Abstract
Patients undergoing HCT are at increased risk of chronic health conditions, including second malignant neoplasms and cardiovascular disease. Little is known about health behaviors and cancer screening practices among HCT survivors that could moderate the risk of these conditions. The BMTSS examined health behaviors and cancer screening practices in individuals who underwent HCT between 1976 and 1998, and survived 2+ years. Health behavior was deemed high-risk if an individual was a current smoker and if they reported risky alcohol intake (≥4 drinks/day [males], ≥ 3 drinks/day [females]) on days of alcohol consumption. Cancer screening assessment was per American Cancer Society recommendations. There were 1040 survivors: 42.7% underwent allogeneic HCT; 43.8% were female; median time from HCT: 7.4 years (range 2.0–27.7 years). Median age at study participation: 43.8 years (range 18.3–73.0 years). Multivariate regression analysis revealed younger age (<35 years) at study participation (Odds Ratio [OR]=4.7; p<0.01) and lower education (<college: OR=2.1; p<0.01) to be significantly associated with high-risk behavior. Survivors were significantly less likely to report high-risk behavior (OR=0.5; p<0.01), and more likely to have had a screening mammogram (OR=2.8; p=0.05) when compared to gender-matched sibling controls (N=309).
INTRODUCTION
Improvements in transplantation strategies have contributed to increments in survival approximating 10% per decade, and long-term survival is an expected outcome for many who undergo HCT.1–3 Patients who survive for 2 years after allogeneic HCT now have survival rates that exceed 80% at 15 years,4,5 and the survival rates approach 70% at 10 years following autologous HCT.6 The growing population of long-term survivors has brought to the medical forefront a host of chronic and debilitating conditions attributed to toxicity from pre-transplantation exposure, transplantation conditioning regimens, chronic immunosuppression and graft versus host disease (GvHD).2,3,7,8 The cumulative incidence of any chronic health condition in these long-term survivors approaches 60% at 10 years, and the incidence is 35% for severe or life-threatening conditions or death from such conditions.9
Appropriate, timely, and risk-based preventive care for these survivors may help attenuate late effects of therapy via preventive strategies that encourage healthy lifestyle behaviors, specialized surveillance and screening, and health risk management, which may ultimately lead to improved quality of life. On the other hand, high risk health behaviors may negatively impact quality of life in long-term survivors, and there are well-described associations between smoking, excessive alcohol intake, and diminished physical activity and various adverse health-related outcomes in cancer survivors.10–13 There is a paucity of data regarding health-behaviors and cancer screening practices in long-term HCT survivors. An estimate of the populations at risk for unhealthy life-style practices, and for non-adherence to current recommendations for cancer screening would inform development of targeted interventions. The current study describes health risk behaviors and cancer screening practices of long-term survivors of HCT, and a healthy comparison group, and identifies sociodemographic and clinical factors associated with increased risk for health risk behaviors and non-adherence to cancer screening recommendations.
MATERIALS AND METHODS
Subjects
BMTSS is a collaborative effort between City of Hope (COH) and University of Minnesota (UMN) to examine the long-term outcomes of individuals who have survived two or more years after HCT. Eligible participants include individuals who received HCT at COH or UMN between 1974 and 1998 for a hematologic malignancy or severe aplastic anemia (SAA); survived at least two years post-transplantation; and were alive and 18 years of age or older at study participation. The Human Subjects Protection committees at the participating institutions approved the study. Informed consent was obtained according to the Declaration of Helsinki.
Of the 1680 survivors who were alive at study participation, 1506 (90%) were successfully contacted, and 1040 (69%) participated. Compared to non-participants, study participants were older at HCT (median age: 34.3 versus 31.1 years, p<0.01), had a shorter period of follow-up after HCT (median: 7.4 versus 9.2 years, p<0.01), were less likely to be female (37% versus 44%, p=0.01), and non-Hispanic white (69% versus 77%, p<0.01). Participation rate did not differ by type of transplant (autologous, allogeneic), diagnosis, institution, or risk of relapse at HCT. Comparison with a non-cancer population was made possible by asking participating survivors to invite a nearest-age sibling to the study. A total of 309 siblings participated in this study.
A 255-item mailed questionnaire was used to collect information from all participants alive at the time of study enrollment, and covered the following general areas: sociodemographics (race/ethnicity, marital status, education, employment, annual household income, and health insurance); utilization of medical care over the past two years; presence of chronic GvHD; concerns regarding their future health, and diagnosis of physical health conditions (endocrinopathies; central nervous system compromise; cardiopulmonary dysfunction; gastrointestinal and hepatic sequelae; musculoskeletal abnormalities; and subsequent malignancies). Health conditions were graded using CTCAE v 3.0 (grade 1–4, ranging from mild to life-threatening/disabling).9 The reliability and validity of the BMTSS questionnaire has been tested, and the results indicate moderate to high agreement between self-reported outcomes and medical records.14
Information regarding primary diagnosis, preparative regimens, source of stem cells (autologous or allogeneic), and risk of relapse at HCT (standard vs. high risk), was obtained from institutional transplantation databases. Patients transplanted in first or second complete remission after acute leukemia (acute myeloid [AML] or lymphoid [ALL]) leukemia), lymphoma (Hodgkin lymphoma [HL] or non-Hodgkin lymphoma [NHL]), in first chronic phase of chronic myeloid leukemia [CML], and all patients with SAA were considered being at standard risk for relapse; all others were considered at high-risk.
Health Behaviors
Smoking
Current smokers were defined as those who reported having smoked at least 100 cigarettes in their lifetime and currently smoked on a regular basis. Former smokers were defined as individuals who reported having smoked at least 100 or more cigarettes in their lifetime but did not currently smoke. Never-smokers included those who did not smoke more than 100 cigarettes in their lifetime.
Alcohol intake
Risky alcohol intake was defined as that which exceeded the National Institute for Alcohol Abuse and Alcoholism (NIAAA) guidelines.15,16 These guidelines include limits on both daily and weekly alcohol intake and define risky drinking for women to exceed three drinks per day or seven drinks per week, and for men more than four drinks per day or 14 drinks per week.
High Risk Behavior
Survivors were considered to be engaging in High Risk Behavior if they reported current smoking and risky alcohol intake.
Cancer Screening Practices
Cancer screening practices were assessed according to recommendations for the general population at the time this survey was conducted, including those of the U.S. Preventive Services Task Force (USPSTF) and the American Cancer Society (ACS).17–20 The guidelines for the general population were chosen because at the time of questionnaire administration, there was no consensus concerning guidelines for cancer screening in conventionally treated21 or HCT22 survivors.
Female participants
For females, breast cancer screening practices were assessed by asking about frequency of self-breast examination as well as recency of clinical breast examination. Self-breast examination was considered to be regular, if a survivor reported at least monthly self-examination. Clinical breast exam was considered recent if it was performed by a physician or health care professional within the past 2 years. Cervical cancer screening was considered to be recent if an individual had a papanicolaou (pap) smear within the past 2 years. In addition, all female participants were asked if they ever had a mammogram.
Male participants
Males were asked about frequency of testicular self examination (TSE); regular testicular cancer screening was defined as TSE of at least once a month.
Statistical Analyses
Survivors and siblings were compared with regards to demographics, socioeconomic variables, healthcare utilization, and concerns regarding their future health using standard parametric and non-standard parametric tests. Prevalence of health behaviors and cancer screening was determined for survivors and siblings. Health behaviors were dichotomized as follows: smoking (current smoker vs. former or never smoked), risky alcohol intake (Females: >3 drinks/day or >7 drinks/week or Males: >4 drinks/day or >14 drinks/week vs. less frequently), high risk behavior (current smoker and risky alcohol intake vs. none). Cancer screening practices were dichotomized as follows: females – self-breast examination (once a month vs. less frequently), clinical breast examination (within the past 2 years vs. >2 years ago), mammogram (at least once vs. none); males – TSE (once a month vs. less frequently). Analyses pertaining to mammography were limited to females ≥ 40 years of age at the time questionnaire per general population recommendations.18,19 Subset analyses were performed using an earlier age cutoff (≥30 years) to compare mammography practices in females treated with mediastinal radiation versus no radiation or siblings sibling controls. Age 30 was selected because at the time of questionnaire administration, it represented a conservative estimate of age at which many authorities agreed mammography should be performed regularly for survivors treated with mediastinal radiation.23,24
HCT survivors vs. sibling controls
Multivariate logistic regression analyses were performed to compare cancer screening practices and health risk behaviors of HCT survivors vs. siblings, after adjusting for potential confounding variables. Independent variables included age at questionnaire, ethnicity, education, household income, marital status, health insurance, level of concern regarding their future health, and gender (limited to health behavior analyses). The results were reported as odds ratios (ORs) with 95% confidence intervals (CIs). Data were analyzed using SAS 9.1 (SAS institute, Cary, NC). All statistical tests were two-sided.
HCT survivors
Multivariate logistic regression analyses were used to identify predictors of cancer screening and health risk behaviors among HCT survivors. The dependent variables for the regression models included: regular breast examination (females), recent clinical breast examination (females), previous mammogram (limited to females ≥ 40 years of age at questionnaire), recent pap smear (females), regular TSE (males), current smoking, risky alcohol intake, and high-risk behavior; independent variables included age at questionnaire, gender (limited to health behavior analysis), ethnicity, marital status, household income, health insurance, education, age at HCT, type of HCT, conditioning regimen, severity of reported chronic health condition, follow-up at Cancer/HCT center, and level of concern regarding their future health.
RESULTS
Demographic and Clinical Characteristics of HCT Survivors and Siblings
The demographic and clinical characteristics of the study participants (HCT survivors, n=1040; siblings, n=309) are presented in Table 1. Compared to siblings, HCT survivors were younger at study participation (median age, 43.8 years versus 45.2 years, p=0.03), less likely to be female (43.8% versus 63.4%, p<0.01), or non-Hispanic white (77.0% versus 87.4%, p<0.01), less likely to be college graduates (43.5% versus 56.3%, p<0.01), less likely to be married (65.5% versus 78.3%, p<0.01), or insured (90.2% versus 94.2%, p=0.03). AML (24.5%), CML (23.9%), NHL (20.0%), and ALL (10.2%) accounted for 78.6% of all primary diagnoses. Total body irradiation (TBI) was used for 78.4%, and 35% of the survivors were at high-risk for relapse at HCT. Fifty-seven percent of the HCT survivors had received an allogeneic HCT; of them, approximately 54% developed chronic GvHD after HCT. The cohort had been followed for a median of 7.4 years from HCT.
Table 1.
Demographic and Clinical Characteristics of Hematopoietic Cell Transplantation Survivors and Siblings
| Characteristic | Survivors (N=1040) | Siblings (n=309) | P value |
|---|---|---|---|
| Age at study participation – year | |||
| Medan (±SD) | 43.8 (12.0) | 45.2 (11.8) | 0.03 |
| Range | 18.3–73.0 | 19.3–78.7 | |
| Gender – no. (%) | |||
| Female | 456 (43.8%) | 196 (63.4%) | <0.01 |
| Race – no. (%) | |||
| Non-Hispanic White | 801 (77.0%) | 270 (87.4%) | <0.01 |
| Education – no. (%) | |||
| <High school | 218 (21.0%) | 38 (12.3%) | |
| High school/Some college or training | 369 (35.5%) | 97 (31.4%) | <0.01 |
| College graduate | 452 (43.5%) | 174 (56.3%) | |
| Marital status – no. (%) | |||
| Married | 681 (65.5%) | 242 (78.3%) | <0.01 |
| Household income - no. (%)* | |||
| >$60,000/yr | 430 (44.4%) | 189 (64.3%) | |
| $20–60,000/yr | 383 (39.6%) | 93 (31.6%) | <0.01 |
| <$20,000/yr | 155 (16.0%) | 12 (4.1%) | |
| Health insurance - no. (%) | |||
| Yes | 938 (90.2%) | 291 (94.2%) | 0.03 |
| Age at HCT – year | |||
| Median (±SD) | 34.3 | NA | |
| Range | 0.4–68.6 | NA | |
| Interval between HCT and study – year | |||
| Median (±SD) | 7.4 | NA | |
| Range | 2.0–27.7 | NA | |
| Primary cancer diagnosis – no. (%) | |||
| Chronic myeloid leukemia | 249 (23.9%) | NA | |
| Acute myeloid leukemia | 255 (24.5%) | NA | |
| Hodgkin lymphoma | 92 (8.8%) | NA | |
| Non-Hodgkin lymphoma | 208 (20.0%) | NA | |
| Acute lymphoblastic leukemia | 106 (10.2%) | NA | |
| Others | 130 (12.5%) | NA | |
| Stem cell donor - no. (%) | |||
| Autologous HCT | 444 (42.7%) | NA | |
| Allogeneic donor | 596 (57.3%) | NA | |
| Chronic GvHD - no. (%) | |||
| Yes | 324 (31.2%) | NA | |
| Risk of Relapse at HCT - no. (%) | |||
| Standard risk | 676 (65.0%) | NA | |
| High risk | 364 (35.0%) | NA | |
| Conditioning regimen - no. (%) | |||
| Chemotherapy | 225 (21.6%) | NA | |
| Chemotherapy + TBI | 815 (78.4%) | NA | |
Information available for 968 Survivors, 294 Siblings
Risky Health Behaviors and Cancer Screening Practices of HCT Survivors and Siblings
Compared to siblings, survivors were significantly more likely to be concerned about their future health (very/somewhat concerned: 51.9% versus 39.7%, p<0.01); the proportion of those concerned about the likelihood of developing cancer was equivalent among survivors (41.9%) and siblings (37.9%; p=0.13). Table 2 summarizes the prevalence of health behaviors and cancer screening practices of survivors and sibling controls. Despite reporting similar rates of having ever smoked >100 cigarettes (40.6% survivors versus 44.7%, p=0.20), survivors were significantly less likely to report current smoking (7.0%) when compared to siblings (12.9%, p<0.01). Of the 73 survivors who reported current smoking, 58 (79.5%) had started smoking prior to HCT, while 15 (20.5%) initiated smoking following HCT. Median rate of smoking was 10 cigarettes per day (range: 1–50), and 53.4% reported attempting to quit in the previous 2 years. Of the 349 survivors who were former smokers, 291 (83.4%) quit prior to undergoing HCT, while 58 (16.6%) quit following HCT. There were no differences in demographics, socioeconomic status, or treatment-related variables between survivors who quit following HCT versus those who continued to smoke after HCT. However, survivors who initiated smoking after HCT were significantly younger at HCT (mean age: 12.5 years versus 27.4 years, p<0.01), and tended to have a longer follow-up period (mean time from HCT: 15.0 years versus 12.3 years, p=0.10), and were less likely to have regular cancer/HCT-related visits (60.0% vs. 77.2%, p=0.13) when compared to survivors who quit after HCT.
Table 2.
Health behaviors and cancer screening practices Hematopoietic Cell Transplantation Survivors and Siblings
| Health Behavior | Survivors (N=1040) | Siblings (n=309) | P value |
|---|---|---|---|
| Smoking, ever – no. (%) | |||
| Yes | 423 (40.6%) | 138 (44.7%) | 0.20 |
| Smoking, current – no. (%) | |||
| Yes | 73 (7.0%) | 40 (12.9%) | <0.01 |
| Risky alcohol intake – no. (%) | |||
| Yes | 99 (9.5%) | 41 (13.3%) | 0.06 |
| High risk behavior – no. (%) | |||
| Yes | 88 (8.5%) | 45 (14.6.0%) | <0.01 |
| Cancer Screening, Females | Survivors (N=456) | Siblings (N=196) | |
| Breast self-examination – no. (%) | |||
| Monthly | 132 (28.9%) | 53 (27.0%) | 0.62 |
| Clinical breast examination – no. (%) | |||
| Within previous 2 years | 403 (88.4%) | 179 (91.3%) | 0.26 |
| Mammogram* – no. (%) | |||
| Ever | 253 (96.2%) | 121 (91.7%) | 0.06 |
| Pap smear – no. (%) | |||
| Within previous 2 years | 390 (85.5%) | 175 (89.3%) | 0.20 |
| Cancer Screening, Males | Survivors (N=584) | Siblings (N=113) | |
| Testicular self-examination – no. (%) | |||
| Monthly | 103 (17.6%) | 23 (20.4%) | 0.49 |
Limited to females ≥ 40 y.o. (survivors, n=263; siblings, n=132)
Compared to siblings, HCT survivors were less likely to report risky alcohol intake (9.5% versus 13.3%, p=0.06); overall, survivors were significantly less likely to report high risk behavior (current smoking and risky alcohol intake [8.5% versus 14.6%, p<0.01]) compared to siblings.
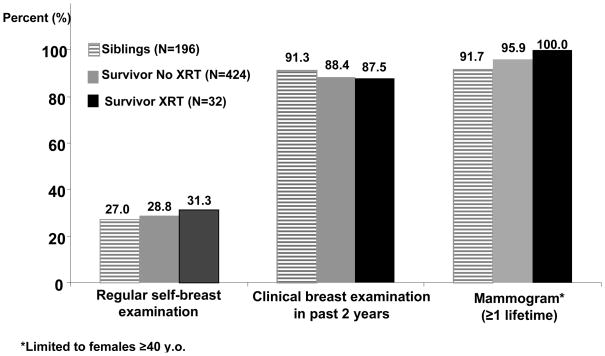
There were no differences in cervical or testicular cancer screening practices between survivors and siblings (table 2). Thirty-two out of 456 (7.0%) female HCT survivors had received chest or mantle radiation as part of their pre-HCT treatment. As seen in figure 1, siblings and survivors reported equally high rates of self- or clinical breast examination or mammography, regardless of radiation exposure. Using a younger age cutoff of ≥ 30 years at the time of questionnaire, we found that survivors previously treated with chest or mantle radiation were significantly more likely to report ever having had a mammogram (95.8%) when compared to survivors not treated with radiation (85.0%) or siblings (76.2%, p=0.01). The prevalence of mammographic screening in the younger cohort (age 25–39 years) was 80.0% for survivors treated with chest radiation, and 58.4% and 25.0% for survivors with no radiation and siblings, respectively (p<0.01) Multivariate logistic regression revealed that survivors were significantly less likely to report current smoking (odds ratio [OR] = 0.4, p<0.01), risky alcohol intake (OR=0.5, p<0.01), or high risk behavior (OR=0.5, p<0.01) when compared to sibling controls (Table 3). Female survivors were nearly three-times as likely to report ever having had a mammogram (OR=2.8, p=0.05) when compared to siblings.
Figure 1.
Reported breast cancer screening among HCT survivors (with and without chest radiation exposure) and siblings.
Table 3.
Odds ratios (OR) for reporting cancer screening practices and health risk behaviors in HCT survivors in comparison with siblings
| Risk Factor | Odds ratio | 95% CI | P-Value |
|---|---|---|---|
| Cancer Screening Practices§ | |||
| Females | |||
| Breast self-examination, monthly | 0.9 | 0.6–1.5 | 0.93 |
| Clinical Breast Examination within previous 2 years | 0.8 | 0.4–1.6 | 0.58 |
| Mammogram, ever | 2.8 | 1.0–8.7 | 0.05 |
| Pap smear within previous 2 years | 0.7 | 0.4–1.2 | 0.20 |
| Males | |||
| Testicular self-examination, monthly | 0.8 | 0.5–1.3 | 0.36 |
| Health Risk Behaviors† | |||
| Smoking, current | 0.4 | 0.3–0.7 | <0.01 |
| Risky alcohol intake | 0.5 | 0.4–0.8 | <0.01 |
| High risk behavior | 0.5 | 0.3–0.7 | <0.01 |
Adjusted for: age at questionnaire, ethnicity, education, household Income, marital status, health Insurance, concern regarding future health.
Adjusted for: age at questionnaire, ethnicity, education, household Income, marital status, health Insurance, concern regarding future health, and gender.
Risky Health Behaviors and Cancer Screening Practices among HCT survivors
Table 4 summarizes the results of multivariate regression analysis restricted to HCT survivors, examining the relevant demographic, clinical, and treatment-related factors associated with self-reported risky health behavior. Younger participants (<35 years) were 5.6-fold more likely to report risky alcohol intake (p<0.01) and nearly 5-fold more likely to report high risk behavior (p<0.01). Having less than college education was associated with a 3.1-fold risk of current smoking (p<0.01), and a 2.1-fold likelihood of risky alcohol intake (OR=2.1, p<0.01) or high risk behavior (OR=2.1, p<0.01). Lack of health insurance was associated with a 2.4-fold risk of current smoking (p=0.02) There were no predictors of cancer screening practices identified among HCT survivors (data not shown).
Table 4.
Predictors of adverse health behavior among HCT survivors
| Risk Factor | Smoking, current (N=73) | Risky Alcohol Intake§ (N=99) | High Risk Behavior† (N=88) |
|---|---|---|---|
| Odds Ratios (95% Confidence Interval) | |||
| Gender | |||
| Female | 1.0 | 1.0 | 1.0 |
| Male | 0.9 (0.6–1.6) | 1.4 (0.9–2.2) | 1.3 (0.8–2.1) |
| Ethnicity | |||
| Others | 1.0 | 1.0 | 1.0 |
| Non-Hispanic white | 1.6 (0.8–3.2) | 1.2 (0.7–2.1) | 1.2 (0.8–2.0) |
| Age at HCT | |||
| < 30 years | 1.0 | 1.0 | 1.0 |
| ≥ 30 years | 1.4 (0.6–3.1) | 0.8 (0.4–1.8) | 1.3 (0.6–2.7) |
| Age at study participation | |||
| > 55 years | 1.0 | 1.0 | 1.0 |
| 35 – 55 years | 1.9 (0.8–4.7) | 1.8 (0.7–4.5) | 1.9 (0.8–3.8) |
| <35 years | 2.8 (0.8–9.2) | 5.6 (1.8–17.7)** | 4.7 (1.4–14.9)** |
| Marital Status | |||
| Married | 1.0 | 1.0 | 1.0 |
| Not married | 1.4 (0.7–4.0) | 1.2 (0.7–2.0) | 1.1 (0.6–1.8) |
| Annual Household Income | |||
| > $60,000 | 1.0 | 1.0 | 1.0 |
| $20,000 – 60,000 | 0.8 (0.3–2.0) | 1.6 (0.7–3.4) | 0.9 (0.4–1.9) |
| < $20,000 | 1.8 (0.8–3.9) | 1.6 (0.8–3.3) | 1.3 (0.6–2.5) |
| Health Insurance | |||
| Yes | 1.0 | 1.0 | 1.0 |
| No | 2.4 (1.1–5.0)* | 1.7 (0.8–3.4) | 1.6 (0.8–3.4) |
| Education | |||
| ≥ College | 1.0 | 1.0 | 1.0 |
| < College | 3.1 (1.6–6.0)** | 2.1 (1.3–3.5)** | 2.1 (1.2–3.6)** |
| HCT Source | |||
| Allogeneic | 1.0 | 1.0 | 1.0 |
| Autologous | 1.3 (0.8–2.3) | 1.0 (0.6–1.7) | 1.2 (0.7–2.0) |
| Conditioning regimen | |||
| Chemotherapy | 1.0 | 1.0 | 1.0 |
| Chemotherapy + TBI | 1.1 (0.6–2.0) | 1.4 (0.8–2.4) | 1.1 (0.6–1.8) |
| Chronic health condition | |||
| Grade 0–2 | 1.0 | 1.0 | 1.0 |
| Grade 3 or 4 | 0.9 (0.5–1.6) | 0.8 (0.5–1.4) | 0.8 (0.5–1.5) |
| Cancer/HCT center follow-up | |||
| Yes | 1.0 | 1.0 | 1.0 |
| No | 0.7 (0.4–1.3) | 1.5 (0.9–2.4) | 1.0 (0.5–1.8) |
| Concern for future health | |||
| Yes | 1.0 | 1.0 | 1.0 |
| No | 0.9 (0.6–1.6) | 1.2 (0.8–2.0) | 0.9 (0.6–1.5) |
Females: >3 drinks/day or 7/week; Males: >4 drinks/day or 14/week
Current smoking and Risky alcohol intake
p<0.05,
p<0.01
DISCUSSION
The overall goals of the current study were to determine the prevalence of cancer screening practices and risky health behaviors in HCT survivors in comparison to a healthy sibling group, and identify populations with poor adherence to screening and health promotion recommendations.15,17–20 We found that compared to siblings, survivors were less likely to report risky health behaviors such as smoking or excessive alcohol intake, and had similar or better cancer screening practices. However, despite the potential for long-term sequelae, younger survivors, those without health insurance, and with lower education continued to engage in high risk behaviors, thus informing education and targeted interventions in the future.
The Institute of Medicine (IOM) recommends that all cancer survivors have regular medical care that is adapted to specific risks due to their previous cancer therapy, lifestyle, or any comorbid health conditions.25 Investigators from the Childhood Cancer Survivors Study (CCSS) have found that despite having similar cancer screening rates as the general population, the frequency and extent of these screenings remains suboptimal for many adult survivors of childhood cancer.10,26,27 On the other hand, studies in survivors of adult onset cancer have reported better than expected cancer screening practices in survivors when compared to age-matched controls.28,29 To date, few studies have examined the cancer screening practices of long-term HCT survivors, a population at risk for developing chronic health conditions, including subsequent malignant neoplasms.9
We found that the vast majority of female survivors reported having had a recent clinical breast examination (88%) or pap smear (85%), and this was better than or comparable to U.S. population-based data from the time of questionnaire administration (breast examination, 60–76%; pap smear, 85–87%) 30,31. The reported low rates of regular BSE (survivors, 29%; siblings, 27%) or TSE (survivors, 18%; siblings 20%) in the current study were similar to those reported in adult survivors of childhood cancer (27% for regular BSE, 17% regular TSE),27 and may reflect ongoing controversies regarding the utility and yield of such screenings.32–35 After adjusting for sociodemographics and level of concern regarding future health, female HCT survivors were nearly three times as likely to report having had at least one mammogram compared to sibling controls. There were no differences in cervical cancer or testicular cancer screening practices between the two groups.
It is now well-recognized that female cancer survivors previously treated with chest or mantle radiation are at increased risk of developing breast cancer years following completion of therapy;26 as a result it is recommended that these women undergo screening at a younger age than general population..21,22,26 In the current study, nearly all women (96%) treated with chest radiation who were 30 years of age or older at the time of questionnaire reported having had at least one screening mammogram, and all had a recent clinical breast examination. As expected, the difference in screening rates between radiation-treated, non-radiation treated, and sibling participants was greatest for the 30–39 year old age group. While none of the consensus guidelines were available at the time of questionnaire administration, it is encouraging that a sizeable number of survivors treated with radiation had undergone regular breast cancer screening, a finding that is likely to continue to improve with increased awareness and greater dissemination of survivorship screening guidelines.
Guidelines of the USPSTF recommend that healthcare providers screen all adults for tobacco use and other high risk behavior, and that cessation interventions be provided for those engaged in such behavior.36 Adverse health related outcomes attributed to excess alcohol or tobacco use have been well-described in the non-oncology community, and there is a growing body of literature describing their modifying effect on adverse outcomes in long-term cancer survivors.10 For patients engaging in high risk behaviors, a diagnosis of cancer or HCT may serve as a catalyst for life change. However, studies have found that most survivors report relatively few changes in their overall health behaviors, and that the lifestyle practices largely remain unchanged in the long-term by the cancer experience.13,37 In the current study, despite having a similar past smoking history, survivors were significantly less likely to report current smoking, and had a higher quit rate when compared to sibling controls. Overall, after adjusting for baseline demographic differences, HCT survivors were 50% less likely to report high risk behavior when compared to sibling controls.
Despite the encouraging health practices of HCT survivors, there were a sizeable number of survivors who continued to engage in risky health behaviors long after undergoing HCT. Consistent with previous research,10,38 we found that younger age, lack of health insurance, and lower education were significant predictors of adverse health behavior. The reasons why cancer survivors chose to engage in these behaviors are not known, but a perception of invulnerability or denial have been proposed as possible factors.39 Survivors who initiated smoking after HCT were younger at HCT, had a longer period of follow-up post-HCT and were less likely to report regular cancer/HCT-related visits when compared to those who quit smoking after HCT, suggesting that the level of engagement in health-related issues may be an important modifier of risk for adverse health behaviors in this population. This highlights the importance of ongoing multi-disciplinary long term follow-up care that places equal emphasis on primary as well as secondary disease prevention.
The results of this study must be interpreted in the context of potential limitations. Approximately one third of the eligible patients did not participate in the current study. Although participants and non-participants were similar in many respects, it is possible that those who participated were more likely to be engaged in health screenings, less likely to have high risk behaviors. If this were to be true, the prevalence rates presented here are more conservative than what would be expected with complete participation. In addition, similar to previous studies, 13,28,29,38,39 information regarding cancer screening and health risk behaviors were ascertained using self-report, potentially contributing to an overestimation of cancer screening practices and underestimation of adverse health behaviors in both survivors and siblings. However, the reported cancer screening rates for siblings were similar to those in the general population at the time of survey, suggesting that despite these limitations, information presented in the current study adequately represented the health behaviors of long-term survivors of HCT.
In summary, HCT survivors had comparable cervical and testicular cancer screening practices, were more likely to have had breast cancer screening by mammography, and were less likely to be engaged in high risk behaviors when compared to healthy sibling controls. We found that despite potential long-term risks, certain subsets of survivors continue to engage in high-risk behaviors such as smoking and excessive alcohol intake, indicating the need for targeted interventions for these high risk populations. Continued vigilance in encouraging appropriate cancer screening and healthy behaviors for HCT survivors will be critical to building on the positive behavioral outcomes described in the current study.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Cohen A, Bekassy AN, Gaiero A, et al. Endocrinological late complications after hematopoietic SCT in children. Bone Marrow Transplant. 2008;41 (Suppl 2):S43–8. doi: 10.1038/bmt.2008.54. [DOI] [PubMed] [Google Scholar]
- 3.Faraci M, Bekassy AN, De Fazio V, et al. Non-endocrine late complications in children after allogeneic haematopoietic SCT. Bone Marrow Transplant. 2008;41 (Suppl 2):S49–57. doi: 10.1038/bmt.2008.55. [DOI] [PubMed] [Google Scholar]
- 4.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–92. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–22. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syrjala KL, Langer SL, Abrams JR, et al. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol. 2005;23:6596–606. doi: 10.1200/JCO.2005.12.674. [DOI] [PubMed] [Google Scholar]
- 8.Ranke MB, Schwarze CP, Dopfer R, et al. Late effects after stem cell transplantation (SCT) in children--growth and hormones. Bone Marrow Transplant. 2005;35 (Suppl 1):S77–81. doi: 10.1038/sj.bmt.1704853. [DOI] [PubMed] [Google Scholar]
- 9.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. doi: 10.1182/blood-2009-06-229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathan PC, Ford JS, Henderson TO, et al. Health behaviors, medical care, and interventions to promote healthy living in the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27:2363–73. doi: 10.1200/JCO.2008.21.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Travis LB, Gospodarowicz M, Curtis RE, et al. Lung cancer following chemotherapy and radiotherapy for Hodgkin’s disease. J Natl Cancer Inst. 2002;94:182–92. doi: 10.1093/jnci/94.3.182. [DOI] [PubMed] [Google Scholar]
- 12.Marks DI, Ballen K, Logan BR, et al. The effect of smoking on allogeneic transplant outcomes. Biol Blood Marrow Transplant. 2009;15:1277–87. doi: 10.1016/j.bbmt.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanchard CM, Courneya KS, Stein K. Cancer survivors’ adherence to lifestyle behavior recommendations and associations with health-related quality of life: results from the American Cancer Society’s SCS-II. J Clin Oncol. 2008;26:2198–204. doi: 10.1200/JCO.2007.14.6217. [DOI] [PubMed] [Google Scholar]
- 14.Louie AD, Robison LL, Bogue M, et al. Validation of self-reported complications by bone marrow transplantation survivors. Bone Marrow Transplant. 2000;25:1191–6. doi: 10.1038/sj.bmt.1702419. [DOI] [PubMed] [Google Scholar]
- 15.National Institute on Alcohol Abuse and Alcoholism. The Physician’s Guide to Helping Patients with Alcohol Problems. Rockville, MD: National Institutes of Health; 1995. [Google Scholar]
- 16.National Institute on Alcohol Abuse and Alcoholism; Services USDoHaH. The Clinician’s Guide to Helping Patients Who Drink Too Much. National Institutes of Health; 2005. [Google Scholar]
- 17.Wilkins W, editor. U.S. Preventive Services Task Force. Guide to clinical preventive services. Baltimore: 1996. [Google Scholar]
- 18.Routine cancer screening. ACOG Committee Opinion: Committee on Gynecologic Practice number 128--October 1993. Int J Gynaecol Obstet. 1993;43:344–8. [PubMed] [Google Scholar]
- 19.American Academy of Family Physicians CoPHaSA. Age charts for periodic health examinations. Kansas City, MO: American Academy of Family Physicians; 1994. [Google Scholar]
- 20.American Urological Association. Testicular Cancer & testicular self-examination. Baltimore, MD: American Urological Association, Inc; 1993. [Google Scholar]
- 21.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–90. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- 22.Rizzo JD, Wingard JR, Tichelli A, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–51. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Kaste SC, Hudson MM, Jones DJ, et al. Breast masses in women treated for childhood cancer: incidence and screening guidelines. Cancer. 1998;82:784–92. doi: 10.1002/(sici)1097-0142(19980215)82:4<784::aid-cncr23>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 24.Powers A, Cox C, Reintgen DS. Breast cancer screening in childhood cancer survivors. Med Pediatr Oncol. 2000;34:210–2. doi: 10.1002/(sici)1096-911x(200003)34:3<210::aid-mpo9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 25.From Cancer Patient to Cancer Survivor. Lost in Transition. Washington, D.C.: National Academy Press; 2005. [Google Scholar]
- 26.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. Jama. 2009;301:404–14. doi: 10.1001/jama.2008.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeazel MW, Oeffinger KC, Gurney JG, et al. The cancer screening practices of adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2004;100:631–40. doi: 10.1002/cncr.20008. [DOI] [PubMed] [Google Scholar]
- 28.Hudson SV, Hahn KA, Ohman-Strickland P, et al. Breast, colorectal and prostate cancer screening for cancer survivors and non-cancer patients in community practices. J Gen Intern Med. 2009;24 (Suppl 2):S487–90. doi: 10.1007/s11606-009-1036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer DK, Terrin NC, Menon U, et al. Screening practices in cancer survivors. J Cancer Surviv. 2007;1:17–26. doi: 10.1007/s11764-007-0007-0. [DOI] [PubMed] [Google Scholar]
- 30.CDC. Prevalence and Trends Data. 2000–2002. Behavioral Risk Factor Surveillance System. [Google Scholar]
- 31.Smith RA, Cokkinides V, von Eschenbach AC, et al. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2002;52:8–22. doi: 10.3322/canjclin.52.1.8. [DOI] [PubMed] [Google Scholar]
- 32.Buetow SA. Testicular cancer: to screen or not to screen? J Med Screen. 1996;3:3–6. doi: 10.1177/096914139600300103. [DOI] [PubMed] [Google Scholar]
- 33.Westlake SJ, Frank JW. Testicular self-examination: an argument against routine teaching. Fam Pract. 1987;4:143–8. doi: 10.1093/fampra/4.2.143. [DOI] [PubMed] [Google Scholar]
- 34.Baxter N. Preventive health care, 2001 update: should women be routinely taught breast self-examination to screen for breast cancer? Cmaj. 2001;164:1837–46. [PMC free article] [PubMed] [Google Scholar]
- 35.Rosolowich V. Breast self-examination. J Obstet Gynaecol Can. 2006;28:728–30. doi: 10.1016/s1701-2163(16)32223-x. [DOI] [PubMed] [Google Scholar]
- 36.Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2009;150:551–5. doi: 10.7326/0003-4819-150-8-200904210-00009. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins NA, Smith T, Zhao L, et al. Health-related behavior change after cancer: results of the American cancer society’s studies of cancer survivors (SCS) J Cancer Surviv. 4:20–32. doi: 10.1007/s11764-009-0104-3. [DOI] [PubMed] [Google Scholar]
- 38.Bishop MM, Lee SJ, Beaumont JL, et al. The Preventive Health Behaviors of Long-Term Survivors Cancer and Hematopoietic Stem Cell Transplantation Compared to Matched Controls. Biol Blood Marrow Transplant. 2009 doi: 10.1016/j.bbmt.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Emmons K, Li FP, Whitton J, et al. Predictors of smoking initiation and cessation among childhood cancer survivors: a report from the childhood cancer survivor study. J Clin Oncol. 2002;20:1608–16. doi: 10.1200/JCO.2002.20.6.1608. [DOI] [PubMed] [Google Scholar]