Abstract
The present study introduces a new three-dimensional (3D) surface image analysis technique in which white light illumination from different incident angles is used to create 3D surfaces with a photometric approach. The three-dimensional features of the surface images created are then used in the characterization of particle size distributions of granules. This surface image analysis method is compared to sieve analysis and a particle sizing method based on spatial filtering technique with nearly 30 granule batches. The aim is also to evaluate the technique in flowability screening of granular materials. Overall, the new 3D imaging approach allows a rapid analysis of large amounts of sample and gives valuable visual information on the granule surfaces in terms of surface roughness and particle shape.
KEY WORDS: 3D image analysis, flowability, particle size measurement, spatial filtering technique, weight variation
INTRODUCTION
Particle size and shape play an important role in the material characterisation, processing and manufacturing of pharmaceutical solid systems. The particle size distribution affects the internal flow and segregation of granules and thus the uniformity of dosage units during tablet compression. Accurate monitoring and control of particle size and shape can lead to significant improvements in the overall operation of manufacturing processes.
Many different methods are available for particle size analysis, e.g. sieving, laser diffraction, Coulter counter, electron microscopy, focused beam reflectance method, spatial filtering technique (SFT), near-infrared spectroscopy (NIRS), acoustic emission and optical microscopy in conjunction with image analysis (1–6). The size characterization of large spherical particles is often relatively easy but challenging for small irregular particles for which the assigned size will depend on the characterization method used.
Until recently, little had been done in the field of powder technology and pharmaceutical development in terms of systematic utilisation of image information apart from static and dynamic image-based particle size analyzers measuring dispersed particles (7–12). There is an enormous unused potential in the image sources produced in various steps of drug development. There are also immense prospects to increase useful imaging approaches. Consequently, the development of this discipline creates a challenge within the characterization pharmaceutical and other solid systems. The attempts in developing image-based particle, agglomerate and surface analysis tools for pharmaceutical powders should strive for reliable, fast and easily usable methods with possibilities of intelligent image feature extraction and feedback control mechanisms for process monitoring situations. Recently, several commercial instruments that make use of high speed imaging have become available and have been utilised in, e.g. pharmaceutical research (13–15).
Methods based on visual information can provide additional information on the appearance of powders which can be relevant in terms of downstream processability. Therefore, the images do not only give information on the particle size and the particle size distribution but also on the particle shape and the surface characteristics of, e.g. granules. Thus, also the flow and the segregation properties of the granules could be estimated based on the images.
The flowability of granules is an important property influencing several drug manufacturing steps. In the tablet manufacturing process, for instance, flowability plays a role in mixing and compaction as well as content and weight uniformity of the final dosage form. Flowability is affected by the physical properties of the granules, such as particle size and shape. Particles larger than 250 μm usually flow freely, while particles below 100 μm are generally cohesive and prone to flowability problems (16). Thus, rapid image-based screening tool providing both particle size and flowability information would be useful in pharmaceutical manufacturing.
Traditional image analysis carried out using optical microscope is often considered as time-consuming and problematic due to the low number of particles it can measure (17). Also, the whole powder stream should preferably be taken into account (18). Recently, a surface imaging approach using colored light sources has been successfully used in the analysis of granules (19). The present study introduces a new 3D-surface image analysis technique in which the white light illumination from different incident angles is used to create 3D surfaces with a photometric approach. The three-dimensional features of the surface images created are then used in the characterization of particle size distributions. The nature of the imaging setup also allows simultaneous evaluation of the powder flow characteristics of the samples. The main goal of the study was to investigate the applicability of the new photometric approach in particle size measurement and flow rate screening. Additionally, the surface roughness information was extracted from the images and used in characterisation of the materials. An ultimate goal was to aim at meaningful image-based feature extraction to support understanding of physical properties of materials and phenomena relevant to pharmaceutical solid dosage form processing.
MATERIALS
The model formulation (batch size 3,500 g) consisted of 175 g (5% w/w) of caffeine (Orion Pharma, Espoo, Finland), 475 g microcrystalline cellulose (Emcocel 50M, Penwest Pharmaceuticals, Nastola, Finland), 2,200 g lactose monohydrate (Pharmatose 200M, DMV Pharma, Veghel, The Netherlands) and 500 g pregelatinized starch (Starch 1,500, Colorcon, Indianapolis, IN). One hundred seventy-five grammes (5% w/w) of polyvinylpyrrolidone (PVP; Kollidon K25, BASF, Ludwigshafen, Germany) was used as a binder in the formulation. Solutions in purified water were prepared using 8.75% w/w of PVP. Moreover, three microcrystalline celluloses, Avicel® PH101, PH102 and PH200 (FMC BioPolymer, Little Island, Ireland) were used as supplied.
METHODS
Granulation
Thirty-nine granulations were made with a bench-scale fluidized bed granulator (Glatt, WSG 5, Glatt Gmbh, Binzen, Germany). The aim was to obtain batches with varying end-point particle mean sizes. Twenty-eight of the batches were used in this study and will be referred to as granules R1–R28. The granulation process has been thoroughly described earlier (20).
Particle Size Measurements
In total, 28 granule batches with varying particle sizes were analysed. The particle size distribution (PSD) of the granules was determined using a 3D-surface image analysis method (Flashsizer FS3D, Intelligent Pharmaceutics Ltd, Helsinki, Finland), SFT (Parsum, Malvern Instruments, Malvern, UK) and sieve analysis as a reference method. Approximately 300 g of each granule batch was analysed by FS3D. After the FS3D measurements, 20 g of the samples was measured by Parsum and sieving (n = 3). The sampling for the granules was made using a rotary sample divider (Fritsch Sample Divider Laborette 27, Idar-Oberstein, Germany), and the representative samples obtained were used for Parsum and sieve analyses. To evaluate the FS3D measurements with narrow size distributions, sieve fractions were prepared from an example formulation.
3D Image Analysis Method (FS3D)
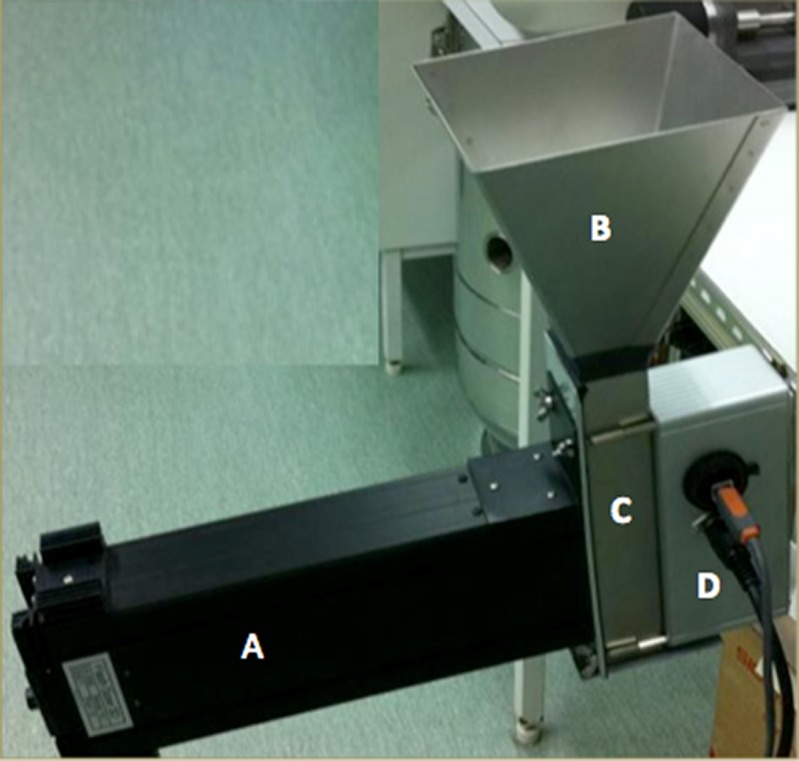
The FS3D image analysis setup consists of a camera connected to a computer and a steel chute with a glass window (Fig. 1). The size of the measurement field is 1,280 × 960 pixels, i.e. 15.1 × 11.3 mm. The dimensions of the rectangular chute are 40 × 40 mm. The samples are fed into a chute through a hopper (orifice diameter 40 mm), and the particle size is recorded with 5 s intervals. The number of images and particles measured depends on the shutter opening velocity and the flow rate of the powder. In this case, on average 30 images were taken per batch. The number of particles per image measured varied from 600 to 1,600. A variant of photometric stereo (21,22) at two lights was used to obtain 3D surface of a sample (Fig. 2). The samples were presented to the instrument in a continuous feed and imaged through a glass window. The camera is situated horizontally to the window and sample surface. The viewing direction is kept constant, but the direction of the incident illumination is varied. In following the method, the light sources were placed 180° from each other in a horizontal plane and the angle of illumination was 30°. The resulting gradient fields obtained with the above-mentioned setup contain direct information about surface normal in xz plane and indirect information about surface normal in yz plane. Line integration was used in horizontal direction to obtain a 3D surface. The cumulative error that is typical for line integration-based methods was removed with a high pass filter. It is assumed that the sample surface is approximately straight on larger scale because samples lay against a straight glass surface of the cuvette/sampler glass window. The high pass filter was constructed from a moving average low pass filter. Peaks on the 3D surface are assumed to be particles. The volume (V)-based particle size (d) is then calculated from the area of peaks (a) in xy direction:
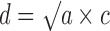 |
1 |
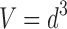 |
2 |
Fig. 1.
a Camera. b Feeding funnel. c Sample chute with glass window towards the camera. d Control unit for the sample shutter
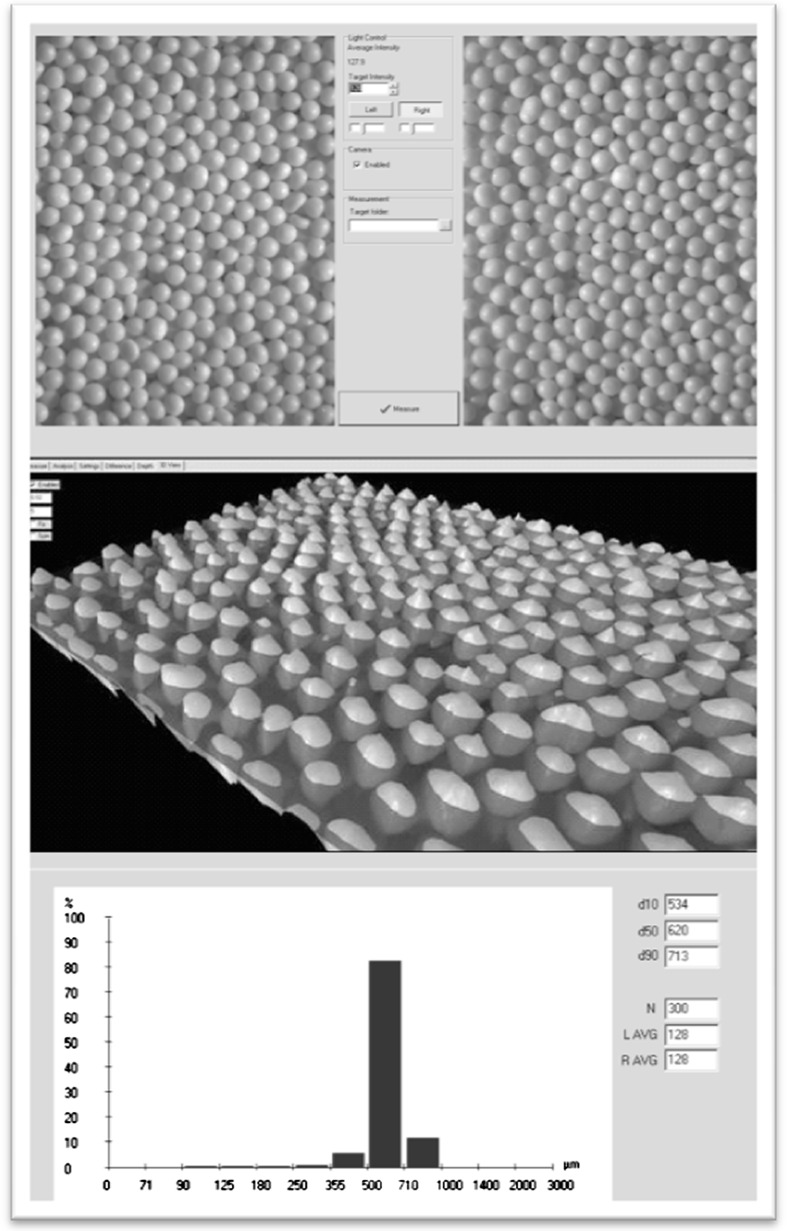
Fig. 2.
Image capture (use of two illumination angles; top). Creation of a 3D surface and particle size data extraction (middle). Presentation of particle size data (bottom)
c in Eq. 1 is calibration constant, calibrated with six different-sized (100–1,400 μm) spherical cellulose particles, cellets (Syntapharm, Mülheim an der Ruhr, Germany). Previous work in the field has investigated the sample preparation procedure, optimal illumination angles, and imaging conditions in general for an imaging system (23,24) similar to the FS3D.
Spatial Filtering Technique (Parsum)
SFT (Parsum, Chemnitz, Germany) is a method that determines the chord length of each particle passing through the laser light beam (3). An SFT apparatus (Parsum IPP 70; Gesellschaft für Partikel-, Strömungs- und Umweltmesstechnik GmbH, Chemnitz, Germany) was installed on a laboratory table, and the sample (20 g) was poured through an orifice (diameter 4 mm) using a funnel. The particles were dispersed by pressurized air. The number particle size (chord length) distribution was transformed to volume particle size distribution for data analysis.
Sieve Analysis
The granules were sieved using Fritsch analysette sieve shaker and sieves (Fritsch GmbH, Idar-Oberstein, Germany). The sieving time was 5 min with the amplitude of 6. The sieve analyses (range 90–1,400 μm with √2 increment) were performed in triplicate.
Tabletting
Twelve granule batches were tabletted for an earlier study with an instrumented (Korsch (EK-0), Germany) single-punch tabletting machine (20). The weight variation of the tablets was measured, and the data was used in the current study.
Flowability Measurements
The flow rate of the 28 granule batches and three grades of microcrystalline cellulose (Avicel PH101, PH102 and PH200) were measured with the FS3D method. The sample was weighed and fed into a cuvette through a hopper, and the images were taken. The bottom of the cuvette was opened for 0.3 s every 2 s. All samples were measured three times. The flow rate of the samples was calculated by dividing the mass of the sample by the time it took the sample to flow through the instrument.
Roughness and Segregation Measurements
The roughness values were obtained from the data recorded by the FS3D instrument during the flow rate measurements. The calculation principles of roughness values has been previously explained (25). Also the segregation tendency of the samples was evaluated based on the relative standard deviations of d50 values obtained with the FS3D measurements. The segregation tendency was then compared to the mean particle size, relative width of particle size distribution and flow rate of the granules.
Multivariate Analysis
Principal component analysis (PCA) was performed to visualise the physical characterisation data obtained from FS3D using the Simca-P software v 10.1. (Umetrics AB, Umeå, Sweden). PCA was used for finding the interdependencies in the data set.
RESULTS AND DISCUSSION
Particle Size
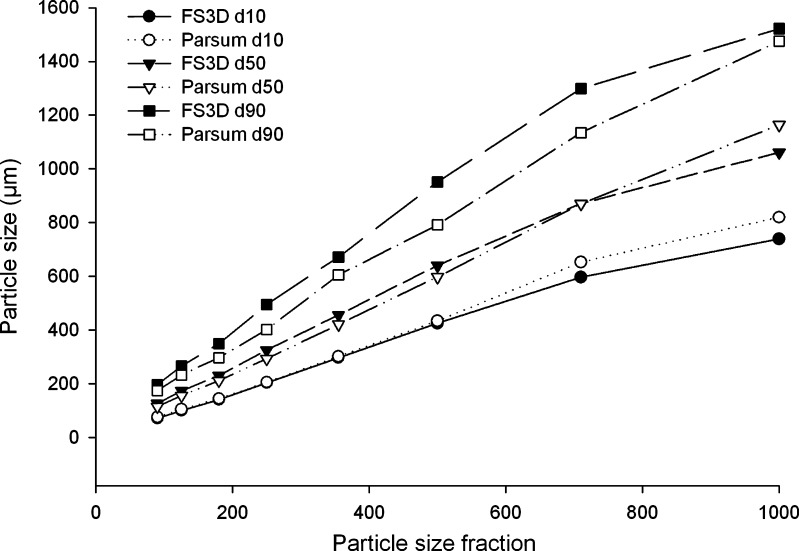
The FS3D method was used for the particle size measurements of both final granules and sieved example fractions. As shown in Fig. 3, the volume size distribution results of the sieve fractions are in good accordance with the expected values measured by Parsum. The results for different granule sizes were determined very accurately, and their Pearson’s correlation value was 1.00 for the d10, d50 and d90 values.
Fig. 3.
Comparison of the d 10, d 50 and d 90 particle size values of sieved granule fractions measured by FS3D and Parsum
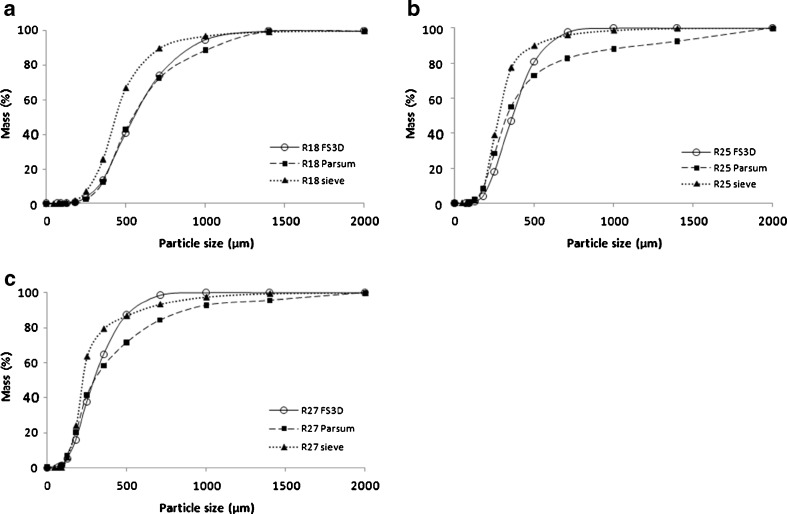
The particle size distributions of the granules measured by sieve analysis, FS3D and Parsum are presented in Table I. The Pearson’s correlation values were calculated to compare the different techniques with regard to the d10, d50 and d90 values, respectively. These were as follows: FS3D vs. sieving (0.55, 0.82, 0.84), FS3D vs. Parsum (0.95, 0.82, 0.34) and Parsum vs. sieving (0.72, 0.64, 0.35). Generally, it can be noticed that the best correlation is between d10 and d50 values between Parsum and FS3D. Correlations between FS3D and sieving are moderately good for the d50 and d90 values but already much poorer for the d10 values. The overall correlation is poorest between Parsum and sieving, d10 and d50 being better than the d90 correlation. It can be clearly seen that comparisons between sizing techniques can be challenging. To further illustrate the data, cumulative particle size distributions of three granule batches (R18, R25 and R27) are compared in Fig. 4a–c. The d50 values and the particle size distributions given by different methods generally correlate rather well. Sieving in general indicates a shift towards the smaller sizes compared to the other techniques used. This is likely to result from diminution of the brittle granules during sieving due to friability observed during handling. Nevertheless, the correlation between Parsum and FS3D is generally rather good, particularly when it comes to the smaller ends of the size range. However, Parsum seems to indicate larger particle sizes at the higher end of the size range than the other methods and the Parsum results of a few granule batches, such as R7 or R16, are significantly larger than the FS3D results. The differences are likely to arise from Parsum interpreting two middle-sized particles as one large granule. Also, the measured chord length in Parsum depends on the orientation and location of a granule (26). Thus, the measured chord length distribution is larger than the actual particle size distribution, which explains why Parsum gives a broader PSD than the other methods. Furthermore, irregular granule shape and particle agglomeration during the Parsum measurements may lead to overestimation of the amount of large granules. By contrast, the FS3D method is able to recognise the agglomerates as different particles. Some of the deviation between the results arises from the different measuring principles of the three methods and the fact that they generally assume the particles to be smooth and spherical (27,28).
Table I.
The Particle Size Distribution of 28 Granule Batches Measured by FS3D, Parsum and Sieve Analysis
| Sample | FS3D (μm) | Parsum (μm) | Sieve (μm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| d 10 | d 50 | d 90 | d 10 | d 50 | d 90 | d 10 | d 50 | d 90 | |
| R1 | 380 | 680 | 1,032 | 286 | 542 | 1,441 | 257 | 390 | 824 |
| R2 | 564 | 928 | 1,304 | 598 | 1,098 | 2,801 | 395 | 662 | 1,171 |
| R3 | 237 | 437 | 733 | 203 | 425 | 1,771 | 192 | 279 | 464 |
| R4 | 332 | 629 | 961 | 256 | 681 | 1,862 | 181 | 279 | 552 |
| R5 | 206 | 397 | 624 | 165 | 380 | 1,238 | 167 | 249 | 494 |
| R6 | 691 | 1,142 | 1,576 | 993 | 1,453 | 2,400 | 728 | 1,085 | 1,679 |
| R7 | 224 | 467 | 790 | 204 | 1,108 | 2,506 | 148 | 236 | 647 |
| R8 | 343 | 656 | 1,051 | 324 | 870 | 1,938 | 151 | 253 | 676 |
| R9 | 310 | 588 | 915 | 269 | 957 | 2,461 | 163 | 285 | 739 |
| R10 | 289 | 507 | 789 | 217 | 504 | 1,291 | 183 | 304 | 646 |
| R11 | 304 | 566 | 908 | 258 | 603 | 1,715 | 182 | 296 | 692 |
| R12 | 520 | 916 | 1,306 | 451 | 1,249 | 2,335 | 257 | 420 | 978 |
| R13 | 468 | 852 | 1,251 | 412 | 1,039 | 2,314 | 193 | 321 | 636 |
| R14 | 539 | 860 | 1,259 | 504 | 921 | 1,445 | 150 | 468 | 953 |
| R15 | 298 | 543 | 863 | 240 | 559 | 1,562 | 188 | 288 | 515 |
| R16 | 132 | 245 | 407 | 137 | 538 | 2,328 | 113 | 198 | 518 |
| R17 | 782 | 1,222 | 1,653 | 810 | 1,346 | 2,056 | 176 | 686 | 1,349 |
| R18 | 473 | 782 | 1,134 | 478 | 767 | 1,446 | 378 | 623 | 1,004 |
| R19 | 205 | 374 | 587 | 175 | 431 | 1,719 | 139 | 238 | 615 |
| R20 | 443 | 752 | 1,126 | 495 | 997 | 2,695 | 383 | 646 | 1,249 |
| R21 | 268 | 534 | 843 | 275 | 1,094 | 3,295 | 147 | 253 | 688 |
| R22 | 722 | 1,152 | 1,645 | 682 | 1,285 | 2,592 | 241 | 616 | 1,334 |
| R23 | 283 | 526 | 871 | 254 | 602 | 1,823 | 197 | 310 | 645 |
| R24 | 298 | 561 | 904 | 248 | 845 | 2,215 | 168 | 268 | 671 |
| R25 | 312 | 519 | 788 | 260 | 467 | 1,480 | 256 | 397 | 711 |
| R26 | 279 | 532 | 879 | 210 | 597 | 1,876 | 163 | 262 | 553 |
| R27 | 228 | 427 | 694 | 199 | 415 | 1,233 | 196 | 319 | 843 |
| R28 | 290 | 513 | 783 | 265 | 613 | 1,491 | 192 | 310 | 600 |
Fig. 4.
Comparison of three particle sizing methods (FS3D, Parsum and sieve analysis) shown as cumulative particle size distributions of three granule batches: a R18, b R25 and c R27
Change in the granule size distribution during the FS3D measurement was also investigated in order to assess the segregation tendency of the batches (Table II). Mostly, only minor changes were observed, but in some batches, the particle size distribution shifted slightly towards larger granules in the beginning of the measurement. The largest particles were generally observed close to the midpoint of the measurement, while the smaller particles were present in the end of the measurement. These findings suggest that segregation took place in some batches, i.e. small particles tended to percolate in the bottom of the hopper below larger granules. Moreover, a growing fraction of small particles was observed towards the end of the measurement due to their poorer flow rate compared to larger particles. In general, difference in particle size is the main reason behind segregation leading to percolation of small particles (29). However, no clear correlation was found between the segregation tendency of the batches and their flow rate, median particle size or relative width of particle size distribution. Nevertheless, the applicability of the instrument for segregation screening should be thoroughly studied in the future.
Table II.
The Flow Rate, Segregation Tendency and Surface Roughness of 28 Granule Batches Measured by FS3D
| Sample | Flow rate (g/s) | SGT (%) | Ra (au) |
|---|---|---|---|
| R1 | 3.9 | 9 | 85 |
| R2 | 3.2 | 7 | 105 |
| R3 | 2.6 | 5 | 63 |
| R4 | 3.7 | 7 | 67 |
| R5 | 2.0 | 10 | 61 |
| R6 | 3.1 | 6 | 102 |
| R7 | 2.1 | 6 | 56 |
| R8 | 2.2 | 13 | 64 |
| R9 | 2.5 | 12 | 73 |
| R10 | 2.3 | 5 | 67 |
| R11 | 2.8 | 5 | 67 |
| R12 | 5.9 | 9 | 93 |
| R13 | 3.0 | 7 | 67 |
| R14 | 2.7 | 18 | 85 |
| R15 | 2.5 | 5 | 68 |
| R16 | 1.6 | 5 | 45 |
| R17 | 3.8 | 18 | 92 |
| R18 | 3.4 | 6 | 104 |
| R19 | 1.5 | 4 | 52 |
| R20 | 3.8 | 6 | 106 |
| R21 | 2.1 | 10 | 60 |
| R22 | 5.2 | 10 | 98 |
| R23 | 3.4 | 5 | 68 |
| R24 | 3.6 | 8 | 64 |
| R25 | 4.7 | 6 | 86 |
| R26 | 3.0 | 5 | 57 |
| R27 | 3.8 | 8 | 69 |
| R28 | 2.7 | 10 | 70 |
SGT segregation tendency, Ra roughness, au arbitrary unit
Figure 5 shows examples of three-dimensional images of batches R18 and R27 generated by the FS3D instrument. The figures demonstrate the usefulness of image information in powder and granule characterization. The figures do not only show the particle size of the granules but also the morphology and surface texture. They also give an idea on the particle size distribution and packing behaviour of the granules. Thus, the FS3D method has a potential for being used also as an in-line or at-line process analytical technology (PAT) tool in pharmaceutical manufacturing processes. Moreover, the FS3D method is a fast and reliable tool for analysing the particle size of granules. One advantage of the method is that a complete granule batch can be measured without sampling and sample preparation in a non-destructive manner.
Fig. 5.
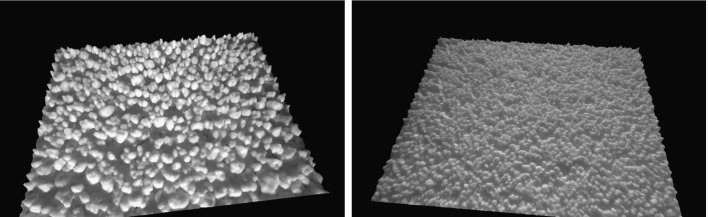
3D images of granule batches R18 (left) and R25 (right) created by the FS3D instrument
Comparison of the Particle Sizing Methods
Several particle size analysis methods have been compared previously (30), and it has been found out that Parsum (SFT) measurements were the most consistent of the studied three techniques (laser diffraction, sieve analysis and SFT). Parsum gave the best goodness of fit and goodness of prediction values, and FS3D was therefore compared with Parsum in this study. Both methods are quite simple to use, but FS3D also gives valuable information on the particle surfaces.
An advantage of the FS3D is that the samples are presented to the instrument in a continuous feed and the entire batch can be measured without sampling. In reality, the complete batch is not measured, but only the particles on the surface of the cuvette window. However, a relatively large amount of the sample can be analysed without any tedious sampling procedures. Moreover, the analysed sample can be used for other purposes after the FS3D analysis since the sample remains undamaged. The sieving process wears down granules and can cause tribocharging of especially small granules leading to errors in the particle size results. On the other hand, shades inside an irregular particle may lead to the instrument interpreting the particle as more than one. Furthermore, transparent or reflective particles, such as glass spheres, cannot be measured with FS3D due to light penetrating the particles instead of scattering from their surface. Also high moisture content, particularly free surface water, can affect the reflective properties of the particles. Nevertheless, the influence of the reflective properties of materials on the FS3D results needs to be looked into in the future studies. The main objective of the study was to find novel ways of using image information in pharmaceutical powder technology and characterisation of pharmaceutical solids.
Flowability and Tabletting Properties
The flow rate of the granules ranged from 1.6 to 5.9 g/s (Table II). However, due to powder arching, the microcrystalline celluloses could not be measured without aiding the flow by vibrating the hopper. Powder arching in hopper can occur if the powder forms a self-supporting arch across the hopper opening (31). The flow rate results of the granules correlate well with the visual observations made during the measurements: the samples with flow rate below 2 g/s exhibited funnel flow while samples with a flow rate higher than 2 g/s generally demonstrated mass flow. The flowability differences can largely be explained by the particle size of the granules. However, the correlation between the particle size and flow rate is not linear. Moreover, the PSD plays a role in the granule flowability; but for example, batches R2 and R12 have fairly similar PSDs but different flow rates. The fact that the flow rate is not directly correlated to the particle size and PSD of the granules is likely to result from the surface properties of the granules, for example roughness. For instance, the R12 granules are spherical while a large amount of the R2 granules seem to be glued to each other leading to highly unsymmetrical granule morphology. Thus, the particle shape is likely to cause the significantly poorer flow rate of R2 granules compared to the R12 batch. In general, it seems that there is a positive correlation between the flow rate and surface roughness of the granules. This is due to the fact that the instrument measures the roughness of the powder bed. Hence, the roughness values are generally higher for powders with larger particle size. This is an issue for further development—information on the roughness of a single particle would also be useful. Based on the results, it was possible to utilise the FS3D method to get an indication of the flow rate of granules in this study. However, the current setup is not suitable for measuring raw material powders as they are generally too cohesive and prone to arching in the hopper to flow into the sample cuvette without mechanical or vibratory assistance. Moreover, tribocharging is likely to influence the flowability of powders more than the flowability of granules due to the smaller particle size of the raw material powders. All in all, the application of the FS3D on flowability measurements needs to be studied more in the future.
It has been previously shown that the weight variation of tablets increases when the particle size of the granules grows (20). Figure 6 shows the correlation plot between the flow rate of the granules and the weight variation of tablets. The plot reveals that the weight variation decreases with improved flowability. However, when the mean particle size of the granules becomes too large, approximately 700 μm, the correlation deteriorates. This can be seen from the two samples in upper right corner of Fig. 6, which have particle sizes of 1,020 and 889 μm. According to the earlier findings, the weight variation increases when the granule size exceeds 500 μm (20). Hence, low weight variation is related to both good flow rate and optimal particle size. Particles with good flowability are able to fill the tablet die completely, while batches with poorer flow rates may not have enough time to flow into the die leaving it partly empty. Moreover, optimal particle size minimises the empty spaces in the die leading to decreased weight variation.
Fig. 6.
Correlation between the flow rate of 12 granule batches and the weight variation of corresponding tablets
Multivariate Analysis
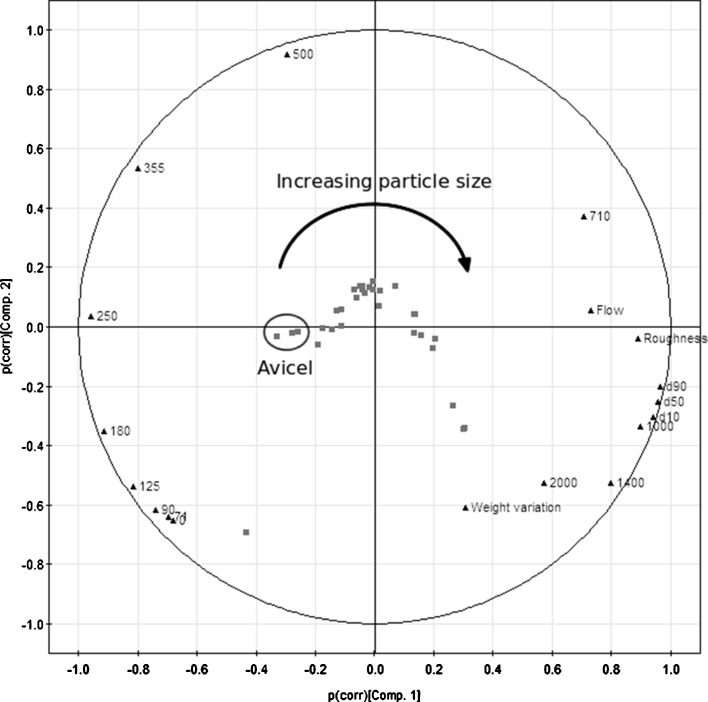
To further get an overview of the data and study the interrelationships between the particle size data, roughness, flowability of the granules and the correlation between the flow rate and weight variation data, principal component analysis was performed. The visualisation is made for the two PCs and is shown as a correlation bi-plot for the scores (samples) and the loadings (measurements; Fig. 7). Generally, the closer the properties and samples (squares) are to each other on the PCA map, the higher the correlation between them. The main information showing the interdependencies of the data are in the first two PCs. The principal component (PC) 1 explains approximately 62% of the variability of data and PC2 23%. The physical properties having the largest impact on the flowability are the surface roughness and the size fraction of 710–1,000 μm. The flow rate is dominated by large granule fractions, while the weight variation of tablets seems to be influenced by both small and large fractions. The PCA map shows that the microcrystalline celluloses are clustered together at the left side of the graph, and the granule batches are spread based on their properties towards the areas that are more on the right side of the PCA map. Generally, it can be seen that the difference along the horizontal direction are influenced by the flow rate and the vertical axis describes differences in weight variation data of the batches analysed.
Fig. 7.
A correlation bi-plot of the FS3D results shows how the granule and Avicel PH101, PH102 and PH200 samples (boxes) are related to the measured physical properties (triangles; PC1 62%, PC2 23%)
CONCLUSIONS
This paper has introduced a novel optical 3D method for particle sizing of granules. The instrument is reliable, easy to operate and also has the potential for rapid flowability screening and extraction of roughness information in the future. The flowability information obtained in the study also correlated well with weight variation of tablets compressed from the studied granules. Nevertheless, further studies on the effect of intraparticle shades and reflective properties of materials on the characterization results should be carried out in the future. All in all, the present imaging technique has a potential for being used as a fast PAT tool in granulation processes, providing also predictive information of material behaviour during dry milling or tablet compression. Overall, the study highlights the challenges in comparing particle size data between different techniques.
REFERENCES
- 1.Halstensen M, de Bakker P, Esbensen K. Acoustic chemometric monitoring of an industrial granulation production process—a PAT feasibility study. Chemom Intell Lab Syst. 2006;84:88–97. doi: 10.1016/j.chemolab.2006.05.012. [DOI] [Google Scholar]
- 2.Hu X, Cunningham JC, Winstead D. Study growth kinetics in fluidized bed granulation with at-line FBRM. Int J Pharm. 2008;347:54–61. doi: 10.1016/j.ijpharm.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 3.Petrak D. Simultaneous measurement of particle size and particle velocity by the spatial filtering technique. Part Part Syst Charact. 2002;19:391–400. doi: 10.1002/ppsc.200290002. [DOI] [Google Scholar]
- 4.Frake P, Greenhalgh D, Grierson SM, Hempenstall JM, Rudd DR. Process control and end-point determination of a fluid bed granulation by application of near infra-red spectroscopy. Int J Pharm. 1997;151:75–80. doi: 10.1016/S0378-5173(97)04894-1. [DOI] [Google Scholar]
- 5.Rantanen J, Räsänen E, Tenhunen J, Känsäkoski M, Mannermaa J-P, Yliruusi J. In-line moisture measurement during granulation with a four-wavelength near infrared sensor: an evaluation of particle size and binder effects. Eur J Pharm Biopharm. 2000;50:271–6. doi: 10.1016/S0939-6411(00)00096-5. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Chen D. Measuring average particle size for fluidized bed reactors by employing acoustic emission signals and neural networks. Chem Eng Technol. 2008;31(1):95–102. doi: 10.1002/ceat.200700176. [DOI] [Google Scholar]
- 7.Hlinak AJ, Kuriyan K, Morris KR, Reklaitis GV, Basu PK. Understanding critical material properties for solid dosage form design. J Pharm Innovation. 2006;1(1):12–7. doi: 10.1007/BF02784876. [DOI] [Google Scholar]
- 8.Bonifazi G, La Marca F, Massacci P. Characterization of bulk particles in real time. Part Part Syst Charact. 2002;19:240–6. doi: 10.1002/1521-4117(200208)19:4<240::AID-PPSC240>3.0.CO;2-#. [DOI] [Google Scholar]
- 9.Huang J, Esbensen KH. Applications of angle measure technique (AMT) in image analysis. Part II. Prediction of powder function properties and mixing components using multivariate AMT regression (MAR) Chemom Intell Lab Syst. 2001;57:37–56. doi: 10.1016/S0169-7439(01)00120-4. [DOI] [Google Scholar]
- 10.Wang XZ, Roberts KJ, Ma C. Crystal growth measurement using 2D and 3D imaging and the perspectives for shape control. Chem Eng Sci. 2008;63(5):1173–84. doi: 10.1016/j.ces.2007.07.018. [DOI] [Google Scholar]
- 11.Eggers J, Kempkes M, Mazzotti M. Measurement of size and shape distributions of particles through image analysis. Chem Eng Sci. 2008;63:5513–21. doi: 10.1016/j.ces.2008.08.007. [DOI] [Google Scholar]
- 12.Liao CW, Tarng YS. On-line automatic optical inspection system for coarse particle size distribution. Powder Technol. 2009;189:508–13. doi: 10.1016/j.powtec.2008.08.013. [DOI] [Google Scholar]
- 13.Sandler N, Wilson D. Prediction of granule packing and flow behavior based on particle size and shape analysis. J Pharm Sci. 2010;99(2):958–68. doi: 10.1002/jps.21884. [DOI] [PubMed] [Google Scholar]
- 14.Yu W, Hancock BC. Evaluation of dynamic image analysis for characterizing pharmaceutical excipient particles. Int J Pharm. 2008;361(1–2):150–7. doi: 10.1016/j.ijpharm.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Patchigolla K, Wilkinson D. Crystal shape characterisation of dry samples using microscopic and dynamic image analysis. Part Part Syst Charact. 2009;26(4):171–8. doi: 10.1002/ppsc.200700030. [DOI] [Google Scholar]
- 16.Staniforth JN. Powder flow. In: Aulton ME, editor. Pharmaceutics—the science of dosage form design. 3. London: Churchill Livingstone; 2007. pp. 168–79. [Google Scholar]
- 17.Andrès C, Bracconi P, Réginault P, Blouquin P, Rochat MH, Pourcelot Y. Assessing the particle size of a broadly dispersed powder by complementary techniques. Int J Pharm. 1998;167(1–2):129–38. doi: 10.1016/S0378-5173(98)00052-0. [DOI] [Google Scholar]
- 18.Allen T. Particle size measurement. 4. London: Chapman and Hall; 1990. [Google Scholar]
- 19.Närvänen T, Seppälä K, Antikainen O, Yliruusi J. A new rapid on-line imaging method to determine particle size distribution of granules. AAPS PharmSciTech. 2008;9(1):282–7. doi: 10.1208/s12249-008-9043-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laitinen N, Rantanen J, Antikainen O, Yliruusi J. New perspectives for visual characterization of pharmaceutical solids. J Pharm Sci. 2004;93(1):165–76. doi: 10.1002/jps.10529. [DOI] [PubMed] [Google Scholar]
- 21.Woodham RJ. Photometric method for determining surface orientation from multiple images. Opt Eng. 1980;19(1):139–44. [Google Scholar]
- 22.Horn BKP. Shape from shading: a method for obtaining the shape of a smooth opaque object from one view. Massachusetts Institute of Technology, PhD Thesis; 1970.
- 23.Laitinen N, Antikainen O, Yliruusi J. Does a powder surface contain all necessary information for particle size distribution analysis? Eur J Pharm Sci. 2002;17(4–5):217–27. doi: 10.1016/S0928-0987(02)00189-6. [DOI] [PubMed] [Google Scholar]
- 24.Laitinen N, Antikainen O, Yliruusi J. Characterisation of particles sizes in bulk pharmaceutical solids using digital image information. AAPS PharmSci Tech. 2003;4(4):383–91. doi: 10.1208/pt040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krogars K, Antikainen O, Heinämäki J, Laitinen N, Yliruusi J. Tablet film-coating with amylose-rich maize starch. Eur J Pharm Sci. 2002;17(1–2):23–30. doi: 10.1016/S0928-0987(02)00134-3. [DOI] [PubMed] [Google Scholar]
- 26.Närvänen T, Lipsanen T, Antikainen O, Räikkönen H, Heinämäki J, Yliruusi J. Gaining fluid bed process understanding by in-line particle size analysis. J Pharm Sci. 2009;98(3):1110–7. doi: 10.1002/jps.21486. [DOI] [PubMed] [Google Scholar]
- 27.Shekunov BY, Chattopadhyay P, Tong HHY, Chow AHL. Particle size analysis in pharmaceutics: principles, methods and applications. Pharm Res. 2007;24(2):203–27. doi: 10.1007/s11095-006-9146-7. [DOI] [PubMed] [Google Scholar]
- 28.Andrès C, Réginault P, Rochat MH, Chaillot B, Pourcelot Y. Particle-size distribution of a powder: comparison of three analytical techniques. Int J Pharm. 1996;144(2):141–6. doi: 10.1016/S0378-5173(96)04737-0. [DOI] [Google Scholar]
- 29.Williams JC. The segregation of particulate materials. A review. Powder Technol. 1976;15(2):245–51. doi: 10.1016/0032-5910(76)80053-8. [DOI] [Google Scholar]
- 30.Närvänen T, Lipsanen T, Antikainen O, Räikkönen H, Yliruusi J. Controlling granule size by granulation liquid feed pulsing. Int J Pharm. 2008;357:132–8. doi: 10.1016/j.ijpharm.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 31.Fitzpatrick JJ, Barringer SA, Iqbal T. Flow property measurement of food powders and sensitivity of Jenike’s hopper design methodology to the measured values. J Food Eng. 2004;61:399–405. doi: 10.1016/S0260-8774(03)00147-X. [DOI] [Google Scholar]