–86. doi: 10.1016/s0006-8993(97)00876-7. [DOI] [PubMed] [Google Scholar]
- 61.Kohno T, Wang H, Amaya F, Brenner GJ, Cheng JK, Ji RR, Woolf CJ. Bradykinin enhances AMPA and NMDA receptor activity in spinal cord dorsal horn neurons by activating multiple kinases to produce pain hypersensitivity. J Neurosci. 2008;28:4533–4540. doi: 10.1523/JNEUROSCI.5349-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- 63.Fang L, Wu J, Lin Q, Willis WD. Protein kinases regulate the phosphorylation of the GluR1 subunit of AMPA receptors of spinal cord in rats following noxious stimulation. Brain Res Mol Brain Res. 2003;118:160–165. doi: 10.1016/j.molbrainres.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Li KC, Zheng JH, Chen J. Involvement of spinal protein kinase C in induction and maintenance of both persistent spontaneous flinching reflex and contralateral heat hyperalgesia induced by subcutaneous bee venom in the conscious rat. Neurosci Lett. 2000;285:103–106. doi: 10.1016/s0304-3940(00)01039-9. [DOI] [PubMed] [Google Scholar]
- 65.Wang Y, Mu X, Wu J, Wu A, Fang L, Li J, Yue Y. Differential Roles of Phosphorylated AMPA Receptor GluR1 Subunits at Serine-831 and Serine-845 Sites in Spinal Cord Dorsal Horn in a Rat Model of Post-Operative Pain. Neurochem Res. 2010 doi: 10.1007/s11064-010-0288-y. [DOI] [PubMed] [Google Scholar]
- 66.Kitada M, Koya D, Sugimoto T, Isono M, Araki S, Kashiwagi A, Haneda M. Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes. 2003;52:2603–2614. doi: 10.2337/diabetes.52.10.2603. [DOI] [PubMed] [Google Scholar]
- 67.Sharma P, Evans AT, Parker PJ, Evans FJ. NADPH-oxidase activation by protein kinase C-isotypes. Biochem Biophys Res Commun. 1991;177:1033–1040. doi: 10.1016/0006-291x(91)90642-k. [DOI] [PubMed] [Google Scholar]
- 68.Dang PM, Morel F, Gougerot-Pocidalo MA, El Benna J. Phosphorylation of the NADPH oxidase component p67(PHOX) by ERK2 and P38MAPK: selectivity of phosphorylated sites and existence of an intramolecular regulatory domain in the tetratricopeptide-rich region. Biochemistry. 2003;42:4520–4526. doi: 10.1021/bi0205754. [DOI] [PubMed] [Google Scholar]
- 69.El Benna J, Faust RP, Johnson JL, Babior BM. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J Biol Chem. 1996;271:6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- 70.Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 71.Li Q, Subbulakshmi V, Fields AP, Murray NR, Cathcart MK. Protein kinase calpha regulates human monocyte O-2 production and low density lipoprotein lipid oxidation. J Biol Chem. 1999;274:3764–3771. doi: 10.1074/jbc.274.6.3764. [DOI] [PubMed] [Google Scholar]
- 72.Remijsen QF, Fontayne A, Verdonck F, Clynen E, Schoofs L, Willems J. The antimicrobial peptide parabutoporin competes with p47(phox) as a PKC-substrate and inhibits NADPH oxidase in human neutrophils. FEBS Lett. 2006;580:6206–6210. doi: 10.1016/j.febslet.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 73.Cheng N, He R, Tian J, Dinauer MC, Ye RD. A critical role of protein kinase C delta activation loop phosphorylation in formyl-methionyl-leucyl-phenylalanine-induced phosphorylation of p47(phox) and rapid activation of nicotinamide adenine dinucleotide phosphate oxidase. J Immunol. 2007;179:7720–7728. doi: 10.4049/jimmunol.179.11.7720. [DOI] [PubMed] [Google Scholar]
- 74.Dang PM, Fontayne A, Hakim J, El Benna J, Perianin A. Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol. 2001;166:1206–1213. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- 75.Zhao X, Xu B, Bhattacharjee A, Oldfield CM, Wientjes FB, Feldman GM, Cathcart MK. Protein kinase Cdelta regulates p67phox phosphorylation in human monocytes. J Leukoc Biol. 2005;77:414–420. doi: 10.1189/jlb.0504284. [DOI] [PubMed] [Google Scholar]
- 76.Bouin AP, Grandvaux N, Vignais PV, Fuchs A. p40(phox) is phosphorylated on threonine 154. serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase c-type kinase in the phosphorylation process. J Biol Chem. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- 77.Raad H, Paclet MH, Boussetta T, Kroviarski Y, Morel F, Quinn MT, Gougerot-Pocidalo MA, Dang PM, El-Benna J. Regulation of the phagocyte NADPH oxidase activity: phosphorylation of gp91phox/NOX2 by protein kinase C enhances its diaphorase activity and binding to Rac2, p67phox, and p47phox. FASEB J. 2009;23:1011–1022. doi: 10.1096/fj.08-114553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fan CY, Katsuyama M, Yabe-Nishimura C. PKCdelta mediates up-regulation of NOX1, a catalytic subunit of NADPH oxidase, via transactivation of the EGF receptor: possible involvement of PKCdelta in vascular hypertrophy. Biochem J. 2005;390:761–767. doi: 10.1042/BJ20050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Doyle T, Bryant L, Muscoli C, Cuzzocrea S, Esposito E, Chen Z, Salvemini D. Spinal NADPH oxidase is a source of superoxide in the development of morphine-induced hyperalgesia and antinociceptive tolerance. Neurosci Lett. 2010 doi: 10.1016/j.neulet.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moriyama T, Higashi T, Togashi K, Iida T, Segi E, Sugimoto Y, Tominaga T, Narumiya S, Tominaga M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol Pain. 2005;1:3. doi: 10.1186/1744-8069-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vellani V, Kinsey AM, Prandini M, Hechtfischer SC, Reeh P, Magherini PC, Giacomoni C, McNaughton PA. Protease activated receptors 1 and 4 sensitize TRPV1 in nociceptive neurones. Mol Pain. 2010;6:61. doi: 10.1186/1744-8069-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andratsch M, Mair N, Constantin CE, Scherbakov N, Benetti C, Quarta S, Vogl C, Sailer CA, Uceyler N, Brockhaus J, Martini R, Sommer C, Zeilhofer HU, Muller W, Kuner R, Davis JB, Rose-John S, Kress M. A key role for gp130 expressed on peripheral sensory nerves in pathological pain. J Neurosci. 2009;29:13473–13483. doi: 10.1523/JNEUROSCI.1822-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sachs D, Villarreal C, Cunha F, Parada C, Ferreira S. The role of PKA and PKCepsilon pathways in prostaglandin E2-mediated hypernociception. Br J Pharmacol. 2009;156:826–834. doi: 10.1111/j.1476-5381.2008.00093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dina OA, Chen X, Reichling D, Levine JD. Role of protein kinase Cepsilon and protein kinase A in a model of paclitaxel-induced painful peripheral neuropathy in the rat. Neuroscience. 2001;108:507–515. doi: 10.1016/s0306-4522(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 86.Chen P, Wang D, Li M, Zhang Y, Quirion R, Hong Y. Modulation of sensory neuron-specific receptors in the development of morphine tolerance and its neurochemical mechanisms. J Neurosci Res. 2010;88:2952–2963. doi: 10.1002/jnr.22448. [DOI] [PubMed] [Google Scholar]
- 87.Fan HC, Zhang X, McNaughton PA. Activation of the TRPV4 ion channel is enhanced by phosphorylation. J Biol Chem. 2009;284:27884–27891. doi: 10.1074/jbc.M109.028803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun RQ, Tu YJ, Lawand NB, Yan JY, Lin Q, Willis WD. Calcitonin gene-related peptide receptor activation produces PKA- and PKC-dependent mechanical hyperalgesia and central sensitization. J Neurophysiol. 2004;92:2859–2866. doi: 10.1152/jn.00339.2004. [DOI] [PubMed] [Google Scholar]
- 89.Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukunaga K, Soderling TR, Miyamoto E. Activation of Ca2+/calmodulin-dependent protein kinase II and protein kinase C by glutamate in cultured rat hippocampal neurons. J Biol Chem. 1992;267:22527–22533. [PubMed] [Google Scholar]
- 91.Hammer B, Parker WD, Jr, Bennett JP., Jr NMDA receptors increase OH radicals in vivo by using nitric oxide synthase and protein kinase C. NeuroReport. 1993;5:72–74. doi: 10.1097/00001756-199310000-00018. [DOI] [PubMed] [Google Scholar]
- 92.Linden DJ, Wong KL, Sheu FS, Routtenberg A. NMDA receptor blockade prevents the increase in protein kinase C substrate (protein F1) phosphorylation produced by long-term potentiation. Brain Res. 1988;458:142–146. doi: 10.1016/0006-8993(88)90506-9. [DOI] [PubMed] [Google Scholar]
- 93.Hsiao HY, Mak OT, Yang CS, Liu YP, Fang KM, Tzeng SF. TNF-alpha/IFN-gamma-induced iNOS expression increased by prostaglandin E2 in rat primary astrocytes via EP2-evoked cAMP/PKA and intracellular calcium signaling. Glia. 2007;55:214–223. doi: 10.1002/glia.20453. [DOI] [PubMed] [Google Scholar]
- 94.Hsieh HL, Wang HH, Wu CY, Jou MJ, Yen MH, Parker P, Yang CM. BK-induced COX-2 expression via PKC-delta-dependent activation of p42/p44 MAPK and NF-kappaB in astrocytes. Cell Signal. 2007;19:330–340. doi: 10.1016/j.cellsig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 95.Hsieh HL, Wu CY, Hwang TL, Yen MH, Parker P, Yang CM. BK-induced cytosolic phospholipase A2 expression via sequential PKC-delta, p42/p44 MAPK, and NF-kappaB activation in rat brain astrocytes. J Cell Physiol. 2006;206:246–254. doi: 10.1002/jcp.20457. [DOI] [PubMed] [Google Scholar]
- 96.Zvalova D, Cordier J, Mesnil M, Junier MP, Chneiweiss H. p38/SAPK2 controls gap junction closure in astrocytes. Glia. 2004;46:323–333. doi: 10.1002/glia.10334. [DOI] [PubMed] [Google Scholar]
- 97.Belcheva MM, Clark AL, Haas PD, Serna JS, Hahn JW, Kiss A, Coscia CJ. Mu and kappa opioid receptors activate ERK/MAPK via different protein kinase C isoforms and secondary messengers in astrocytes. J Biol Chem. 2005;280:27662–27669. doi: 10.1074/jbc.M502593200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Furukawa A, Kita K, Toyomoto M, Fujii S, Inoue S, Hayashi K, Ikeda K. Production of nerve growth factor enhanced in cultured mouse astrocytes by glycerophospholipids, sphingolipids, and their related compounds. Mol Cell Biochem. 2007;305:27–34. doi: 10.1007/s11010-007-9524-4. [DOI] [PubMed] [Google Scholar]
- 99.Kalandadze A, Wu Y, Robinson MB. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem. 2002;277:45741–45750. doi: 10.1074/jbc.M203771200. [DOI] [PubMed] [Google Scholar]
- 100.Zhou J, Sutherland ML. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J Neurosci. 2004;24:6301–6306. doi: 10.1523/JNEUROSCI.1404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang JM, Li H, Liu B, Brull SJ. Acute topical application of tumor necrosis factor alpha evokes protein kinase A-dependent responses in rat sensory neurons. J Neurophysiol. 2002;88:1387–1392. doi: 10.1152/jn.2002.88.3.1387. [DOI] [PubMed] [Google Scholar]
- 102.Kopp UC, Cicha MZ, Smith LA. PGE(2) increases release of substance P from renal sensory nerves by activating the cAMP-PKA transduction cascade. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1618–1627. doi: 10.1152/ajpregu.00701.2001. [DOI] [PubMed] [Google Scholar]
- 103.Smith JA, Davis CL, Burgess GM. Prostaglandin E2-induced sensitization of bradykinin-evoked responses in rat dorsal root ganglion neurons is mediated by cAMP-dependent protein kinase A. Eur J Neurosci. 2000;12:3250–3258. doi: 10.1046/j.1460-9568.2000.00218.x. [DOI] [PubMed] [Google Scholar]
- 104.Amadesi S, Cottrell GS, Divino L, Chapman K, Grady EF, Bautista F, Karanjia R, Barajas-Lopez C, Vanner S, Vergnolle N, Bunnett NW. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RWt. cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron. 2002;35:721–731. doi: 10.1016/s0896-6273(02)00802-4. [DOI] [PubMed] [Google Scholar]
- 106.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 107.Chen Y, Luo F, Yang C, Kirkmire CM, Wang ZJ. Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice. J Pharmacol Exp Ther. 2009;330:650–659. doi: 10.1124/jpet.109.152165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Luo F, Yang C, Chen Y, Shukla P, Tang L, Wang LX, Wang ZJ. Reversal of chronic inflammatory pain by acute inhibition of Ca2+/calmodulin-dependent protein kinase II. J Pharmacol Exp Ther. 2008;325:267–275. doi: 10.1124/jpet.107.132167. [DOI] [PubMed] [Google Scholar]
- 109.Larsson M, Broman J. Translocation of GluR1-containing AMPA receptors to a spinal nociceptive synapse during acute noxious stimulation. J Neurosci. 2008;28:7084–7090. doi: 10.1523/JNEUROSCI.5749-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larsson M, Broman J. Pathway-specific bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II at spinal nociceptive synapses after acute noxious stimulation. J Neurosci. 2006;26:4198–4205. doi: 10.1523/JNEUROSCI.0352-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Y, Yang C, Wang ZJ. Ca2+/calmodulin-dependent protein kinase II alpha is required for the initiation and maintenance of opioid-induced hyperalgesia. J Neurosci. 2010;30:38–46. doi: 10.1523/JNEUROSCI.4346-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fan GH, Wang LZ, Qiu HC, Ma L, Pei G. Inhibition of calcium/calmodulin-dependent protein kinase II in rat hippocampus attenuates morphine tolerance and dependence. Mol Pharmacol. 1999;56:39–45. doi: 10.1124/mol.56.1.39. [DOI] [PubMed] [Google Scholar]
- 113.Lou L, Zhou T, Wang P, Pei G. Modulation of Ca2+/calmodulin-dependent protein kinase II activity by acute and chronic morphine administration in rat hippocampus: differential regulation of alpha and beta isoforms. Mol Pharmacol. 1999;55:557–563. [PubMed] [Google Scholar]
- 114.Liang DY, Li X, Clark JD. Formalin-induced spinal cord calcium/calmodulin-dependent protein kinase II alpha expression is modulated by heme oxygenase in mice. Neurosci Lett. 2004;360:61–64. doi: 10.1016/j.neulet.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 115.Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hongpaisan J, Winters CA, Andrews SB. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J Neurosci. 2004;24:10878–10887. doi: 10.1523/JNEUROSCI.3278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lai B, Zhang L, Dong LY, Zhu YH, Sun FY, Zheng P. Impact of inhibition of Qo site of mitochondrial complex III with myxothiazol on persistent sodium currents via superoxide and protein kinase C in rat hippocampal CA1 cells. Neurobiol Dis. 2006;21:206–216. doi: 10.1016/j.nbd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 118.Balafanova Z, Bolli R, Zhang J, Zheng Y, Pass JM, Bhatnagar A, Tang XL, Wang O, Cardwell E, Ping P. Nitric oxide (NO) induces nitration of protein kinase Cepsilon (PKCepsilon ), facilitating PKCepsilon translocation via enhanced PKCepsilon -RACK2 interactions: a novel mechanism of no-triggered activation of PKCepsilon. J Biol Chem. 2002;277:15021–15027. doi: 10.1074/jbc.M112451200. [DOI] [PubMed] [Google Scholar]
- 119.Robles-Flores M, Melendez L, Garcia W, Mendoza-Hernandez G, Lam TT, Castaneda-Patlan C, Gonzalez-Aguilar H. Posttranslational modifications on protein kinase c isozymes. Effects of epinephrine and phorbol esters. Biochim Biophys Acta. 2008;1783:695–712. doi: 10.1016/j.bbamcr.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 120.Knapp LT, Kanterewicz BI, Hayes EL, Klann E. Peroxynitrite-induced tyrosine nitration and inhibition of protein kinase C. Biochem Biophys Res Commun. 2001;286:764–770. doi: 10.1006/bbrc.2001.5448. [DOI] [PubMed] [Google Scholar]
- 121.Chakraborti T, Das S, Chakraborti S. Proteolytic activation of protein kinase Calpha by peroxynitrite in stimulating cytosolic phospholipase A2 in pulmonary endothelium: involvement of a pertussis toxin sensitive protein. Biochemistry. 2005;44:5246–5257. doi: 10.1021/bi0477889. [DOI] [PubMed] [Google Scholar]
- 122.Oh-hashi K, Maruyama W, Yi H, Takahashi T, Naoi M, Isobe K. Mitogen-activated protein kinase pathway mediates peroxynitrite-induced apoptosis in human dopaminergic neuroblastoma SH-SY5Y cells. Biochem Biophys Res Commun. 1999;263:504–509. doi: 10.1006/bbrc.1999.1237. [DOI] [PubMed] [Google Scholar]
- 123.Bolanos JP, Cidad P, Garcia-Nogales P, Delgado-Esteban M, Fernandez E, Almeida A. Regulation of glucose metabolism by nitrosative stress in neural cells. Mol Aspects Med. 2004;25:61–73. doi: 10.1016/j.mam.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 124.Bolanos JP, Heales SJ, Land JM, Clark JB. Effect of peroxynitrite on the mitochondrial respiratory chain: differential susceptibility of neurones and astrocytes in primary culture. J Neurochem. 1995;64:1965–1972. doi: 10.1046/j.1471-4159.1995.64051965.x. [DOI] [PubMed] [Google Scholar]
- 125.Foresti R, Sarathchandra P, Clark JE, Green CJ, Motterlini R. Peroxynitrite induces haem oxygenase-1 in vascular endothelial cells: a link to apoptosis. Biochem J. 1999;339(Pt 3):729–736. [PMC free article] [PubMed] [Google Scholar]
- 126.Guidarelli A, Cerioni L, Tommasini I, Brune B, Cantoni O. A downstream role for protein kinase Calpha in the cytosolic phospholipase A2-dependent protective signalling mediated by peroxynitrite in U937 cells. Biochem Pharmacol. 2005;69:1275–1286. doi: 10.1016/j.bcp.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 127.Jope RS, Zhang L, Song L. Peroxynitrite modulates the activation of p38 and extracellular regulated kinases in PC12 cells. Arch Biochem Biophys. 2000;376:365–370. doi: 10.1006/abbi.2000.1728. [DOI] [PubMed] [Google Scholar]
- 128.Sommer D, Coleman S, Swanson SA, Stemmer PM. Differential susceptibilities of serine/threonine phosphatases to oxidative and nitrosative stress. Arch Biochem Biophys. 2002;404:271–278. doi: 10.1016/s0003-9861(02)00242-4. [DOI] [PubMed] [Google Scholar]
- 129.Namgaladze D, Shcherbyna I, Kienhofer J, Hofer HW, Ullrich V. Superoxide targets calcineurin signaling in vascular endothelium. Biochem Biophys Res Commun. 2005;334:1061–1067. doi: 10.1016/j.bbrc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 130.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 131.Watkins LR, Hutchinson MR, Johnston IN, Maier SF. Glia: novel counter-regulators of opioid analgesia. Trends Neurosci. 2005;28:661–669. doi: 10.1016/j.tins.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 132.Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Watkins LR, Milligan ED, Maier SF. Glial activation: a driving force for pathological pain. Trends Neurosci. 2001;24:450–455. doi: 10.1016/s0166-2236(00)01854-3. [DOI] [PubMed] [Google Scholar]
- 134.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 135.Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem. 2002;383:401–409. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- 136.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 137.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 139.Irie Y, Saeki M, Kamisaki Y, Martin E, Murad F. Histone H1.2 is a substrate for denitrase, an activity that reduces nitrotyrosine immunoreactivity in proteins. Proc Natl Acad Sci U S A. 2003;100:5634–5639. doi: 10.1073/pnas.1131756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mishra OP, Delivoria-Papadopoulos M. Cellular mechanisms of hypoxic injury in the developing brain. Brain Res Bull. 1999;48:233–238. doi: 10.1016/s0361-9230(98)00170-1. [DOI] [PubMed] [Google Scholar]
- 141.Zanelli SA, Ashraf QM, Delivoria-Papadopoulos M, Mishra OP. Peroxynitrite-induced modification of the N-methyl-D-aspartate receptor in the cerebral cortex of the guinea pig fetus at term. Neurosci Lett. 2000;296:5–8. doi: 10.1016/s0304-3940(00)01608-6. [DOI] [PubMed] [Google Scholar]
- 142.Zanelli SA, Ashraf QM, Mishra OP. Nitration is a mechanism of regulation of the NMDA receptor function during hypoxia. Neuroscience. 2002;112:869–877. doi: 10.1016/s0306-4522(02)00141-0. [DOI] [PubMed] [Google Scholar]
- 143.Trotti D, Rolfs A, Danbolt NC, Brown RH, Jr, Hediger MA. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat Neurosci. 1999;2:848. doi: 10.1038/12227. [DOI] [PubMed] [Google Scholar]
- 144.Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976–5979. doi: 10.1074/jbc.271.11.5976. [DOI] [PubMed] [Google Scholar]
- 145.Minana MD, Kosenko E, Marcaida G, Hermenegildo C, Montoliu C, Grisolia S, Felipo V. Modulation of glutamine synthesis in cultured astrocytes by nitric oxide. Cell Mol Neurobiol. 1997;17:433–445. doi: 10.1023/A:1026339428059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gorg B, Qvartskhava N, Bidmon HJ, Palomero-Gallagher N, Kircheis G, Zilles K, Haussinger D. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52:256–265. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gorg B, Qvartskhava N, Voss P, Grune T, Haussinger D, Schliess F. Reversible inhibition of mammalian glutamine synthetase by tyrosine nitration. FEBS Lett. 2007;581:84–90. doi: 10.1016/j.febslet.2006.11.081. [DOI] [PubMed] [Google Scholar]
- 148.Gorg B, Wettstein M, Metzger S, Schliess F, Haussinger D. Lipopolysaccharide-induced tyrosine nitration and inactivation of hepatic glutamine synthetase in the rat. Hepatology. 2005;41:1065–1073. doi: 10.1002/hep.20662. [DOI] [PubMed] [Google Scholar]
- 149.Schliess F, Gorg B, Fischer R, Desjardins P, Bidmon HJ, Herrmann A, Butterworth RF, Zilles K, Haussinger D. Ammonia induces MK-801-sensitive nitration and phosphorylation of protein tyrosine residues in rat astrocytes. FASEB J. 2002;16:739–741. doi: 10.1096/fj.01-0862fje. [DOI] [PubMed] [Google Scholar]
- 150.Bidmon HJ, Gorg B, Palomero-Gallagher N, Schleicher A, Haussinger D, Speckmann EJ, Zilles K. Glutamine synthetase becomes nitrated and its activity is reduced during repetitive seizure activity in the pentylentetrazole model of epilepsy. Epilepsia. 2008;49:1733–1748. doi: 10.1111/j.1528-1167.2008.01642.x. [DOI] [PubMed] [Google Scholar]
- 151.Xu X, Wang P, Zou X, Li D, Fang L, Gong K, Lin Q. The effects of sympathetic outflow on upregulation of vanilloid receptors TRPV(1) in primary afferent neurons evoked by intradermal capsaicin. Exp Neurol. 2010;222:93–107. doi: 10.1016/j.expneurol.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mayer DJ, Mao J, Holt J, Price DD. Cellular mechanisms of neuropathic pain, morphine tolerance, and their interactions. Proc Natl Acad Sci U S A. 1999;96:7731–7736. doi: 10.1073/pnas.96.14.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mao J, Price DD, Hayes RL, Lu J, Mayer DJ. Differential roles of NMDA and non-NMDA receptor activation in induction and maintenance of thermal hyperalgesia in rats with painful peripheral mononeuropathy. Brain Res. 1992;598:271–278. doi: 10.1016/0006-8993(92)90193-d. [DOI] [PubMed] [Google Scholar]
- 154.Mayer DJ, Mao J, Price DD. The association of neuropathic pain, morphine tolerance and dependence, and the translocation of protein kinase C. NIDA Res Monogr. 1995;147:269–298. [PubMed] [Google Scholar]
- 155.Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. [DOI] [PubMed] [Google Scholar]
- 156.Elliott K, Minami N, Kolesnikov YA, Pasternak GW, Inturrisi CE. The NMDA receptor antagonists, LY274614 and MK-801, and the nitric oxide synthase inhibitor, NG-nitro-L-arginine, attenuate analgesic tolerance to the mu-opioid morphine but not to kappa opioids. Pain. 1994;56:69–75. doi: 10.1016/0304-3959(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 157.Mao J, Mayer DJ. Spinal cord neuroplasticity following repeated opioid exposure and its relation to pathological pain. Ann N Y Acad Sci. 2001;933:175–184. doi: 10.1111/j.1749-6632.2001.tb05823.x. [DOI] [PubMed] [Google Scholar]
- 158.Marek P, Ben-Eliyahu S, Gold M, Liebeskind JC. Excitatory amino acid antagonists (kynurenic acid and MK-801) attenuate the development of morphine tolerance in the rat. Brain Res. 1991;547:77–81. doi: 10.1016/0006-8993(91)90576-h. [DOI] [PubMed] [Google Scholar]
- 159.McCarthy RJ, Kroin JS, Tuman KJ, Penn RD, Ivankovich AD. Antinociceptive potentiation and attenuation of tolerance by intrathecal co-infusion of magnesium sulfate and morphine in rats. Anesth Analg. 1998;86:830–836. doi: 10.1097/00000539-199804000-00028. [DOI] [PubMed] [Google Scholar]
- 160.Lutfy K, Shen KZ, Kwon IS, Cai SX, Woodward RM, Keana JF, Weber E. Blockade of morphine tolerance by ACEA-1328, a novel NMDA receptor/glycine site antagonist. Eur J Pharmacol. 1995;273:187–189. doi: 10.1016/0014-2999(94)00716-k. [DOI] [PubMed] [Google Scholar]
- 161.Trujillo KA, Akil H. Inhibition of opiate tolerance by non-competitive N-methyl-D-aspartate receptor antagonists. Brain Res. 1994;633:178–188. doi: 10.1016/0006-8993(94)91538-5. [DOI] [PubMed] [Google Scholar]
- 162.Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wong CS, Cherng CH, Luk HN, Ho ST, Tung CS. Effects of NMDA receptor antagonists on inhibition of morphine tolerance in rats: binding at mu-opioid receptors. Eur J Pharmacol. 1996;297:27–33. doi: 10.1016/0014-2999(95)00728-8. [DOI] [PubMed] [Google Scholar]
- 164.Fairbanks CA, Wilcox GL. Acute tolerance to spinally administered morphine compares mechanistically with chronically induced morphine tolerance. J Pharmacol Exp Ther. 1997;282:1408–1417. [PubMed] [Google Scholar]
- 165.Guo RX, Zhang M, Liu W, Zhao CM, Cui Y, Wang CH, Feng JQ, Chen PX. NMDA receptors are involved in upstream of the spinal JNK activation in morphine antinociceptive tolerance. Neurosci Lett. 2009;467:95–99. doi: 10.1016/j.neulet.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 166.Adam F, Dufour E, Le Bars D. The glycine site-specific NMDA antagonist (+)-HA966 enhances the effect of morphine and reverses morphine tolerance via a spinal mechanism. Neuropharmacology. 2008;54:588–596. doi: 10.1016/j.neuropharm.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 167.Mao J, Price DD, Lu J, Mayer DJ. Antinociceptive tolerance to the mu-opioid agonist DAMGO is dose-dependently reduced by MK-801 in rats. Neurosci Lett. 1998;250:193–196. doi: 10.1016/s0304-3940(98)00472-8. [DOI] [PubMed] [Google Scholar]
- 168.Dunbar S, Yaksh TL. Concurrent spinal infusion of MK801 blocks spinal tolerance and dependence induced by chronic intrathecal morphine in the rat. Anesthesiology. 1996;84:1177–1188. doi: 10.1097/00000542-199605000-00020. [DOI] [PubMed] [Google Scholar]
- 169.Ossipov MH, Lopez Y, Nichols ML, Bian D, Porreca F. The loss of antinociceptive efficacy of spinal morphine in rats with nerve ligation injury is prevented by reducing spinal afferent drive. Neurosci Lett. 1995;199:87–90. doi: 10.1016/0304-3940(95)12022-v. [DOI] [PubMed] [Google Scholar]
- 170.Kreeger JS, Yukhananov R, Larson AA. Increased N-methyl-D-aspartate (NMDA) activity in the mouse spinal cord following morphine does not mediate opioid withdrawal. Brain Res. 1994;663:101–106. doi: 10.1016/0006-8993(94)90467-7. [DOI] [PubMed] [Google Scholar]
- 171.Gutstein HB, Trujillo KA. MK-801 inhibits the development of morphine tolerance at spinal sites. Brain Res. 1993;626:332–334. doi: 10.1016/0006-8993(93)90597-g. [DOI] [PubMed] [Google Scholar]
- 172.Kest B, Mogil JS, Shamgar BE, Kao B, Liebeskind JC, Marek P. The NMDA receptor antagonist MK-801 protects against the development of morphine tolerance after intrathecal administration. Proc West Pharmacol Soc. 1993;36:307–310. [PubMed] [Google Scholar]
- 173.Tiseo PJ, Cheng J, Pasternak GW, Inturrisi CE. Modulation of morphine tolerance by the competitive N-methyl-D-aspartate receptor antagonist LY274614: assessment of opioid receptor changes. J Pharmacol Exp Ther. 1994;268:195–201. [PubMed] [Google Scholar]
- 174.Tiseo PJ, Inturrisi CE. Attenuation and reversal of morphine tolerance by the competitive N-methyl-D-aspartate receptor antagonist, LY274614. J Pharmacol Exp Ther. 1993;264:1090–1096. [PubMed] [Google Scholar]
- 175.Trujillo KA. Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacology (Berl) 2000;151:121–141. doi: 10.1007/s002130000416. [DOI] [PubMed] [Google Scholar]
- 176.Davis AM, Inturrisi CE. d-Methadone blocks morphine tolerance and N-methyl-D-aspartate-induced hyperalgesia. J Pharmacol Exp Ther. 1999;289:1048–1053. [PubMed] [Google Scholar]
- 177.Lutfy K, Doan P, Weber E. ACEA-1328, a NMDA receptor/glycine site antagonist, acutely potentiates antinociception and chronically attenuates tolerance induced by morphine. Pharmacol Res. 1999;40:435–442. doi: 10.1006/phrs.1999.0538. [DOI] [PubMed] [Google Scholar]
- 178.Marek P, Ben-Eliyahu S, Vaccarino AL, Liebeskind JC. Delayed application of MK-801 attenuates development of morphine tolerance in rats. Brain Res. 1991;558:163–165. doi: 10.1016/0006-8993(91)90736-f. [DOI] [PubMed] [Google Scholar]
- 179.Wong CS, Hsu MM, Chou YY, Tao PL, Tung CS. Morphine tolerance increases [3H]MK-801 binding affinity and constitutive neuronal nitric oxide synthase expression in rat spinal cord. Br J Anaesth. 2000;85:587–591. doi: 10.1093/bja/85.4.587. [DOI] [PubMed] [Google Scholar]
- 180.Zhao M, Joo DT. Enhancement of spinal N-methyl-D-aspartate receptor function by remifentanil action at delta-opioid receptors as a mechanism for acute opioid-induced hyperalgesia or tolerance. Anesthesiology. 2008;109:308–317. doi: 10.1097/ALN.0b013e31817f4c5d. [DOI] [PubMed] [Google Scholar]
- 181.Liaw WJ, Zhang B, Tao F, Yaster M, Johns RA, Tao YX. Knockdown of spinal cord postsynaptic density protein-95 prevents the development of morphine tolerance in rats. Neuroscience. 2004;123:11–15. doi: 10.1016/j.neuroscience.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 182.Liaw WJ, Zhu XG, Yaster M, Johns RA, Gauda EB, Tao YX. Distinct expression of synaptic NR2A and NR2B in the central nervous system and impaired morphine tolerance and physical dependence in mice deficient in postsynaptic density-93 protein. Mol Pain. 2008;4:45. doi: 10.1186/1744-8069-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gudehithlu KP, Bhargava HN. Differential binding of [3H]MK-801 to brain regions and spinal cord of mice treated chronically with morphine. Gen Pharmacol. 1996;27:91–94. doi: 10.1016/0306-3623(95)00110-7. [DOI] [PubMed] [Google Scholar]
- 184.Lin SL, Tsai RY, Shen CH, Lin FH, Wang JJ, Hsin ST, Wong CS. Co-administration of ultra-low dose naloxone attenuates morphine tolerance in rats via attenuation of NMDA receptor neurotransmission and suppression of neuroinflammation in the spinal cords. Pharmacol Biochem Behav. 2010;96:236–245. doi: 10.1016/j.pbb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 185.Lim G, Wang S, Zeng Q, Sung B, Yang L, Mao J. Expression of spinal NMDA receptor and PKCgamma after chronic morphine is regulated by spinal glucocorticoid receptor. J Neurosci. 2005;25:11145–11154. doi: 10.1523/JNEUROSCI.3768-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu JB, Yao YX, Jiang W. Inhibitory effects of Group I metabotropic glutamate receptors antagonists on the expression of NMDA receptor NR1 subunit in morphine tolerant rats. Neurosci Lett. 2009;452:268–272. doi: 10.1016/j.neulet.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 187.Mao J, Price DD, Phillips LL, Lu J, Mayer DJ. Increases in protein kinase C gamma immunoreactivity in the spinal cord of rats associated with tolerance to the analgesic effects of morphine. Brain Res. 1995;677:257–267. doi: 10.1016/0006-8993(95)00161-i. [DOI] [PubMed] [Google Scholar]
- 188.South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulavsky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Brenner GJ, Ji RR, Shaffer S, Woolf CJ. Peripheral noxious stimulation induces phosphorylation of the NMDA receptor NR1 subunit at the PKC-dependent site, serine-896, in spinal cord dorsal horn neurons. Eur J Neurosci. 2004;20:375–384. doi: 10.1111/j.1460-9568.2004.03506.x. [DOI] [PubMed] [Google Scholar]
- 190.Shimoyama N, Shimoyama M, Davis AM, Monaghan DT, Inturrisi CE. An antisense oligonucleotide to the N-methyl-D-aspartate (NMDA) subunit NMDAR1 attenuates NMDA-induced nociception, hyperalgesia, and morphine tolerance. J Pharmacol Exp Ther. 2005;312:834–840. doi: 10.1124/jpet.104.074856. [DOI] [PubMed] [Google Scholar]
- 191.Lee IO, Yukhananov R, Standaert DG, Crosby G. NMDA-R1 antisense oligodeoxynucleotides modify formalin-induced nociception and spinal c-Fos expression in rat spinal cord. Pharmacol Biochem Behav. 2004;79:183–188. doi: 10.1016/j.pbb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 192.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 193.Harris JA. Using c-fos as a neural marker of pain. Brain Res Bull. 1998;45:1–8. doi: 10.1016/s0361-9230(97)00277-3. [DOI] [PubMed] [Google Scholar]
- 194.Bullitt E. Expression of c-fos-like protein as a marker for neuronal activity following noxious stimulation in the rat. J Comp Neurol. 1990;296:517–530. doi: 10.1002/cne.902960402. [DOI] [PubMed] [Google Scholar]
- 195.Dai JL, Zhu YH, Li KY, Huang DK, Xu SF. Central expression of c-fos protein after peripheral noxious thermal stimulation in awake rats. Zhongguo Yao Li Xue Bao. 1993;14:306–311. [PubMed] [Google Scholar]
- 196.Herdegen T, Kovary K, Leah J, Bravo R. Specific temporal and spatial distribution of JUN, FOS, and KROX-24 proteins in spinal neurons following noxious transsynaptic stimulation. J Comp Neurol. 1991;313:178–191. doi: 10.1002/cne.903130113. [DOI] [PubMed] [Google Scholar]
- 197.Menetrey D, Gannon A, Levine JD, Basbaum AI. Expression of c-fos protein in interneurons and projection neurons of the rat spinal cord in response to noxious somatic, articular, and visceral stimulation. J Comp Neurol. 1989;285:177–195. doi: 10.1002/cne.902850203. [DOI] [PubMed] [Google Scholar]
- 198.Coggeshall RE. Fos, nociception and the dorsal horn. Prog Neurobiol. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 199.Rohde DS, Detweiler DJ, Basbaum AI. Spinal cord mechanisms of opioid tolerance and dependence: Fos-like immunoreactivity increases in subpopulations of spinal cord neurons during withdrawal [corrected] Neuroscience. 1996;72:233–242. doi: 10.1016/0306-4522(95)00529-3. [DOI] [PubMed] [Google Scholar]
- 200.Vera-Portocarrero LP, Zhang ET, King T, Ossipov MH, Vanderah TW, Lai J, Porreca F. Spinal NK-1 receptor expressing neurons mediate opioid-induced hyperalgesia and antinociceptive tolerance via activation of descending pathways. Pain. 2007;129:35–45. doi: 10.1016/j.pain.2006.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Le Guen S, Catheline G, Besson JM. Effects of NMDA receptor antagonists on morphine tolerance: a c-Fos study in the lumbar spinal cord of the rat. Eur J Pharmacol. 1999;373:1–11. doi: 10.1016/s0014-2999(99)00272-1. [DOI] [PubMed] [Google Scholar]
- 202.Zou X, Lin Q, Willis WD. Enhanced phosphorylation of NMDA receptor 1 subunits in spinal cord dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. J Neurosci. 2000;20:6989–6997. doi: 10.1523/JNEUROSCI.20-18-06989.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Caudle RM, Perez FM, King C, Yu CG, Yezierski RP. N-methyl-D-aspartate receptor subunit expression and phosphorylation following excitotoxic spinal cord injury in rats. Neurosci Lett. 2003;349:37–40. doi: 10.1016/s0304-3940(03)00700-6. [DOI] [PubMed] [Google Scholar]
- 204.Caudle RM, Perez FM, Del Valle-Pinero AY, Iadarola MJ. Spinal cord NR1 serine phosphorylation and NR2B subunit suppression following peripheral inflammation. Mol Pain. 2005;1:25. doi: 10.1186/1744-8069-1-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hertz L. Functional interactions between neurons and astrocytes I. Turnover and metabolism of putative amino acid transmitters. Prog Neurobiol. 1979;13:277–323. doi: 10.1016/0301-0082(79)90018-2. [DOI] [PubMed] [Google Scholar]
- 206.Choi BH. Radial glia of developing human fetal spinal cord: Golgi, immunohistochemical and electron microscopic study. Brain Res. 1981;227:249–267. doi: 10.1016/0165-3806(81)90112-7. [DOI] [PubMed] [Google Scholar]
- 207.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- 208.Brustovetsky T, Purl K, Young A, Shimizu K, Dubinsky JM. Dearth of glutamate transporters contributes to striatal excitotoxicity. Exp Neurol. 2004;189:222–230. doi: 10.1016/j.expneurol.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 209.Jabaudon D, Scanziani M, Gahwiler BH, Gerber U. Acute decrease in net glutamate uptake during energy deprivation. Proc Natl Acad Sci U S A. 2000;97:5610–5615. doi: 10.1073/pnas.97.10.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–9251. doi: 10.1523/JNEUROSCI.19-21-09242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Semba J, Wakuta MS. Regional differences in the effects of glutamate uptake inhibitor L-trans-pyrrolidine-2,4-dicarboxylic acid on extracellular amino acids and dopamine in rat brain: an in vivo microdialysis study. Gen Pharmacol. 1998;31:399–404. doi: 10.1016/s0306-3623(98)00047-0. [DOI] [PubMed] [Google Scholar]
- 212.Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- 213.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 214.Arriza JL, Kavanaugh MP, Fairman WA, Wu YN, Murdoch GH, North RA, Amara SG. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- 215.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 216.Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- 217.Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, Koepsell H, Storm-Mathisen J, Seeberg E, Kanner BI. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- 218.Robinson MB, Dowd LA. Heterogeneity and functional properties of subtypes of sodium-dependent glutamate transporters in the mammalian central nervous system. Adv Pharmacol. 1997;37:69–115. doi: 10.1016/s1054-3589(08)60948-5. [DOI] [PubMed] [Google Scholar]
- 219.Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hughes DI, Polgar E, Shehab SA, Todd AJ. Peripheral axotomy induces depletion of the vesicular glutamate transporter VGLUT1 in central terminals of myelinated afferent fibres in the rat spinal cord. Brain Res. 2004;1017:69–76. doi: 10.1016/j.brainres.2004.05.054. [DOI] [PubMed] [Google Scholar]
- 221.Liaw WJ, Stephens RL, Jr, Binns BC, Chu Y, Sepkuty JP, Johns RA, Rothstein JD, Tao YX. Spinal glutamate uptake is critical for maintaining normal sensory transmission in rat spinal cord. Pain. 2005;115:60–70. doi: 10.1016/j.pain.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 222.Lievens JC, Bernal F, Forni C, Mahy N, Kerkerian-Le Goff L. Characterization of striatal lesions produced by glutamate uptake alteration: cell death, reactive gliosis, and changes in GLT1 and GADD45 mRNA expression. Glia. 2000;29:222–232. doi: 10.1002/(sici)1098-1136(20000201)29:3<222::aid-glia4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 223.Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 225.Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur J Pharmacol. 2001;419:39–45. doi: 10.1016/s0014-2999(01)00965-7. [DOI] [PubMed] [Google Scholar]
- 226.Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wen ZH, Wu GJ, Chang YC, Wang JJ, Wong CS. Dexamethasone modulates the development of morphine tolerance and expression of glutamate transporters in rats. Neuroscience. 2005;133:807–817. doi: 10.1016/j.neuroscience.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 228.Tai YH, Wang YH, Wang JJ, Tao PL, Tung CS, Wong CS. Amitriptyline suppresses neuroinflammation and up-regulates glutamate transporters in morphine-tolerant rats. Pain. 2006;124:77–86. doi: 10.1016/j.pain.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 229.Schulz JB, Matthews RT, Muqit MM, Browne SE, Beal MF. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995;64:936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- 230.Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK. Glutathione, iron and Parkinson’s disease. Biochem Pharmacol. 2002;64:1037–1048. doi: 10.1016/s0006-2952(02)01174-7. [DOI] [PubMed] [Google Scholar]
- 231.Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem. 2003;84:1332–1339. doi: 10.1046/j.1471-4159.2003.01630.x. [DOI] [PubMed] [Google Scholar]
- 233.Shanker G, Allen JW, Mutkus LA, Aschner M. The uptake of cysteine in cultured primary astrocytes and neurons. Brain Res. 2001;902:156–163. doi: 10.1016/s0006-8993(01)02342-3. [DOI] [PubMed] [Google Scholar]
- 234.Himi T, Ikeda M, Yasuhara T, Nishida M, Morita I. Role of neuronal glutamate transporter in the cysteine uptake and intracellular glutathione levels in cultured cortical neurons. J Neural Transm. 2003;110:1337–1348. doi: 10.1007/s00702-003-0049-z. [DOI] [PubMed] [Google Scholar]
- 235.Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol. 1996;493(Pt 2):419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Aoyama K, Matsumura N, Watabe M, Nakaki T. Oxidative stress on EAAC1 is involved in MPTP-induced glutathione depletion and motor dysfunction. Eur J Neurosci. 2008;27:20–30. doi: 10.1111/j.1460-9568.2007.05979.x. [DOI] [PubMed] [Google Scholar]
- 237.Suarez I, Bodega G, Fernandez B. Glutamine synthetase in brain: effect of ammonia. Neurochem Int. 2002;41:123–142. doi: 10.1016/s0197-0186(02)00033-5. [DOI] [PubMed] [Google Scholar]
- 238.Kennedy AJ, Voaden MJ, Marshall J. Glutamate metabolism in the frog retina. Nature. 1974;252:50–52. doi: 10.1038/252050a0. [DOI] [PubMed] [Google Scholar]
- 239.Brown DR. Neurons depend on astrocytes in a coculture system for protection from glutamate toxicity. Mol Cell Neurosci. 1999;13:379–389. doi: 10.1006/mcne.1999.0751. [DOI] [PubMed] [Google Scholar]
- 240.Rosenberg PA, Amin S, Leitner M. Glutamate uptake disguises neurotoxic potency of glutamate agonists in cerebral cortex in dissociated cell culture. J Neurosci. 1992;12:56–61. doi: 10.1523/JNEUROSCI.12-01-00056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Waniewski RA. Physiological levels of ammonia regulate glutamine synthesis from extracellular glutamate in astrocyte cultures. J Neurochem. 1992;58:167–174. doi: 10.1111/j.1471-4159.1992.tb09292.x. [DOI] [PubMed] [Google Scholar]
- 242.Waniewski RA, Martin DL. Exogenous glutamate is metabolized to glutamine and exported by rat primary astrocyte cultures. J Neurochem. 1986;47:304–313. doi: 10.1111/j.1471-4159.1986.tb02863.x. [DOI] [PubMed] [Google Scholar]
- 243.Chen Z, Muscoli C, Doyle T, Bryant L, Cuzzocrea S, Mollace V, Mastroianni R, Masini E, Salvemini D. NMDA-receptor activation and nitroxidative regulation of the glutamatergic pathway during nociceptive processing. Pain. 2010;149:100–106. doi: 10.1016/j.pain.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Muscoli C, Visalli V, Colica C, Nistico R, Palma E, Costa N, Rotiroti D, Nistico G, Mollace V. The effect of inflammatory stimuli on NMDA-related activation of glutamine synthase in human cultured astroglial cells. Neurosci Lett. 2005;373:184–188. doi: 10.1016/j.neulet.2004.09.079. [DOI] [PubMed] [Google Scholar]
- 245.Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rose C, Kresse W, Kettenmann H. Acute insult of ammonia leads to calcium-dependent glutamate release from cultured astrocytes, an effect of pH. J Biol Chem. 2005;280:20937–20944. doi: 10.1074/jbc.M412448200. [DOI] [PubMed] [Google Scholar]
- 247.Schliess F, Gorg B, Haussinger D. Pathogenetic interplay between osmotic and oxidative stress: the hepatic encephalopathy paradigm. Biol Chem. 2006;387:1363–1370. doi: 10.1515/BC.2006.171. [DOI] [PubMed] [Google Scholar]
- 248.Korn T, Magnus T, Jung S. Autoantigen specific T cells inhibit glutamate uptake in astrocytes by decreasing expression of astrocytic glutamate transporter GLAST: a mechanism mediated by tumor necrosis factor-alpha. FASEB J. 2005;19:1878–1880. doi: 10.1096/fj.05-3748fje. [DOI] [PubMed] [Google Scholar]
- 249.Watkins LR, Maier SF. Immune regulation of central nervous system functions: from sickness responses to pathological pain. J Intern Med. 2005;257:139–155. doi: 10.1111/j.1365-2796.2004.01443.x. [DOI] [PubMed] [Google Scholar]
- 250.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10:23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Wang Z, Ma W, Chabot JG, Quirion R. Cell-type specific activation of p38 and ERK mediates calcitonin gene-related peptide involvement in tolerance to morphine-induced analgesia. FASEB J. 2009;23:2576–2586. doi: 10.1096/fj.08-128348. [DOI] [PubMed] [Google Scholar]
- 252.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 253.Watkins LR, Hutchinson MR, Rice KC, Maier SF. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci. 2009;30:581–591. doi: 10.1016/j.tips.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cao L, Tanga FY, Deleo JA. The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain. Neuroscience. 2009;158:896–903. doi: 10.1016/j.neuroscience.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bettoni I, Comelli F, Rossini C, Granucci F, Giagnoni G, Peri F, Costa B. Glial TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia. 2008;56:1312–1319. doi: 10.1002/glia.20699. [DOI] [PubMed] [Google Scholar]
- 256.Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci U S A. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Hutchinson MR, Zhang Y, Shridhar M, Evans JH, Buchanan MM, Zhao TX, Slivka PF, Coats BD, Rezvani N, Wieseler J, Hughes TS, Landgraf KE, Chan S, Fong S, Phipps S, Falke JJ, Leinwand LA, Maier SF, Yin H, Rice KC, Watkins LR. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav Immun. 2009 doi: 10.1016/j.bbi.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lewis SS, Hutchinson MR, Rezvani N, Loram LC, Zhang Y, Maier SF, Rice KC, Watkins LR. Evidence that intrathecal morphine-3-glucuronide may cause pain enhancement via toll-like receptor 4/MD-2 and interleukin-1beta. Neuroscience. 2010;165:569–583. doi: 10.1016/j.neuroscience.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology. 2004;113:153–162. doi: 10.1111/j.1365-2567.2004.01976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Goggel R, Winoto-Morbach S, Vielhaber G, Imai Y, Lindner K, Brade L, Brade H, Ehlers S, Slutsky AS, Schutze S, Gulbins E, Uhlig S. PAF-mediated pulmonary edema: a new role for acid sphingomyelinase and ceramide. Nat Med. 2004;10:155–160. doi: 10.1038/nm977. [DOI] [PubMed] [Google Scholar]
- 262.Delogu G, Famularo G, Amati F, Signore L, Antonucci A, Trinchieri V, Di Marzio L, Cifone MG. Ceramide concentrations in septic patients: a possible marker of multiple organ dysfunction syndrome. Crit Care Med. 1999;27:2413–2417. doi: 10.1097/00003246-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 263.Petrache I, Natarajan V, Zhen L, Medler TR, Richter AT, Cho C, Hubbard WC, Berdyshev EV, Tuder RM. Ceramide upregulation causes pulmonary cell apoptosis and emphysema-like disease in mice. Nat Med. 2005;11:491–498. doi: 10.1038/nm1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Masini E, Bani D, Vannacci A, Pierpaoli S, Mannaioni PF, Comhair SA, Xu W, Muscoli C, Erzurum SC, Salvemini D. Reduction of antigen-induced respiratory abnormalities and airway inflammation in sensitized guinea pigs by a superoxide dismutase mimetic. Free Radic Biol Med. 2005;39:520–531. doi: 10.1016/j.freeradbiomed.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 265.Claus RA, Bunck AC, Bockmeyer CL, Brunkhorst FM, Losche W, Kinscherf R, Deigner HP. Role of increased sphingomyelinase activity in apoptosis and organ failure of patients with severe sepsis. FASEB J. 2005;19:1719–1721. doi: 10.1096/fj.04-2842fje. [DOI] [PubMed] [Google Scholar]
- 266.Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, Spasojevic I, Salvemini D. Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance. Neuroscience. 2009 doi: 10.1016/j.neuroscience.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cuzzocrea S, Salvemini D. Molecular mechanisms involved in the reciprocal regulation of cyclooxygenase and nitric oxide synthase enzymes. Kidney Int. 2007;71:290–297. doi: 10.1038/sj.ki.5002058. [DOI] [PubMed] [Google Scholar]
- 268.Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol Rev. 2005;57:217–252. doi: 10.1124/pr.57.2.1. [DOI] [PubMed] [Google Scholar]
- 269.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yang T, Zhang A, Pasumarthy A, Zhang L, Warnock Z, Schnermann JB. Nitric oxide stimulates COX-2 expression in cultured collecting duct cells through MAP kinases and superoxide but not cGMP. Am J Physiol Renal Physiol. 2006;291:F891–895. doi: 10.1152/ajprenal.00512.2005. [DOI] [PubMed] [Google Scholar]
- 271.Cheng HF, Zhang MZ, Harris RC. Nitric oxide stimulates cyclooxygenase-2 in cultured cTAL cells through a p38-dependent pathway. Am J Physiol Renal Physiol. 2006;290:F1391–1397. doi: 10.1152/ajprenal.00315.2005. [DOI] [PubMed] [Google Scholar]
- 272.Markey CM, Alward A, Weller PE, Marnett LJ. Quantitative studies of hydroperoxide reduction by prostaglandin H synthase. Reducing substrate specificity and the relationship of peroxidase to cyclooxygenase activities. J Biol Chem. 1987;262:6266–6279. [PubMed] [Google Scholar]
- 273.Landino LM, Crews BC, Timmons MD, Morrow JD, Marnett LJ. Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 1996;93:15069–15074. doi: 10.1073/pnas.93.26.15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.von Knethen A, Brune B. Cyclooxygenase-2: an essential regulator of NO-mediated apoptosis. FASEB J. 1997;11:887–895. [PubMed] [Google Scholar]
- 275.Habib A, Bernard C, Lebret M, Creminon C, Esposito B, Tedgui A, Maclouf J. Regulation of the expression of cyclooxygenase-2 by nitric oxide in rat peritoneal macrophages. J Immunol. 1997;158:3845–3851. [PubMed] [Google Scholar]
- 276.Eligini S, Habib A, Lebret M, Creminon C, Levy-Toledano S, Maclouf J. Induction of cyclo-oxygenase-2 in human endothelial cells by SIN-1 in the absence of prostaglandin production. Br J Pharmacol. 2001;133:1163–1171. doi: 10.1038/sj.bjp.0704163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Perkins DJ, Kniss DA. Blockade of nitric oxide formation down-regulates cyclooxygenase-2 and decreases PGE2 biosynthesis in macrophages. J Leukoc Biol. 1999;65:792–799. doi: 10.1002/jlb.65.6.792. [DOI] [PubMed] [Google Scholar]
- 278.Kim SF, Huri DA, Snyder SH. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 2005;310:1966–1970. doi: 10.1126/science.1119407. [DOI] [PubMed] [Google Scholar]
- 279.Trostchansky A, O’Donnell VB, Goodwin DC, Landino LM, Marnett LJ, Radi R, Rubbo H. Interactions between nitric oxide and peroxynitrite during prostaglandin endoperoxide H synthase-1 catalysis: a free radical mechanism of inactivation. Free Radic Biol Med. 2007;42:1029–1038. doi: 10.1016/j.freeradbiomed.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 280.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 281.Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- 282.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 283.Pingle SC, Matta JA, Ahern GP. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb Exp Pharmacol. 2007:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 284.Valtschanoff JG, Rustioni A, Guo A, Hwang SJ. Vanilloid receptor VR1 is both presynaptic and postsynaptic in the superficial laminae of the rat dorsal horn. J Comp Neurol. 2001;436:225–235. [PubMed] [Google Scholar]
- 285.Carlton SM, Coggeshall RE. Peripheral capsaicin receptors increase in the inflamed rat hindpaw: a possible mechanism for peripheral sensitization. Neurosci Lett. 2001;310:53–56. doi: 10.1016/s0304-3940(01)02093-6. [DOI] [PubMed] [Google Scholar]
- 286.Mezey E, Toth ZE, Cortright DN, Arzubi MK, Krause JE, Elde R, Guo A, Blumberg PM, Szallasi A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97:3655–3660. doi: 10.1073/pnas.060496197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Cui Y, Chen Y, Zhi JL, Guo RX, Feng JQ, Chen PX. Activation of p38 mitogen-activated protein kinase in spinal microglia mediates morphine antinociceptive tolerance. Brain Res. 2006;1069:235–243. doi: 10.1016/j.brainres.2005.11.066. [DOI] [PubMed] [Google Scholar]
- 288.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- 289.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 290.Palazzo E, de Novellis V, Marabese I, Cuomo D, Rossi F, Berrino L, Maione S. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur J Pharmacol. 2002;439:69–75. doi: 10.1016/s0014-2999(02)01367-5. [DOI] [PubMed] [Google Scholar]
- 291.Jin YH, Bailey TW, Li BY, Schild JH, Andresen MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci. 2004;24:4709–4717. doi: 10.1523/JNEUROSCI.0753-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Marinelli S, Vaughan CW, Christie MJ, Connor M. Capsaicin activation of glutamatergic synaptic transmission in the rat locus coeruleus in vitro. J Physiol. 2002;543:531–540. doi: 10.1113/jphysiol.2002.022863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Musella A, De Chiara V, Rossi S, Prosperetti C, Bernardi G, Maccarrone M, Centonze D. TRPV1 channels facilitate glutamate transmission in the striatum. Mol Cell Neurosci. 2009;40:89–97. doi: 10.1016/j.mcn.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 294.Yang K, Kumamoto E, Furue H, Yoshimura M. Capsaicin facilitates excitatory but not inhibitory synaptic transmission in substantia gelatinosa of the rat spinal cord. Neurosci Lett. 1998;255:135–138. doi: 10.1016/s0304-3940(98)00730-7. [DOI] [PubMed] [Google Scholar]
- 295.Li HB, Mao RR, Zhang JC, Yang Y, Cao J, Xu L. Antistress effect of TRPV1 channel on synaptic plasticity and spatial memory. Biol Psychiatry. 2008;64:286–292. doi: 10.1016/j.biopsych.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 296.Wong GY, Gavva NR. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res Rev. 2009;60:267–277. doi: 10.1016/j.brainresrev.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 297.Schumacher MA. Transient receptor potential channels in pain and inflammation: therapeutic opportunities. Pain Pract. 2010;10:185–200. doi: 10.1111/j.1533-2500.2010.00358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Palazzo E, Luongo L, de Novellis V, Berrino L, Rossi F, Maione S. Moving towards supraspinal TRPV1 receptors for chronic pain relief. Mol Pain. 2010;6:66. doi: 10.1186/1744-8069-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Pitchford S, Levine JD. Prostaglandins sensitize nociceptors in cell culture. Neurosci Lett. 1991;132:105–108. doi: 10.1016/0304-3940(91)90444-x. [DOI] [PubMed] [Google Scholar]
- 300.Varga A, Bolcskei K, Szoke E, Almasi R, Czeh G, Szolcsanyi J, Petho G. Relative roles of protein kinase A and protein kinase C in modulation of transient receptor potential vanilloid type 1 receptor responsiveness in rat sensory neurons in vitro and peripheral nociceptors in vivo. Neuroscience. 2006;140:645–657. doi: 10.1016/j.neuroscience.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 301.Numazaki M, Tominaga T, Toyooka H, Tominaga M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J Biol Chem. 2002;277:13375–13378. doi: 10.1074/jbc.C200104200. [DOI] [PubMed] [Google Scholar]
- 302.Cesare P, Dekker LV, Sardini A, Parker PJ, McNaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- 303.Rathee PK, Distler C, Obreja O, Neuhuber W, Wang GK, Wang SY, Nau C, Kress M. PKA/AKAP/VR-1 module: A common link of Gs-mediated signaling to thermal hyperalgesia. J Neurosci. 2002;22:4740–4745. doi: 10.1523/JNEUROSCI.22-11-04740.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca2+/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 305.Pall ML, Anderson JH. The vanilloid receptor as a putative target of diverse chemicals in multiple chemical sensitivity. Arch Environ Health. 2004;59:363–375. doi: 10.3200/AEOH.59.7.363-375. [DOI] [PubMed] [Google Scholar]
- 306.Gazzieri D, Trevisani M, Springer J, Harrison S, Cottrell GS, Andre E, Nicoletti P, Massi D, Zecchi S, Nosi D, Santucci M, Gerard NP, Lucattelli M, Lungarella G, Fischer A, Grady EF, Bunnett NW, Geppetti P. Substance P released by TRPV1-expressing neurons produces reactive oxygen species that mediate ethanol-induced gastric injury. Free Radic Biol Med. 2007;43:581–589. doi: 10.1016/j.freeradbiomed.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 307.Schultz HD, Ustinova EE. Capsaicin receptors mediate free radical-induced activation of cardiac afferent endings. Cardiovasc Res. 1998;38:348–355. doi: 10.1016/s0008-6363(98)00031-5. [DOI] [PubMed] [Google Scholar]
- 308.Westlund KN, Kochukov MY, Lu Y, McNearney TA. Impact of central and peripheral TRPV1 and ROS levels on proinflammatory mediators and nociceptive behavior. Mol Pain. 2010;6:46. doi: 10.1186/1744-8069-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Schilling T, Eder C. Stimulus-dependent requirement of ion channels for microglial NADPH oxidase-mediated production of reactive oxygen species. J Neuroimmunol. 2010;225:190–194. doi: 10.1016/j.jneuroim.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 310.Starr A, Graepel R, Keeble J, Schmidhuber S, Clark N, Grant A, Shah AM, Brain SD. A reactive oxygen species-mediated component in neurogenic vasodilatation. Cardiovasc Res. 2008;78:139–147. doi: 10.1093/cvr/cvn012. [DOI] [PubMed] [Google Scholar]
- 311.Leonelli M, Martins DO, Britto LR. TRPV1 receptors are involved in protein nitration and Muller cell reaction in the acutely axotomized rat retina. Exp Eye Res. 2010;91:755–768. doi: 10.1016/j.exer.2010.08.026. [DOI] [PubMed] [Google Scholar]
- 312.Hu F, Sun WW, Zhao XT, Cui ZJ, Yang WX. TRPV1 mediates cell death in rat synovial fibroblasts through calcium entry-dependent ROS production and mitochondrial depolarization. Biochem Biophys Res Commun. 2008;369:989–993. doi: 10.1016/j.bbrc.2008.02.155. [DOI] [PubMed] [Google Scholar]
- 313.Grant ER, Dubin AE, Zhang SP, Zivin RA, Zhong Z. Simultaneous intracellular calcium and sodium flux imaging in human vanilloid receptor 1 (VR1)-transfected human embryonic kidney cells: a method to resolve ionic dependence of VR1-mediated cell death. J Pharmacol Exp Ther. 2002;300:9–17. doi: 10.1124/jpet.300.1.9. [DOI] [PubMed] [Google Scholar]
- 314.Kim S, Moon A. Capsaicin-induced apoptosis of H-ras-transformed human breast epithelial cells is Rac-dependent via ROS generation. Arch Pharm Res. 2004;27:845–849. doi: 10.1007/BF02980177. [DOI] [PubMed] [Google Scholar]
- 315.Lee YS, Kang YS, Lee JS, Nicolova S, Kim JA. Involvement of NADPH oxidase-mediated generation of reactive oxygen species in the apototic cell death by capsaicin in HepG2 human hepatoma cells. Free Radic Res. 2004;38:405–412. doi: 10.1080/10715760410001665262. [DOI] [PubMed] [Google Scholar]
- 316.Qiao S, Li W, Tsubouchi R, Haneda M, Murakami K, Yoshino M. Involvement of peroxynitrite in capsaicin-induced apoptosis of C6 glioma cells. Neurosci Res. 2005;51:175–183. doi: 10.1016/j.neures.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 317.Amantini C, Mosca M, Nabissi M, Lucciarini R, Caprodossi S, Arcella A, Giangaspero F, Santoni G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J Neurochem. 2007;102:977–990. doi: 10.1111/j.1471-4159.2007.04582.x. [DOI] [PubMed] [Google Scholar]
- 318.Ma F, Zhang L, Westlund KN. Reactive oxygen species mediate TNFR1 increase after TRPV1 activation in mouse DRG neurons. Mol Pain. 2009;5:31. doi: 10.1186/1744-8069-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Ustinova EE, Schultz HD. Activation of cardiac vagal afferents by oxygen-derived free radicals in rats. Circ Res. 1994;74:895–903. doi: 10.1161/01.res.74.5.895. [DOI] [PubMed] [Google Scholar]
- 320.Cerutti PA. Mechanisms of action of oxidant carcinogens. Cancer Detect Prev. 1989;14:281–284. [PubMed] [Google Scholar]
- 321.Gopalakrishna R, Jaken S. Protein kinase C signaling and oxidative stress. Free Radic Biol Med. 2000;28:1349–1361. doi: 10.1016/s0891-5849(00)00221-5. [DOI] [PubMed] [Google Scholar]
- 322.Keeble JE, Bodkin JV, Liang L, Wodarski R, Davies M, Fernandes ES, Coelho Cde F, Russell F, Graepel R, Muscara MN, Malcangio M, Brain SD. Hydrogen peroxide is a novel mediator of inflammatory hyperalgesia, acting via transient receptor potential vanilloid 1-dependent and independent mechanisms. Pain. 2009;141:135–142. doi: 10.1016/j.pain.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 323.Schilling T, Eder C. Importance of the non-selective cation channel TRPV1 for microglial reactive oxygen species generation. J Neuroimmunol. 2009;216:118–121. doi: 10.1016/j.jneuroim.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 324.Chuang HH, Lin S. Oxidative challenges sensitize the capsaicin receptor by covalent cysteine modification. Proc Natl Acad Sci U S A. 2009;106:20097–20102. doi: 10.1073/pnas.0902675106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Susankova K, Tousova K, Vyklicky L, Teisinger J, Vlachova V. Reducing and oxidizing agents sensitize heat-activated vanilloid receptor (TRPV1) current. Mol Pharmacol. 2006;70:383–394. doi: 10.1124/mol.106.023069. [DOI] [PubMed] [Google Scholar]
- 326.Suzukawa K, Miura K, Mitsushita J, Resau J, Hirose K, Crystal R, Kamata T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J Biol Chem. 2000;275:13175–13178. doi: 10.1074/jbc.275.18.13175. [DOI] [PubMed] [Google Scholar]
- 327.Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, Uhlson C, Murphy RC, Hargreaves KM. Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest. 2010;120:1617–1626. doi: 10.1172/JCI41678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Patwardhan AM, Scotland PE, Akopian AN, Hargreaves KM. Activation of TRPV1 in the spinal cord by oxidized linoleic acid metabolites contributes to inflammatory hyperalgesia. Proc Natl Acad Sci U S A. 2009;106:18820–18824. doi: 10.1073/pnas.0905415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Salvemini D, Wang ZQ, Bourdon DM, Stern MK, Currie MG, Manning PT. Evidence of peroxynitrite involvement in the carrageenan-induced rat paw edema. Eur J Pharmacol. 1996;303:217–220. doi: 10.1016/0014-2999(96)00140-9. [DOI] [PubMed] [Google Scholar]
- 330.Salvemini D, Wang ZQ, Wyatt PS, Bourdon DM, Marino MH, Manning PT, Currie MG. Nitric oxide: a key mediator in the early and late phase of carrageenan-induced rat paw inflammation. Br J Pharmacol. 1996;118:829–838. doi: 10.1111/j.1476-5381.1996.tb15475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Urban MO, Coutinho SV, Gebhart GF. Involvement of excitatory amino acid receptors and nitric oxide in the rostral ventromedial medulla in modulating secondary hyperalgesia produced by mustard oil. Pain. 1999;81:45–55. doi: 10.1016/s0304-3959(98)00265-6. [DOI] [PubMed] [Google Scholar]
- 332.Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- 333.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Urban MO, Gebhart GF. Central mechanisms in pain. Med Clin North Am. 1999;83:585–596. doi: 10.1016/s0025-7125(05)70125-5. [DOI] [PubMed] [Google Scholar]
- 335.Wei F, Guo W, Zou S, Ren K, Dubner R. Supraspinal glial-neuronal interactions contribute to descending pain facilitation. J Neurosci. 2008;28:10482–10495. doi: 10.1523/JNEUROSCI.3593-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 337.Vanegas H. To the descending pain-control system in rats, inflammation-induced primary and secondary hyperalgesia are two different things. Neurosci Lett. 2004;361:225–228. doi: 10.1016/j.neulet.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 338.Kim HK, Park SK, Zhou JL, Taglialatela G, Chung K, Coggeshall RE, Chung JM. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain. 2004;111:116–124. doi: 10.1016/j.pain.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 339.Ji G, Neugebauer V. Reactive oxygen species are involved in group I mGluR-mediated facilitation of nociceptive processing in amygdala neurons. J Neurophysiol. 2010;104:218–229. doi: 10.1152/jn.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Terayama R, Guan Y, Dubner R, Ren K. Activity-induced plasticity in brain stem pain modulatory circuitry after inflammation. NeuroReport. 2000;11:1915–1919. doi: 10.1097/00001756-200006260-00022. [DOI] [PubMed] [Google Scholar]
- 341.Carlson JD, Maire JJ, Martenson ME, Heinricher MM. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222–13231. doi: 10.1523/JNEUROSCI.3715-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Ren K, Dubner R. Pain facilitation and activity-dependent plasticity in pain modulatory circuitry: role of BDNF-TrkB signaling and NMDA receptors. Mol Neurobiol. 2007;35:224–235. doi: 10.1007/s12035-007-0028-8. [DOI] [PubMed] [Google Scholar]
- 343.Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Miki K, Zhou QQ, Guo W, Guan Y, Terayama R, Dubner R, Ren K. Changes in gene expression and neuronal phenotype in brain stem pain modulatory circuitry after inflammation. J Neurophysiol. 2002;87:750–760. doi: 10.1152/jn.00534.2001. [DOI] [PubMed] [Google Scholar]
- 345.Terayama R, Dubner R, Ren K. The roles of NMDA receptor activation and nucleus reticularis gigantocellularis in the time-dependent changes in descending inhibition after inflammation. Pain. 2002;97:171–181. doi: 10.1016/s0304-3959(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 346.Budai D, Khasabov SG, Mantyh PW, Simone DA. NK-1 receptors modulate the excitability of ON cells in the rostral ventromedial medulla. J Neurophysiol. 2007;97:1388–1395. doi: 10.1152/jn.00450.2006. [DOI] [PubMed] [Google Scholar]
- 347.Roberts J, Ossipov MH, Porreca F. Glial activation in the rostroventromedial medulla promotes descending facilitation to mediate inflammatory hypersensitivity. Eur J Neurosci. 2009;30:229–241. doi: 10.1111/j.1460-9568.2009.06813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Lim G, Wang S, Lim JA, Mao J. Activity of adenylyl cyclase and protein kinase A contributes to morphine-induced spinal apoptosis. Neurosci Lett. 2005;389:104–108. doi: 10.1016/j.neulet.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 349.Bonfoco E, Krainc D, Ankarcrona M, Nicotera P, Lipton SA. Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with N-methyl-D-aspartate or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci U S A. 1995;92:7162–7166. doi: 10.1073/pnas.92.16.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Guo Q, Sebastian L, Sopher BL, Miller MW, Ware CB, Martin GM, Mattson MP. Increased vulnerability of hippocampal neurons from presenilin-1 mutant knock-in mice to amyloid beta-peptide toxicity: central roles of superoxide production and caspase activation. J Neurochem. 1999;72:1019–1029. doi: 10.1046/j.1471-4159.1999.0721019.x. [DOI] [PubMed] [Google Scholar]
- 351.Yamamoto T, Maruyama W, Kato Y, Yi H, Shamoto-Nagai M, Tanaka M, Sato Y, Naoi M. Selective nitration of mitochondrial complex I by peroxynitrite: involvement in mitochondria dysfunction and cell death of dopaminergic SH-SY5Y cells. J Neural Transm. 2002;109:1–13. doi: 10.1007/s702-002-8232-1. [DOI] [PubMed] [Google Scholar]
- 352.Vieira HL, Belzacq AS, Haouzi D, Bernassola F, Cohen I, Jacotot E, Ferri KF, El Hamel C, Bartle LM, Melino G, Brenner C, Goldmacher V, Kroemer G. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene. 2001;20:4305–4316. doi: 10.1038/sj.onc.1204575. [DOI] [PubMed] [Google Scholar]
- 353.Qi X, Lewin AS, Sun L, Hauswirth WW, Guy J. Mitochondrial protein nitration primes neurodegeneration in experimental autoimmune encephalomyelitis. J Biol Chem. 2006;281:31950–31962. doi: 10.1074/jbc.M603717200. [DOI] [PubMed] [Google Scholar]
- 354.Susin SA, Lorenzo HK, Zamzami N, Marzo I, Brenner C, Larochette N, Prévost M-C, Alzari PM, Kroemer G. Mitochondrial Release of Caspase-2 and -9 during the Apoptotic Process. The Journal of Experimental Medicine. 1999;189:381–394. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Mancini M, Nicholson DW, Roy S, Thornberry NA, Peterson EP, Casciola-Rosen LA, Rosen A. The Caspase-3 Precursor Has a Cytosolic and Mitochondrial Distribution: Implications for Apoptotic Signaling. The Journal of Cell Biology. 1998;140:1485–1495. doi: 10.1083/jcb.140.6.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Shacka JJ, Sahawneh MA, Gonzalez JD, Ye YZ, D’Alessandro TL, Estevez AG. Two distinct signaling pathways regulate peroxynitrite-induced apoptosis in PC12 cells. Cell Death Differ. 2006;13:1506–1514. doi: 10.1038/sj.cdd.4401831. [DOI] [PubMed] [Google Scholar]
- 357.Zhuang S, Simon G. Peroxynitrite-induced apoptosis involves activation of multiple caspases in HL-60 cells. Am J Physiol Cell Physiol. 2000;279:C341–351. doi: 10.1152/ajpcell.2000.279.2.C341. [DOI] [PubMed] [Google Scholar]
- 358.Virag L, Marmer DJ, Szabo C. Crucial role of apopain in the peroxynitrite-induced apoptotic DNA fragmentation. Free Radic Biol Med. 1998;25:1075–1082. doi: 10.1016/s0891-5849(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 359.Zamzami N, Susin SA, Marchetti P, Hirsch T, Gómez-Monterrey I, Castedo M, Kroemer G. Mitochondrial control of nuclear apoptosis. The Journal of Experimental Medicine. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. The Journal of Experimental Medicine. 1996;184:1331–1341. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Marzo I, Brenner C, Zamzami N, Jurgensmeier JM, Susin SA, Vieira HL, Prevost MC, Xie Z, Matsuyama S, Reed JC, Kroemer G. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 362.Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- 363.Bai P, Bakondi E, Szabo E, Gergely P, Szabo C, Virag L. Partial protection by poly(ADP-ribose) polymerase inhibitors from nitroxyl-induced cytotoxity in thymocytes. Free Radic Biol Med. 2001;31:1616–1623. doi: 10.1016/s0891-5849(01)00756-0. [DOI] [PubMed] [Google Scholar]
- 364.Virag L, Szabo C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev. 2002;54:375–429. doi: 10.1124/pr.54.3.375. [DOI] [PubMed] [Google Scholar]
- 365.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 366.Virag L, Szabo E, Gergely P, Szabo C. Peroxynitrite-induced cytotoxicity: mechanism and opportunities for intervention. Toxicol Lett. 2003;140–141:113–124. doi: 10.1016/s0378-4274(02)00508-8. [DOI] [PubMed] [Google Scholar]
- 367.Boulares AH, Yakovlev AG, Ivanova V, Stoica BA, Wang G, Iyer S, Smulson M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J Biol Chem. 1999;274:22932–22940. doi: 10.1074/jbc.274.33.22932. [DOI] [PubMed] [Google Scholar]
- 368.Niwa Y, Somiya K, Michelson AM, Puget K. Effect of liposomal-encapsulated superoxide dismutase on active oxygen-related human disorders. A preliminary study. Free Radic Res Commun. 1985;1:137–153. doi: 10.3109/10715768509056547. [DOI] [PubMed] [Google Scholar]
- 369.Flohe L. Superoxide dismutase for therapeutic use: clinical experience, dead ends and hopes. Mol Cell Biochem. 1988;84:123–131. doi: 10.1007/BF00421046. [DOI] [PubMed] [Google Scholar]
- 370.Goebel KM, Storck U, Neurath F. Intrasynovial orgotein therapy in rheumatoid arthritis. Lancet. 1981;1:1015–1017. doi: 10.1016/s0140-6736(81)92185-1. [DOI] [PubMed] [Google Scholar]
- 371.Goebel KM, Storck U. Effect of intra-articular orgotein versus a corticosteroid on rheumatoid arthritis of the knees. Am J Med. 1983;74:124–128. doi: 10.1016/0002-9343(83)91128-2. [DOI] [PubMed] [Google Scholar]
- 372.Lund-Olesen K, Menander-Huber KB. Intra-articular orgotein therapy in osteoarthritis of the knee. A double-blind, placebo-controlled trial. Arzneimittelforschung. 1983;33:1199–1203. [PubMed] [Google Scholar]
- 373.Gammer W, Broback LG. Clinical comparison of orgotein and methylprednisolone acetate in the treatment of osteoarthrosis of the knee joint. Scand J Rheumatol. 1984;13:108–112. doi: 10.3109/03009748409100372. [DOI] [PubMed] [Google Scholar]
- 374.Mazieres B, Masquelier AM, Capron MH. A French controlled multicenter study of intraarticular orgotein versus intraarticular corticosteroids in the treatment of knee osteoarthritis: a one-year followup. J Rheumatol Suppl. 1991;27:134–137. [PubMed] [Google Scholar]
- 375.McIlwain H, Silverfield JC, Cheatum DE, Poiley J, Taborn J, Ignaczak T, Multz CV. Intra-articular orgotein in osteoarthritis of the knee: a placebo-controlled efficacy, safety, and dosage comparison. Am J Med. 1989;87:295–300. doi: 10.1016/s0002-9343(89)80154-8. [DOI] [PubMed] [Google Scholar]
- 376.Huskisson EC, Scott J. Orgotein in osteoarthritis of the knee joint. Eur J Rheumatol Inflamm. 1981;4:212–218. [PubMed] [Google Scholar]
- 377.Lund-Olesen K, Menander KB. Orgotein: a new anti-inflammatory metalloprotein drug: preliminary evaluation of clinical efficacy and safety in degenerative joint disease. Curr Ther Res Clin Exp. 1974;16:706–717. [PubMed] [Google Scholar]
- 378.Lin Y, Pape HD, Friedrich R. Use of superoxide dismutase (SOD) in patients with temporomandibular joint dysfunction--a preliminary study. Int J Oral Maxillofac Surg. 1994;23:428–429. doi: 10.1016/s0901-5027(05)80038-4. [DOI] [PubMed] [Google Scholar]
- 379.Pascu O, Dejica D. Oxygen free radicals and duodenal ulcer pain. Preliminary data. Med Interne. 1987;25:81–84. [PubMed] [Google Scholar]
- 380.Primus G. Orgotein in the treatment of plastic induration of the penis (Peyronie’s disease) Int Urol Nephrol. 1993;25:169–172. [PubMed] [Google Scholar]
- 381.Schieroni MP, Revello MP, Colombo M, Randone DF, Rolle L. [Orgotein iontophoresis in the therapy of induratio penis plastica] Minerva Med. 1985;76:1085–1088. [PubMed] [Google Scholar]
- 382.Calabro A, Pegoraro V, Aragona F, Tasca A, Artibani W, Lembo A. [Peyronie disease: observations on 40 cases treated with orgotein] Arch Esp Urol. 1985;38:529–532. [PubMed] [Google Scholar]
- 383.Altomare GF, Pigatto PD, Polenghi MM, Fioroni A. [Intra-focal orgotein in the treatment of plastic penile induration] G Ital Dermatol Venereol. 1986;121:289–291. [PubMed] [Google Scholar]
- 384.Bagis S, Tamer L, Sahin G, Bilgin R, Guler H, Ercan B, Erdogan C. Free radicals and antioxidants in primary fibromyalgia: an oxidative stress disorder? Rheumatol Int. 2005;25:188–190. doi: 10.1007/s00296-003-0427-8. [DOI] [PubMed] [Google Scholar]
- 385.Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, Rowlands BJ. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. J Gastrointest Surg. 2006;10:499–503. doi: 10.1016/j.gassur.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 386.Campana F, Zervoudis S, Perdereau B, Gez E, Fourquet A, Badiu C, Tsakiris G, Koulaloglou S. Topical superoxide dismutase reduces post-irradiation breast cancer fibrosis. J Cell Mol Med. 2004;8:109–116. doi: 10.1111/j.1582-4934.2004.tb00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]