Abstract
Nicotine (NCT) buccal tablets consisting of sodium alginate (SA) and nicotine–magnesium aluminum silicate (NCT–MAS) complexes acting as drug carriers were prepared using the direct compression method. The effects of the preparation pH levels of the NCT–MAS complexes and the complex/SA ratios on NCT release, permeation across mucosa, and mucoadhesive properties of the tablets were investigated. The NCT–MAS complex-loaded SA tablets had good physical properties and zero-order release kinetics of NCT, which indicate a swelling/erosion-controlled release mechanism. Measurement of unidirectional NCT release and permeation across porcine esophageal mucosa using a modified USP dissolution apparatus 2 showed that NCT delivery was controlled by the swollen gel matrix of the tablets. This matrix, which controlled drug diffusion, resulted from the molecular interactions of SA and MAS. Tablets containing the NCT–MAS complexes prepared at pH 9 showed remarkably higher NCT permeation rates than those containing the complexes prepared at acidic and neutral pH levels. Larger amounts of SA in the tablets decreased NCT release and permeation rates. Additionally, the presence of SA could enhance the mucoadhesive properties of the tablets. These findings suggest that SA plays the important role not only in controlling release and permeation of NCT but also for enhancing the mucoadhesive properties of the NCT–MAS complex-loaded SA tablets, and these tablets demonstrate a promising buccal delivery system for NCT.
Key words: buccal tablets, magnesium aluminum silicate, nicotine, release and permeation, sodium alginate
INTRODUCTION
Nicotine (NCT), obtained from tobacco plants, is a volatile and strongly alkaline liquid. NCT is highly soluble not only in water but also in hydrophobic solvents (1). It has well-separated pKa values; pKa1 and pKa2 are 3.04 and 7.84, respectively (2). This leads to the formation of diprotonated, monoproptonated, and neutral NCT at acidic, neutral, and basic pH levels, respectively. NCT has been widely used in smoking cessation therapy for relieving addiction symptoms. NCT is absorbed through skin and mucosal membranes, such as buccal and nasal membranes (2). The free base form of NCT is volatile and susceptible to oxidative degradation. Several researchers have sought an NCT-adsorbing material to prevent the evaporation and improve stability. NCT-adsorbent complexes are employed to carry the drug in powdered form. Cellulose powders were employed as such adsorbent complexes, but in water, NCT adsorbed to these complexes was observed to quickly and completely dissociate within 1 h (3). In addition, adsorption onto a cation exchange resin was employed to deliver NCT both in a chewing gum (4) and in powder formulations intended for nasal delivery (5).
Magnesium aluminum silicate (MAS) is a mixture of montmorillonite and saponite clays (6), both of which have silicate layer structures. Each layer comprised tetrahedrally coordinated silica atoms fused into an edge-shared octahedral plane, with either aluminum hydroxide or magnesium hydroxide (6,7). The silicate layers of MAS have weakly positively charged edges and negatively charged surfaces. The negatively charged surfaces of the silicate layers strongly interact with NCT at different pH levels (8), leading to the formation of NCT–MAS complexes. This allows NCT to intercalate into the silicate layers of MAS that was investigated using a powder X-ray diffractometry in the previous study (9). The NCT–MAS complex particles enhance NCT’s thermal stability and were shown to sustain NCT release in distilled water and phosphate buffer with a pH 6 (9). Additionally, MAS forms a microcomposite material with sodium alginate (SA), a negatively charged polysaccharide, via intermolecular hydrogen bonding (10,11). SA is a sodium salt of alginic acid, a linear polysaccharide found in marine brown algae. SA has been widely used as a food and pharmaceutical additive as well as a tablet disintegrant and gelling agent. Additionally, it has been employed as a bioadhesive material and drug release modifier for intraoral drug delivery dosage forms such as tablets (12–16) and films (17).
Buccal drug delivery offers many advantages in comparison to oral delivery. For example, buccal delivery allows drugs to avoid first-pass hepatic metabolism, leading to higher bioavailability and facilitating drug withdrawal (18). NCT is a candidate for buccal delivery due to its low bioavailability after oral administration (1) and its ability to permeate across the buccal mucosa (2,19,20). For these reasons, buccal tablets using NCT hydrogen tartrate, a salt form of NCT with high solubility in water, were previously developed (16,21). Thus, it is interesting that usage of NCT–MAS complex particles as drug carriers in SA tablets offers several advantages. First, SA tablets would offer the advantageous physical properties of tablets. Furthermore, interaction of MAS with SA in the swollen gel matrix tablets may aid in controlling NCT release and permeation across the mucosa. Finally, SA may improve the mucoadhesive properties of the tablets for buccal delivery.
The objective of this study was to prepare and characterize NCT–MAS complex-loaded SA tablets for buccal delivery of NCT. The effects of complex preparation pH and the complex/SA ratio on the properties of the complex-loaded SA tablets were investigated. The tablets were prepared using a direct compression method. The NCT release kinetics and mucoadhesive properties of the tablets were investigated. Unidirectional NCT release and permeation was measured using a modified USP dissolution apparatus 2; this is the first report for the use of such an experimental setup with buccal tablets. In addition, molecular interaction of SA with MAS in the swollen gel matrix was examined using Fourier transform infrared (FTIR) spectroscopy.
MATERIALS AND METHODS
Materials
MAS (Veegum®HV) and NCT were obtained from R.T. Vanderbilt Company, Inc. (Norwalk, CT, USA) and Fluka (Buchs, Switzerland), respectively. SA (Manugel®DMF) was obtained from ISP Thailand Ltd. (Bangkok, Thailand). Magnesium stearate (Mallinckrodt Inc., St Louis, MO) was used as a lubricant for tabletting. All other reagents used were of analytical grade and were used as received.
Preparation of NCT–MAS Complexes
A 4% (w/v) MAS suspension was prepared using hot water and was cooled to room temperature prior to use. An NCT solution (2.0%, w/v) was prepared using deionized water as solvent. Fifty milliliters of the 4% (w/v) MAS suspension was then mixed with 50 ml of the 2% (w/v) NCT solution in an Erlenmeyer flask. The pH levels of all NCT–MAS dispersions were adjusted by adding small amounts of 1 M HCl or 1 M NaOH into the flask while swirling until the final pH of the dispersions was either at 4, 7, or 9, as measured with a pH meter (WalkLAB TI9000, Singapore). To achieve equilibrium of NCT adsorption onto MAS, the dispersions were then incubated with shaking at 37°C for 24 h (8). Following incubation, the NCT–MAS complexes were separated from the filtrates by filtration, washed twice using 20 ml of deionized water, and dried at 50°C for 24 h. The dry NCT–MAS complexes were ground using a mortar and pestle, sieved through a 180-μm sieve, and stored in a desiccator.
Characterization of the NCT–MAS Complexes
Determination of NCT Content
Twenty-five milligrams of the NCT–MAS complexes were weighed and dispersed in 100 ml of 2 M HCl for 12 h. The supernatant was then collected and filtered and its NCT content analyzed using a UV–visible spectrophotometer (Shimadzu UV1201, Kyoto, Japan) at a wavelength of 259 nm.
Particle Size Determination
The particle sizes of the NCT–MAS complexes prepared at different pH levels were measured using a laser diffraction particle size analyzer (Mastersizer2000 Model Hydro2000SM, Malvern Instrument Ltd., UK). The samples were dispersed in 70 ml of pH 6 phosphate buffer in a small volume sample dispersion unit and stirred at a rate of 50 Hz for 30 s before the measurement. The particle sizes (volume-weighted mean diameter) were reported.
In Vitro Release Studies
A USP dissolution apparatus 1 (basket method, VanKel VK200, USA) was used to characterize the release of NCT from the complexes. In each case, the amount of NCT–MAS complex used was sufficient to contain 15 mg of NCT. The complex particles were placed into the basket. The bottom of the basket was coated with a 0.45-μm cellulose acetate membrane to retain the complex particles during the test. The studies were performed in 500 ml of phosphate buffer, pH 6, at 37.0 ± 0.5°C and a rotation speed of 50 rpm. Samples (7 ml) were collected and replaced with fresh medium at various time intervals. The amount of NCT released was analyzed using a UV–visible spectrophotometer (Shimadzu UV1201) at a wavelength of 259 nm.
Preparation of NCT–MAS Complex-Loaded SA Tablets
All tablets were prepared using a direct compression method. The tablets consisted of NCT–MAS complexes, SA, and magnesium stearate. The effects of the complexes’ preparation pH levels and the complex/SA ratios on the characteristics of the tablets were investigated. The tablet components are listed in Tables I and II. The NCT–MAS complexes were mixed with SA in a rotomixer for 3 min; magnesium stearate was then blended with the mixture for 1 min before tabletting. Tablets were prepared by placing each 202-mg mix into 10-mm flat-faced punches and dies and then applying 23 MPa with a hydrostatic press (model 3126, Shimadzu) without holding time. The tablets obtained were stored in a desiccator until the measurements.
Table I.
Component of NCT–MAS Complex-loaded SA Tablets Using Different Preparation pH Levels of Complexes
| Component | Preparation pH of complexes | ||
|---|---|---|---|
| pH 4 | pH 7 | pH 9 | |
| NCT–MAS complexes (mg) (equivalent to 15 mg NCT) | 166.0 | 129.0 | 120.0 |
| SA (mg) | 34.0 | 71.0 | 80.0 |
| Magnesium stearate (mg) | 2.0 | 2.0 | 2.0 |
| Complex/SA ratio | 1:0.20 | 1:0.55 | 1:0.67 |
Table II.
Component of NCT–MAS Complex-Loaded SA Tablets Using Different Complex/SA Ratios
| Component | Complex/SA ratio | |||
|---|---|---|---|---|
| 1:4 | 1:1.5 | 1:0.67 | 1:0 | |
| NCT–MAS complexes prepared at pH 9 (mg) | 40.0 | 80.0 | 120.0 | 200.0 |
| SA (mg) | 160.0 | 120.0 | 80.0 | 0.0 |
| Magnesium stearate (mg) | 2.0 | 2.0 | 2.0 | 2.0 |
| Amount of NCT (mg) | 5 | 10 | 15 | 26.3 |
Characterization of NCT–MAS Complex-Loaded SA Tablets
Thickness and Hardness
The thicknesses of the tablets were measured using a Vernier caliper (Mitutoyo, Japan). The hardness of the tablets was measured with a Stokes tablet hardness tester.
In Vitro Release Studies
NCT release from the NCT–MAS complex-loaded SA tablets was studied using two apparatus. NCT release from the whole tablets was studied using a USP dissolution apparatus 1 (basket method, VanKel VK200). The tablets were placed into the basket with a rotation speed of 50 rpm. The release medium was 500 ml of pH 6 phosphate buffer at 37.0 ± 0.5°C. Samples (7 ml) were collected and replaced with fresh medium at various time intervals. The amount of NCT released was analyzed using a UV–visible spectrophotometer (Shimadzu UV1201) at a wavelength of 259 nm.
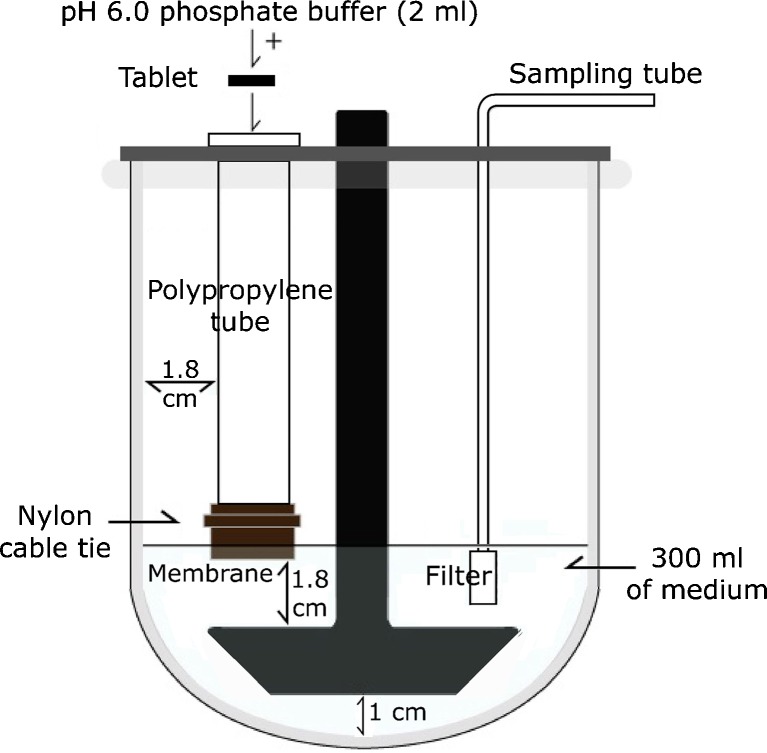
Unidirectional release of NCT from the tablets was characterized using a modified USP dissolution apparatus 2, shown in Fig. 1. A 0.45-μm cellulose acetate membrane which had been hydrated in pH 6 phosphate buffer for 12 h was tightly attached at the lowest point of a polypropylene tube (inner diameter = 1.8 cm) using a nylon cable tie. This tube was vertically placed in a dissolution vessel containing 300 ml of pH 6 phosphate buffer at 37.0 ± 0.5°C. The tube position was adjusted so that the membrane was wetted and in contact with the medium. The distance between the paddle and vessel bottom was set to 1 cm, and the rotation speed of the paddle was set to 50 rpm. The tablets were placed in the tube and wetted using 2 ml of phosphate buffer, pH 6. Samples (7 ml) were collected and replaced with fresh medium at various time intervals. The amount of NCT released was quantified with high-performance liquid chromatography (HPLC).
Fig. 1.
Schematic presentation of the modified USP dissolution apparatus 2 for characterizing unidirectional NCT release and permeation of the buccal tablets
In Vitro Permeation Studies
NCT permeation of the tablets was also performed using a modified USP dissolution apparatus 2 (Fig. 1). Porcine esophageal mucosa was employed in this study because it has a lipid composition similar to porcine buccal mucosa, but a simpler preparation method (22). Esophageal mucosa of crossbred pig (hybrid kinds of Duroc Jersey–Landrace–Large White) with 80- to 100-kg weight was obtained from a local slaughterhouse (Non Muang Village, Khon Kaen, Thailand). The porcine esophageal tube was opened longitudinally and immersed in 0.9% sodium chloride at 60°C for 1 min (22,23). The epithelium was then peeled away from the connective tissue and stored at −20°C. The frozen mucosal membranes were brought to room temperature by immersion in pH 7.4 isotonic phosphate buffer for 15 min. The mucosal membrane was then mounted and tightly attached to the end of a polypropylene tube. The dissolution vessel contained 300 ml of pH 7.4 isotonic phosphate buffer at 37.0 ± 0.5°C; the methods and experimental conditions were the same as the previous release study.
Analysis of Release and Permeation Data
The mechanisms of NCT release were determined both with a semi-empirical equation and a power law (24,25), shown in Eqs. 1 and 2, respectively, as follows:
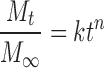 |
1 |
and
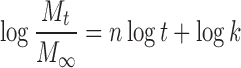 |
2 |
where Mt/M∞ is the fractional NCT release at time t, k is the kinetic constant, and n is the release exponent indicative of the drug release mechanism. A release exponent of n = 0.5 indicates a diffusion-controlled drug release (Fickian diffusion), whereas a release exponent of n = 1 corresponds to a polymer swelling/erosion-controlled release mechanism. Thus, release exponents between these two extreme values indicate a so-called anomalous transport—a complex transport mechanism that is a mixture of both drug diffusion and swelling/erosion of polymer (26).
The NCT release and permeation rates of the tablets were analyzed using both zero-order and Higuchi models (27), which can be expressed as Eqs. 3 and 4, respectively, as follows:
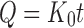 |
3 |
and
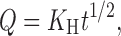 |
4 |
where Q is the amount of NCT released, t is time, and K0 and KH are the zero-order and Higuchi release rates, respectively.
Determination of Mucoadhesive Properties
The mucoadhesive properties of the tablets were measured using a texture analyzer (TA.XT plus, Stable Micro Systems, UK) with a 50-N load cell equipped with a bioadhesive test rig.
The tablet was attached to a 10-mm diameter cylindrical probe using a two-sided adhesive tape. Esophageal mucosa of pig was also obtained from a local slaughterhouse (Non Muang Village, Khon Kaen, Thailand). The mucosal membrane from the porcine esophagus (about 2 × 2 cm) without heat treatment and elimination of the connective tissue that had been hydrated with pH 7.4 isotonic phosphate buffer for 20 min was placed on the stage of bioadhesive holder and gently blotted with tissue paper to remove excess water on the surface of the mucosal membrane. Next, 200 μl of pH 6 phosphate buffer was pipetted onto the membrane surface before testing. The probe and attached tablets were moved down at a constant speed of 1 mm s−1 with 0.5-N contact force and 2-min contact time. Immediately afterwards, the probe was moved upwards with a constant speed of 0.5 mm s−1. The relationship between the force and tablet displacement was plotted. The maximum detachment force (Fmax) and work of adhesion (Wad, the area under the force versus distance curve) were calculated using the Texture Exponent 32 program version 4.0.9.0 (Stable Micro Systems).
HPLC Condition for NCT Analysis
The concentration of NCT was determined using HPLC (Perkin Elmer Series, USA). Reversed-phase HPLC using a C-18 column (Waters Spherisorb® S5 ODS2, 5-μm particle size, 4.6 × 250 mm, Ireland) connected with a guard column was employed. The mobile phase was 0.05 M sodium acetate/methanol/triethylamine (88:12:0.5, v/v), and the final pH was adjusted to 4.2 with glacial acetic acid. The flow rate of the mobile phase was 1 ml min−1, and the detector was a UV–visible detector at a wavelength of 259 nm. The retention time of NCT was approximately 7.0 min. Under these conditions, good linearity and reproducibility were shown over the range 1.0–100.0 μg ml−1 NCT.
FTIR Spectroscopy
The molecular interactions between SA and MAS in the tablets were investigated using an FTIR spectrophotometer (Spectrum One, Perkin Elmer, Norwalk, CT) and the KBr disc method. The residual mass of the swollen gel matrix tablets on the cellulose acetate membrane after the NCT release testing with the modified USP dissolution apparatus 2 (“In Vitro Release Studies”) was collected, dried at 50°C, and gently ground with a mortar and pestle. Each sample was pulverized, gently triturated with KBr powder in a weight ratio of 1:100, and then pressed in a hydrostatic press at a pressure of 10 tons for 5 min. The disc was placed in the sample holder and scanned from 4,000 to 450 cm−1 at a resolution of 4 cm−1.
RESULTS AND DISCUSSION
Characteristics of NCT–MAS Complexes
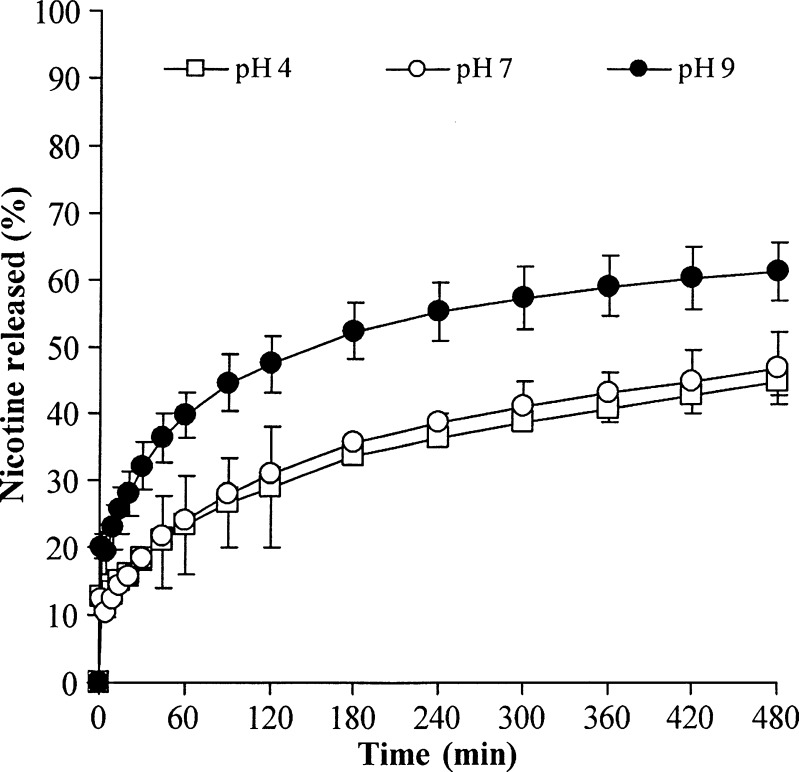
Due to the molecular interactions between NCT and MAS via electrostatic force and hydrogen bonding (9), NCT–MAS complexes were formed and successfully prepared at pH 4, 7, and 9 using an adsorption method. The sizes of the NCT–MAS complex particles obtained fell into the range of 82.1–94.0 μm (Table III). The NCT content in the NCT–MAS complexes increased with increasing preparation pH (Table III). This was due to the denser matrix structure of the NCT–MAS complexes formed at acidic and neutral pH levels (8), resulting in a reduction of surface area for NCT adsorption. The NCT release profile of the complexes at different preparation pH levels is presented in Fig. 2. The NCT release percentage showed good agreement with the Higuchi model (R2 higher than 0.99). This indicates that the NCT release kinetics from the complex particles is controlled by a matrix/particle diffusion mechanism (9). The complexes prepared at pH 9 gave a higher NCT release rate than those prepared at neutral and acidic pH levels (Table III). It is possible that the higher NCT content of the complexes prepared at pH 9 brought about a greater NCT concentration gradient in the complex particles, leading to faster NCT release. Moreover, the denser matrix formation of the complexes at pH 4 and 7 led to a lower NCT release rate than that of the complexes at pH 9.
Table III.
Characteristics of NCT–MAS Complexes Prepared at Different pH Levels
| NCT–MAS complexes | Particle size (μm) | NCT content (% w/w) | NCT release rate (% min−1/2) |
|---|---|---|---|
| pH 4 | 94.0 ± 2.6 | 9.50 ± 0.03 | 1.84 ± 0.10 (R 2 = 0.993) |
| pH 7 | 82.1 ± 2.4 | 12.20 ± 0.02 | 2.22 ± 0.06 (R 2 = 0.993) |
| pH 9 | 93.2 ± 1.8 | 13.20 ± 0.04 | 3.54 ± 0.08 (R 2 = 0.991) |
Data are the mean ± SD, n = 3
Fig. 2.
NCT release profiles of the NCT–MAS complex particles prepared at different pH levels. Each point is the mean ± SD, n = 3
Physical Properties of NCT–MAS Complex-Loaded SA Tablets
All mixtures prepared were easily compressed into tablets using a direct compression method. The thicknesses of the NCT–MAS complex-loaded SA tablets were in the range of 1.46–1.71 mm and are listed in Table IV. The tablets thus obtained were acceptable upon visual inspection, and acceptable hardness ranges from 39.2 to 70.6 N. Moreover, the hardness of pure SA and MAS tablets was found to be 14.7 and 36.3 N, respectively, suggesting that both materials have acceptable compressibility under pressure. Additionally, it was observed that pure MAS tablets had lower hardness than the pH 9 NCT–MAS complex tablets (1:0 complex/SA ratio), suggesting that the NCT adsorbed onto MAS may alter the surface interfacial properties of the MAS particles. This may lead to higher cohesive force at the contact surface after deformation of the NCT–MAS complex particles under compression.
Table IV.
Physical Properties and NCT Release Characteristics of NCT–MAS Complex-Loaded SA Tablets
| Tablets | Thickness (mm) | Hardness (N) | NCT release | |
|---|---|---|---|---|
| Release exponent, n | Release rate (% min−1) | |||
| Preparation pH of complexes | ||||
| pH 4 | 1.46 ± 0.02 | 70.6 ± 2.7 | 1.05 ± 0.07 (R 2 = 0.992) | 0.14 ± 0.01 (R 2 = 0.962) |
| pH 7 | 1.58 ± 0.02 | 58.8 ± 0.1 | 0.92 ± 0.05 (R 2 = 0.990) | 0.15 ± 0.02 (R 2 = 0.980) |
| pH 9 | 1.61 ± 0.01 | 60.8 ± 2.7 | 0.98 ± 0.07 (R 2 = 0.992) | 0.15 ± 0.03 (R 2 = 0.997) |
| Complex/SA ratio | ||||
| 1:4 | 1.71 ± 0.02 | 48.8 ± 3.2 | 0.77 ± 0.03 (R 2 = 0.992) | 0.14 ± 0.02 (R 2 = 0.996) |
| 1:1.5 | 1.69 ± 0.02 | 39.2 ± 0.1 | 0.85 ± 0.04 (R 2 = 0.992) | 0.12 ± 0.01 (R 2 = 0.999) |
| 1:0.67 | 1.61 ± 0.01 | 60.8 ± 2.7 | 0.98 ± 0.07 (R 2 = 0.992) | 0.15 ± 0.03 (R 2 = 0.997) |
| 1:0 | 1.42 ± 0.01 | 68.6 ± 0.1 | 0.46 ± 0.01 (R 2 = 0.972) | 3.20 ± 0.08a (R 2 = 0.988) |
| Pure SA | 1.87 ± 0.02 | 14.7 ± 3.5 | ND | ND |
| Pure MAS | 1.31 ± 0.01 | 36.3 ± 4.4 | ND | ND |
Data are the mean ± SD, n = 3
ND not determined
aCalculated from Higuchi model (unit, % min−1/2)
NCT Release Characteristics of the Tablets
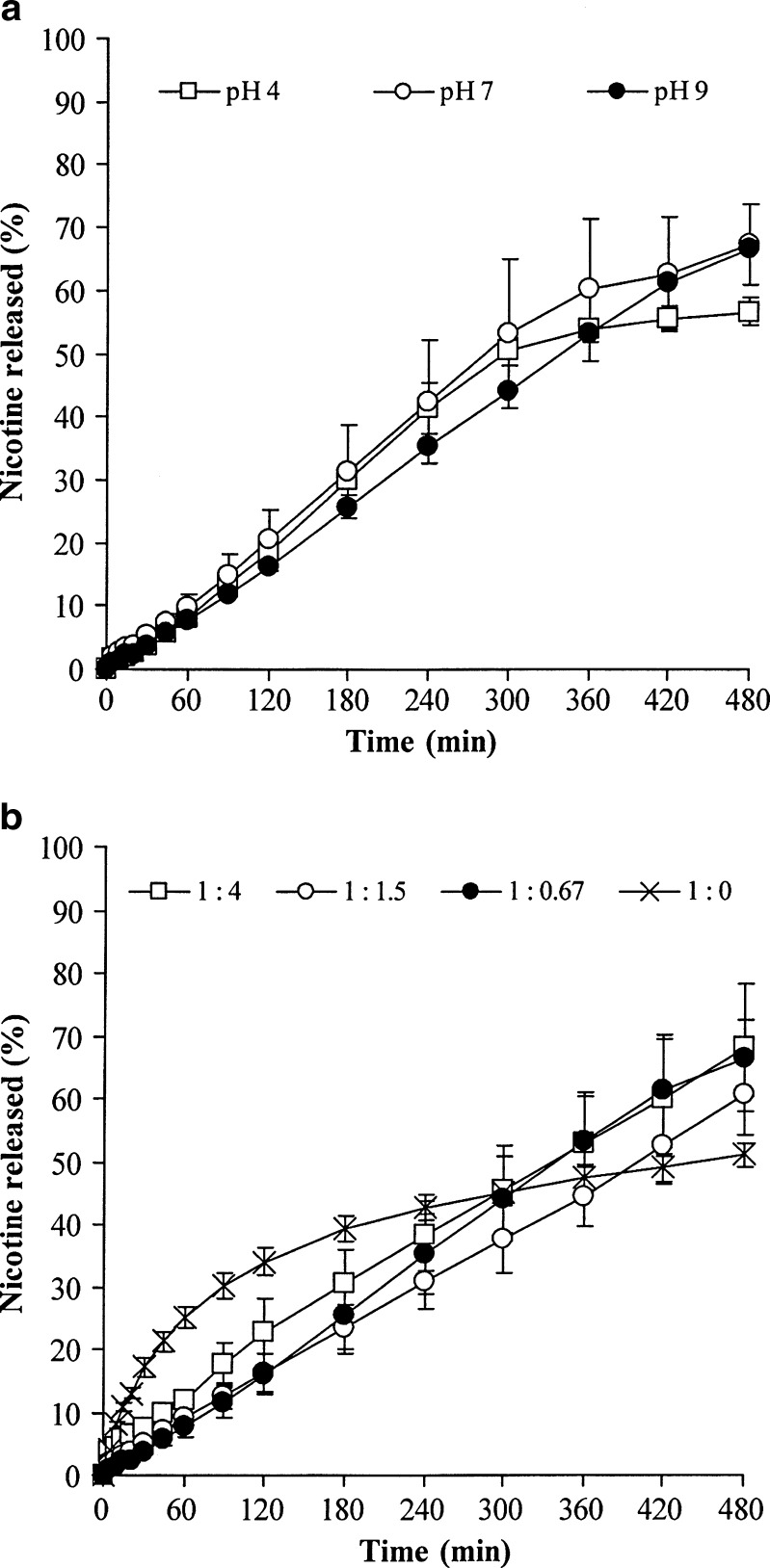
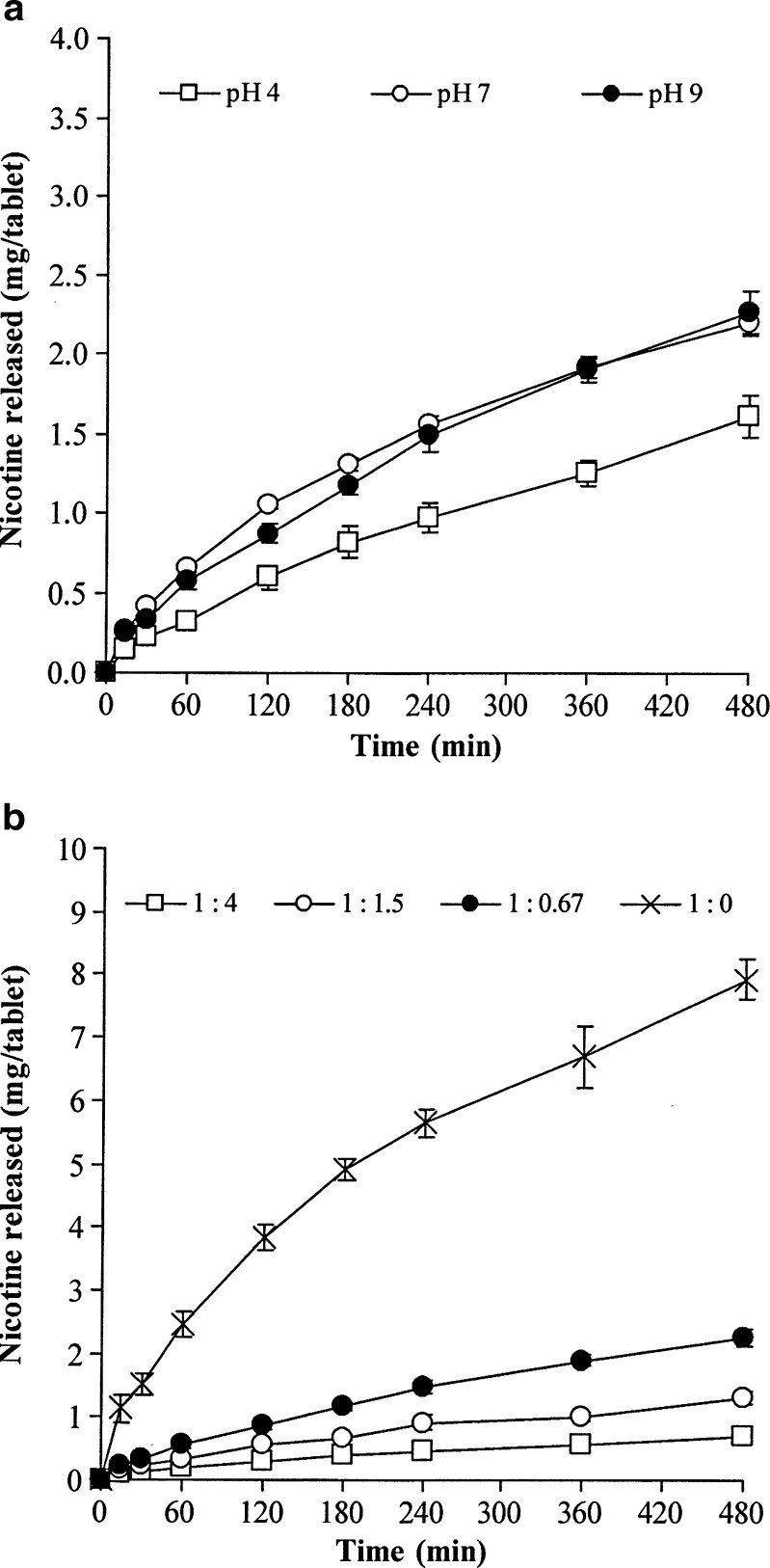
The NCT release profiles of the whole tablets in pH 6 phosphate buffer are shown in Fig. 3. The measured release of NCT from the NCT–MAS complex-loaded SA tablets (Table IV) fits well with the power law (Eq. 2; R2 more than 0.99). The exponent n of the NCT–MAS complex-loaded SA tablets at all preparation pH levels was close to unity. This suggests that the NCT release was controlled by a polymer swelling/erosion mechanism and can be described using zero-order release kinetics. Additionally, increasing the SA ratio in the tablets led to a lower exponent value, particularly in the 1:4 and 1:1.5 complex/SA ratios. This indicates that the NCT release involved both a diffusion process through the swollen matrix and a swelling/erosion process of the SA. Moreover, the release process of the pH 9 NCT–MAS complex tablets without SA (1:0 complex/SA ratio) had an exponent value of 0.46, the lowest that was found, suggesting that the NCT release was Fickian diffusion. Apart from NCT release mechanism, the NCT release rate of the whole tablets was calculated using the zero-order model (Eq. 3), as shown in Table IV. It was observed that the effect of preparation pH and the complex/SA ratio did not influence the NCT release rate. In contrast, the NCT release rate of the NCT–MAS complex tablets were well described by the Higuchi model, but not the zero-order model. The release rate of NCT from the tablets was lower than that of the complex particles (Table III) because of complex tablets’ lower surface area for NCT release.
Fig. 3.
NCT release profiles of the NCT–MAS complex-loaded SA tablets prepared using different preparation pH levels of complexes (a) and various complex/SA ratios (b). Each point is the mean ± SD, n = 3
In this study, the observed NCT release from the NCT–MAS complex tablets without SA indicates a matrix/particle diffusion-controlled mechanism. Incorporation of a small amount of SA into the tablets could control the NCT release to achieve the zero-order release kinetics that was clearly observed in the tablets containing the complexes prepared at different pH levels. This indicates that the NCT release was controlled not only by matrix diffusion of the complex particles but also by the swollen gel matrix of SA formed around the tablets. However, the higher ratio of SA in the tablets caused the NCT release to be dominated by a matrix diffusion mechanism. We hypothesize that the greater amount of SA in the tablets may lead to higher viscosity and thickness in the swollen gel matrix during NCT release. Furthermore, it is interesting that all NCT–MAS complex-loaded SA tablets measured had a similar NCT release rate despite very different complex/SA ratios. It is possible that the swollen gel matrix may have changed during NCT release because of interactions between SA with MAS. We therefore investigated the molecular interactions of the tablet’s components after drug release testing using FTIR spectroscopy.
Molecular Interaction of SA with MAS
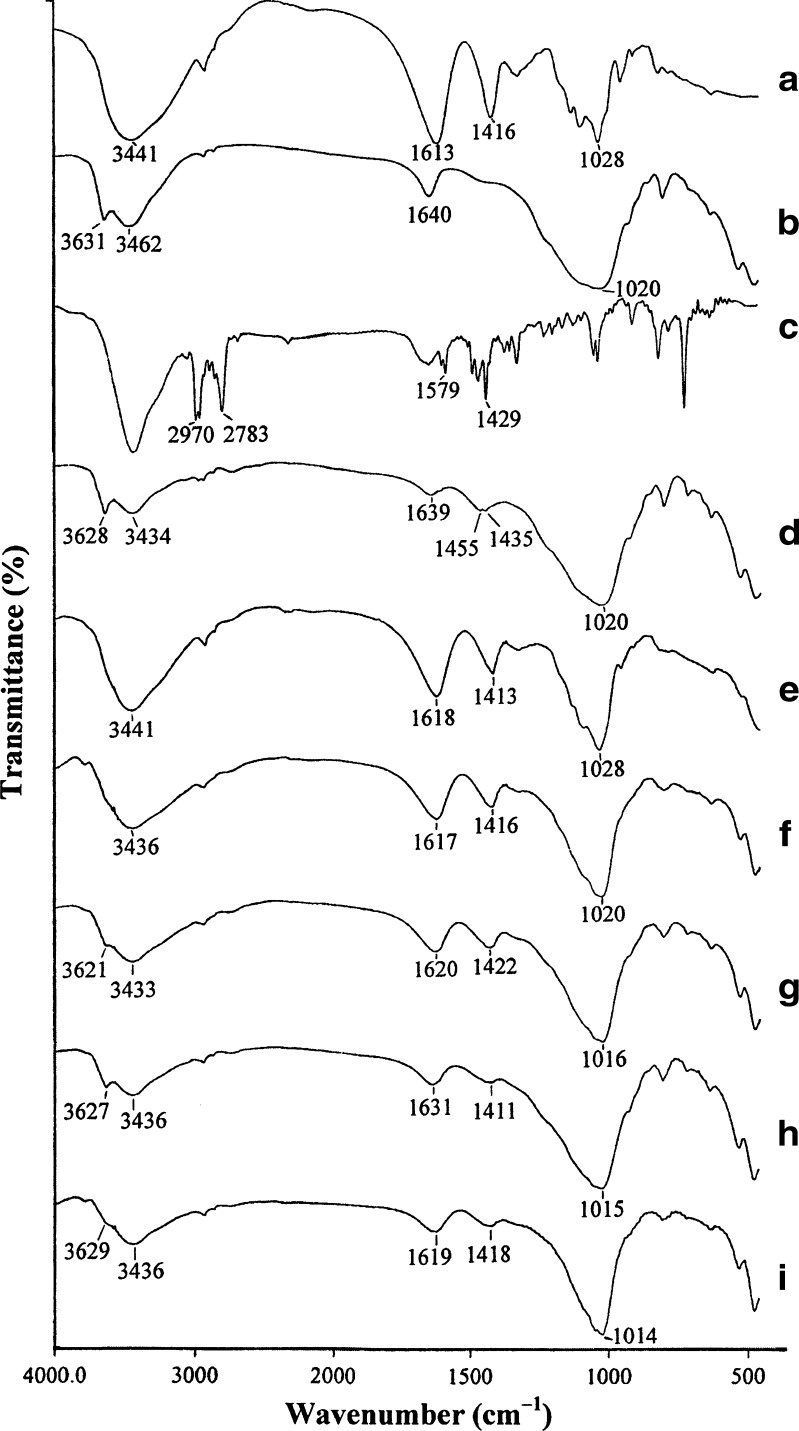
The molecular interaction of SA with MAS in the swollen gel matrix was investigated in this study. SA showed stretching peaks from OH, COO− (symmetric), and COO− (asymmetric) at 3,441, 1,613, and 1,416 cm−1, respectively (Fig. 4a). Figure 4b shows the peaks of MAS, such as hydroxyl stretching in SiOH (3,631 cm−1), hydroxyl stretching in hydrogen-bonded water (3,462 cm−1), hydroxyl bending (1,640 cm−1), and stretching of Si–O–Si (1,020 cm−1) (28). In the FTIR spectra of the pH 9 NCT–MAS complex tablets (Fig. 4d), a shift of the hydroxyl stretching peak of water hydrogen bonded to MAS to a lower wavenumber (3,462 to 3,434 cm−1) was observed. A shift of the hydroxyl stretching peak of SiOH from 3,631 to 3,628 cm−1 was also observed. Moreover, the C–H bending on the pyridine ring of NCT was of low intensity and shifted from 1,429 cm−1 (Fig. 4c) to a higher wavenumber of 1,435 cm−1 (Fig. 4d). These results suggest that the amine group of the pyridine ring could interact with MAS via electrostatic forces and intermolecular hydrogen bonding (9). FTIR spectra of the pH 9 NCT–MAS complex-loaded SA tablets showed a shift of the SA COO− stretching peaks (Fig. 4e–g) to a higher wavenumber, suggesting that the negative charge of the carboxyl groups of SA interacted electrostatically with the positively charged sites in the edges of MAS and could also create intermolecular hydrogen bonding with MAS silanol groups (11). Furthermore, the shift of the MAS Si–O–Si stretching peak to a lower wavenumber (from 1,020 to 1,016 cm−1) could indicate intermolecular hydrogen bonding between SA and MAS. The pH 4 and 7 NCT–MAS complex-loaded SA tablets (Fig. 4h, i, respectively) also had spectra similar to those loaded with the pH 9 complexes. Unfortunately, the interaction of SA with NCT could not be clearly examined in this study. This may be due to a small amount of NCT remaining in the swollen gel matrix tablets. However, it is possible that NCT and SA could interact electrostatically because of their opposite charges, as well as through previously reported intermolecular hydrogen bonding (29). These findings indicate that SA can interact with MAS in the swollen gel matrix tablets, resulting in a denser matrix formation, thus modifying NCT release from the tablets. This was the reason describing why a small amount of SA could control the NCT release to achieve zero-order release kinetics. Additionally, the interaction of NCT with SA in the swollen gel matrix led to NCT release that was mainly controlled by swelling and erosion of the tablets.
Fig. 4.
FTIR spectra of SA tablet (a), MAS tablet (b), NCT (c), pH 9 NCT–MAS complex tablet (d), pH 9 NCT–MAS complex-loaded SA tablets using 1:4 (e), 1:1.5 (f) and 1:0.67 (g)complex/SA ratios, and NCT–MAS complex-loaded SA tablets using pH 4 (h) and pH 7 (i) complexes
Unidirectional Release and Permeation of NCT
Studies of the unidirectional NCT release and permeation of the NCT–MAS complex-loaded SA tablets were performed using 0.45-μm cellulose acetate membrane and porcine esophageal membrane, respectively. The USP dissolution apparatus 2 was modified for measuring the NCT release and permeation of the buccal tablets. The advantages of this modified apparatus were its convenience of operation, high stirring efficiency, and large medium volume for maintaining the sink condition. The NCT release profiles of the NCT–MAS complex-loaded SA tablets from this study are shown in Fig. 5. The NCT release data fit well with the power law equation (Eq. 2), with R2 >0.99 (Table V). The exponent obtained was in the range of 0.58–0.70, suggesting an anomalous release mechanism, i.e., that drug diffusion and polymer swelling mechanisms controlled NCT release from the tablets. The exponent values obtained in this study were lower than those obtained from the release testing of whole tablets. This is due to the difference in release conditions and the surface area of the tablets exposed to the medium. Moreover, in the current study, erosion of the swollen gel matrix could not occur because of the use of cellulose acetate membrane as a barrier. The NCT release rates of the tablets were also calculated using the zero-order and Higuchi models, the results of which are listed in Table V. The NCT release rates showed a better fit with the Higuchi model than the zero-order model, as shown by the determination coefficient (R2). The NCT release rate of the tablets increased with increasing preparation pH, even though the amount of SA used in the tablets using the complexes prepared at pH 4 was less than that in the complexes prepared at pH 7 and 9. This is due to the influence of the NCT release of the complex particles that was previously described in “Characteristics of NCT–MAS Complexes.” An effect of the complex/SA ratio on NCT release was observed. Increasing the amount of complex in the tablets led to higher NCT release rates (Table V) by increasing the NCT concentration gradient for diffusion process. Moreover, the reduction of SA in the tablets decreased the swelling in the gel matrix that acted as a diffusion barrier for NCT. For this reason, the highest NCT release rate was found in the pH 9 NCT–MAS complex tablets without SA.
Fig. 5.
Effect of preparation pH (a) and complex/SA ratio (b) on NCT release of NCT–MAS complex-loaded SA tablets using cellulose acetate as a membrane, measured using a modified USP dissolution apparatus 2. Each point is the mean ± SD, n = 3
Table V.
NCT Release and Permeation Rate of NCT–MAS Complex-Loaded SA Tablets Tested Using Modified USP Dissolution Apparatus 2
| Tablets | Release exponent, n | NCT release ratea | NCT permeation rateb | ||
|---|---|---|---|---|---|
| K 0 (μg min−1) | K H (μg min−1/2) | K 0 (μg min−1) | K H (μg min−1/2) | ||
| Preparation pH of complexes | |||||
| pH 4 | 0.70 ± 0.01 (R 2 = 0.990) | 3.18 ± 0.26 (R 2 = 0.984) | 77.2 ± 6.6 (R 2 = 0.981) | 0.97 ± 0.10 (R 2 = 0.922) | 43.1 ± 2.6 (R 2 = 0.963) |
| pH 7 | 0.61 ± 0.01 (R 2 = 0.997) | 4.26 ± 0.13 (R 2 = 0.955) | 105.6 ± 2.7 (R 2 = 0.994) | 3.10 ± 0.28 (R 2 = 0.979) | 84.6 ± 5.3 (R 2 = 0.980) |
| pH 9 | 0.66 ± 0.04 (R 2 = 0.993) | 4.51 ± 0.23 (R 2 = 0.979) | 109.8 ± 5.2 (R 2 = 0.986) | 4.88 ± 0.30 (R 2 = 0.980) | 131.6 ± 7.4 (R 2 = 0.993) |
| Complex/SA ratio | |||||
| 1:4 | 0.58 ± 0.04 (R 2 = 0.989) | 1.30 ± 0.07 (R2 = 0.992) | 32.7 ± 1.7 (R2 = 0.989) | 1.90 ± 0.24 (R2 = 0.982) | 52.5 ± 6.6 (R2 = 0.989) |
| 1:1.5 | 0.63 ± 0.05 (R 2 = 0.991) | 2.49 ± 0.23 (R 2 = 0.966) | 62.3 ± 5.5 (R 2 = 0.981) | 3.11 ± 0.23 (R 2 = 0.986) | 85.7 ± 5.8 (R 2 = 0.990) |
| 1:0.67 | 0.66 ± 0.04 (R 2 = 0.993) | 4.51 ± 0.23 (R 2 = 0.979) | 109.8 ± 5.2 (R 2 = 0.986) | 4.88 ± 0.30 (R 2 = 0.980) | 131.6 ± 7.4 (R 2 = 0.993) |
| 1:0 | 0.58 ± 0.03 (R 2 = 0.991) | 15.27 ± 0.66 (R 2 = 0.937) | 382.3 ± 15.8 (R 2 = 0.995) | 7.90 ± 1.30 (R 2 = 0.955) | 208.9 ± 32.6 (R 2 = 0.996) |
Data are mean ± SD, n = 3
aNCT release using cellulose acetate membrane
bNCT permeation using porcine esophageal membrane
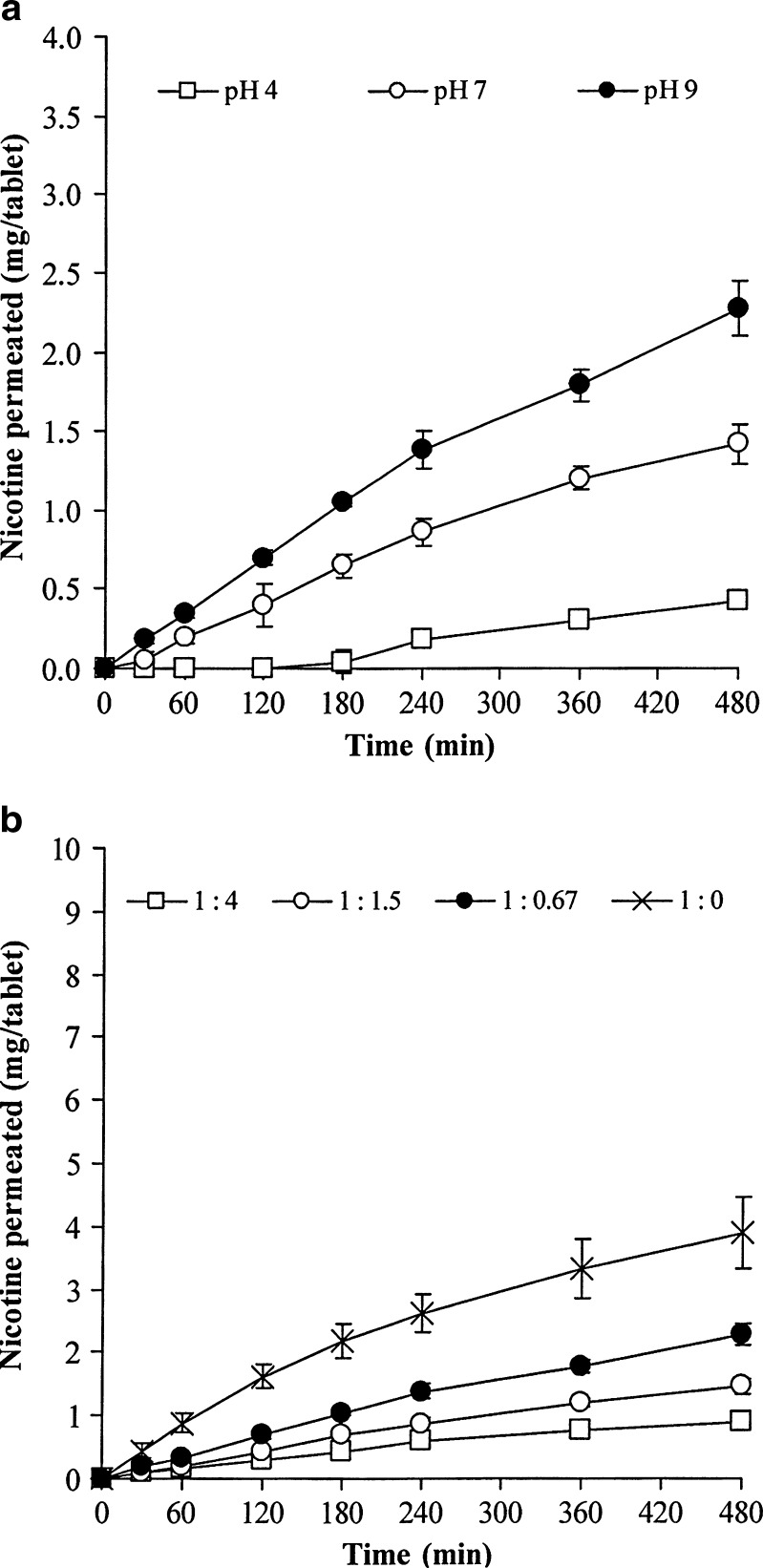
The permeation profiles of NCT from the NCT–MAS complex-loaded SA tablets across the mucosal membrane are shown in Fig. 6. NCT permeation rate was calculated using the zero-order and Higuchi models. The Higuchi model provided described the data better than the zero-order model (Table V), as was the case with the NCT release. This suggests that the rate-limiting step was not permeation across the mucosal membrane but NCT diffusion in the swollen gel matrix was. The preparation pH of NCT–MAS complexes had a clear effect on the NCT permeation rate (Fig. 6a and Table V). The tablets using the complexes prepared at pH 4 had the lowest NCT permeation rate with a very long lag time (the point of intersection with time axis); the highest NCT permeation rate was obtained from the complexes prepared at pH 9. This phenomenon occurred because the NCT molecules in the complexes formed at pH 4 were the protonated species (8) that possess lower permeability across the mucosal membranes (19,20). However, at pH 9, NCT is in its neutral species, which has higher mucosal permeability. Moreover, increasing the amount of SA in the tablets led to lower permeation rates of NCT (Table V). This was due to a slower NCT release in tablets with a higher ratio of SA. It was also observed that the permeation rates of NCT were greater than the NCT release rates in the pH 9 NCT–MAS complex-loaded SA tablets. This could be explained by the fact that the tablets composed of SA could absorb the medium through the pore channels of the cellulose acetate membrane used, whereas tablets rarely absorbed the medium when using the mucosal membrane due to the membrane’s low water permeability. This resulted in increased swelling of the tablets during the release testing and led to the reduction of the concentration gradient and increase of the path length for NCT diffusion. Thus, the NCT release rate was possibly lower than the NCT permeation rate in this study.
Fig. 6.
Effect of preparation pH (a) and complex/SA ratio (b) on NCT permeation across porcine esophageal membranes of NCT–MAS complex-loaded SA tablets, measured using a modified USP dissolution apparatus 2. Each point is the mean ± SD, n = 3
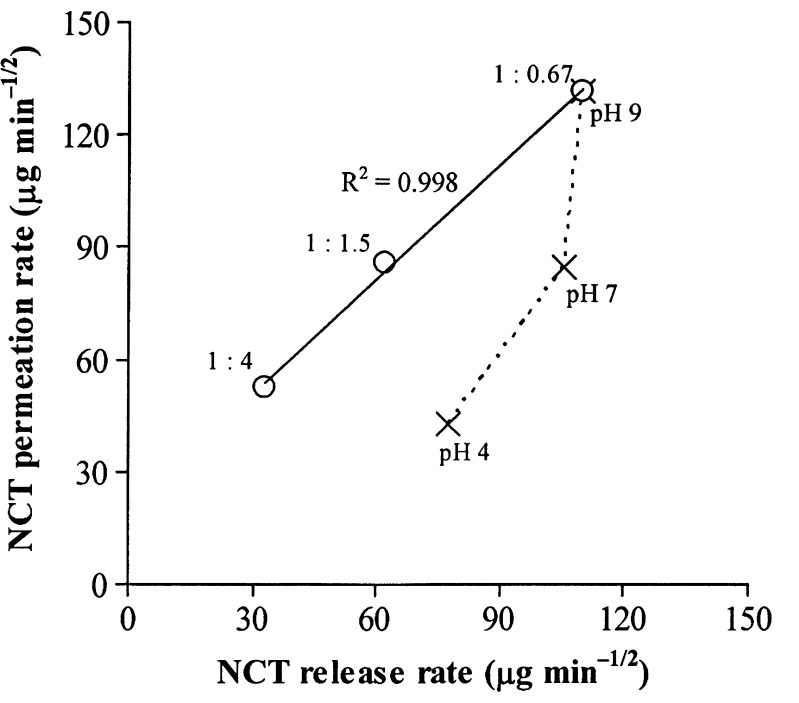
Relationship between the release rate and the permeation rate of NCT from the matrix tablets with various complex/SA ratios showed good linearity with R2 higher than 0.99, as shown in Fig 7. It can be seen that the NCT permeation rate increased with increasing NCT release rate from the swollen matrix tablets. This suggested that the greater the NCT release rate, the higher the NCT concentration gradient on the surface of the mucosal membrane. This led to a higher NCT permeation rate as well. In contrast, the matrix tablets using NCT–MAS complexes prepared at different pH levels had not a linear relationship of both parameters. This was due to the low permeability across the mucosal membrane of protonated NCT released from the complexes prepared at acidic and neutral pH levels.
Fig. 7.
Relationship between release rate and permeation rate of NCT from NCT–MAS complex-loaded SA tablets prepared using different preparation pH levels of complexes and complex/SA ratios
It is preferable to control the delivery of drugs via the drug delivery system rather than via the mucosal membrane. This study showed that the pH 9 NCT–MAS complex tablets without SA gave remarkably higher NCT release rates when compared with the NCT permeation rate, indicating that the permeation of NCT was controlled via the mucosal membrane. In contrast, the pH 9 NCT–MAS complex-loaded SA tablets gave similar release and permeation rates to NCT, suggesting that the delivery of NCT across the mucosal membrane was mainly controlled by the swollen gel matrix of the NCT–MAS complex-loaded SA tablets. This resulted from the molecular interaction of SA with MAS to form the gel matrix structure for controlling drug delivery.
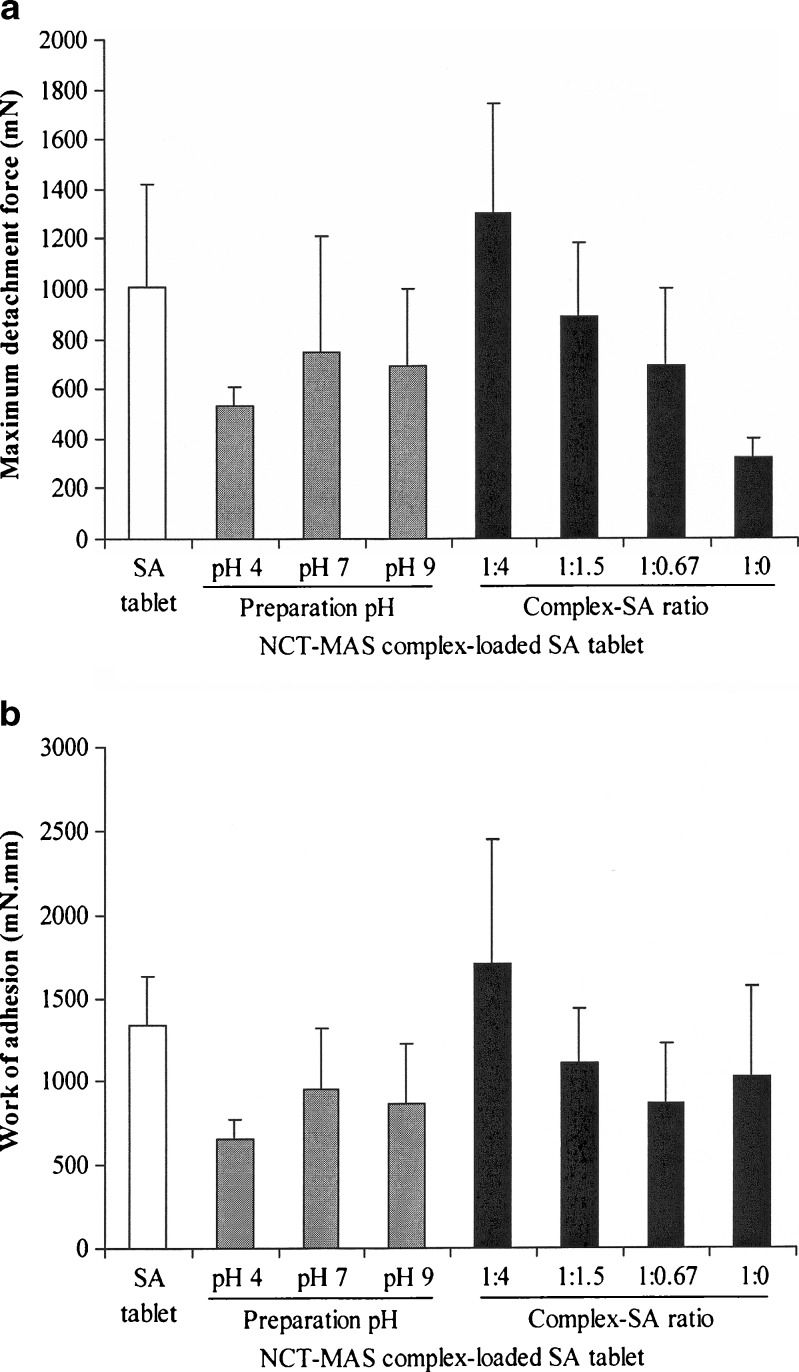
Mucoadhesive Properties of the Tablets
The mucoadhesive properties, maximum detachment force (Fmax), and work of adhesion (Wad) of the tablets are presented in Fig. 8. The NCT–MAS complex-loaded SA tablets with different preparation pH levels had comparable Fmax and Wad values and did not differ from the mucoadhesive properties of SA tablets, although the amount of SA incorporated was quite low in the tablets. However, the pH 4 NCT–MAS complex-loaded SA tablets seemed to display the lowest Fmax and Wad. This is likely because these tablets used the smallest amount of SA. The complex/SA ratios used in the tablets affected the mucoadhesive properties. The Fmax and Wad of the tablets tended to decrease with decreasing SA amount in the tablets. Surprisingly, the pH 9 NCT–MAS complex tablets without SA showed large enough Fmax and Wad values for adhesion onto the mucosal membrane.
Fig. 8.
Maximum detachment force (a) and work of adhesion (b) of the tablets. Each bar is the mean ± SD, n = 5
MAS has a silicate layer surface containing numerous hydroxyl groups which could possibly adhere to the mucosal membrane via hydrogen bonding with mucus. The NCT–MAS complex tablets possess mucoadhesive properties, suggesting that MAS still has enough hydroxyl groups to interact with mucus after the complexation with NCT. SA is a polysaccharide that possesses a mucoadhesive property (18,30,31) because it contains numerous hydrogen bond-forming groups, i.e., carboxyl and hydroxyl groups. It has been proposed that the interaction between the mucus on mucosal membrane and hydrophilic polymers occurs by physical entanglement and chemical interactions, such as hydrogen bonding (31). Due to the mucoadhesive properties of SA and NCT–MAS complexes, the NCT–MAS complex-loaded SA tablets were sufficiently mucoadhesive for adhesion onto the mucosal membrane. However, the reduction of SA amount in the tablets caused a decrease of Fmax and Wad, suggesting that the swelling and physical entanglement of SA on the tablet surface was an important process for promoting the interaction with mucus.
CONCLUSION
The NCT–MAS complex-loaded SA tablets prepared using the direct compression method had favorable physical properties and gave a zero-order NCT release kinetic controlled by a swelling/erosion mechanism. The matrix tablets containing the NCT–MAS complexes prepared at pH 9 showed obviously greater NCT permeation rates than those containing the complexes prepared at acidic and neutral conditions. NCT release and permeation rates decreased with increasing SA amounts in the tablets. The use of the NCT–MAS complexes prepared at pH 9 in the tablets provided a highly effective NCT delivery across the mucosal membrane, which was controlled by the swollen gel matrix of the tablets. This resulted from the molecular interaction of SA with MAS to form the gel matrix structure that controlled the drug diffusion. Moreover, the presence of SA in the tablets could enhance the mucoadhesive properties of the tablets. This study suggests that SA could play an important role for controlling NCT release and enhancing the mucoadhesive properties of NCT–MAS complex-loaded SA tablets, and these tablets demonstrate strong potential for use as a buccal delivery system for NCT.
Acknowledgments
The authors would like to thank the Thailand Research Fund (Bangkok, Thailand) for research funding (grant no. RSA5280013) and the Faculty of Pharmaceutical Sciences, Khon Kaen University (Khon Kaen, Thailand) for technical support. We are very pleased to acknowledge the Graduate School, Khon Kaen University (Khon Kaen, Thailand) for a scholarship for Sopaphan Kanjanabat.
References
- 1.Dollery SC. Therapeutic drugs. Edinburgh: Churchill Livingstone; 1991. pp. N65–N72. [Google Scholar]
- 2.Nair MA, Chetty DJ, Ho H, Chien YW. Biomembrane permeation of nicotine: mechanistic studies with porcine mucosae and skin. J Pharm Sci. 1997;86:257–62. doi: 10.1021/js960095w. [DOI] [PubMed] [Google Scholar]
- 3.Mihranyan A, Andersson SB, Ek R. Sorption of nicotine to cellulose powders. Eur J Pharm Sci. 2004;22:279–86. doi: 10.1016/j.ejps.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Borodkin PB. Ion exchange resins and sustained release. In: Swarbrick J, Boylan JC, editors. Encyclopedia of pharmaceutical technology. New York: Marcel Dekker; 1993. pp. 203–16. [Google Scholar]
- 5.Cheng YH, Watts P, Hinchcliffe M, Hotchkiss R, Nankervis R, Faraj NF, Smith A, Davis SS, Illum DL. Development of a novel nasal nicotine formulation comprising an optimal pulsatile and sustained plasma nicotine profile for smoking cessation. J Control Release. 2000;79:243–54. doi: 10.1016/S0168-3659(01)00553-3. [DOI] [PubMed] [Google Scholar]
- 6.Kibbe HA. Handbook of pharmaceutical excipients. 3. Washington: American Pharmaceutical Association; 2000. pp. 295–8. [Google Scholar]
- 7.Alexandre M, Dubois P. Polymer-layered silicate nanocomposites: preparation, properties and uses of a new class of materials. Mater Sci Eng. 2000;28:1–63. doi: 10.1016/S0927-796X(00)00012-7. [DOI] [Google Scholar]
- 8.Suksri H, Pongjanyakul T. Interaction of nicotine with magnesium aluminum silicate at different pHs: characterization of flocculate size, zeta potential and nicotine adsorption behavior. Colloids Surf B. 2008;65:54–60. doi: 10.1016/j.colsurfb.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 9.Pongjanyakul T, Khunawattanakul W, Puttipipatkhachorn S. Physicochemical characterizations and release studies of nicotine–magnesium aluminum silicate complexes. Appl Clay Sci. 2009;44:242–50. doi: 10.1016/j.clay.2009.03.004. [DOI] [Google Scholar]
- 10.Pongjanyakul T, Priprem A, Puttipipatkhachorn S. Investigation of novel alginate–magnesium aluminum silicate microcomposite films for modified-release tablets. J Control Release. 2005;107:343–56. doi: 10.1016/j.jconrel.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Pongjanyakul T. Alginate–magnesium aluminum silicate films: importance of alginate block structures. Int J Pharm. 2009;365:100–8. doi: 10.1016/j.ijpharm.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Boyapally H, Nukala RK, Bhujbal P, Douroumis D. Controlled release from directly compressible theophylline buccal tablets. Colloids Surf B. 2010;77:227–33. doi: 10.1016/j.colsurfb.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Miyazaki S, Nakayama A, Oda M, Takada M, Attwood D. Chitosan and sodium alginate based bioadhesive tablets for intraoral drug delivery. Biol Pharm Bull. 1994;17:745–7. doi: 10.1248/bpb.17.745. [DOI] [PubMed] [Google Scholar]
- 14.Choi HG, Kim CK. Development of omeprazole buccal adhesive tablets with stability enhancement in human saliva. J Control Release. 2000;68:397–404. doi: 10.1016/S0168-3659(00)00276-5. [DOI] [PubMed] [Google Scholar]
- 15.Choi H, Jung J, Yong CS, Rhee C, Lee M, Han J, Park K, Kim C. Formulation and in vivo evaluation of omeprazole buccal adhesive tablet. J Control Release. 2000;68:405–12. doi: 10.1016/S0168-3659(00)00275-3. [DOI] [PubMed] [Google Scholar]
- 16.Ìkinci G, Senel S, Wilson CG, Şumnu M. Development of buccal bioadhesive nicotine tablet formulation for smoking cessation. Int J Pharm. 2004;277:173–8. doi: 10.1016/j.ijpharm.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Save T, Shah UM, Ghamande AR, Venkatachalam P. Comparative study of buccoadhesive formulations and sublingual capsules of nifedipine. J Pharm Pharmacol. 1994;46:192–5. doi: 10.1111/j.2042-7158.1994.tb03776.x. [DOI] [PubMed] [Google Scholar]
- 18.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J Control Release. 2006;114:15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Chen LH, Chetty DJ, Chien YW. A mechanistic analysis to characterize oramucosal permeation properties. Int J Pharm. 1999;184:63–72. doi: 10.1016/S0378-5173(99)00091-5. [DOI] [PubMed] [Google Scholar]
- 20.Adrian CL, Olin HBD, Dalhoff K, Jacobsen J. In vivo human buccal permeability of nicotine. Int J Pharm. 2006;311:196–202. doi: 10.1016/j.ijpharm.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Park CR, Munday DL. Development and evaluation of a biphasic buccal adhesive tablet for nicotine replacement therapy. Int J Pharm. 2002;237:215–26. doi: 10.1016/S0378-5173(02)00041-8. [DOI] [PubMed] [Google Scholar]
- 22.Diaz-del Consuelo I, Jacques Y, Pizzolato G, Guy RH, Falson F. Comparison of the lipid composition of porcine buccal and esophageal permeability barriers. Arch Oral Biol. 2005;50:981–7. doi: 10.1016/j.archoralbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Diaz-del Consuelo I, Falson F, Guy RH, Jacques Y. Ex vivo evaluation of bioadhesive films for buccal delivery of fentanyl. J Control Release. 2007;122:135–40. doi: 10.1016/j.jconrel.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110–1. [PubMed] [Google Scholar]
- 25.Siepmann J, Siepmann F. Mathematical modeling of drug delivery. Int J Pharm. 2009;364:328–43. doi: 10.1016/j.ijpharm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Ritger PL, Peppas NA. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36. doi: 10.1016/0168-3659(87)90034-4. [DOI] [PubMed] [Google Scholar]
- 27.Costa P, Lobo JMS. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2001;13:123–33. doi: 10.1016/S0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 28.Katti KS, Sikdar D, Katti DR, Ghosh P, Verma D. Molecular interaction in intercalated organically modified clay and clay–polycaprolactam nanocomposites: experiments and modeling. Polymers. 2006;47:403–14. doi: 10.1016/j.polymer.2005.11.055. [DOI] [Google Scholar]
- 29.Pongjanyakul T, Suksri H. Alginate–magnesium aluminum silicate films for buccal delivery of nicotine. Colloids Surf B. 2009;74:103–13. doi: 10.1016/j.colsurfb.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 30.Batchelor HK, Banning D, Dettmar PW, Hampson FC, Jolliffe IG, Craig DCM. An in vitro mucosal model for prediction of bioadhesion of alginate solutions to the oesophagus. Int J Pharm. 2002;238:123–32. doi: 10.1016/S0378-5173(02)00062-5. [DOI] [PubMed] [Google Scholar]
- 31.Salamat-Miller N, Chittchang M, Johnston TP. The use of mucoadhesive polymers in buccal drug delivery. Adv Drug Deliv Rev. 2005;57:1666–91. doi: 10.1016/j.addr.2005.07.003. [DOI] [PubMed] [Google Scholar]