Abstract
A monoclonal antibody (mAb) product development case study is presented to address some of the issues faced during developing a pre-filled syringe (PFS) product for a biotherapeutic. In particular, issues involving incompatibility with silicone oil and a stability-based approach for selection of PFS barrel and tip cap components have been discussed. Silicone spiking studies followed by exposure to agitation stress or accelerated temperature conditions were used to check for incompatibilities of the mAb with silicone oil, a necessary product contact material in PFS. In addition, screening studies to compare various closure materials as well as syringe barrel processing methods were used to select the optimum closure materials as well as the correct syringe processing method. Results indicate that the model mAb formulation used was sensitive to high levels of silicone oil especially under accelerated temperature conditions resulting in formation of protein–silicone particles in the solution for samples that were spiked with the silicone oil. Agitation stress did not have any significant impact on the quality attributes tested. Samples stored in syringe barrels that were processed with sprayed-on silicone had higher levels of subvisible particles as compared to those that were processed with the baked-on process. The tip cap comparability study resulted in one tip cap material having superior compatibility among the three that were tested. The quality attribute that was most impacted by the tip cap materials was mAb oxidation. An approach for evaluation of primary packaging components during the development of pre-filled syringe presentations for biotechnology-based compounds has been highlighted.
Key words: formulation development, PFS, pre-filled syringes, primary container closure system, protein development, silicone oil compatibility, syringe processing, tip cap selection
INTRODUCTION
The development of biotechnology products such as therapeutic proteins or vaccines in pre-filled syringes (PFS) has attracted considerable interest from pharmaceutical and biotechnology companies in the past 5 to 7 years. This can be attributed mainly to several benefits that PFS provide including reduction of medical dosing errors, minimized risk for microbial contamination by limiting manipulations prior to dosing, and enhanced convenience and ease of use (1). Pre-filled syringes represent a win-win scenario for end-users as well as manufacturers as they encourage and simplify self-administration by the patient or caregiver and provide reduced therapy and injection cost. A lower “dead volume” in PFS results in a greater number of filled units for a given batch compared to vials hence increasing overall process yield during manufacturing.
In spite of these aforementioned advantages, the development of biotechnology products in PFS presents its own set of distinctive challenges. Most of them are due to the complex and amphiphilic nature of proteins and impact of the container closure on protein stability. Protein stability in PFS over shelf life is a concern due to potential incompatibility of these types of molecules with various contact surfaces and interactions with leachables of the PFS system. Prolonged exposure of these leachables can also increase the potential for various forms of protein degradation including conformational changes, reduced activity, and increased immunogenicity. In addition to protein stability, there are several other technical challenges that are faced by manufacturers including functionality issues (break loose, glide force, and tip cap removal force), filling issues such as “wet plungers”, plunger movement during shipping, and container closure integrity of PFS system.
In this paper, we have highlighted an approach that we used for assessing the suitability of a PFS system for development of a monoclonal antibody (mAb) product. In particular, issues involving incompatibility with silicone oil and a stability-based approach for selection of PFS barrel and tip cap components have been discussed.
PFS systems essentially consist of a glass barrel made up of type I borosilicate glass that is typically siliconized for syringe functionality (2). Several vendors have the option of a non-glass (plastic) barrel available, but this technology has not attracted a lot of interest and use of glass barrels is still dominant in the industry. Typically during the barrel formation, the glass cane is cut into the required length and formed into the flange end and tip (luer tip). A tungsten pin (other newer metal pins are being explored as well) is used to bore the fluid path through the glass barrel tip. In the case of staked-in needle syringes, once the fluid path is formed by the tungsten pin, a needle is securely placed in that fluid path by means of an adhesive. During this entire process when the tungsten pin is heated to extremely high temperatures, it could form tungsten oxide which in turn can potentially interact with the glass to form complex tungsten polyanions (paratungstenate-A or paratungstenate-B) which could be left behind as tungsten-related residue in the fluid path of the syringes. These residual tungsten polyanions can interact with the protein and lead to further instability issues (3). The amount of residual tungsten polyanion left in the syringe which is available to interact with the formulation is more in the luer-tip syringes compared to the staked-in needle syringes because in the case of the latter, the needle and adhesive minimize the exposed surface area of the actual fluid path to the formulation. Once this fluid path is created, the syringe is further processed for siliconization (followed by baking if required) and washing. Following this, a needle shield (for staked-in needle) or tip cap (for luer slip) is placed on the tip end of the syringe. The overall system is then sterilized and used for fill-finish activities where once filling is completed, a plunger is placed in the syringe and then sent for further finishing operations such as visual inspection, etc.
Based on the above-mentioned process, description, and several literature reports (4–6), the critical parts of the pre-filled syringe system which constitute product contact surface are the glass barrel, elastomeric material used for tip cap/needle shield and plunger, silicone oil (Si-oil) used for siliconization, residual tungsten from boring the liquid path into the tip and the needle, and any residual glue in case of the staked-in needle syringe. Hence, it is important to understand the effect of these contact parts on the stability of protein therapeutics (3).
Although several biotech products such as Enbrel, Prevnar, Epogen, Aranesp, Humira, Pegasys, and Peg-Intron are available in PFS, common approaches taken to develop biotechnology products are not readily available, and there is very little published literature available that describe the issues of protein product development through key approaches and case studies. A few reports on the issues that are faced by the industry during the development of protein therapeutics in PFS, especially shedding light on silicone oil-related issues, have recently been reported. Jones et al. investigated the propensity of model proteins to aggregate in the presence of Si-oil and found that all the proteins tested aggregated in the presence of 0.5% Si-oil irrespective of molecular weight and isoelectric point of the protein (7). Thirumangalathu et al. studied Si-oil induced aggregation of an IgG1 monoclonal antibody as a function of agitation, temperature, pH, ionic strength, and excipient levels such as polysorbate 20 and sucrose. Although agitation-induced aggregation was observed at higher speeds, addition of polysorbate 20 inhibited the aggregate formation (8). Overcashier et al. described some technical considerations for development of protein therapeutics in PFS including selection of primary components such as plungers and needle shields based on syringe functionality experiments (1).
In this paper, we have provided general guidance on the development of biotechnology products in PFS. We have systematically evaluated the effect of Si-oil on the compatibility of a mAb formulation through spiking studies and accelerated temperature stability studies. A study to compare two types of syringe barrels differentiated by the siliconization process has been presented. Furthermore, a strategy for screening primary components has been provided for determining optimum primary components for PFS systems.
MATERIALS AND METHODS
Materials
An in-house IgG2 mAb solution at 5 or 10 mg/mL in a buffer system at pH 6.0 containing surfactant and a disaccharide as excipients was used as a model protein formulation for this study. Si-oil (Dimithicone, 350 cs) was purchased from Dow Corning, Midland, MI, USA. The vials and syringes used in the study were made up of type I glass. Syringes were sourced from multiple vendors each using different processing methodologies to process and siliconize the barrels. Elastomeric closure components which can be sterilized by autoclaving (plungers) or ETO sterilization (tip caps) were used in this study. These were sourced from multiple vendors and are free of natural rubber and molded from standard formulations that meet requirements of the major pharmacopoeias.
Silicone Oil–Water Emulsion Preparation
A stock solution of silicone oil/water emulsion was prepared as described earlier by Jones et al. (7). Si-oil was added in deionized water in a 50-mL centrifuge tube to obtain Si-oil concentration in water of 30 mg/mL. This solution was sonicated (Misonix S-4000) for approximately 5 min at 50% amplitude to obtain the emulsion which was opalescent due to fine dispersed Si-oil droplets. The stock solution was diluted to obtain emulsions at concentrations of 10 and 20 mg/mL Si-oil in water. The freshly prepared solutions were used to spike Si-oil into the mAb formulations.
Agitation and Real-Time Stability Study Setup for the Spiked Samples
Type 1 glass vials (2 mL) were filled with 1 mL of the mAb formulation at 10 mg/mL and were spiked with 0.5, 1.0, or 1.5 mg equivalent of Si-oil. A control arm containing vials that were not spiked with Si-oil was included. The vials were placed in a box in the horizontal orientation to maximize the air–water interface. The boxes were then placed on orbital shakers at 5°C and room temperature and agitated at 300 rpm for 5 and 3 days, respectively. A vial from each group (control, 0.5, 1.0, and 1.5 mg/mL Si-oil) was removed after days 1, 2, and 3 at room temperature and after days 1, 3, and 5 at 5°C. In addition to the agitation stress study, a separate real-time and accelerated temperature stability study at 25°C and 40°C was initiated for vials that were spiked with the various levels of Si-oil. All stability vials were placed in the inverted orientation to maximize contact with the closure as a worse-case scenario.
Stability Study Using Pre-filled Syringes
Two types of syringes, each with a different processing method, were used. The first type of syringe used was a luer-tip syringe with baked-on silicone. This syringe was subjected to a “baking” process post-siliconization wherein the syringes are exposed to a specific temperature for an appropriate time resulting in longer chains of Si that are more closely adhered to the surfaces they coat, thus resulting in a reduced concentration of free silicone in these syringes and lower chemical reactivity (9). The second type of syringe evaluated was one with the staked-in needle with silicone sprayed-on. The mAb formulations were filled into these syringes with a nominal fill of 1 mL, and plungers were placed using vacuum stoppering resulting in minimal (<1 mm) headspace between the liquid and the plunger. Syringes were then placed on real-time and accelerated temperature stability at 5°C, 25°C, and 40°C. All stability syringes were placed in the horizontal orientation to maximize contact with both the tip cap as well as the plunger stopper. For the purpose of this study, the two different types of syringes are referred to as baked-on or sprayed-on, respectively, in the “RESULTS” and “DISCUSSION” sections.
Tip Cap Compatibility
Three different tip cap materials from two vendors were used in this study. An accelerated stability study at 25°C was initiated to discern the difference between tip cap materials and any potential effect on the stability of the mAb. Formulations at 5 mg/mL were used for this study. Each tip cap was cut into four pieces and added to a vial containing 1 mL of the mAb formulation. The surface area-to-volume ratio of the tip cap area exposed to liquid in the vial was kept constant for all three tip caps. A control arm was also added to the study where no tip cap material was added to the vials.
Analytical Methods for Analysis
Protein concentration was calculated by using the extinction coefficient of the mAb and measuring the UV absorbance at 240 nm using a Cary 400 UV spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). Formation of soluble aggregates was measured by SEC-HPLC using an Agilent 1100 system equipped with G3000SWXL and G2000SWXL columns in series. The detection wavelength was 214 nm using a diode array detector and a nominal protein concentration of 1 mg/mL. The protein was eluted isocratically with a 200-mM sodium phosphate (pH 7.0) mobile phase at a flow rate of 0.7 mL/min. Oxidation was assessed using a product-specific reversed-phase HPLC using an Agilent 1100 system equipped with two C18 columns in series. Enzymatic digestion was used to digest the mAb, and the met-256 containing fragment and its oxidized counterpart were monitored at 214 nm using a diode array detector and a nominal protein concentration of 1 mg/mL. All the aforementioned analytical assay methods had been validated specifically for the mAb that was studied. Subvisible particle analysis was performed using a HIAC 9703 equipped with an HRLD-150 sensor. A 15-μm Duke latex count standard was run as a system suitability check prior to any sample analysis. In addition, a water blank was analyzed before each sample to ensure the background counts were appropriate for testing. All samples were equilibrated to room temperature before analysis.
RESULTS
The aim of this report was to highlight the issues we experienced and approaches we took when developing a mAb product in PFS presentation. This should also provide some recommendations on approaches that can be used for development of similar products in PFS.
Silicone Oil Compatibility: Spiking Approach
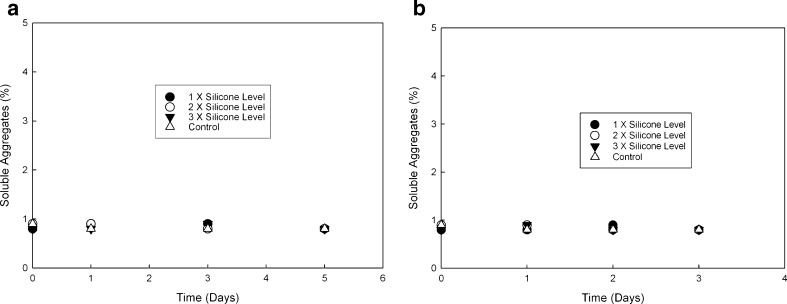
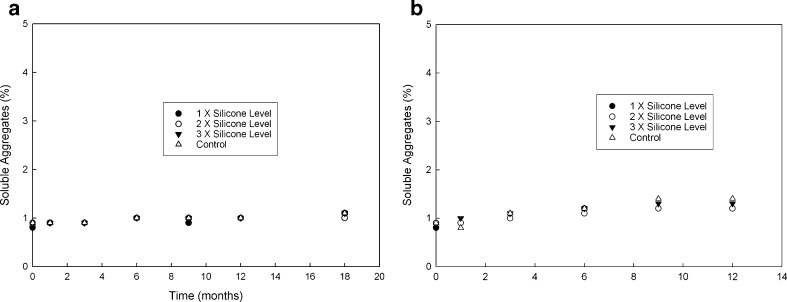
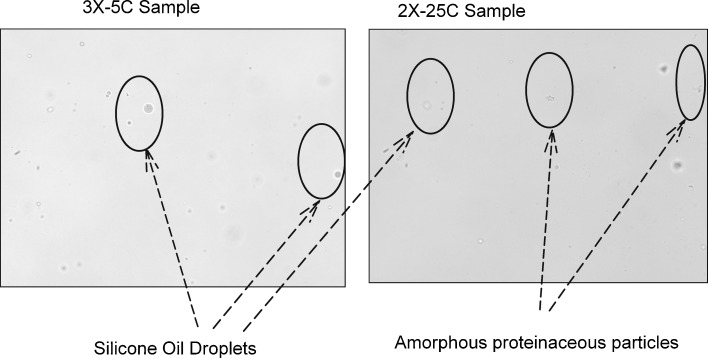
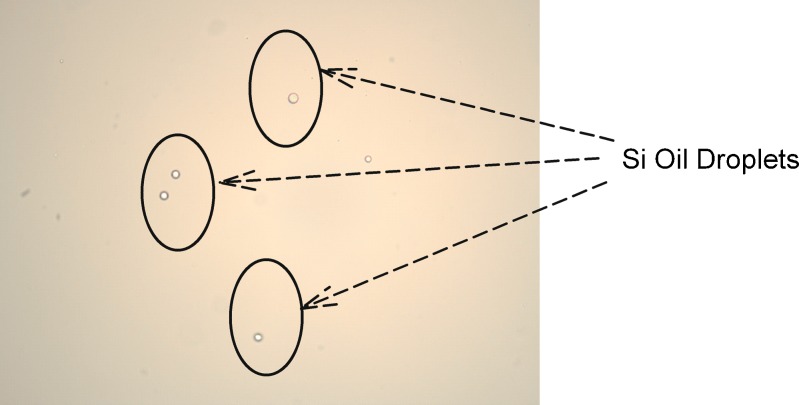
Si-oil levels typically observed (based on input from multiple syringe manufacturers) for staked-in needle syringes (0.5 to 0.8 mg/mL) were used for this study as a realistic representation of Si-oil. The mAb solution was spiked with stock Si-oil emulsion to achieve 0.5, 1.0, and 1.5 mg/mL silicone oil in mAb formulation as described in the “MATERIALS AND METHODS” section. These samples were subjected to an agitation stress study and a real-time and accelerated temperature stability study. A control arm consisting of formulations that were not spiked was also included in the study. The results for agitation stress study are shown in Fig. 1a, b for 5°C and 25°C, respectively. Soluble aggregate levels as measured by SEC did not show any appreciable difference when compared with control at 5°C and 25°C for up to 5 and 3 days, respectively, at an agitation speed of 300 rpm. Furthermore, no differences in appearance, protein concentration, or pH were observed. The stability study results, presented in Fig. 2a, b, show no significant change in soluble aggregate levels as a function of time and silicone oil spiking levels at 5°C. At the accelerated temperature condition of 25°C, a slight increase in soluble aggregates over 12 months was observed. Varying Si-oil spiking levels did not impact rate of formation of soluble aggregates. At 5°C, there was no observed increase in soluble aggregates even after 1 year of storage irrespective of silicone oil spiking level. In addition, no changes in color, clarity, pH, and protein concentration were observed over the duration of the study at either of the two temperatures for all the arms including control. All of the Si-oil spiked samples stored at 25°C had visible particle formation at the 1-year time point. This was also seen for samples spiked at the 1.5-mg Si-oil level at 5°C at the 1-year time point. No visible particle formation was observed for control samples at either temperature. Further investigation and particle characterization using a combination of optical microscopy (polarized light) and Fourier transform infrared spectroscopy (FTIR) was conducted. Results from optical microscopy are shown in Fig. 3. The particle morphology and reflectivity/opacity properties under varying light conditions suggest that the particles seen for samples at 25°C are a mixture of Si-oil droplets along with very small amorphous particles that resemble protein particulate matter. The shape and morphology of the silicone oil droplet observed was similar to that reported by Narhi et al. (10). Further characterization of these particles using FTIR spectroscopy confirmed that the amorphous particles observed were proteinaceous in nature. It can be hypothesized that, in the presence of Si-oil, prolonged exposure to accelerated temperature conditions has the potential to cause small amounts of protein to precipitate on the Si-oil droplets resulting in visible particles containing Si-oil and protein matter (Si-protein particle). It is important to note that the formation of these visible particles did not have significant impact on either protein concentration or soluble aggregate formation, indicating that the amount of protein precipitating was very small and within the assay variation for the concentration assay and that the formation of soluble aggregates was independent of the amount of Si-oil in the sample. Particle characterization of samples stored at 5°C, which had visible particle formation at the 1-year time point, indicated that they were Si-oil droplets without the presence of any protein-related matter. This suggests that samples containing high levels of Si-oil may be challenging for visual inspection due to the potential for the Si-oil to coalesce over time resulting in higher levels of rejects of the drug product.
Fig. 1.
Soluble aggregate levels for silicone oil spiked samples when subjected to agitation stress at a 5°C and b 25°C, respectively
Fig. 2.
Soluble aggregate levels for silicone oil spiked samples at a 5°C and b 25°C, respectively, when subjected to real-time and accelerated temperature conditions, as a function of time
Fig. 3.
Optical microscopy photographs showing silicone oil droplets and amorphous proteinaceous particles for 25°C sample spiked with 1 mg silicone oil and only silicone oil droplets for 5°C samples spiked with 1.5 mg silicone oil spiking
Selection of Primary Components: Type of Syringe Barrel and Siliconization Process
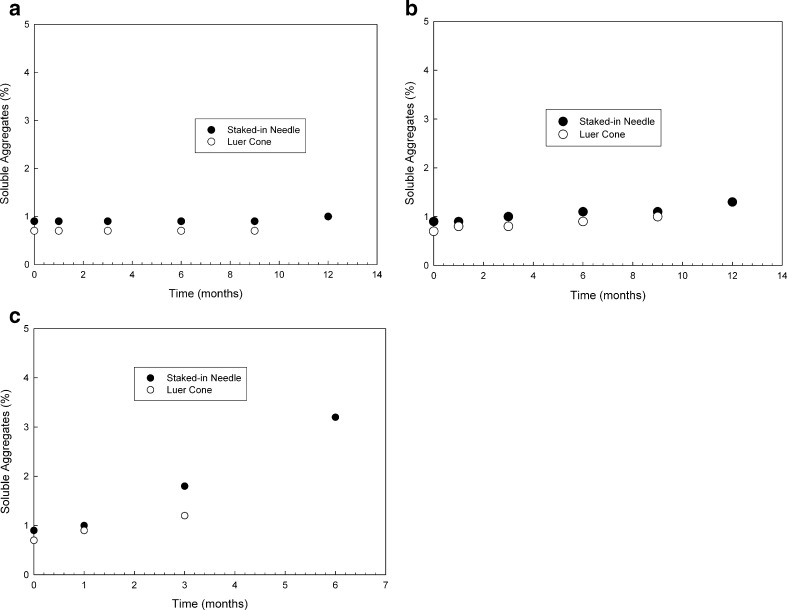
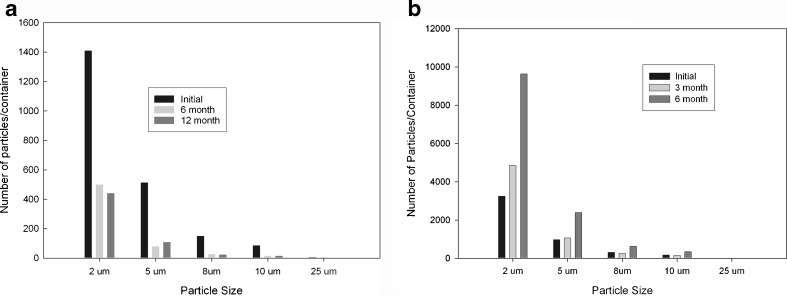
The selection of primary components such as syringe barrel, plunger, and tip cap and/or needle shield is an important decision from a container closure standpoint. Oftentimes, several options are available, and it is important to choose the right combination of primary packaging material that can provide adequate stability for the molecule of interest as well as maintain the desired functionality properties over the product shelf life. This section highlights a study we conducted for choosing the correct type of syringe barrel for a mAb product. Two types of syringes, each with a different processing method, were used as described in the “METHODS AND MATERIALS” section. Real-time and accelerated temperature studies of the model mAb formulation in PFS were initiated. Based on extraction studies performed in our labs (data not shown), it was determined that the sprayed-on syringes contained higher residual-free silicone compared to baked-on silicone syringes. Figure 4a–c depicts soluble aggregate levels obtained for the 10-mg/mL mAb formulation stored in either sprayed-on or baked-on syringes at 5°C, 25°C, or 40°C, respectively. No specific trends were observed in the stability profiles for both syringe types in the case of samples that were stored at 5°C, although a slight increase in soluble aggregate level was observed for the sprayed-on syringes stored at the accelerated temperature condition of 25°C. At 40°C, a significant increase in soluble aggregate formation was observed for the sprayed-on syringes. All the samples were visually free of particulate matter. In addition to this, the samples were evaluated for subvisible particle counts per USP <788> at both 5°C and 25°C. For this assay, in addition to the USP-specified channels of 10 and 25 μm, particle counts at lower size-range channels such as 2, 5, and 8 μm were also measured to gather additional information in order to determine trends. As expected, the results from the subvisible particulate analysis on samples stored in baked-on syringes were within the USP <788> limits for 10 and 25 μm, and no specific trends were observed at the lower size channels as a function of time (Fig. 5a). Subvisible particle count levels for sprayed-on syringes were significantly different than those seen for the baked-on syringes especially for the lower particle channels (Fig. 5b) with an increase in subvisible particles for size channels 2, 5, and 8 μm observed for the sprayed-on syringes. It is also worthwhile to note that particle counts for samples analyzed at the 12-month time point stored at 5°C and 3- and 6-month time points stored at 25°C (data not shown) were so high that they saturated the detector, and no measurements could be made for those samples. Further particle characterization using optical microscopy indicated that the particles were identical to those seen in the Si-oil spiking study, and the shape and morphology of the particles was correlated very nicely with that reported by Narhi et al. (10) and was therefore attributed to small Si-oil droplets (Fig. 6). Overall data suggested that the baked-on silicone process was better suited for protein formulation development in PFS as it represented a lesser degree of risk for the formation of subvisible particulate matter as well as minimized any potential for protein precipitation on the Si-oil droplets.
Fig. 4.
Soluble aggregate levels for spayed-on and baked-on syringes at a 5°C, b 25°C, and c 40°C as a function of time
Fig. 5.
Subvisible particle levels for a baked-on and b sprayed-on syringes (respectively) at 5°C as a function of time
Fig. 6.
Optical microscopy image showing that the subvisible particles obtained from sprayed-on silicone syringes
Selection of Primary Components: Tip Cap Compatibility
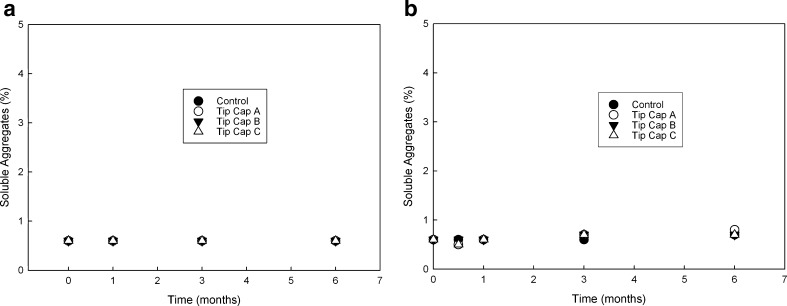
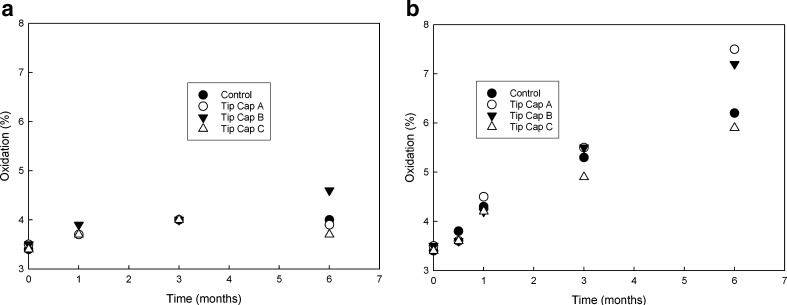
In this section, we have provided an example of an approach that was used for choosing the correct type of tip cap for development of a mAb product in PFS system. Although tip cap material is considered to have minimal product contact, it is imperative that optimum material is chosen. Three different tip cap materials from two vendors were evaluated against the mAb formulation. Tip cap samples were cut and placed in vials containing mAb formulation as described in the “MATERIALS AND METHODS” section. These samples were evaluated for changes in quality attributes over 6 months when stored at 5°C and 25°C. A control arm with no tip cap in the formulation was also placed on stability. Figure 7a, b shows no substantial difference in soluble aggregate level between the different tip cap materials as compared to the control at both temperatures, with all the materials fairing equally. Figure 8a, b shows oxidation level changes as a function of time and tip cap material at 5°C and 25°C, respectively. Increase in oxidation as a function of time was observed for both 5°C and 25°C samples over 6 months for materials A and B as compared to the control. Material C did not show any significant increase in oxidation over the same time period when compared with the control samples. This observation was significant and prompted the choice of material C for the tip cap.
Fig. 7.
Soluble aggregate levels for mAb formulation samples at a 5°C and b 25°C (respectively) when in contact with various tip cap materials
Fig. 8.
Oxidation levels for mAb formulation samples at a 5°C and b 25°C (respectively) when in contact with various tip cap materials
DISCUSSION
In this paper, we have limited our investigation to two broad aspects: stability of a model mAb formulation in the presence of practically relevant levels of Si-oil and a stability-based approach for selection of primary packaging components (syringe barrel and tip cap). Discussions on the selection of the plunger stopper were not in scope of this study since the elastomer resin selected for the plunger stopper for this mAb product was identical in composition to that of the vial stopper for the mAb product in vials. Hence, no additional compatibility work was required prior to the stopper selection. However, an approach similar to the one taken for the tip can also be used for the plunger stopper.
Silicone Oil Compatibility
Silicone oil is an essential component of PFS due to its role as lubricant to achieve desired functionality (e.g., maintain a certain break-loose force and glide force over shelf life). Proteins are complex molecules and due to their amphiphilic nature can interact with a variety of surfaces. These interactions can result in structural changes in the protein leading to denaturation. Presence of a silicone oil layer as the main contact surface in PFS systems can have adverse effects on protein stability (7). Under typical conditions, protein solutions in pre-filled syringes can be exposed to several types of stress factors (shaking during shipping, temperature excursions, etc.) while in contact with silicone oil–water interface over its shelf life. Therefore, it is imperative that the compatibility of protein formulation with silicone oil be assessed for the development of protein formulations in PFS. In this paper, we have presented an approach for evaluating the compatibility of protein formulation with silicone oil. A silicone oil/water emulsion was prepared and used to spike the required amount of silicone to the mAb formulation in vials to represent a scenario of silicone oil level which is typically seen in PFS. The silicone oil level can vary depending on the syringe manufacturer, syringe size, and processing conditions. A survey of leading PFS manufactures resulted in our finding that the typical levels reported vary between 0.5 and 1 mg silicone per syringe. Hence, we selected 0.5, 1, and 1.5 mg as the varying levels of silicone oil for our spiking study. Once the samples were spiked with the determined amounts of silicone, our objective was to simulate and/or exaggerate the types of stress that a typical syringe would experience during its lifetime on the shelf. Agitation and temperature were identified as the major stress factors to be evaluated. Results from the agitation stress study suggest that no deleterious impact on any of the quality attributes monitored was seen when samples were agitated with speeds of up to 300 rpm. In particular, there was no increase in soluble aggregates and no changes in appearance of the solution during the entire agitation study duration of up to 5 days at 5°C for all the samples including control. Placing vials in the horizontal orientation during the agitation study helped maximize the air–water interfacial area available for protein adsorption. The results follow similar trends as those seen with the previous study presented by Thirumangalathu et al., where authors observed that aggregation of the monoclonal antibody was promoted at agitation speeds of ≥350 rpm. Interestingly, the authors also reported that there was no aggregation seen at agitation speeds of less than or equal to 200 rpm (8). The agitation speed of 300 rpm used in this study is quite vigorous and, from our visual observations, thought to be equivalent to (if not more rigorous) expected agitation experienced during shipment or under recommended storage and shipping conditions.
Although no deleterious effect on protein quality in terms of soluble aggregates was noted at the preferred storage condition of 5°C over 12 months, formation of Si-protein particles was observed at the accelerated condition (25°C). No changes in soluble aggregate levels or appearance were observed at 5°C over the duration of the study (12 months). These observations are consistent with those made by Jones et al. where significantly higher levels of Si-oil than the ones used in our study (5 mg/mL) was tested with lysozyme, bovine serum albumin, con A, and ribonuclease A proteins (7). They reported that the aggregation propensity measured at 45°C indicated faster kinetics in the presence of Si. The observation of Si-oil droplets in samples stored at 5°C in the form of subvisible particulates is similar to the findings of Ludwig et al. where the authors reported that Si-oil emulsion stability itself was different in the presence of protein, sucrose, sodium chloride, and polysorbate 20 when compared to silicone oil emulsion alone. In the presence of sucrose and sodium chloride, the emulsion was destabilized while addition of polysorbate 20 stabilized the emulsion. This emulsion instability in the presence of formulation excipients may lead to formation of subvisible/visible particles (11). Therefore, careful control of silicone levels is needed for the incoming primary packaging components as they are the largest source of Si-oil for the product. In addition, it is important to note that short and quick stress studies such as the agitation study may not provide an immediate indication of protein susceptibility to denature in the presence of silicone oil and more elaborate stability monitoring at accelerated as well as preferred storage condition may be required.
Selection of Primary Components
Selection of primary packaging components for PFS systems often presents unique challenges due to the optimal balance that is required from both syringe functionality and protein stability perspectives. An ideal way of selecting primary components is to perform stability studies using various primary packaging components and select the best performing components from protein stability and PFS functionality standpoints. Oftentimes, this approach is not feasible due to short development timelines, lack of material for stability, and timely availability of primary packaging components in the desired configuration. We have presented an approach for selection of primary packaging components through syringe barrel and tip cap material selection case study illustrations.
A screening study in PFS systems using both the sprayed-on silicone and baked-on silicone barrels provided actual evidence of stability and how it is impacted by the syringe barrel process method. Although the spiking study provided an early warning for silicone oil when the level of silicone was higher than 0.5 mg/syringe, stability study in PFS provided actual evidence of issues that could have a significant impact on certain quality attributes of the drug product. It was clear that sprayed-on silicone syringes resulted in the formation of silicone oil droplets which saturated the sensor during subvisible particle measurement. This is most likely due to the fact that over time, silicone oil droplets can coalesce resulting into the formation of bigger droplets and hence giving the appearance of visible particles. In combination with our findings from the silicone spiking studies (where there was evidence that the protein could precipitate onto these oil droplets and lead to the formation of Si-protein particulates), this could result in deviations on particulate matter formation during shelf storage resulting in potential cost impact to the product. On the other hand, baked-on syringes, which approximately contain ten-fold less free silicone oil, showed no deleterious impact on product quality and no increase in subvisible particles formation over the study duration of 12 months and hence represent a lesser risk from a development perspective when decisions on container closure systems need to be made under very aggressive timelines.
Using the material screening approach, we investigated three different tip cap materials through accelerated temperature stability testing and were able to tease out the differences between the three materials. Tip cap material C performed the best among the three materials tested resulting in the lowest propensity for oxidation. Although determination of a cause of this increased oxidation was not in the scope of the study, it can be hypothesized that the tip cap resin can potentially contain leachables that can initiate or catalyze protein oxidation resulting in the higher oxidation level. Such observations of leachables influencing protein stability for various biologics have been summarized previously by Markovic (12)
Although some of the approaches used in this study can provide “watch outs” on stability of the protein formulation, it is recommended that long-term stability studies be carried out on the actual PFS system to be used including components such as the syringe barrel, plunger, and tip cap/needle shield to get more definitive information and support any regulatory filings.
CONCLUSIONS
Overall, this paper describes an approach that can be taken to develop biotechnology products in PFS systems. In addition, although there is awareness within the field of incompatibilities with PFS systems such as tungsten, silicone oil, etc., there is not a lot of literature or examples of case studies highlighting actual PFS system development and issues encountered. Although the purpose of this paper is to provide general guidance on development of protein therapeutics in PFS, it is important to point out that there could be several approaches that can be taken depending upon resources, previous or platform knowledge, project timeline constraints, etc. These case studies can be used as examples for developing biotechnology products in syringes.
Acknowledgments
Authors wish to thank Maria Toler and Julie Stambek for the subvisible particle analysis as well as the particle characterization and the Pharmaceutical Characterization and Assay Support team lead by Jolie Ziemba for all the analytical assay support.
References
- 1.Overcashier DE, Chan EK, Hsu CC. Technical considerations in the development of prefilled syringes for protein products. Am. Pharm. Rev. 2006;9(7):77–83. [Google Scholar]
- 2.Eakins M. The design and construction of pre-filled syringes. Am. Pharm. Review. 2007;10(6):47–51. [Google Scholar]
- 3.Wen Z-Q, Torraca G, Yee C, Li G. Investigation of contaminants in protein pharmaceuticals in pre-filled syringes by multiple micro-spectroscopies. Am. Pharm. Rev. 2007;10(5):101–7. [Google Scholar]
- 4.Jiang Y, Nashed-Samuel Y, Li C, Liu W, Pollastrini J, Mallard D, et al. Tungsten-induced protein aggregation: solution behavior. J. Pharm. Sci. 2009;98(12):4695–710. doi: 10.1002/jps.21778. [DOI] [PubMed] [Google Scholar]
- 5.Liu W, Swift R, Torraca G, Nashed-Samuel Y, Wen Z-Q, Jiang Y, Vance A, Mire-Sluis A, Freund E, Davis J, Narhi L. Root cause analysis of tungsten-induced protein aggregation in pre-filled syringes. PDA J. Pharm. Sci. Technol. 2010;64(1):11–9. [PubMed] [Google Scholar]
- 6.Bee JS, Nelson SA, Freund E, Carpenter JF, Randolph TW. Precipitation of a monoclonal antibody by soluble tungsten. J. Pharm. Sci. 2009;98(9):3290–301. doi: 10.1002/jps.21707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones LS, Kaufmann A, Middugh CR. Silicone oil induced aggregation of proteins. J. Pharm. Sci. 2005;94(4):918–27. doi: 10.1002/jps.20321. [DOI] [PubMed] [Google Scholar]
- 8.Thirumangalathu R, Krishnan S, Ricci MS, Brems DN, Theodore W, Randolph JFC. Silicone oil- and agitation-induced aggregation of a monoclonal antibody in aqueous solution. J. Pharm. Sci. 2009;98(9):3167–81. doi: 10.1002/jps.21719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furness G. Innovations that meet the growing demand. Newtimber: ONdrugDelivery; 2005. [Google Scholar]
- 10.Narhi LO, Jiang Y, Cao S, Benedek K, Shnek D. A critical review of analytical methods for subvisible and visible particles. Curr. Pharm. Biotechnol. 2009;10(4):373–81. doi: 10.2174/138920109788488905. [DOI] [PubMed] [Google Scholar]
- 11.Ludwig DB, Carpenter JF, Hamel JB, Randolph TW. Protein adsorption and excipient effects on kinetic stability of silicone oil emulsions. J. Pharm. Sci. 2010;99(4):1721–33. doi: 10.1002/jps.21982. [DOI] [PubMed] [Google Scholar]
- 12.Markovic I. Challenges associated with extractable and/or leachable substances in therapeutic biologic protein products. Am. Pharm. Review. 2006;9(6):20–7. doi: 10.1517/14740338.6.5.487. [DOI] [PubMed] [Google Scholar]