Abstract
The porcine esophageal mucosa has been proposed as a substitute for the buccal mucosa barrier on ex vivo permeability studies mainly due to its large surface area as well as its easier preparation. Therefore, this study compared the ex vivo permeability parameters of two drugs (carmabazepine (CBZ) and triamcinolone acetonide (TAC)) with different permeabilities and physicochemical properties through buccal and esophageal mucosae using a Franz diffusion cell system and HPLC as detection method. The freezing effects on drug permeability parameters were also evaluated by comparing them when fresh and frozen tissues were used. The barrier properties were not affected by the freezing process since the obtained parameters for both drugs were similar in frozen and fresh tissues (buccal and esophageal mucosae). However, an increase of CBZ retention was shown in frozen tissues. Fresh and frozen esophageal mucosae provided higher permeation of TAC than on buccal mucosae while the obtained permeability parameters for CBZ were similar on both mucosae. According to our results, porcine esophageal mucosa could be used as a substitute for buccal mucosa on ex vivo studies involving CBZ but not TAC. Frozen tissues could be used as substitute for fresh tissues in both cases. However, any substitution should be done with care and only if previous tests were performed, because the results could differ depending on the tested drug.
KEY WORDS: buccal mucosa, carbamazepine, esophageal mucosa, permeability, triamcinolone acetonide
INTRODUCTION
The buccal cavity provides a potential site for oral administration of drugs for both systemic and local actions (1). Direct targeting to the systemic circulation through the internal jugular vein may promote an increase in bioavailability whereas it avoids the hepatic first-pass metabolism (2).
Several methods have been proposed to evaluate the permeation profile and absorption kinetics. While in vivo methods are often more appropriate to assess bioavailability via this route, in vitro and ex vivo methods have been helpful for preclinical drug screening as well as elucidating mechanisms of transport across the buccal mucosa. Another application of these systems includes the evaluation of potential chemical penetration enhancers for improvement of buccal transport (3).
Most reported transbuccal permeation studies were carried out ex vivo due to their advantages over in vivo studies (4). Ex vivo studies can be easily set up using various types of diffusion apparatus, and the experimental conditions can be controlled. Additionally, ex vivo studies are cheaper than in vivo testing since only small pieces of animal tissues are used in these studies (5).
The porcine buccal mucosa has been considered a suitable model for ex vivo permeability studies given that it presents a non-keratinized mucosa, an inexpensive maintenance cost, as well as because its structure and composition are more similar to that of the human buccal mucosa than to any other animal model (6). However, the separation of buccal mucosa from the underlying tissues is very difficult and time-consuming. For these reasons, the porcine esophageal mucosa has been proposed as a substitute for the buccal barrier (7). The esophagus is also covered by a squamous, stratified, and non-keratinized epithelium, similarly to the buccal mucosa, but it offers a larger surface area and it is much easier to prepare. Furthermore, buccal and esophageal epithelia present a similar lipid composition with elevated levels of glycosylceramides and low amounts of ceramides (7).
The storage of excised tissues represents another problem associated to the development of an ex vivo model to study transmucosal drug permeability. Although it is assumed that freshly excised buccal tissues retains most of the barrier properties of the original mucosa, the availability of fresh tissues is often limited by the used methodology, the experimentation schedule, and by the amount of tissues supplied (8).
The main objective of this study is to understand whether it is possible to substitute the buccal mucosa by the esophageal mucosa and fresh tissue by the frozen one. Therefore, in this study, we compared the ex vivo permeability parameters of two drugs (carmabazepine (CBZ) and triamcinolone acetonide (TAC)) with different physicochemical characteristics through fresh and frozen buccal and esophageal mucosae. The experiments were carried out in Franz diffusion cells and HPLC was used as detection method.
MATERIALS AND METHODS
Tissue Preparation
Fresh porcine buccal and esophageal mucosae, obtained from a local slaughterhouse, were used at least within 4 h after the animals were slaughtered. These mucosae (epithelium + connective tissue) were separated from the underlying tissues by surgical scissors. During transport and processing, the mucosae were equilibrated with Krebs–Ringer buffer prior to permeation studies. The histological studies were carried out within 2 h after slaughtering. Some samples of these tissues were stored at −80°C (9), no longer than a month, to evaluate the freezing effects of tissues and storage on permeability and barrier integrity. All studies were performed using tissues of different animals in order to check inter-animal variations and to increase the experimental precision.
Histological Evaluation
Before the permeation studies, fresh and frozen buccal and esophageal mucosae were evaluated in order to verify if the low temperature could cause histological changes. The mucosae were fixed in 10% neutral-buffered formalin (Sigma, Brazil). Each section was dehydrated using a graded series of ethanol (Merck, Brazil) and embedded in paraffin wax (Inlab, Brazil). Tissues were separated into small pieces (about 4 μm in thickness) and stained with hematoxylin and eosin (Sigma, Brazil). All sections were examined by light microscope (Nikon, Japan). After 1 month storage, samples of all frozen tissues were also fixed and examined as described above. Three slides per tissue sample were prepared and examined.
Permeation Studies
Prior to conducting the diffusion experiments, the frozen mucosae were thawed to room temperature to minimize tissue damage, and then immersed in freshly prepared phosphate buffer (PBS) to remove the residual Krebs from the tissues. This same procedure was performed for fresh tissues. These mucosae were mounted between the donor and receiver chambers of Franz diffusion cells with an available diffusion area of 1.77 cm2. The receiver chambers were filled with 10 ml of simulated saliva buffer: ethanol (50:50 for TCA and 80:20 for CBZ), and the donor chambers were filled with 2 ml of each drug solution (1 mg ml−1). The simulated saliva buffer was prepared by dissolving 2.38 g Na2HPO4, 0.19 g KH2PO4, and 8.00 g NaCl in 1 L of distilled water (adjusted to pH 6.75 with phosphoric acid) (10). Ethanol was added to maintain sink conditions and to solubilize the drugs. The permeation studies were undertaken at 37°C with stirring (13.58 ×g). At regular time intervals (each 1 h), samples (400 μl) were withdrawn from the receptor compartments and replaced with the same volume of fresh buffer. The amount of drug permeated across the mucosae was determined by HPLC as explained below. The cumulative amount of the permeated drug from the mucosa was plotted versus time, and the steady-state flux was determined from the slope of the linear part of the curve, and lag time as the intercept using linear-regression analysis. Detected changes in the permeability of frozen tissues in relation to fresh tissues could indicate loss of integrity due to the storage conditions.
At the end of the permeation experiments, the amount of CBZ retained in the different mucosae was also investigated, since this evaluation was not found in the literature for this drug. The mucosae (frozen and fresh) were placed in separate pre-weighed tubes to determine the amount of CBZ in each tissue sample collected. The drug was extracted from the tissues with 5 mL of methanol (Merck, Brazil), vortexed for 10 min (Quimis, Brazil), sonicated for 15 min (Fisher Scientific, USA), and filtered through cellulose membranes (0.45 μm; Millipore). The extraction method was previously validated in blank experiments and by spiking the mucosae with known amount of CBZ. The amount of drug retained in mucosae was determined by HPLC as explained below.
Instrumentation and Analytical Procedure
The apparatus used for HPLC analysis was a Shimadzu system (Kyoto, Japan) equipped with a LC-10AD Shimadzu pump and Shimadzu SPD-10A UV detector (Kyoto, Japan). C18 columns (Phenomenex—4.0 × 300 mm, 5 μm; and Kromasil®—4.6 × 150 mm, 5 μm for TAC and CBZ, respectively) were employed. The mobile phase used (at a flow rate of 1 ml min−1) for TAC and CBZ was a mixture of acetonitrile: water (55:45 v/v), and methanol: water (70:30 v/v), respectively. HPLC-grade acetonitrile and methanol were obtained from Tedia. The mobile phases were tailored to provide a retention time greater than 4 min for each drug since biological components eluted out of the buccal tissue from 1 to 3 min (data not shown). The UV detector was set at 254 (TA) and 286 nm (CBZ). Peak areas were plotted and integrated using Shimadzu CLASS-VP software. The analytical method was previously validated according to ICH Guidelines (11).
Statistical Analysis
The results were expressed as mean ± standard deviation, and statistical differences were determined by using one-way ANOVA test and Student–Newman–Keuls test (SNK) for multiple comparisons with a significance level of p < 0.05. The software GraphPad Instat® from Graph Pad Software Inc. (San Diego, USA) was used for statistical analysis.
RESULTS AND DISCUSSION
Histological Evaluation
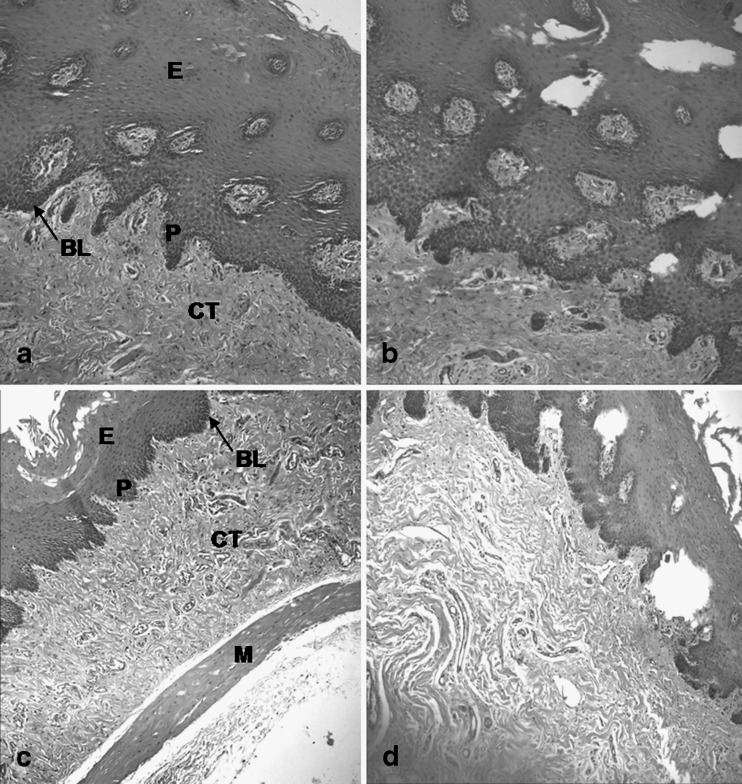
Structural similarities between buccal and esophageal mucosae were shown (Fig. 1) with a stratified squamous epithelium supported by a fibrous connective tissue (lamina propria and submucosa). These mucosae were differentiated by a layer of muscle fibers in the esophageal mucosa. Furthermore, the total thickness of buccal epithelium was bigger than the esophageal epithelium, and the papillae of esophageal epithelium were smaller and more regular than those of buccal epithelium.
Fig. 1.
Porcine buccal and esophageal mucosae before (a and c, respectively) and after (b and d, respectively) freezing process (magnification, ×40) E stratified squamous epithelium; BL basal lamina; CT connective tissue; P papilla; M muscularis mucosa (present only in the esophagus)
The freezing at −80°C promoted discrete morphological alterations in both mucosae, probably due to the formation of ice crystals in these tissues. According to Hadzija et al. (12), when the tissue undergoes freezing process in an uncontrolled manner, ice crystals are formed within the tissue, which could damage the intracellular matrix and cell membrane, altering the barrier properties of these tissues. A superficial desquamation was also observed in the frozen tissues.
Permeability of Carbamazepine through Fresh and Frozen Buccal and Esophageal Mucosae
CBZ is currently administered by peroral route, with an irregular and slow absorption when immediate-release tablets are administered (13). The bioavailability and absorption of this drug are limited due to their low solubility in water (14), and thus alternative routes for the CBZ delivery have recently been proposed. The oral cavity could be an interesting site for transbuccal CBZ delivery due to two properties: slow administration of constant low drug doses and less dose-related side effects (15), hence this drug was selected for the tests.
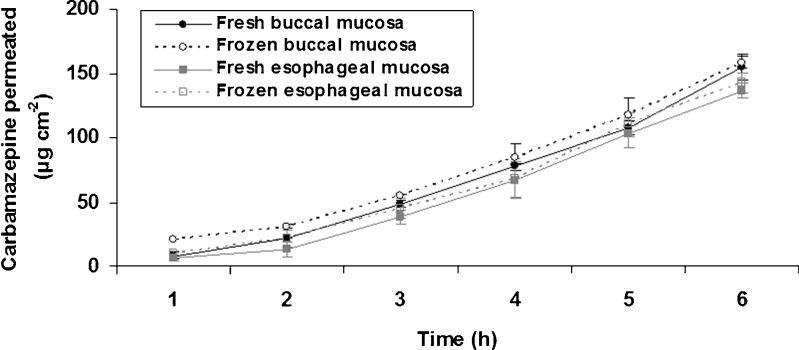
The diffusion kinetics obtained in this study (Fig. 2) showed a linear increase of permeated CBZ amount in time course. For periods longer than 3 h, the amounts of permeated CBZ in different tissues (buccal vs. esophageal, frozen vs. fresh) had similar values (p > 0.05, SNK).
Fig. 2.
Permeability of carbamazepine through fresh and frozen porcine buccal and esophageal mucosae. Data are presented as mean ± standard deviation (n = 4)
The permeability parameters obtained from diffusion kinetics are shown in Table I. The lag time was relatively short (less than 1.19 h), which provides a rapid diffusion of CBZ through the mucosae (epithelium and connective tissue). According to Shah (16), the permeability experiments should be carried out in a period corresponding to at least three lag times to achieve a steady state. So, when an experiment lasts less than three lag times, the flux and lag time estimated may be controversial. The lag time of CBZ was approximately six times lower than total experiment time demonstrating that the equilibrium was rapidly achieved and thus the estimated permeability parameters were consistent.
Table I.
Permeability Parameters of Carbamazepine
| Parameter | Fresh buccal mucosa | Fresh esophageal mucosa | Frozen buccal mucosa | Frozen esophageal mucosa |
|---|---|---|---|---|
| J S (mean ± SD) (μg/cm2.h)i | 29.203 ± 2.449(a) | 27.142 ± 1.879(a) | 28.002 ± 0.527(a) | 27.312 ± 1.869(a) |
| T L (mean ± SD) (h)ii | 1.090 ± 0.128(ab) | 1.185 ± 0.089(b) | 0.718 ± 0.071(a) | 1.071 ± 0.217(ab) |
| P (mean ± SD) (cm/h)i | 2.92 × 10−2 ± 2.45 × 10−3(a) | 2.71 × 10−2 ± 1.87 × 10−3(a) | 2.80 × 10−2 ± 5.27 × 10−4(a) | 2.73 × 10−2 ± 1.87 × 10−3(a) |
ANOVA and SNK tests (p < 0.05) were carried out as appropriate (n = 4). Identical letters mean absence of statistical differences among treatments. Each permeability parameter was analyzed separately
i F = 0.635, p = 0.619
ii F = 4.413, p = 0.058
In regard to the flux values (J) and permeability coefficients (P; Table I), the different mucosae had similar values when separately analyzed (p > 0.05, SNK). The flux values obtained for the CBZ were approximately 1.5 times higher than those found by Giannola et al. (17), who also used porcine tissues. The effect of ethanol used as an enhancer and/or the different concentrations of CBZ in donor solution (1 mg ml−1vs. 0.4 mg ml−1) could explain this difference. Furthermore, the obtained permeability coefficients were approximately 1.5 times lower than those obtained by the same authors due to the fact that the calculating parameter includes the initial drug concentration in donor solution. The type and thickness of tissue used in the experiments could also contribute to explain the difference found between the P values since these authors used only epithelial tissue (fat and connective tissue were removed). Nicolazzo et al. (18) also showed that the presence of connective tissue promotes a reduction in drug permeability (e.g., caffeine and estradiol), as it enhances the permeability barrier and increases the lag time. Therefore, the connective tissue presence is desirable in ex vivo permeability experiments in order to allow greater correlation with in vivo data.
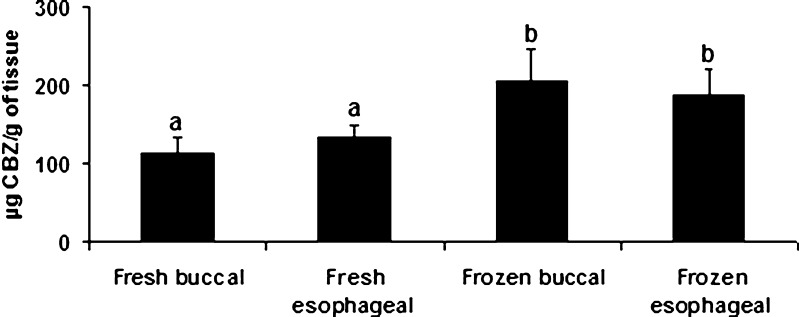
Since different studies have shown a reservoir function for buccal mucosa (19, 20), the amounts of CBZ retained in frozen and fresh buccal and esophageal mucosae were also compared (Fig. 3). This drug showed similar retention amounts (p > 0.05, SNK) in the both mucosae; however, frozen mucosae showed a greater retention capacity over the fresh mucosae (F = 8.5, p = 0.0033). When these results are correlated with those from histological studies, it is possible to conclude that the presence of vacuoles in the frozen mucosae could be the reason for a greater drug retention.
Fig. 3.
Carbamazepine amount retained in fresh and frozen porcine buccal and esophageal mucosae. Data are presented as mean ± standard deviation (n = 4). ANOVA/SNK tests (p < 0.05) were carried out as appropriate. Identical letters mean absence of statistical differences among treatments
In summary, the freezing process does not seem to compromise the barrier properties of buccal and esophageal mucosae for CBZ since the flux values (J) and permeability coefficients (P) were not statiscally different between frozen and fresh mucosae. On the other hand, an increase in drug retention was shown for frozen tissues, probably due to the accumulation of CBZ within the vacuoles observed in these tissues. When buccal and esophageal mucosae are compared, the retention profile as well as the permeability coefficients is similar.
Permeability of Triamcinolone Acetonide through Buccal and Esophageal Mucosa (Fresh and Frozen)
TAC is a long-acting synthetic glucocorticoid used for the local treatment of some diseases such as oral lichen planus (21). Even though some studies have evaluated the distribution and permeability of TAC through the buccal mucosa (22, 23), the present study aimed to optimize the permeability of TAC in order to calculate its permeability parameters (flux, permeability coefficient, lag time) with greater reliability and also to reduce the experimental time. According to Nicolazzo et al. (22), the permeated amount of this drug is reduced and close to the quantification limit when absorption enhancers are not used. Thus, ethanol was tested to this purpose. The retention analysis of TAC in buccal mucosa was not evaluated in this study given that it was already reported (22).
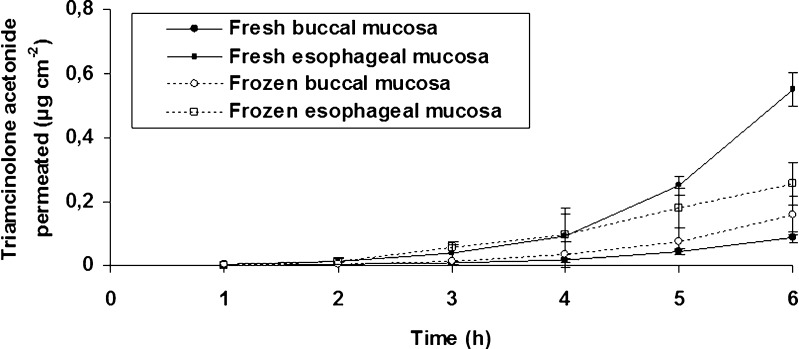
TAC permeability parameters without ethanol were initially evaluated (Fig. 4) and a lag time higher than 3 h was found for all tested mucosae. According to Ho (24), lipophilic compounds (e.g., TAC) would have a rapid uptake by partitioning into the buccal epithelium, followed by tissue retention, and slow transfer into the systemic circulation. The same behavior was observed by Nicolazzo et al. (22), who found a disappearance rate of TAC from the donor chamber higher than the appearance rate in the receptor chamber (approximately 18.8 times), indicating a high retention of this drug in buccal mucosa. The addition of ethanol in the receptor chamber increased the throughput of TAC from the underlying layers of the epithelium since a lag time reduction (within the range of 0.65 to 1.29 h) was shown (Table II).
Fig. 4.
Permeability of triamcinolone acetonide through fresh and frozen porcine buccal and esophageal mucosae when simulated saliva buffer (without ethanol) was used as acceptor fluid. Data are presented as mean ± standard deviation (n = 4)
Table II.
Permeability Parameters of Triamcinolone Acetonide
| Parameter | Fresh buccal mucosa | Fresh esophageal mucosa | Frozen buccal mucosa | Frozen esophageal mucosa |
|---|---|---|---|---|
| J S (mean ± SD) (μg/cm2 h)i | 10.806 ± 1.484(a) | 23.840 ± 3.015(b) | 13.427 ± 1.709(a) | 24.757 ± 0.287(b) |
| T L (mean ± SD) (h)ii | 1.280 ± 0.189(a) | 1.131 ± 0.128(a) | 1.296 ± 0.071(a) | 0.653 ± 0.160(b) |
| P (mean ± SD) (cm/h)i | 1.08 × 10−2 ± 1.48 × 10−3(a) | 2.38 × 10−2 ± 3.02 × 10−3(b) | 1.34 × 10−2 ± 1.71 × 10−3(a) | 2.48 × 10−2 ± 2.87 × 10−4(b) |
ANOVA and SNK tests (p < 0.05) were carried out as appropriate (n = 4). Identical letters mean absence of statistical differences among treatments. Each permeability parameter was analyzed separately
i F = 25.511, p = 0.0004
ii F = 11.276, p = 0.0045
The freezing process did not affect the barrier properties of the esophageal and buccal mucosae since the flux values of TAC were similar between frozen and fresh tissues (p > 0.05, SNK, Table II). This finding suggests that in this case the mucosa barrier properties are not affected by freezing process. Van Eyk and Van Der Biijl (25) also compared the permeability of β-estradiol through fresh and frozen mucosae, which present structural and physicochemical properties similar to those of TAC. Even though these authors carried out experiments in a period relatively longer (about 24 h), the flux values of β-estradiol did not achieve a steady state, and the estimated flux values in frozen mucosae were 24% higher than in fresh mucosae. However, the analysis of permeability curves (permeated β-estradiol vs. time) showed no statistical differences between fresh and frozen tissues (F test).
Nicolazzo et al. (18) also used estradiol to evaluate the freezing effect (−20°C) on its permeability through porcine epithelium and buccal mucosa (epithelium + connective tissue), and they reported that freezing did not affect the flux and the permeated estradiol amount through epithelium. Although it has not been possible to calculate the stradiol flux values when the diffusion barrier was enhanced (epithelium + connective tissue), the analysis of permeated estradiol showed a similar profile between frozen and fresh tissues.
In this study, the obtained flux values of TAC for esophageal mucosa were higher than for buccal mucosa (F = 25.51, p = 0.0004). Fresh esophageal mucosa presented a flux value 2.2 times higher than that of fresh buccal mucosa, and this ratio was 1.84 times higher when the different frozen mucosae (buccal and esophageal mucosae) were compared. The barrier properties of these mucosae were also studied by Consuelo et al. (7) using fentanyl citrate (FC) as a drug model, and the flux values obtained were similar for both mucosae. The different physicochemical characteristics of these drugs (TAC and FC) could be the reason for the observed divergence, since TAC is much more lipophilic than FC. Furthermore, FC is potentially ionizable at the pH value selected for the study, and thus it followed predominantly the paracellular route.
In summary, these findings indicate that the porcine esophageal mucosa cannot be used as a substitute for the porcine buccal mucosa in ex vivo permeability studies of TAC since the obtained permeability parameters in these mucosae were different. On the other hand, frozen mucosae could be an alternative to fresh mucosae since the freezing process did not affect the barrier properties for TAC.
CONCLUSION
Although the freezing process has caused histological changes, the barrier properties of esophageal and buccal mucosae were not affected as the permeability parameters for the tested drugs were similar in frozen and fresh tissues. Therefore, frozen porcine mucosae could be substituted by fresh porcine mucosae in permeability studies involving CBZ and TAC. On the other hand, a retention increase for CBZ was shown in frozen tissues. Esophageal mucosa could be used as a substitute for buccal mucosa only in ex vivo experiments involving CBZ where the objective is not to assess the retention of this drug in the mucosa. These findings suggest that the drug type and its physicochemical characteristics are relevant during the development of an ex vivo model to study buccal drug permeability.
Acknowledgments
The authors would like to thank Mr. Victor Antunes and Dr. Claudia Pinto Figueiredo for their assistance in processing tissue samples for microscopic studies, as well as Dr. Flávio Henrique Reginatto and Dr. Letícia Scherer Koester for their help with data analysis. This study was supported by CAPES/MEC and CNPq/MCT.
References
- 1.Kurosaki Y, Kimura T. Regional variation in oral mucosal drug permeability. Crit Rev Ther Drug Carrier Syst. 2000;17:467–508. [PubMed] [Google Scholar]
- 2.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Nicolazzo JA, Finnin BC. In vivo and in vitro models for assessing drug absorption across the buccal mucosa. In: Ehrhardt C, Kim K-J, editors. Drug absorption studies—in situ, in vitro, and in silico models. New York, NY: Springer; 2008. pp. 89–111. [Google Scholar]
- 4.Zhang H, Robinson JR. In vitro methods for measuring permeability of the oral mucosa. In: Rathbone M, editor. Oral mucosal drug delivery. New York, NY: Marcel Dekker; 1996. pp. 85–100. [Google Scholar]
- 5.Xiang J, Fang X, Li X. Transbuccal delivery of 2′,3′-dideoxycytidine: in vitro permeation study and histological investigation. Int J Pharm. 2002;231:57–66. doi: 10.1016/S0378-5173(01)00865-1. [DOI] [PubMed] [Google Scholar]
- 6.Squier CA, Kremer MJ. Biology of oral mucosa and esophagus. J Natl Cancer Inst Monogr. 2001;29:7–15. doi: 10.1093/oxfordjournals.jncimonographs.a003443. [DOI] [PubMed] [Google Scholar]
- 7.Consuelo DI, Pizzolato G-P, Falson F, Guy RH, Jacques Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J Pharm Sci. 2005;94:2777–2787. doi: 10.1002/jps.20409. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Lee SK, Choi YW. The effect of storage conditions on the permeability of porcine buccal mucosa. Arch Pharm Res. 2002;25:546–549. doi: 10.1007/BF02976616. [DOI] [PubMed] [Google Scholar]
- 9.Lesch CA, Squier CA, Cruchley A, Williams DM, Speight P. The permeability of human oral mucosa and skin to water. J Dent Res. 1989;68:1345–1349. doi: 10.1177/00220345890680091101. [DOI] [PubMed] [Google Scholar]
- 10.Peh KK, Wong CF. Polymeric films as vehicle for buccal belivery: swelling, mechanical, and bioadhesive properties. J Pharm Pharmaceut Sci. 1999;2:53–61. [PubMed] [Google Scholar]
- 11.ICH (International Conference on Harmonization). 1997. Validation of analytical procedures: text and methodology. http://www.ich.org/cache/compo/363-272-1.html. Accessed 10 Feb 2010.
- 12.Hadzija BW, Ruddy SB, Ballenger ES. Effect of freezing on iontophoretic transport through hairless rat skin. J Pharm Pharmacol. 1992;44:387–390. doi: 10.1111/j.2042-7158.1992.tb03630.x. [DOI] [PubMed] [Google Scholar]
- 13.Barakat NS, Elbagory IM, Almurshedi AS. Formulation, release characteristics and bioavailability study of oral monolithic matrix tablets containing carbamazepine. AAPS PharmSciTech. 2008;9:931–938. doi: 10.1208/s12249-008-9108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nair R, Gonen S, Hoag SW. Influence of polyethylene glycol and povidone on the polymorphic transformation and solubility of carbamazepine. Int J Pharm. 2002;240:11–22. doi: 10.1016/S0378-5173(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 15.Campisi G, Paderni C, Saccone R, Siragusa MG, Lo ML, Tripodo C, et al. Carbamazepine transbuccal delivery: the histo-morphological features of reconstituted human oral epithelium and buccal porcine mucosae in the transmucosal permeation. Int J Immunopathol Pharmacol. 2008;21:903–910. doi: 10.1177/039463200802100414. [DOI] [PubMed] [Google Scholar]
- 16.Shah J. Analysis of permeation data: evaluation of lag time method. Int J Pharm. 1993;90:161–169. doi: 10.1016/0378-5173(93)90152-6. [DOI] [Google Scholar]
- 17.Giannola LI, De Caro V, Giandalia G, Siragusa MG, D'angelo M, Lo ML, et al. Transbuccal tablets of carbamazepine: formulation, release and absorption pattern. Int J Immunopathol Pharmacol. 2005;18:21–31. [PubMed] [Google Scholar]
- 18.Nicolazzo JA, Reed BL, Finnin BC. The effect of various in vitro conditions on the permeability characteristics of the buccal mucosa. J Pharm Sci. 2003;92:2399–2410. doi: 10.1002/jps.10505. [DOI] [PubMed] [Google Scholar]
- 19.Kremer MJ, Squier CA, Wertz PW. Absorption and release of topical triamcinolone in oral mucosa. J Dent Res. 1997;76:361. [Google Scholar]
- 20.Squier CA, Kremer MJ, Bruskin A, Rose A, Haley JD. Oral mucosal permeability and stability of transforming growth factor beta-3 in vitro. Pharm Res. 1999;16:1557–1563. doi: 10.1023/A:1015052520467. [DOI] [PubMed] [Google Scholar]
- 21.Zegarelli EV, Kutscher AH. Triamcinolone acetonide in the treatment of erosive lichen planus of the oral mucosae. Arch Dermatol. 1960;82:1010–1011. doi: 10.1001/archderm.1960.01580060166033. [DOI] [PubMed] [Google Scholar]
- 22.Nicolazzo JA, Reed BL, Finnin BC. Enhancing the buccal mucosal uptake and retention of triamcinolone acetonide. J Control Release. 2005;105:240–248. doi: 10.1016/j.jconrel.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Shin SC, Kim JY. Enhanced permeation of triamcinolone acetonide through the buccal mucosa. Eur J Pharm Biopharm. 2000;50:217–220. doi: 10.1016/S0939-6411(00)00101-6. [DOI] [PubMed] [Google Scholar]
- 24.Ho NFH. Biophysical kinetic modeling of buccal absorption. Adv Drug Deliv Rev. 1993;12:61–97. doi: 10.1016/0169-409X(93)90041-2. [DOI] [Google Scholar]
- 25.Van Eyk AD, Van Der Biijl P. Comparative permeability of fresh and frozen/thawed porcine buccal mucosa towards various chemical markers. SADJ. 2006;61:200–203. [PubMed] [Google Scholar]