Abstract
In this study, nanovesicles were developed for brimonidine tartrate by film hydration technique and dispersed in viscous carbopol solution for ocular delivery. Scanning electron microscopy revealed spherical shape of the vesicles. As high as 32.27% drug entrapment efficiency was achieved depending upon the surfactant/cholesterol molar ratio (7:4 to 7:8). The vesicles were in the size range of 298.0–587.9 nm. Release study showed a biphasic drug-release pattern for the lyophilized vesicular formulation in buffered saline solution, i.e., initial burst release followed by gradual release over the period of 8 h. On contrary, the isolated vesicles reduced the burst effect in 3 h by two to three times and the drug release was comparatively slower at the intermediate ratio in both cases. With variation in cholesterol content, the drug release followed either first order or Higuchi’s kinetics. Physically the lyophilized vesicular formulations were more stable at refrigerated temperature. DSC and X-RD analyses indicated loss of drug crystallinity in the vesicles. FTIR spectroscopy did not reveal any interaction between drug and excipients. The lyophilized formulation showed better ocular hypotensive activity than marketed drops on albino rabbits and in vivo efficacy was sustained up to 7.5 h. Furthermore, the formulation was found to be non-irritant to the rabbit eye. Hence, the lyophilized vesicles, when dispersed in viscous carbopol solution, had the potential in reducing dosing frequency and could improve patient compliance.
KEY WORDS: entrapment efficiency, hypotensive activity, nanovesicles, ocular drug delivery, release kinetics
INTRODUCTION
Glaucoma is a disease with a characteristic of higher level of intraocular pressure (an IOP of 20.5 mmHg or higher) and progressive optic nerve damage, which might progressively hurt visibility (1,2). Patients under chronic treatment with ocular antihypertensive drops predominantly suffer from ocular surface disorders because of changes in number of goblet cells and epithelial cells (3,4). Instillation of eye drops can lead to increased tear production and subsequent secretion of tear (5). The ocular residence time of conventional drops is reduced to few minutes (6), and the pre-corneal half-life of drugs from these pharmaceutical formulations become short about 1–3 min due to high tear fluid turnover (7). As a consequence, ocular bioavailability of these drops is considered less than 5% and often less than 1% (8).
Drug absorption occurs as a massive pulse entry followed by rapid decline due to systemic absorption via conjunctiva and nasal mucosa (9), which may result in some undesirable side effects (10). Thus to minimize the problems associated with conventional eye drops, formulation chemists are in search for the better ophthalmic drug delivery system. In recent years, different ocular drug delivery devices have been investigated such as insert (11), emulsion (12), in situ gel (13), spray (14), nanoparticles (15), and vesicular systems (16,17). Currently, vesicular systems such as liposomes (phospholipid vesicles) and niosomes (non-ionic surfactant vesicles) are getting considerable attention as a potential ocular drug delivery system. Vesicular systems not only provide sustained and controlled release of the medication at the corneal surface but also prevent metabolism of the drug at tear/corneal epithelium surface by various enzymes including esterases, oxidoreductases (18,19). Vesicles have the advantages of drops but have the ability of localizing and maintaining drug activity at its site of action (19). However, non-ionic surfactant vesicles are considered more advantageous than phospholipid vesicles because of their low cost of preparation, chemical stability, biodegradability, biocompatibility, and non-immunogenicity (20,21).
Chemically, brimonidine tartrate is 5-bromo-6-(2- imidazolidinylideneamino) quinoxaline L-tartrate. It is a water soluble α2 adrenergic agonist which works by reducing aqueous humor production, and by increasing aqueous humor outflow through the trabecular meshwork. Brimonidine binds extensively and reversibly to melanin in ocular tissues without any untoward effects (22). Recent studies have suggested that brimonidine can promote survival of injured retinal ganglion nerve cells by activation of the α2-adrenoceptor in retina and/or optic nerve (23). Hence, brimonidine tartrate is a potential anti-glaucoma agent. Patients continuously taking brimonidine generally suffer from sub-clinical inflammation in conjunctiva and ocular allergy (24). Because brimonidine tartrate is available in the market only in solution form as drops (Alphagan Z 0.1%), the research should be continued for better drug delivery options that allows slow and sustained release of the drug. Under such circumstances, non-ionic surfactant vesicles could be a possible alternative ocular delivery system for the drug.
Many research workers have developed mucoadhesive polymer (Carbopol 934P) coated or uncoated niosomal formulation of timolol maleate (25,26), acetazolamide (27,28), in order to obtain controlled intraocular pressure lowering activity. To the best of our knowledge, no research report is available with non-ionic surfactant vesicles of brimonidine.
Therefore, the objective of our present investigation was (a) to formulate and characterize mucoadhesive nanovesicular system of brimonidine tartrate, and (b) to evaluate its in vivo potential in comparison to a marketed formulation. The physical state of the drug, and the interaction between drug and excipients in the vesicles were evaluated by differential scanning calorimetry (DSC) and Fourier transform infrared (FTIR) spectroscopy.
MATERIALS AND METHODS
Materials
Brimonidine tartrate was a gift sample from Sun Pharmaceutical Industries Ltd., Gujarat, India. Span 60 was purchased from Burgoyne Burridges and Co., Mumbai, India. Cholesterol, Carbopol 940 (Acrylic acid copolymer/poly (acrylic acid)) was purchased from Loba Chemie Pvt Ltd., Mumbai, India. Acetone, chloroform, sodium chloride, potassium dihydrogen phosphate, and disodium hydrogen phosphate were procured from Qualigens Fine Chemicals., Mumbai, India. These commercially obtained analytical reagents were used as received.
Preparation of Nanovesicular Formulation
Nanovesicles were prepared by thin film hydration method as reported earlier (29). Span 60 and cholesterol in different molar ratios of 7:4, 7:6, and 7:8 were dissolved in 10 ml of chloroform in a round bottom flask. The flask was then attached to a rotary vacuum evaporator (Rolex India, Mumbai, India) and the temperature of water bath was maintained at 60°C. The flask was rotated at 150 rpm for 2 h. The combination of heat and suction evaporated chloroform and resulted in the formation of a thin film. The film was kept overnight in vacuum desiccator for the removal of chloroform. Accurately weighed, 50 mg of brimonidine tatrate was dissolved in 50 ml of 10 mM phosphate-buffered saline solution (PBS, pH 7.4) and sterilized through 0.22 μm membrane filter. The drug solution was added to the flask containing the film and was rotated at 140 rpm for another 2 h to peel off the surfactant/cholesterol film. The hydration of film led to the formation of vesicles. The vesicles were sonicated for 1 h (Enertech Electronics Pvt. Ltd., Mumbai, India) and were kept overnight at 4°C. The formulation was lyophilized at a surfactant:cryoprotectant (sucrose) ratio of 1:2.5 after standard pre-freezing at −20°C (20). The whole operation was carried out under aseptic condition in order to achieve sterile products. The glass wares were sterilized by autoclaving at 121°C for 15 min. The viscous nanovesicles were prepared by dispersing lyophilized product and isolated nanovesicles in 0.05% (w/v) carbopol solution in PBS to reach final drug concentration to 0.1% (w/v). The carbopol solution was sterilized by membrane filtration.
Determination of Residual Chloroform in Lyophilized Nanovesicles
The level of residual chloroform in the optimized lyophilized nanovesicles was determined by gas chromatography/mass spectrometry (SHIMADZU GCMS-QP5050A, GCMS-EI analysis). Approximately, 15 mg of vesicles was dispersed in 50 ml of N, N-dimethyl formamide, sonicated, filtered through 0.22-μm membrane filter and 20 μl of the filtrate was injected directly into a capillary column. Calculation was based on a standard curve constructed with standard chloroform solution.
Scanning Electron Microscopy
An aqueous dispersion of the vesicles was placed on NEM adhesive tape, vacuum dried, and photographed under scanning electron microscope at 30,000–100,000 magnification (JEOL JSM-6360, Serial No: G5/Il/42/08, JEOL Datum Ltd., Japan).
Particle Size Determination
The size of nanovesicles was determined using Zetasizer (Ver. 6.00, Malvern Instruments Ltd., UK). The vesicular size was measured based on light-scattering technique.
Determination of Un-entrapped and Entrapped Drug
Accurately weighed, 10 mg of the freeze-dried product was dispersed in 10 ml PBS solution and cold centrifuged (Remi Cooling Centrifuge, Mumbai, India) at 16,500 rpm for 1 h. The clear supernatant was analyzed spectrophotometrically (UV1, Thermo Spectronic, UK) at 252 nm for un-entrapped drug.
The vesicles were separated from the supernatant, washed with PBS solution (2 × 5 ml), and centrifuged for another hour. The drug entrapment efficiency of isolated vesicles was determined by a slight modification of the technique reported earlier (27). The amount of entrapped drug was estimated by lysis of the vesicles. Few drops of chloroform were added to the centrifuge tube to disrupt the isolated vesicles. Certain volume of PBS solution was added to extract the entrapped drug. Simultaneously, the tube was maintained at a temperature of 60°C to evaporate the organic solvent. The PBS solution containing entrapped drug was filtered and analyzed for drug content with a spectrophotometer (UV1, Thermo Spectronic, UK) at 252 nm. The drug entrapment efficiency was calculated by the following equation:
 |
Viscosity Measurement
The viscosity of all formulations was determined by Brookfield viscometer (Model DV-II + Pro, Middleboro, USA) at 25°C. The spindle CPE 41 was rotated at different angular velocity from 0.5 to 2 rpm. Each determination was carried out in triplicate.
In Vitro Drug Release Study
In vitro drug release from freeze-dried product and isolated nanovesicles was carried out by dialysis method. Certain amount of freeze-dried product and isolated nanovesicles were reconstituted in pH 7.4 PBS solution to obtain a drug concentration of 0.1%. One milliliter of the vesicular dispersion was taken into dialysis sac (HiMedia LA398-30MT dialysis membrane-135, molecular weight cut-off 12–14 kDa), and both end of the sac was tightly bound with threads. The sac was placed in a 100-ml Borosil beaker that served as the receptor cell. Fifty milliliters of PBS solution (pH 7.4) was added to the receptor cell and the solution was agitated magnetically at 300 rpm. Care was taken so that the portion of the sac containing the formulation dipped completely in the buffer. The temperature of the system was maintained at 37 ± 1°C. Five milliliters of the sample was withdrawn from the receptor cell at specified time intervals and at each time, the medium was compensated with equal volume of fresh PBS solution. The samples were analyzed by UV spectrophotometer (UV1, Thermo Spectronic, UK) at 252 nm.
Kinetics of Drug Release
The data obtained from in vitro drug-release study were fitted to different kinetic models: zero order (percentage drug released vs. time), first order (logarithm of percentage drug remaining to be released vs. time), and Higuchi’ model (percentage of drug released vs. square root of time). Linear regression analysis was performed to detect the best-fit kinetic model.
Fourier Transform Infrared Spectroscopy
KBr pellets of pure drug, physical mixture of cholesterol/drug/Span 60, dried blank lyophilized vesicles, and lyophilized optimized formulation were made in a hydraulic press (Kimaya Engineers, Maharastra, India). A pressure of 125 kg/cm2 was applied for 5 min and the pellets were scanned in the range of 4,000–400 cm−1 using PerkinElmer instrument (Spectrum RXI-5.31, UK).
Differential Scanning Calorimetry Analysis
DSC thermograms of the samples were traced with PerkinElmer instrument (Pyris Diamond TG/DTA, Singapore) in an inert atmosphere of nitrogen (150 ml/min). Samples of 3–7 mg were heated from 30°C to 230°C at 15°C/min. Platinum crucible with alpha alumina powder was used as a reference standard.
X-Ray Diffraction Analysis
A wide angle X-Ray diffractometer (Ultima III, D/Max 2200, Japan) was used for X-ray diffraction (X-RD) analysis of the samples under the following operating conditions: Cu-Kα radiation; tube voltage of 40 kV; current of 30 mA; and at a scanning rate of 5°/min. The diffraction patterns were recorded over a diffraction angle range of 10–30°.
Stability Study
The stability study of the reconstituted optimized vesicular formulation was conducted at different conditions of temperature (room temperature and refrigerated temperature) for 3 months. Different parameters such as vesicular fusion, pH variation, size distribution, drug leaching were examined.
In Vivo Intraocular Pressure Measurement on Rabbit
The optimized vesicular formulation was tested for its intraocular pressure lowering activity on normotensive albino rabbits (2–3 kg) and the results were compared to that of a marketed brimonidine solution (0.1%). The animal experiment was conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations, as per the spirit of ethics committee. The animals were housed at controlled temperature (25 ± 2°C), and humidity (60 ± 5%), with a 12/12-h light-dark cycle. They had free access to food and water. Institutional Animal Ethics Committee (IAEC) (Registration no. 955/a/06 CPCSEA) approved the pharmacodynamic study. The dispersion of lyophilized product in carbopol solution, containing both entrapped and un-entrapped drug was used in this study and adjusted to a concentration of 0.1% brimonidine. The animals were divided into two groups, each containing six rabbits. Group I received vesicular formulation and Group II was treated with the marketed formulation. The IOP was measured at different intervals with a standardized tonometer (ShiØtz, Germany). A single 50-μl dose of 0.1% brimonidine preparation was instilled onto the corneal surface of rabbit’s left eye; (30) then after 30 min, and subsequently every 1 h interval, the IOP was measured for a period up to 7.5 h. The right eye was left as a control in all the experimental animals. The ocular pressure lowering activity was expressed similarly to that reported by Winum and his associates (31) as the average difference in IOP between the treated and control eye.
Eye Irritation Test
Three healthy albino rabbits (2.5–3 kg) were selected to evaluate the ocular irritancy effects of the optimized niosomal formulations. The animals were housed at a temperature and relative humidity of 20 ± 3°C and 50–60%, respectively, with 12-h light and 12-h dark cycle. For feeding, conventional laboratory diets were used with an unrestricted supply of drinking water. A single dose of 100-μl niosomal formulation was instilled into the conjunctival sac of left eye of each animal (initially to one animal) and the untreated eye served as a control. Each of the animals was observed visually with a slit lamp for the severity of ocular reactions such as corneal ulceration, iritis, conjunctival redness, and conjunctival edema at various intervals of 1, 24, 48, and 72 h. The lesions were scored at specific intervals according to Organisation for Economic Co-operation and Development guideline 405 for the testing of chemicals. The animal experiment was conducted in full compliance with local, national, ethical, and regulatory principles and local licensing regulations, as per the spirit of ethics committee. This study was approved by IAEC (Registration no. 955/a/06 CPCSEA).
Statistical Analysis
The differences in drug-release rate and drug entrapment efficiency of nanovesicles was tested by one-way analysis of variance (ANOVA): single factor using Microsoft Excel software 2002. Difference was considered significant when p < 0.05.
RESULTS AND DISCUSSION
Physicochemical Characterization of Brimonidine Nanovesicular Formulation
It has been reported that ester type surfactant is less toxic than ether type because ester-type surfactant generally gets degraded by esterase to triglycerides and fatty acid in vivo (32). Hence, the anti-glaucomatic vesicles have been formulated using Span 60 as a surfactant. It has also been stated that a suitable molecular geometry and hydrophobicity for bilayer vesicles formation could be achievable with the addition of cholesterol into surfactants (33). The vesicles were characterized for drug entrapment efficiency, size, shape, and in vitro drug release. It was observed that increase in surfactant/cholesterol ratio from 7:4 to 7:6 increased the drug entrapment efficiency from 20.71% to 32.27%; however, above the ratio of 7:6, the drug entrapment efficiency of the vesicles decreased to 25.24% (Table I). Such differences in drug entrapment efficiencies as well as drug content were found to be statistically significant (p < 0.05). Guinedi and his co-workers (34) reported that an increase in Span 60/cholesterol ratio from 7:4 to 7:6 increased acetazolamide entrapment efficiency from 21.48% to 32.21%; however, the same was decreased to 21.36% with further increase in Span 60/cholesterol ratio to 7:7. On contrary, the z-average diameter (the mean diameter based upon the intensity of scattered light) of the vesicles gradually increased from 298.0 to 587.9 nm with the increase in surfactant/cholesterol ratio from 7:4 to 7:6. Further increase in span/cholesterol ratio decreased the vesicle size to 316.7 nm (Table I). Polydispersity index is a parameter that gives an estimate of the width of distribution of the vesicles. The higher the polydispersity index, the wider is the size distribution. This parametric value was in the range of 0.478–0.607 (Table I), and was found to follow bimodal intensity size distribution irrespective of the ratio of Span 60 and cholesterol. Cholesterol generally abolishes the gel to sol transition (35) resulting in vesicles that are less leaky (36). Increase in cholesterol content results in higher microviscosity which is indicative of more rigidity of vesicular lamella (37). However, cholesterol content beyond a certain extent starts disrupting the regular bilayer structure leading to lower drug entrapment efficiency (38). Kapadia et al. (39) reported that increase in Span 60/cholesterol ratio from 7:4 to 7:6 increased the size of acyclovir-loaded vesicles from 3.69 to 3.76 μm; however at ratio of 7:7, the same was found to decrease to 3.73 μm. In this study, we noticed that the size of nanovesicles was gradually increased with the increase in cholesterol content. It has long been realized that incorporation of more cholesterol into the vesicles would yield larger particles because of the reduction in fluidity of the bilayer. This would enhance the rigidity of bilayer membrane above the phase transition temperature, resulting in an increased elastic modulus, which inhibited curving of the bilayer (40,41). The larger size may also contribute to the higher drug entrapment efficiency of the vesicles (37,42). Scanning electron microscopy revealed that the vesicles were spherical in shape and existed in disperse and aggregate collections at all ratios. A representative photograph of the vesicles has been displayed in Fig. 1.
Table I.
The Effect of Span/Cholesterol Ratio on Vesicle Size, its Distribution, and Drug Entrapment Efficiency
| Span/cholesterol molar ratio | z-average diameter (nm) | PDI | Drug content in 10 mg lyophilized powder | Entrapment efficiency (%) mean ± SD | ||
|---|---|---|---|---|---|---|
| Theoretical amount of drug (mg) | Un-entrapped drug (mg) | Entrapped drug (mg) | ||||
| 7:4 | 298.0 | 0.607 | 0.1724 | 0.1368 ± 0.0004 | 0.0357 ± 0.0061 | 20.71 ± 3.52 |
| 7:6 | 587.9 | 0.504 | 0.1613 | 0.1105 ± 0.0007 | 0.0521 ± 0.0035 | 32.27 ± 2.18 |
| 7:8 | 316.7 | 0.478 | 0.1515 | 0.1085 ± 0.0025 | 0.0382 ± 0.0011 | 25.24 ± 0.73 |
Fig. 1.
Scanning electron micrographs of the brimonidine tartrate nanovesicles having Span 60/cholesterol ratio of 7:6
The viscosity of ophthalmic solutions is often increased to prolong their retention in precorneal area because the rate of solution drainage decreases with increasing viscosity. Part of the viscous solution is also incorporated in the precorneal tear film and in the marginal tear strip (43). It is also true that the products with a high viscosity are not well tolerated in the eye, causing lacrimation and blinking until the original viscosity of tear is regained. The drug diffusion from a viscous formulation into eye becomes difficult. Finally, the ocular administration is not so easy. A recent report indicated that the niosomal suspension having an optimal viscosity of 1.20 cps could prolong the ocular residence time, compared to solutions; and will not create lacrimation and blinking or blurred vision (33). We examined that the dispersion of lyophilized product in a merely viscous carbopol (0.05%) solution offer viscosity of 3.38 ± 0.16, 1.46 ± 0.27, and 4.90 ± 0.26 cps, respectively, with their increasing cholesterol content and therefore, the ocular niosomal formulation obtained at the intermediate ratio could be most suitable for ocular administration. Rheological study of the formulations containing 0.05% carbopol exhibited pseudoplastic behavior as evidenced by shear thinning and a decrease in viscosity with increased angular velocity.
In Vitro Drug Release Behaviors
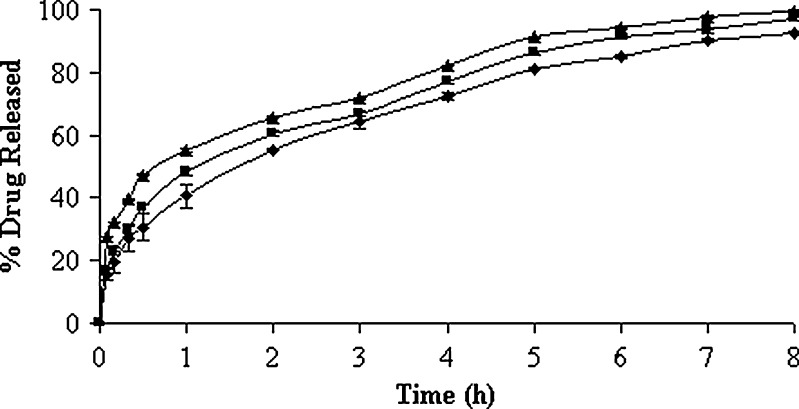
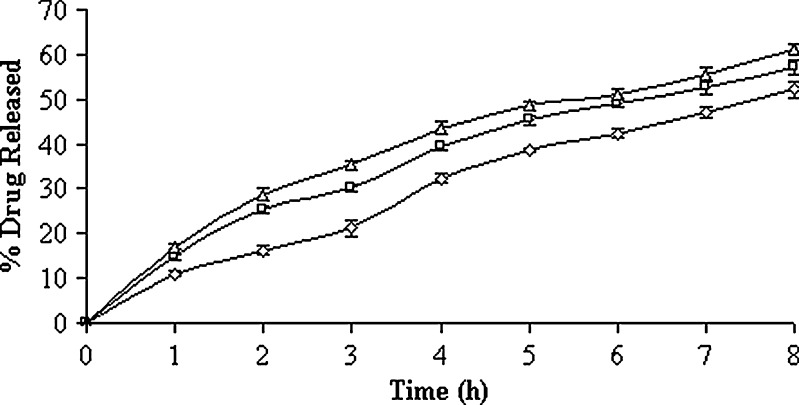
In vitro drug-release profiles of the 0.05% (w/v) carbopol solution containing both un-entrapped and entrapped drug (lyophilized product) have been illustrated in Fig. 2. The time-point approach (t50%) was adopted to compare the drug-release potential. The t50% values (i.e., the time required for the release of 50% drugs) were 1.08 ± 0.08, 1.58 ± 0.18, and 0.80 ± 0.10 h, respectively, for the formulations with their increasing surfactant:cholesterol ratio (Table II). Higher percentage of drug release at the initial stages could be due to the presence of higher percentage of un-entrapped drug in the formulations. A statistically significant difference was observed in their t50% values and, hence, in their drug-release potential (p < 0.05). Following burst release, the vesicles continued to liberate its content comparatively at a slower rate up to 8 h. The formulation with 7:6 M ratios of Span 60 and cholesterol exhibited slower and extended drug-release profile. The trend was followed by vesicular formulation of Span 60 and cholesterol in the molar ratios of 7:4 and 7:8, respectively. This is in good agreement with the fact that cholesterol causes a decrease in density of head groups at the interfaces of bilayer, and an increase in the package of phospholipid tails in the middle of bilayer, thereby reducing their permeability to encapsulated compound (44). Moreover, it is reported that cholesterol in phospholipid vesicular preparations could reduce the leakage of encapsulated material by decreasing the membrane fluidity (45). However, cholesterol beyond 50% starts disrupting the vesicular membrane which serves as the reason for faster drug release from the vesicles. In case of formulations with low and high surfactant/cholesterol ratio, it was revealed that the release kinetics of drug appeared to follow Higuchi’s release kinetics (r2 > 0.9874) because high correlation coefficient was observed in the Higuchi’s plot rather than zero-order and first-order models (Table II). But in case of formulation with intermediate surfactant/cholesterol ratio, first-order kinetic model predominated (r2 = 0.9970) and this indicated that the drug release was dependent on concentration of drug entrapped. The drug-release profiles of isolated vesicles in PBS (pH 7.4) solution have been represented in Fig. 3. The drug-release profile ran always slower at intermediate surfactant/cholesterol ratio than those at low and high ratios. The t50% values of the vesicles differ widely and such differences were also statistically significant (p < 0.05). It was revealed that the release kinetics of drug appeared to follow the mixed release kinetics of first order (r2 > 0.9882) as well as Higuchi’s release kinetics (r2 > 0.9802). However, first-order release kinetics (r2 = 0.9908) predominated at the intermediate ratio (Table III). On the other hand, the best fit with higher correlation (r2 > 0.9935) was found with the Higuchi’s equation at low and high ratios, i.e., the drug release was proportional to square root of time, indicating that the drug release from vesicles was diffusion controlled.
Fig. 2.
In vitro drug-release profiles of lyophilized vesicular formulation composed of different cholesterol content in pH 7.4 PBS solution. Key: Span 60/cholesterol ratio: (black triangle) 7:8, (black square) 7:4, and (black diamond) 7:6
Table II.
Effect of Span/Cholesterol Ratio on Drug Release Characteristics of Lyophilized Powder in pH 7.4 PBS Solution and Kinetic Modeling of Drug Release Data
| Span/cholesterol molar ratio | t 50% (h) mean ± SD | Zero order | First order | Higuchi model | |||
|---|---|---|---|---|---|---|---|
| K 0 (%/h) | r 2 | K 1 (h−1) | r 2 | K H (h−1/2) | r 2 | ||
| 7:4 | 1.08 ± 0.08 | 9.7317 | 0.9132 | 0.379304 | 0.9832 | 31.609 | 0.9885 |
| 7:6 | 1.58 ± 0.18 | 9.6543 | 0.9287 | 0.297548 | 0.9970 | 31.191 | 0.9947 |
| 7:8 | 0.80 ± 0.10 | 8.8244 | 0.9150 | 0.534066 | 0.9239 | 28.616 | 0.9874 |
K 0 zero order, K 1 first order, K H Higuchi’s rate constant, r 2 is the correlation coefficient
Fig. 3.
In vitro drug-release profiles of isolated vesicles composed of different cholesterol content in pH 7.4 PBS solution. Key: Span 60/cholesterol ratio: (white triangle) 7:8, (white square) 7:4, (white diamond) 7:6
Table III.
Effect of Span/Cholesterol Ratio on Drug Release Characteristics of Isolated Nanovesicles in pH 7.4 PBS Solution and Kinetic Modeling of Drug Release Data
| Span/cholesterol molar ratio | t 50% (h) mean ± SD | Zero order | First order | Higuchi model | |||
|---|---|---|---|---|---|---|---|
| K 0 (%/h) | r 2 | K 1 (h−1) | r 2 | K H (h−1/2) | r 2 | ||
| 7:4 | 6.25 ± 0.31 | 5.8994 | 0.9698 | 0.096956 | 0.9915 | 23.286 | 0.9945 |
| 7:6 | 7.53 ± 0.23 | 6.1033 | 0.9834 | 0.091659 | 0.9908 | 23.751 | 0.9802 |
| 7:8 | 5.35 ± 0.15 | 5.9164 | 0.9621 | 0.102944 | 0.9882 | 23.435 | 0.9935 |
K 0 zero order, K 1 first order, K H Higuchi’s rate constant, r 2 is the correlation coefficient
Formulation Stability
The drug content of the formulations were determined and it was observed that the percentage of drug leaching (percent of un-entrapped drug) was increased by 8.43% and 24.13%, respectively, for the preparations stored at refrigerated temperature and room temperature. In addition, the pH shifted from 7.35 to 6.69 and from 7.40 to 7.15, respectively, for the formulations stored at room temperature and refrigerated temperature. Thus no appreciable change in pH was noted for the formulation stored at cold temperature; however, the formulation stored at room temperature exhibited a considerable shift in pH of the nanovesicular suspension. Later, the formulation stored at cold temperature was subjected to scanning electron microscopy and vesicular size analysis. The z-average diameter of the vesicles was found to increase from 587.9 to 627.8 nm and the PDI increased slightly from 0.504 to 0.589. Furthermore, the size distribution of vesicles based on intensity of scattered light was found to follow bimodal distribution (data not shown). Hence, this result could not be considered as an evidence of vesicular fusion because an increase in vesicle size and PDI did not vary widely. Thus, we could suggest that the nanovesicular formulation should be stored at refrigerated temperature to maintain its better physical stability. This formulation was considered optimum because of its comparatively higher drug entrapment efficiency, slower drug-release profile and good physical stability. Residual chloroform in the optimized lyophilized nanovesicles was determined to be 7 ppm and the value is well below the International Conference on Harmonisation chloroform limit of 60 ppm.
Compatibility of Drug in Nanovesicles
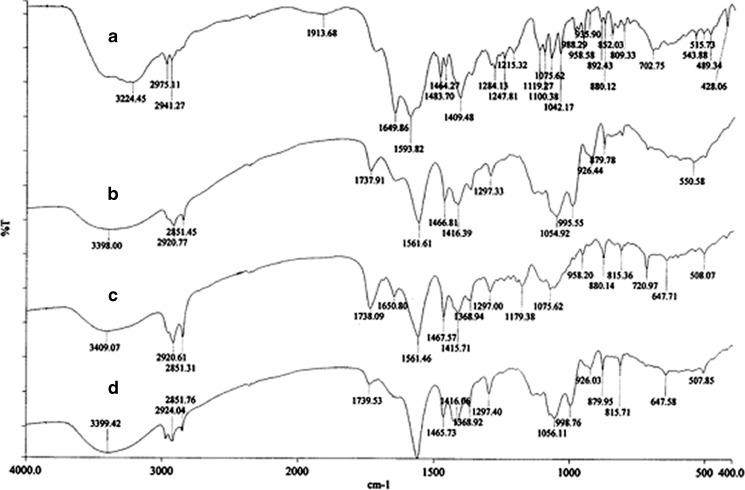
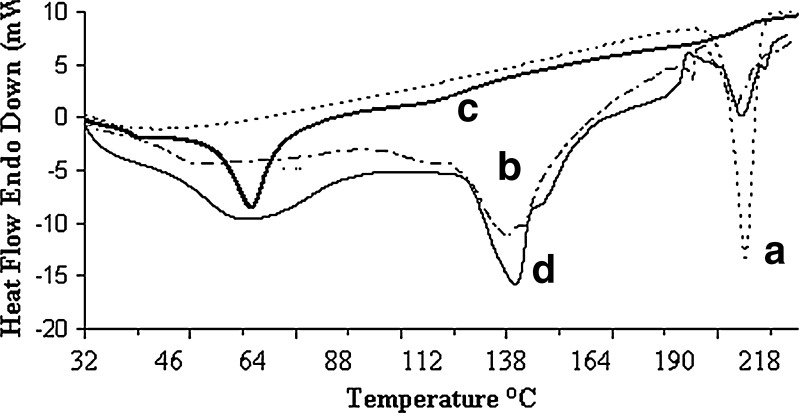
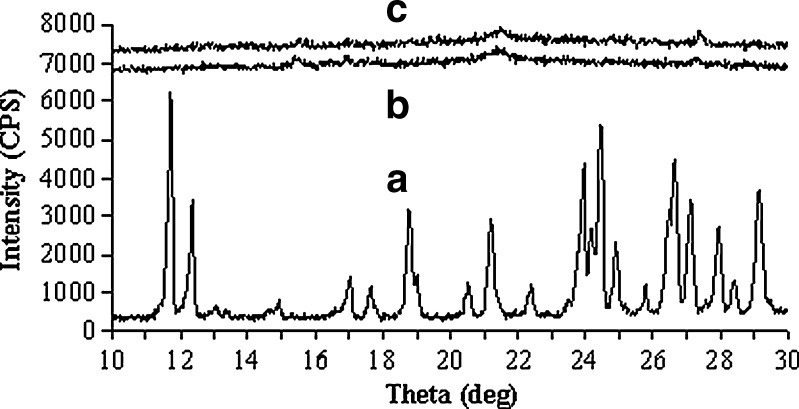
The interaction of drug with excipients in the optimized formulation was studied by FTIR spectroscopy. The IR spectra have been depicted in Fig. 4. The characteristics peaks of pure brimonidine tartrate were found for –NH stretching at 3,224.45 cm−1, –CN stretching at 1,284.13 cm−1, carboxylate ions at 1,593.82 cm−1, –C=O stretching at 1,649.86 cm−1 (11). Similar peaks were identified in the spectrum of optimized formulation and physical mixture with minor differences in frequencies. Hence, it can be said that the drug had no interaction with excipients of the vesicles. The physical state of drug in the optimized vesicles was examined through DSC and X-RD analysis. The melting endothermic transition of pure brimonidine was observed at 212.84°C, which was very close to its reported melting point (207–208°C) (46). In the thermogram of optimized formulation, the melting endothermic peak appeared near the melting temperature of brimonidine but it was comparatively weak in nature (Fig. 5). X-RD analysis revealed that the characteristics intense peaks of pure drug appeared at scattering angles (2θ) of 11.72°, 12.36°, 18.78°, 21.2°, 23.92°, 24.42°, and 26.62° (Fig. 6). However, no such intense peaks were found at these diffraction angles except those that signaled weakly at 21.2°. Thus, the thermal behavior coupled with the crystallographic data suggested that the crystallinity of pure brimonidine has been reduced in its entrapped form.
Fig. 4.
FTIR spectrum of a pure drug, b optimized vesicular formulation, c physical mixture, and d blank vesicles
Fig. 5.
DSC thermograms of a pure drug, b vesicular formulation, c blank vesicles, and d physical mixture
Fig. 6.
X-Ray diffraction pattern of a pure drug, b blank vesicles, and c vesicular formulation
In Vivo IOP Assessment
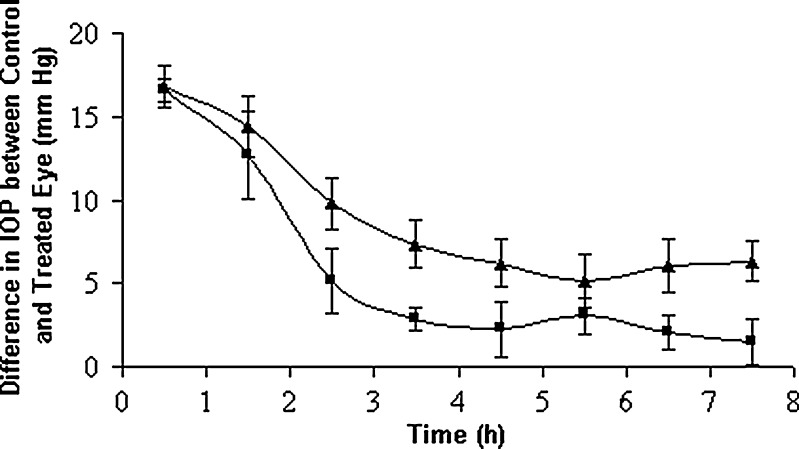
It is usual that un-entrapped drug along with the entrapped one may consolidate the efficacy of vesicular systems in terms of biphasic bio-distribution profile. It could provide initial burst effect followed by slow sustained release (47). Williams and his co-researchers (48) exploited this concept in formulating vesicles with its inherent un-entrapped drug (sodium stibogluconate) producing better therapeutic effect. Hence, the hypotensive effect of optimized lyophilized vesicular suspension was compared with a marketed formulation having the same concentration (0.1%) of brimonidine tartrate. The decrease in IOP was measured as a function of time. The ocular hypotensive activity was expressed as the average difference in IOP between the treated and control eye (Fig. 7). A difference in IOP of 16.55 ± 0.69 mmHg was observed initially for the marketed formulation and the difference reduced rapidly to 2.85 ± 0.67 mmHg at 3.5 h; however, the optimized vesicular formulation did that in a slower and controlled manner. A decrease in IOP was 16.77 ± 1.25 mmHg initially, and the drug action continued up to 7.5 h where a decrease in IOP of 6.3 ± 1.48 mmHg was noted for the optimized nanovesicular formulation. It was evident from in vitro release study that this formulation released its 68% content in 3.5 h. As a consequence, there was a quick fall in IOP (16.77 ± 1.25 to 7.36 ± 1.40 mmHg in 3.5 h) and then showed a sustained fall of IOP. Thus it was obvious that the tested vesicular formulation showed significant IOP-lowering activity for a prolonged period compared to that of a marketed solution. It was believed that the vesicles promoted absorption by preferentially modifying the permeability characteristics of drug through the cornea. Further, the drug release from the vesicles, that follows first-order kinetics, will increase the local concentration gradually at corneal surface, depending on passive diffusion of drug molecule across the corneal barrier. Surfactants (the chief constituent of vesicles) act as a penetration enhancer as they can remove the mucous layer and break junction complexes (49). In addition, carbopol solution being viscous may increase the ocular residence time of the vesicles leading to higher bioavailability of drug.
Fig. 7.
Comparative in vivo intraocular pressure lowering activity of marketed drops and optimized vesicular formulation. Key: (black triangle) optimized vesicular formulation, (white square) marketed formulation
Potential to Ocular Irritation
Initially, the optimum lyophilized formulation was instilled into the left eye of one animal and was observed for 3 days and the test did not reveal any corrosive or severe irritant effects. Therefore, a confirmatory test was conducted with two additional animals. However, the animals did not develop any ocular lesions over the study period of 3 days and the scores totalled zero for all the animals. Thus, it was concluded that the optimized niosomal formulation was non-irritant under the experimental conditions adopted.
CONCLUSIONS
In this study, we have developed nanovesicles of Span 60 by film hydration technique to deliver brimonidine tartrate topically for the management of glaucoma. The variation in Span 60/cholesterol molar ratio affected the properties of vesicles. It was found that above a ratio of 7:6, the drug entrapment efficiency and z-average diameter of vesicles decreased. Highest drug entrapment efficiency of 32.27% was achievable at a ratio of 7:6. At this composition, the drug release from 0.05% carbopol-coated lyophilized vesicular formulation in 10 mM PBS solution (pH 7.4) was comparatively slower and could be extended up to 8 h. However, the drug-release rate was faster at the initial stages of the in vitro study at all ratios of surfactant and cholesterol, and this was solely due to the un-entrapped drug present in the formulation. Even this formulation exhibited a solution viscosity of 1.46 cps and therefore could be applied easily to the eye. Whereas the drug release from isolated vesicles suppressed the burst effect as observed with lyophilized formulation and the suppression was comparatively higher at the intermediate ratio. Here, the advantage of initial faster release followed by slower drug release of the lyophilized formulation at the intermediate ratio of surfactant/cholesterol was exploited in order to achieve a better therapeutic effect. Stability study indicated that this formulation when stored at refrigerated temperature offered maximum physical stability. FTIR spectroscopy revealed that the drug was relatively stable in this formulation. DSC and X-RD analysis suggested that the crystallinity of drug was reduced in the vesicles. The optimized formulation showed a prolonged IOP-lowering activity in albino rabbits compared to the marketed formulation and exhibited no ocular irritation effects. Finally, it can be concluded that viscous lyophilized nanovesicular formulation could be an alternative drug delivery system for the treatment of glaucoma.
ACKNOWLEDGMENTS
The authors wish to thank all the management members of Gupta College of Technological Sciences, Asansol, West Bengal, India and the authority of Jadavpur University, Department of Pharmaceutical Technology, Kolkata, India for their kind co-operation and facilities provided to carry out the present research work.
REFERENCES
- 1.Chiang C-H. Ocular drug delivery systems of antiglaucoma agents. J Med Sci. 1991;12:157–170. [Google Scholar]
- 2.Thylefors B, Négrel AD. The global impact of glaucoma. Bull World Health Org. 1994;72:323–326. [PMC free article] [PubMed] [Google Scholar]
- 3.Pisella PJ, Pouliquen P, Baudouin C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br J Ophthalmol. 2002;86:418–423. doi: 10.1136/bjo.86.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baffa LP, Ricardo JR, Dias AC, Módulo CM, Braz AM, Paula JS, et al. Tear film and ocular surface alterations in chronic users of anti-glaucoma medications. Arq Bras Oftalmol. 2008;71:18–21. doi: 10.1590/S0004-27492008000100004. [DOI] [PubMed] [Google Scholar]
- 5.Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269:1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Sanzgiri YD, Mashi S, Crescenzi V, Callegaro L, Topp EM, Stella VJ. Gellan-based systems for ophthalmic sustained delivery of methylprednisolone. J Control Rel. 1993;26:195–201. doi: 10.1016/0168-3659(93)90186-9. [DOI] [Google Scholar]
- 7.Zignani M, Tabatabay C, Gurny R. Topical semi-solid drug delivery: kinetics and tolerance of ophthalmic hydrogels. Adv Drug Deliv Rev. 1995;16:51–60. doi: 10.1016/0169-409X(95)00015-Y. [DOI] [Google Scholar]
- 8.Urtti A. Ocular drug delivery. Adv Drug Deliv Rev. 2006;58:1129–1130. doi: 10.1016/j.addr.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Saettone MF, Giannaccini B, Chetoni P, Torracca MT, Monti D. Evaluation of high- and low-molecular-weight fractions of sodium hyaluronate and an ionic complex as adjuvants for topical ophthalmic vehicles containing pilocarpine. Int J Pharm. 1991;72:131–139. doi: 10.1016/0378-5173(91)90051-O. [DOI] [Google Scholar]
- 10.Kumar S, Haglund BO, Himmelstein KJ. In situ-forming gels for ophthalmic drug delivery. J Ocular Pharmacol. 1994;10:47–56. doi: 10.1089/jop.1994.10.47. [DOI] [PubMed] [Google Scholar]
- 11.Patel D, Patel MM, Patel NM, Patel M. Preparation and evaluation of ocular insert containing brimonidine tratrate. Int J Pharm Clin Res. 2009;1:19–22. [Google Scholar]
- 12.Chan J, El Maghraby GMM, Craig JP, Alany RG. Ocular delivery of pilocarpine hydrochloride from phase transition microemulsions: in vitro in vivo evaluation. Int J Pharm. 2007;328:65–71. doi: 10.1016/j.ijpharm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Jain SP, Shah SP, Rajadhyaksha NS, Pal Singh PSP, Amin PD. In situ ophthalmic gel of ciprofloxacin hydrochloride for once a day sustained delivery. Drug Dev Ind Pharm. 2008;34:445–452. doi: 10.1080/03639040701831710. [DOI] [PubMed] [Google Scholar]
- 14.Patel GM, Patel MM. Recent advances and challenges in ocular drug delivery system. Pharma Times. 2007;39:21–25. [Google Scholar]
- 15.Agnihotri SA, Aminabhavi TM. Chitosan nanoparticles for prolonged delivery of timolol maleate. Drug Dev Ind Pharm. 2007;33:1254–1262. doi: 10.1080/03639040701384942. [DOI] [PubMed] [Google Scholar]
- 16.Nagarsenkar MS, Londhe VY, Nadkarni GD. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int J Pharm. 1999;190:63–71. doi: 10.1016/S0378-5173(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 17.Saettone MF, Perini G, Carafa M, Santucci E, Alhaique F. Non-ionic surfactant vesicles as ophthalmic carriers for cyclopentolate a preliminary evaluation. STP Pharm Sci. 1996;94:98. [Google Scholar]
- 18.Moroi SE, Lichter PR. Ocular pharmacology. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman’s: the pharmacological basis of therapeutics. New York: The McGraw Hill; 2001. pp. 1821–1848. [Google Scholar]
- 19.Abraham S, Furtado S, Bharath S, Basavaraj BV, Deveswaran R, Madhavan V. Sustained ophthalmic delivery of ofloxacin from an ion-activated in situ gelling system. Pak J Pharm Sci. 2009;22:175–179. [PubMed] [Google Scholar]
- 20.Mukherjee B, Patra B, Layek B, Mukherjee A. Sustained release of acyclovir from nano-liposomes and nano-niosomes: an in vitro study. Int J Nanomedicine. 2007;2:213–225. [PMC free article] [PubMed] [Google Scholar]
- 21.Uchegbu IF, Florence AT. Nonionic surfactant vesicles (niosomes)—physical and pharmaceutical chemistry. Adv Colloid Interf Sci. 1995;58:1–55. doi: 10.1016/0001-8686(95)00242-I. [DOI] [Google Scholar]
- 22.McKinnon SJ, Goldberg LD, Peeples P, Walt JG, Bramley TG. Current management of glaucoma and the need for complete therapy. Am J Manag Care. 2008;14:S20–S27. [PubMed] [Google Scholar]
- 23.Chang JN, Spada LP, Blanda WM, Orilla WC, Bruke JA, Hughes PM. Alpha-2-agonist-polymeric-drug-delivery-systems. US Patent Application 20060233860, 2006.
- 24.Kamath AP, Satyanarayana S, Rodrigues CFEA. Ocular surface changes in primary open angle glaucoma with long term topical antiglaucoma medication. MJAFI. 2007;63:341–345. doi: 10.1016/S0377-1237(07)80011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vyas SP, Mysore N, Jaitely V, Venkatesan N. Discoidal niosome based controlled ocular delivery of timolol maleate. Pharmazie. 1998;53:466–469. [PubMed] [Google Scholar]
- 26.Aggarwal D, Kaur IP. Improved pharmacodynamics of timolol maleate from a mucoadhesive niosomal ophthalmic drug delivery system. Int J Pharm. 2005;290:155–159. doi: 10.1016/j.ijpharm.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 27.Aggarwal D, Garg A, Kaur IP. Development of a topical niosomal preparation of acetazolamide: preparation and evaluation. J Pharm Pharmacol. 2004;56:1509–1517. doi: 10.1211/0022357044896. [DOI] [PubMed] [Google Scholar]
- 28.Kaur IP, Aggarwal D, Mitra KA. Development of a vesicular system for effective ocular delivery of acetazolamide: a comprehensive approach and successful venture. J Drug Deliv Sci Technol. 2007;17:33–41. [Google Scholar]
- 29.Agarwal R, Katare OP, Vyas SP. Preparation and in vitro evaluation of liposomal/niosomal delivery systems for antipsoriatic drug dithranol. Int J Pharm. 2001;228:43–52. doi: 10.1016/S0378-5173(01)00810-9. [DOI] [PubMed] [Google Scholar]
- 30.Kaur IP, Singh M, Kanwar M. Formulation and evaluation of ophthalmic preparation of acetazolamide. Int J Pharm. 2000;199:119–127. doi: 10.1016/S0378-5173(00)00359-8. [DOI] [PubMed] [Google Scholar]
- 31.Winum J, Casini A, Mincione F, Starnotti M, Montero J, Scozzafava A, et al. Carbonic anhydrase inhibitors: N-(p-sulfamoyl phenyl)-d-glycopyransylamines as topically acting antiglaucoma agents in hypertensive rabbits. Bioorg Med Lett. 2004;14:225–229. doi: 10.1016/j.bmcl.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 32.Hunter CA, Dolan TF, Coombs GH, Baillie AJ. Vesicular system (niosomes and liposomes) for delivery of sodium stibogluconate in experimental murine visceral leishmaniasis. J Pharm Pharmacol. 1988;40:161–165. doi: 10.1111/j.2042-7158.1988.tb05210.x. [DOI] [PubMed] [Google Scholar]
- 33.Pandey VP, Deivasigamani K. Preparation and characterisation of ofloxacin non-ionic surfactant vesicles for ophthalmic use. J Pharm Res. 2009;2:1330–1334. [Google Scholar]
- 34.Guinedi AS, Mortada ND, Mansour S, Hathout RM. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int J Pharm. 2005;306:71–82. doi: 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 35.New RRC. Introduction. In: New RRC, editor. Liposomes: a practical approach. New York: Oxford University Press; 1990. pp. 1–32. [Google Scholar]
- 36.Rogerson A, Baillie AJ, Florence AT. Some properties of non-ionic surfactant vesicles and their component mono and di-alkyl non-ionic polyglycerol surfactants. In: Mittal K, editor. Surfactants in Solution. New York: Plenum Press; 1989. pp. 305–318. [Google Scholar]
- 37.Manosroi A, Wongtrakul P, Manosroi J, Sakai H, Sugawara F, Yuasa M, et al. Characterization of vesicles prepared with various non ionic surfactants mixed with cholesterol. Colloids Surf B Biointerfaces. 2003;30:129–138. doi: 10.1016/S0927-7765(03)00080-8. [DOI] [Google Scholar]
- 38.Redziniak G, Perrier P. Cosmetic applications of liposomes. In: Benita S, editor. Microencapsulation, Methods and Industrial Applications. New York: Marcel Dekker; 1996. p. 580.
- 39.Kapadia R, Khambete H, Katara R, Ramteke S. A novel approach for ocular delivery of acyclovir via niosomes entrapped in situ hydrogel system. J Pharm Res. 2009;2:745–751. [Google Scholar]
- 40.Poznansky MJ, Juliano RL. Biological approaches to the controlled delivery of drugs: A critical review. Pharmacol Rev. 1984;36:277–336. [PubMed] [Google Scholar]
- 41.Witoonsaridsilp W, Panyarachun B, Sarisuta N, Mueller-Goymann CC. Influence of microenvironment and liposomal formulation on secondary structure and bilayer interaction of lysozyme. Colloid Surf B. 2010;75:501–509. doi: 10.1016/j.colsurfb.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 42.Yoshioka T, Sternberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span-85) Int J Pharm. 1994;105:1–6. doi: 10.1016/0378-5173(94)90228-3. [DOI] [Google Scholar]
- 43.Sasaki H, Yamamura K, Nishida K, Nakamura J, Ichikawa M. Delivery of drugs to the eye by topical application. Prog Retin Eye Res. 1996;15:583–620. doi: 10.1016/1350-9462(96)00014-6. [DOI] [Google Scholar]
- 44.Jedlovsky P, Mezei M. Effect of cholesterol on the properties of phospholipid membranes. 1. Structural features. J Phys Chem B. 2003;107:5311–5321. doi: 10.1021/jp0219505. [DOI] [Google Scholar]
- 45.Peschka-Suss R, Dennehy C, Szoka F. A simple in vitro model to study the release kinetics of liposome encapsulated material. J Control Rel. 1998;56:41–51. doi: 10.1016/S0168-3659(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 46.Singh KH, Shinde UA. Development and evaluation of novel polymeric nanoparticles of brimonidine tartrate. Curr Drug Deliv. 2010;7:244–251. doi: 10.2174/156720110791561008. [DOI] [PubMed] [Google Scholar]
- 47.Uchegbu IF, Vyas SP. Non-ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70. doi: 10.1016/S0378-5173(98)00169-0. [DOI] [Google Scholar]
- 48.Williams DM, Carter KC, Baillie AJ. Visceral leishmaniasis in the BALB/c mouse: a comparison of the in vivo activity of five non-ionic surfactant vesicle preparations of sodium stibogluconate. J Drug Target. 1995;3:1–7. doi: 10.3109/10611869509015926. [DOI] [PubMed] [Google Scholar]
- 49.Kaur IP, Smitha R. Penetration enhancers and ocular bioadhesives: two new avenues for ophthalmic drug delivery. Drug Dev Ind Pharm. 2002;28:353–369. doi: 10.1081/DDC-120002997. [DOI] [PubMed] [Google Scholar]