Abstract
In the sublingual (SL) cavity, compared with the gastrointestinal tract, tablets are subjected to minimal physiological agitation, and a limited volume of saliva is available to facilitate disintegration and dissolution. None of the official compendial dissolution apparatuses and methods simulate these SL conditions. In this study, a custom-made dissolution apparatus was constructed, and a novel in vitro method that simulates SL conditions was evaluated. Several epinephrine 40 mg SL tablet formulations under development and two commercial SL tablets, isosorbide dinitrate 5 mg and nitroglycerin 0.6 mg, were studied. The dissolution medium was 2 mL of distilled water at 25°C. Dissolution was measured at 60 and 120 s. The novel in vitro method was validated for accuracy, reproducibility, and discrimination capability, and was compared with the official US Pharmacopeia (USP) dissolution method using apparatus 2 (Paddle). The data obtained following the novel in vitro method were accurate and reproducible. This method was capable of detecting minor changes in SL formulations that could not be detected using other in vitro tests. Results from the official USP dissolution method and our novel in vitro method were significantly different (p < 0.05). Results reflecting the dissolution of rapidly disintegrating tablets using simulated SL conditions were obtained using the novel in vitro dissolution method.
Key words: custom-made dissolution apparatus, development and validation, dissolution testing, novel in vitro dissolution method, sublingual tablets
INTRODUCTION
Dissolution testing is a critical and mandatory in vitro quality control procedure for solid dosage forms such as tablets. Such testing confirms that a tablet has released the labeled quantity of active pharmaceutical ingredient (API) into solution within a designated time interval. It demonstrates that the API will be readily available for absorption after oral administration. Ideally, sublingual (SL) tablets such as nitroglycerin should release the total quantity of API within seconds, for maximum absorption via the SL veins into the systemic circulation. The SL route is highly useful for an API, such as epinephrine, that is inactivated in the gastrointestinal tract (GIT) due to extensive metabolism (1). Conversely, buccal formulations are intended to release their APIs over an extended time period. Although buccal and SL delivery both take place within the oral cavity, they differ in aspects such as specific location, mucosa permeability, and intended duration of release of medication (2–4).
The SL route of administration has not been extensively studied because relatively few SL commercial products are currently available. The SL cavity is characterized by unique anatomical and physiological conditions compared with other segments of the GIT such as the stomach and small intestine. A tablet that is swallowed will be subjected to GIT peristalsis in the presence of relatively large volumes of digestive fluids secreted throughout the GIT, facilitating tablet disintegration and drug dissolution. In the SL cavity, tablets are exposed to minimal physiological agitation; moreover, a limited volume of saliva, 0.3 mL/min resting flow rate up to 1 mL/min stimulated flow rate (5), is available to facilitate tablet disintegration and drug dissolution. Currently, the available pharmacopeias’ dissolution apparatuses and methods (6–9) do not simulate these unique characteristics for testing rapidly disintegrating SL tablets. For example, the US Pharmacopeia (USP) dissolution method recommended for isosorbide dinitrate SL tablets uses apparatus 2 (Paddle), 900 mL of water, and 50 rotations per minute (rpm) to achieve not less than 80% of the labeled amount dissolved in 20 min (10). Upon reviewing other pharmacopeia such as the European Pharmacopeia (8) and Japanese Pharmacopeia (9), it is readily apparent that none of the official compendia dissolution apparatuses or methods are designed to evaluate the release of API from a rapidly disintegrating SL tablet dosage form under simulated SL conditions.
The few new non-compendial in vitro methods cited for dissolution testing of SL tablets, utilize similar compendial apparatuses under modified conditions (11,12). Smaller volumes of dissolution medium have been proposed, but they are still larger than the volume of saliva secreted in the SL cavity within 2 min. For example, a mini-paddle apparatus, which can accommodate a minimum operational volume of approximately 30 mL of dissolution medium, has been introduced (13–15). However, the fluid hydrodynamics of these apparatuses are still not appropriate for modeling dissolution within the SL cavity.
Custom-made dissolution apparatuses and more bio-relevant methods are needed to evaluate rapidly disintegrating tablets intended for SL administration. In addition, an in vitro dissolution method should be capable of detecting and discriminating among minor changes in SL tablet formulations (16). Due to the short residence time within the SL cavity, we propose that the minor changes in formulations might have major effects on the rate and the extent of SL absorption (17,18). It is therefore mandatory to develop a dissolution method that meets these requirements.
We designed and constructed a custom-made apparatus suitable for measuring the dissolution of rapidly disintegrating SL tablets under simulated SL conditions. This novel in vitro method was evaluated for accuracy, reproducibility, and discrimination capability, and was compared with an official USP dissolution method.
MATERIALS AND METHODS
Parts of the Custom-Made Apparatus Unit
Parts purchased included Nalgene 180 vacuum tubing and automatic shut-off, quick-disconnect coupling inserts (Sigma-Aldrich Inc., Oakville, ON, Canada) and Millipore 25 mm glass microanalysis vacuum filter holders and supports, Whatman 0.45 μm nylon filter membranes, and 4 mL polystyrene disposable plastic tubes (Fisher Scientific Co., Nepean, ON, Canada).
APIs Used Throughout the Study
Epinephrine bitartrate (EB) (Sigma-Aldrich Inc., St. Louis, MO, USA) was used in the preparation of standard epinephrine (E) solutions and E SL tablets formulated in the tablet manufacturing laboratory of the Faculty of Pharmacy (19,20). Diluted isosorbide dinitrate and diluted nitroglycerin were purchased as standards from the US Pharmacopeia (Rockville, MD, USA) and used to construct their corresponding calibration curves. The commercial generic isosorbide dinitrate 5 mg (Apo-ISDN) and brand nitroglycerin 0.6 mg (Nitrostat®) were purchased from the University Centre Pharmacy (Winnipeg, MB, Canada).
Components of E SL Tablets Under Development
All epinephrine SL tablet formulations (Ea–g) evaluated were formulated in our laboratory. Non-medicinal ingredients (NMIs) incorporated into the E SL tablets included microcrystalline cellulose (Asahi Kasei Chemicals Corp, Tokyo, Japan), mannitol (Roquette America Inc., Keokuk, IA, USA), citric acid (Fisher Scientific Co., Fair Lawn, NJ, USA), low-substituted hydroxypropyl cellulose (Shin-Etsu Chemical Co., Tokyo, Japan), and magnesium stearate (Mallinckrodt Baker, Phillipsburg, NJ, USA).
Construction of One-Unit and Six-Unit Custom-Made Apparatus
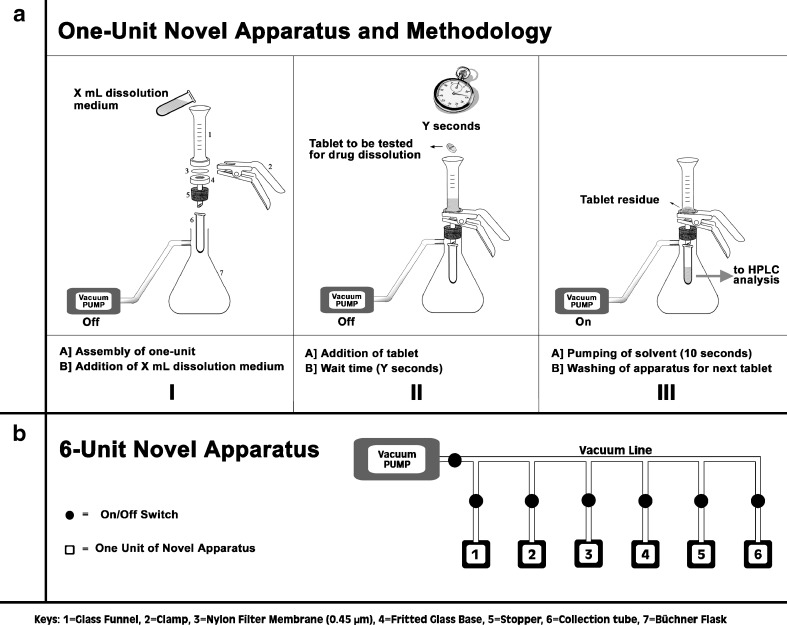
For each dissolution test, a 0.45-μm nylon filter membrane was pre-wetted with 50 μL of distilled water and placed between the 15 mL glass funnel and the fritted glass base, which were clamped and inserted into Büchner flask (Fig. 1a). A 4-mL disposable plastic collection tube was placed at the outlet tip of the clamped unit to collect the filtrate. The Büchner flask was connected to a vacuum line controlled by automatic shut-off, quick-disconnect coupling inserts (on/off switches).
Fig. 1.
Schematic diagram showing the a one-unit and b six-unit custom-made apparatus with brief description of the novel in vitro dissolution method
A six-unit custom-made apparatus was created by joining six individual assemblies to a common vacuum line. The vacuum for each individual unit was controlled by its own on/off switch (Fig. 1b).
The Novel In Vitro Dissolution Method
A volume of 2 mL of distilled water, as the dissolution medium, was measured into the 15 mL glass funnel at 25°C. The tablet was placed into the dissolution medium undisturbed for each specified time. The time-points, ranging from 15 to 120 s (stopwatch), were used initially to assess the dissolution profile of a representative formulation of E 40 mg SL tablets. Based on these results, the 60 and 120 s time-points were selected for subsequent experiments. At each appropriate time-point, the full vacuum was applied by opening the on/off switch causing the total volume of dissolution medium to be withdrawn instantly through a 0.45-μm filter membrane into the collection tube and terminating any further dissolution. The membrane prevented the passage of any undissolved particles and was replaced by a new membrane for each dissolution analysis. The API content in each sample was measured by HPLC with UV detection (Waters Corp) according to the official USP assays for epinephrine injection (21,22), nitroglycerin sublingual tablets (23), and isosorbide dinitrate sublingual tablets (10). To obtain the percent of drug released (DR%), the API content (mg) in the filtrate was compared with the mean content uniformity of ten individual dosage forms of the SL tablets being tested.
Assessment of E Adsorption to Apparatus Components
Using EB, the standard solutions equivalent to E 5, 10, 20, and 40 mg/mL (E5, E10, E20, and E40, respectively) were prepared; and 10 mL of each was filtered through the apparatus corresponding to 50, 100, 200, and 400 mg of E passing through the filter membrane and the fritted glass base to evaluate any loss of E through adsorption to apparatus components. The E content in the four standard solutions before and after filtration was measured. The 0.45-μm filter membrane was soaked in 10 mL distilled water to extract any E residues retained in the membrane for quantification. In addition, the fritted glass base was washed with five 10 mL aliquots of distilled water to detect any E residue remaining from the E5 and E40 solutions after filtration.
Formulation of E SL Tablets and Evaluation of All Tablets
The representative first and second generations of E SL tablet formulations (Ea–g) under development using direct compression (19) were available for dissolution testing. In all E formulations, EB was used to prepare SL tablets equivalent to E 40 mg. NMIs incorporated into these formulations included several grades of microcrystalline cellulose (MCC, diluent), mannitol (powder flow enhancer), citric acid (flavor) (24), low-substituted hydroxypropyl cellulose (disintegrant), and magnesium stearate (lubricant). These formulations differed by grade and proportion of MCC, by proportion of mannitol, and by the compression forces used to maintain uniform hardness (H) and disintegration times (DTs) (19,20). All the representative E SL tablet formulations, as well as the commercial Apo-ISDN and Nitrostat®, were evaluated for weight variation (WV) and content uniformity (CU) according to USP specifications (25).
Assessment of Dissolution Profile Using Representative E 40 mg SL Tablets
The dissolution testing of a representative formulation of E 40 mg SL tablets was evaluated at 15, 30, 45, 60, 75, 90, 105, and 120 s. The minimum time evaluated was 15 s, the time required for complete disintegration of the E SL tablets irrespective of E dose (17); the maximum time evaluated was 120 s, the time recommended for patients to retain the commercially available SL tablets under the tongue (26). Appropriate time-points were selected accordingly for subsequent experiments.
Assessment of Reproducibility
To assess day-to-day variability, dissolution testing at 60 and 120 s was performed and compared between day 1 and day 2 using one commercial SL tablet, Nitrostat®, and three representative E SL tablet formulations, Ea, Eb, and Ec.
Assessment of the Discrimination Ability
To assess the ability to discriminate among SL tablet formulations, dissolution testing at 60 and 120 s was performed using two commercial SL tablets, Nitrostat® and Apo-ISDN, and one representative E 40 mg SL tablet formulation, Ed. The dissolution testing of Apo-ISDN was also evaluated in 10 mL of dissolution medium due to the limited solubility of ISDN. The dissolution testing at 60 s of ten representative E 40 mg SL tablet formulations with DT of ≤15 s was also evaluated.
Dissolution Testing Using the Six-Unit Apparatus
The six-unit apparatus (Fig. 1b) was constructed and used to test the dissolution of six tablets simultaneously, using three E SL tablet formulations, Ee, Ef, and Eg. Results were compared with previous dissolution data collected using the one-unit apparatus.
Dissolution Testing Using the Official USP Apparatus and Method
The official USP apparatus 2 (Paddle) and method for ISDN SL Tablets (10) were used as a control for dissolution testing of two E SL tablet formulations, Ec and Ed, and for two commercial SL tablets, Apo-ISDN and Nitrostat®. The dissolution medium was 900 mL of distilled water at 37 ± 1°C. The paddle rotations were set at 50 rpm, and the samples were withdrawn as recommended at the 20 min time-point for analysis of the API content.
Data Analysis
Results were presented as means ± standard errors of means (SEM) of at least three replicate experiments and statistically analyzed by one-way ANOVA using Microsoft Excel software. The differences were considered significant at p < 0.05.
RESULTS
Assessment of E Adsorption to Apparatus Components
The E content was slightly lower after filtration of 10 mL standard E solutions than before filtration (Table I). The difference in E content before and after filtration, which represents the E retention in both the filter membrane and the fritted glass base, increased with increasing E concentration but did not exceed 10%.
Table I.
Epinephrine (E) Retention (mg) in the Custom-Made Apparatus Following the Filtration of 10 mL of Standard E 5, 10, 20, and 40 mg/mL Solutions (E5, E10, E20, and E40, Respectively)
| Mean ± SEMa | Standard E Solutions | |||
|---|---|---|---|---|
| E5 | E10 | E20 | E40 | |
| E before filtration | 48.8 ± 1.3 | 100.5 ± 0.4 | 200.9 ± 1.5 | 399.9 ± 1.2 |
| E after filtration | 45.9 ± 0.9 | 90.8 ± 5.1 | 187.6 ± 1.5 | 360.2 ± 2.1 |
| E differenceb (%)c | 2.9 (5.9) | 9.7 (9.7) | 13.3 (6.6) | 39.7 (9.9) |
| E residue in filter | 0.9 ± 0.1 | 6.7 ± 1.7 | 11.4 ± 1.4 | 34.8 ± 2.5 |
aMeans ± standard error of means (SEM) of E content (mg) of at least three replicates
bE difference = mean E before filtration − mean E after filtration
cPercent difference = (E difference/mean E before filtration) × 100
After filtration of E5, >88% of the retained E residue in the fritted glass base was washed out after the first wash. The following washes removed >97% of the E residue in the fritted glass base. When filtering standard E solution with higher concentration (E40), >94% of the E residue was removed from the fritted glass base after the first wash and >99% after the fourth wash.
Evaluation of All SL Tablets Used in This Study
The compression forces applied in the manufacturing of representative first and second generation E SL tablet formulations resulted in a uniform H of 1.31 ± 0.04 kg (mean ± SEM). All SL tablets, including the commercial Apo-ISDN and Nitrostat® SL tablets, resulted in a DT of ≤15 s and were within USP limits of WV and CU.
Assessment of Dissolution Profile Using Representative E 40 mg SL Tablets
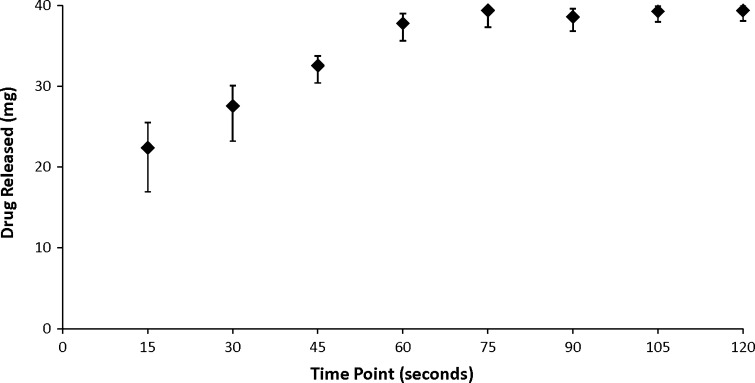
More than 50% of E (22.40 ± 3.14 mg) was released from the SL tablet and dissolved in the dissolution medium after 15 s and >90% (37.78 ± 1.22 mg) after 60 s (Fig. 2). The DR% increased linearly with time from 15 to 60 s. The DR% appears to reach an asymptote after more than 75 s, achieving the values of 96% (39.38 ± 1.18 mg) at 75 s and 98% (39.38 ± 0.72 mg) at 120 s.
Fig. 2.
Dissolution profile of a representative E 40 mg SL tablet formulation over 120 s. Data represent means ± standard error of means (SEM) of at least three replicates
Assessment of Reproducibility
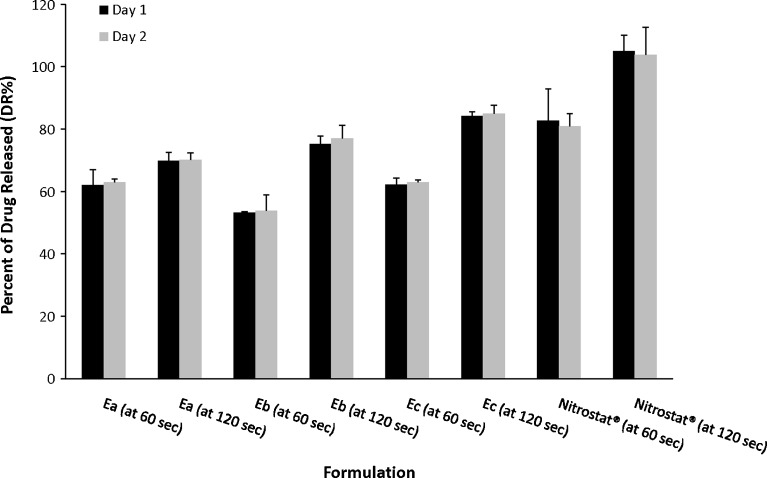
The DR% of E at 60 s from formulations Ea, Eb, and Ec ranged from 53.16% to 62.18% on day 1 and from 53.77% to 63.01% on day 2 (Fig. 3). The DR% of E at 120 s from formulations Ea, Eb, and Ec ranged from 69.81% to 84.14% on day 1 and from 70.15% to 84.96% on day 2. Following dissolution testing of Nitrostat®, the DR% at 60 s was 82.70% and 80.95% for day 1 and day 2, respectively, and 105.06% and 103.89% at 120 s. No significant difference was found between the results from days 1 and 2 of any SL tablet formulation.
Fig. 3.
Percent of drug released (DR%) at 60 and 120 s from Ea, Eb, Ec, and Nitrostat® SL tablets on days 1 and 2. Data represent means±standard error of means (SEM) of at least three replicates. No significant differences (p > 0.05) were found between the adjacent bars
Assessment of the Discrimination Ability
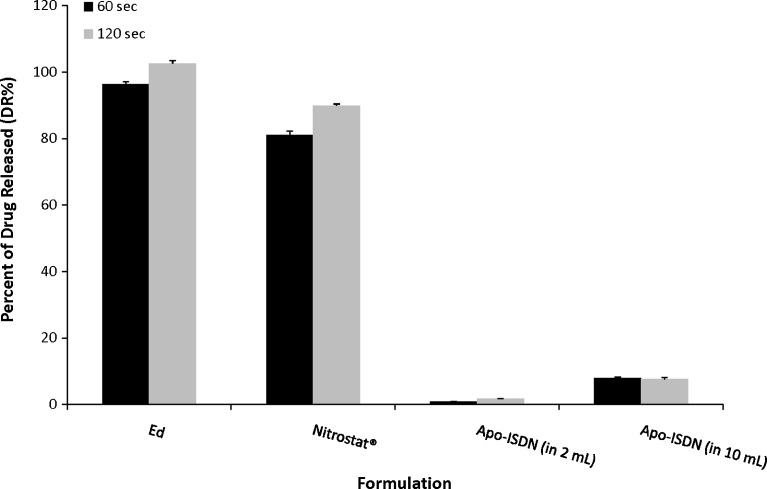
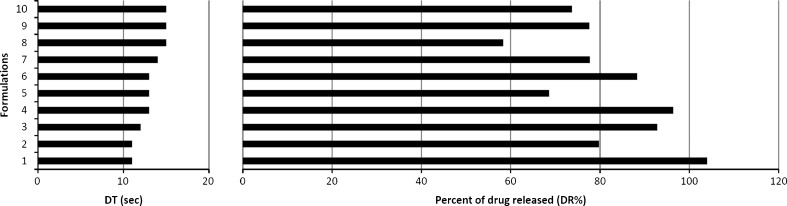
The DR% of E from Ed was 96.41 ± 0.75% at 60 s and 102.62 ± 0.86% at 120 s (Fig. 4). The DR% of nitroglycerin from Nitrostat® SL tablets was 75.1 ± 1.12% at 60 s and 89.94 ± 0.46% at 120 s. Only 0.90 ± 0.06% of Apo-ISDN was released after 60 s and 1.71 ± 0.14% after 120 s in 2 mL of dissolution medium. Compared to the DR% of ISDN from Apo-ISDN in 2 mL, the DR% in 10 mL increased significantly (p < 0.0001) to 8.04 ± 0.22% at 60 s and to 7.62 ± 0.57% at 120 s (Fig. 4). Ten representative formulations with similar DTs (11 to 15 s) were evaluated for dissolution at 60 s which resulted in a DR% ranging from 58% to 104% (Fig. 5).
Fig. 4.
Percent of drug released (DR%) at 60 and 120 s from Ed, Apo-ISDN, and Nitrostat® SL tablets. In addition, DR% from Apo-ISDN SL tablets was tested in 10 mL of dissolution medium. Data represent means ± standard error of means (SEM) of at least three replicates
Fig. 5.
A comparison between the disintegration times (DT) and corresponding percent of drug released in 60 s (DR%) of ten different E 40 mg SL tablet formulations
Dissolution Testing Using the Six-Unit Apparatus
Results obtained using the six-unit apparatus did not differ significantly (p > 0.05) from previous dissolution results of the same three E SL tablet formulations (Ee, Ef, and Eg) using the single-unit apparatus.
Dissolution Testing Using the Official USP Apparatus and Method
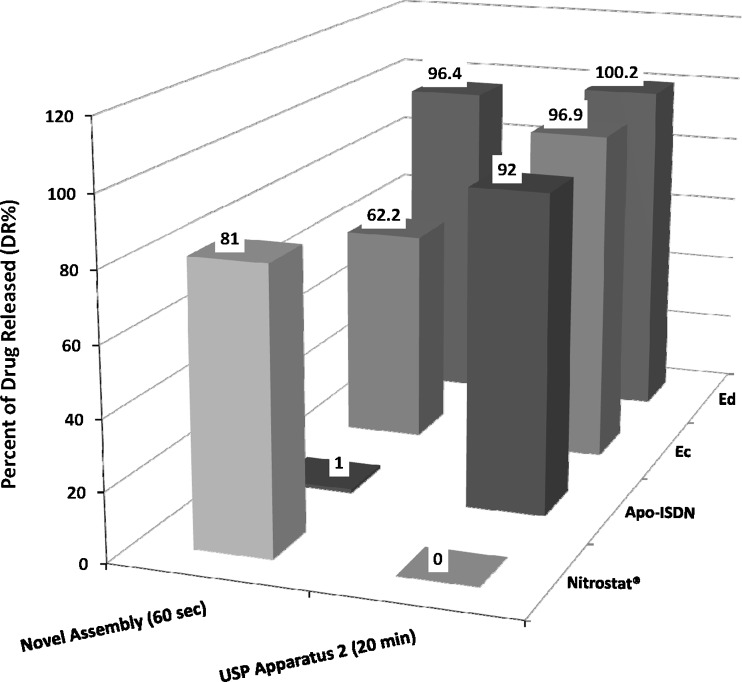
The DR% was >90% after 20 min for all Ec, Ed, and Apo-ISDN (Fig. 6). In contrast, DR% differed significantly (p < 0.05) between Ec, Ed, and Apo-ISDN (62.2%, 96.4%, and 1%, respectively) when dissolution was tested using the custom-made apparatus and the novel in vitro method. Using the official USP apparatus and method, the API released from Nitrostat® was not detected by UV, but was >80% after 60 s using the novel in vitro method.
Fig. 6.
Percent of drug released (DR%) from Ec, Ed, Apo-ISDN, and Nitrostat® SL tablets at 60 s using the custom-made apparatus and novel in vitro method, and at 20 min using the official USP apparatus 2 (Paddle) and method. Data represent means of at least three replicates
DISCUSSION
The official compendia apparatus and method for testing dissolution, and even disintegration (27), of SL tablets includes the use of large volumes of dissolution medium with constant agitation surrounding the tablets. These conditions do not simulate those in the SL cavity. More bio-relevant dissolution tests for SL tablets were proposed in the 1970s using methods based on in situ concentration measurement (28), but few advances have been reported in recent years. Due to the limited number of SL tablet formulations now commercially available, there is limited focus for dissolution testing of this dosage form. In contrast, buccal formulations have been studied extensively, leading to advances in dissolution apparatus, procedures, and techniques (29). Although both buccal and SL formulations are intended to be administered within the oral cavity, there are significant differences in design of these formulations which in turn lead to different physicochemical and release characteristics. It is “bio-irrelevant” to apply one single apparatus and method to test the API release of two or more different dosage form categories, i.e., oral versus SL tablets. Instead, the guidelines of the International Pharmaceutical Federation/American Association of Pharmaceutical Sciences recommend that “different apparatus should be employed on a case-by-case basis, and the method should be specific to the dosage form category, formulation type, or even to a particular, individual product” (30). The custom-made apparatus and the novel in vitro method proposed here were specifically designed to evaluate the dissolution of rapidly disintegrating SL tablets.
The dissolution testing consists of two main steps: the dissolution of the dosage form when the API is released and dissolved into the medium, and the measurement of the API content in the samples. These two steps are separated by a filtration step (15) which ensures that the sample for analysis contains only the API in solution, dissolved in the specified time period. The design of our apparatus was based on these concepts. The API was released and dissolved in the dissolution medium inside the glass funnel, and the samples were instantaneously collected at the specified time-point in the collecting tube following filtration to prevent the passage of undissolved API solid particles and to terminate dissolution.
The reusable parts that came into contact with the API including the glass funnel and the fritted glass base (Fig. 1) were thoroughly cleaned between tests. Any API remaining in the dissolution apparatus was totally removed prior to the next dissolution test in order to maintain the integrity of testing.
Five washings were sufficient to remove virtually 100% of any residual E left in the fritted glass base after the filtration of either low or high concentrations of standard E solutions. Similarly, the potential problem of minimal amounts of E that might have been adsorbed to the nylon filter membrane (31) was solved by replacing the membrane after each test to prevent carry-over to the next test. Other filter membrane materials (32) could be evaluated for possible reduction in the adsorption of API. The recovery of API could also be improved by discarding a partial fixed volume of filtrate before analysis. This step insures that the available active adsorption sites in the filter membrane are saturated and the subsequent filtration does not further decrease the API concentration in the filtrate (31).
The percent of drug released and dissolved increased with time from 15 to 60 s and was virtually 100% at 120 s for the representative E 40 mg SL tablet (Fig. 2). The source of variability among time-points could be partially due to variability in tablet content. Since individual SL tablets had to be used for each time-point as replicates, greater variability was expected due to content variability among tablets, which were always within the USP limits. However, use of the novel in vitro method resulted in reproducible dissolution data, with a coefficient of variation of 5.6% at 60 s and 3.2% at 120 s. Time-points ≥60 s were associated with less variability than those <60 s; consequently, the 60 and 120 s intervals were selected for dissolution testing of SL tablets.
Between-days reproducibility was achieved for both the commercial tablets and the E 40 mg SL tablets under development (Fig. 3). This ensured that the dissolution testing variability among different tablets within the test and among different test days resulted in acceptable consistency using the novel in vitro method.
Our custom-made apparatus required a different tablet to test dissolution at each time-point. Dissolution testing at a range of time-points is required only for modified release formulations and the demonstration of an API release profile over time (33). This apparatus is intended to evaluate SL tablets at the single time-point recommended for SL tablet administration (26). When assessing drug release from rapidly disintegrating SL tablets, the major objective is to ensure virtually total release of the API for absorption within 120 s or less after insertion into the SL cavity.
The sensitivity of the novel in vitro method to evaluate different SL tablet formulations was tested using both commercial tablets and representative E SL tablets under development (Fig. 4). It was shown that Apo-ISDN SL tablets released about 1% of the label content after 60 s and not more than 2% after 120 s. The low DR% of Apo-ISDN could result from a poor SL formulation as reported previously for one of the ISDN SL products of slow and/or incomplete disintegration resulting in lack of therapeutic effectiveness (34). The Apo-ISDN disintegration time was less than 10 s; therefore, the low DR% of Apo-ISDN was anticipated because of the low water solubility of ISDN. Subsequently, the volume of dissolution medium for Apo-ISDN was increased from 2 mL to 10 mL, a volume that is more than sufficient to dissolve the 5 mg dose of ISDN, but is still below “sink” conditions. Even in the presence of 10 mL of dissolution medium, only 8% (0.4 mg out of 5 mg) of ISDN was dissolved (Fig. 4) and would theoretically be available for absorption within the SL cavity. The remaining dose is swallowed and metabolized for ongoing activity via the ISDN metabolites, isosorbide mononitrates, following oral administration. The systemic availability of ISDN after SL and oral tablets was previously reported to be similar, based on plasma levels and area under the plasma concentration versus time curve of the ISDN metabolites (35). The only advantage of a SL tablet of ISDN therefore seems to be the rapid disintegration and fast onset of action of the initial 8% of the dose.
The novel in vitro method was also evaluated for its ability to discriminate among representative E 40 mg SL tablets which have similar in vitro DTs. In previous in vitro and in vivo studies, E SL tablet formulations with similar DTs had different bioavailabilities (18). Although the DT test ensures tablet breakdown into smaller particles, it does not evaluate the rate and extent of the API release, and in general, disintegration has been proved to be a poor indicator of bioavailability (36). The dissolution assessment is a more selective in vitro test than disintegration for predition of in vivo behavior. For tablets showing a narrow range of DTs (11–15 s), the novel in vitro method was sufficiently sensitive to identify a significant difference in DR% at 60 s ranging from 58% to 104% (Fig. 5). For example, formulations 4, 5, and 6 showed identical DT (13 s), but the novel in vitro method was able to detect formulation differences resulting in DR% that ranged from 69% to 96% after 60 s.
Using the official USP dissolution method, the concentrations of nitroglycerin could not be detected for Nitrostat® (nitroglycerin 0.6 mg), due to the sensitivity limit of the UV detector for a 0.6 mg in 900 mL dissolution medium (Fig. 6). In 900 mL, a minimum of 50 Nitrostat® SL tablets would be required for UV detection, which is impractical. This small-volume custom-made apparatus has the advantage of detecting low dose APIs in commercial SL tablets like Nitrostat®. Using the novel in vitro method, the quantitative levels of nitroglycerin from individual tablets were obtained that could be quantified by UV analysis.
With regard to Apo-ISDN (ISDN 5 mg), a procedure for testing a pooled sample of six tablets in the same dissolution vessel was followed by using the USP dissolution apparatus 2 (Paddle; 10). This resulted in 92% of the drug being released after 20 min, which does not necessarily represent the dissolution within 120 s in 2 mL of saliva in the SL cavity. Using the novel in vitro method, individual Apo-ISDN SL tablets were tested in replicate, and 1% of the drug was released at 60 s (Fig. 6). Since only 2 mL of dissolution medium was available for Apo-ISDN in the custom-made apparatus, ISDN solubility was considered to be the rate-limiting step in this process. To provide acceptable “sink” conditions for ISDN 5 mg, at least 15 mL of saliva should be available within the SL cavity which is larger than the normal physiological secretions of saliva in 2 min.
This small-volume custom-made apparatus offers the advantage of testing dissolution in volumes of dissolution medium as low as 2 mL, similar to the volume of saliva normally secreted over 2 min (5). After administration, the SL tablet is maintained in a relatively quiescent environment under the tongue as simulated in this apparatus. The lack of agitation of dissolution medium in our apparatus eliminates the problem of unstable hydrodynamics of small-volume dissolution apparatus, which is a major concern with USP Basket or Paddle apparatuses (14,37–39).
The current design of the apparatus only permits the operation of dissolution testing at room temperature (25°C), but it could be modified to provide the testing of physiological temperatures of 37°C. However, since SL tablets are only exposed to the 2 mL of dissolution medium for ≤120 s, the increased temperature effect on drug dissolution is anticipated to be minimal.
The multi-unit apparatus (Fig. 1b) facilitates testing the dissolution of six SL tablets simultaneously similar to the official USP dissolution apparatus. This novel in vitro method demonstrated day-to-day reproducibility and discrimination among formulations.
CONCLUSION
A novel in vitro method is proposed specifically for the assessment of dissolution of rapidly disintegrating SL tablet dosage forms. Data obtained were accurate, reproducible, and significantly different from the data obtained by using the USP method. The effects of minimal changes in formulations on tablet dissolution were readily detected and measured. This novel in vitro method is potentially useful for dissolution testing of rapidly disintegrating tablets using simulated SL conditions.
Acknowledgment
Ousama Rachid would like to acknowledge the financial support received from the Manitoba Health Research Council/Manitoba Institute of Child Health (MHRC/MICH) Studentship Award and the MPhA/William G. Eamer Graduate Scholarship.
References
- 1.Lefkowitz RJ, Hoffman BB, Taylor P. Neurotransmission: the autonomic and somatic motor nervous systems. In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, editors. Goodman & Gilman’s the pharmacological basis of therapeutics. 9. New York: McGraw-Hill Companies, Inc.; 1996. pp. 105–139. [Google Scholar]
- 2.Campisi G, Paderni C, Saccone R, Di Fede O, Wolff A, Giannola LI. Human buccal mucosa as an innovative site of drug delivery. Curr Pharm Des. 2010;16(6):641–652. doi: 10.2174/138161210790883778. [DOI] [PubMed] [Google Scholar]
- 3.Madhav NV, Shakya AK, Shakya P, Singh K. Orotransmucosal drug delivery systems: a review. J Control Release. 2009;140(1):2–11. doi: 10.1016/j.jconrel.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Zhang J, Streisand JB. Oral mucosal drug delivery: clinical pharmacokinetics and therapeutic applications. Clin Pharmacokinet. 2002;41(9):661–680. doi: 10.2165/00003088-200241090-00003. [DOI] [PubMed] [Google Scholar]
- 5.Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14(1):33–47. doi: 10.1111/j.1741-2358.1997.00033.x. [DOI] [PubMed] [Google Scholar]
- 6.USP 33/NF 28 Reissue . Chapter 711: dissolution. Rockville: Convention, Inc; 2010. p. R-76. [Google Scholar]
- 7.USP 32/NF 27 . Chapter 724: drug release. Rockville: Convention, Inc; 2009. p. 272. [Google Scholar]
- 8.Ph Eur . Chapter 2.9.3. Dissolution test for solid dosage forms. 6. Strasbourg: EMEA; 2008. p. 228. [Google Scholar]
- 9.JP . Chapter 6.10. Dissolution test. 15. Tokyo: Maruzen Company, Ltd; 2006. p. 116. [Google Scholar]
- 10.USP 32/NF 27 . Official monograph: isosorbide dinitrate sublingual tablets. Rockville: Convention, Inc; 2009. p. 2716. [Google Scholar]
- 11.Hunt JP, Shah VP, Prasad VK, Schuirmann DJ, Cabana BE. Dissolution profiles and specifications for dihydroergotoxine sublingual tablets using a new in vitro method. J Pharm Sci. 1981;70(7):796–798. doi: 10.1002/jps.2600700722. [DOI] [PubMed] [Google Scholar]
- 12.Das NGC, Das Sudip K, Surapaneni Madhu S. Dissolution testing of solid dosage forms intended to be administered in the oral cavity. US patent 7.331.251. 2006.
- 13.Klein S. The mini paddle apparatus—a useful tool in the early developmental stage? Experiences with immediate-release dosage forms. Dissolution Technology. 2006;13:6–11. [Google Scholar]
- 14.Klein S, Shah VP. A standardized mini paddle apparatus as an alternative to the standard paddle. AAPS PharmSciTech. 2008;9(4):1179–1184. doi: 10.1208/s12249-008-9161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crist GB. 2009 Trends in small-volume dissolution apparatus for low-dose compounds. Dissolution Technology. 2009;16:19–22. [Google Scholar]
- 16.Fortunato D. Dissolution method development for immediate release solid oral dosage forms. Dissolution Technology. 2005;12:12–14. [Google Scholar]
- 17.Rawas-Qalaji MM, Simons FER, Simons KJ. Sublingual epinephrine tablets versus intramuscular injection of epinephrine: dose equivalence for potential treatment of anaphylaxis. J Allergy Clin Immunol. 2006;117(2):398–403. doi: 10.1016/j.jaci.2005.12.1310. [DOI] [PubMed] [Google Scholar]
- 18.Rawas-Qalaji MM, Simons FER, Simons KJ. Epinephrine for the treatment of anaphylaxis: do all 40 mg sublingual epinephrine tablet formulations with similar in vitro characteristics have the same bioavailability? Biopharm Drug Dispos. 2006;27(9):427–435. doi: 10.1002/bdd.519. [DOI] [PubMed] [Google Scholar]
- 19.Rawas-Qalaji MM, Simons FER, Simons KJ. Fast-disintegrating sublingual tablets: effect of epinephrine load on tablet characteristics. AAPS PharmSciTech. 2006;7(2):E41. doi: 10.1208/pt070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawas-Qalaji MM, Simons FER, Simons KJ. Fast-disintegrating sublingual epinephrine tablets: effect of tablet dimensions on tablet characteristics. Drug Dev Ind Pharm. 2007;33(5):523–530. doi: 10.1080/03639040600897150. [DOI] [PubMed] [Google Scholar]
- 21.USP 32/NF 27 . Official monograph: epinephrine injection. Rockville: Convention, Inc; 2009. p. 2261. [Google Scholar]
- 22.Rawas-Qalaji MM, Simons FER, Collins D, Simons KJ. Long-term stability of epinephrine dispensed in unsealed syringes for the first-aid treatment of anaphylaxis. Ann Allergy Asthma Immunol. 2009;102(6):500–503. doi: 10.1016/S1081-1206(10)60124-X. [DOI] [PubMed] [Google Scholar]
- 23.USP 32/NF 27 . Official monograph: nitroglycerin sublingual tablets. Rockville: Convention, Inc; 2009. p. 3097. [Google Scholar]
- 24.Rachid O, Simons FER, Rawas-Qalaji M, Simons KJ. An electronic tongue: evaluation of the masking efficacy of sweetening and/or flavoring agents on the bitter taste of epinephrine. AAPS PharmSciTech. 2010;11(2):550–557. doi: 10.1208/s12249-010-9402-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.USP 33/NF 28 Reissue . Chapter 905: uniformity of dosage units. Rockville: Convention, Inc; 2010. p. R-86. [Google Scholar]
- 26.Repchinsky C. Compendium of pharmaceuticals and specialties: the Canadian drug reference for health professionals. 44. Toronto: Canadian Pharmacist Association; 2009. [Google Scholar]
- 27.Abdelbary G, Eouani C, Prinderre P, Joachim J, Reynier J, Piccerelle P. Determination of the in vitro disintegration profile of rapidly disintegrating tablets and correlation with oral disintegration. Int J Pharm. 2005;292(1–2):29–41. doi: 10.1016/j.ijpharm.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 28.Gaglia CA, Jr, Lomner JJ, Leonard BL, Chafetz L. Dissolution testing of nitroglycerin tablets. J Pharm Sci. 1976;65(11):1691–1692. doi: 10.1002/jps.2600651132. [DOI] [PubMed] [Google Scholar]
- 29.Azarmi S, Roa W, Löbenberg R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int J Pharm. 2007;328(1):12–21. doi: 10.1016/j.ijpharm.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Siewert M, Dressman J, Brown C, Shah V. FIP/AAPS guidelines to dissolution/in vitro release testing of novel/special dosage forms. AAPS PharmSciTech. 2003;4(1):E7. doi: 10.1208/pt040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiehm K, Dressman JB. Evaluation of drug adsorption to membrane filters under biowaiver test conditions. Dissolution Technology. 2008;15:13–17. [Google Scholar]
- 32.Lindenberg M, Wiegand C, Dressman JB. Comparison of the adsorption of several drugs to typical filter materials. Dissolution Technology. 2005;12:22–25. [Google Scholar]
- 33.Garbacz G, Blume H, Weitschies W. Investigation of the dissolution characteristics of nifedipine extended-release formulations using USP apparatus 2 and a novel dissolution apparatus. Dissolution Technology. 2009;16:7–13. [Google Scholar]
- 34.Weda M, van Riet-Nales DA, van Aalst P, de Kaste D, Lekkerkerker JF. Disintegration of sublingual tablets: proposal for a validated test method and acceptance criterion. Pharmeur Sci Notes. 2006;2006(2):41–44. [PubMed] [Google Scholar]
- 35.Straehl P, Galeazzi RL. Isosorbide dinitrate bioavailability, kinetics, and metabolism. Clin Pharmacol Ther. 1985;38(2):140–149. doi: 10.1038/clpt.1985.150. [DOI] [PubMed] [Google Scholar]
- 36.Kumar V. Dissolution. In: Hendrickson R, editor. Remington: the science and practice of pharmacy. 21. Baltimore: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 37.Scholz A, Kostewicz E, Abrahamsson B, Dressman JB. Can the USP paddle method be used to represent in-vivo hydrodynamics? J Pharm Pharmacol. 2003;55(4):443–451. doi: 10.1211/002235702946. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Kildsig DO, Ghaly ES. Effect of hydrodynamic environment on tablets dissolution rate. Pharm Dev Technol. 2004;9(1):25–37. doi: 10.1081/PDT-120027415. [DOI] [PubMed] [Google Scholar]
- 39.Brown CK, Buhse L, Friedel HD, Keitel S, Kraemer J, Morris JM, Stickelmeyer M, Yomota C, Shah VP. FIP position paper on qualification of paddle and basket dissolution apparatus. AAPS PharmSciTech. 2009;10(3):924–927. doi: 10.1208/s12249-009-9291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]