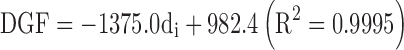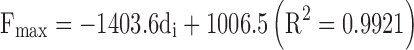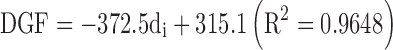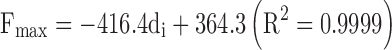Abstract
The current work aimed to propose a system of scoring to rationalize and support the selection of the optimal diameter and length of needles. Four formulations at different viscosity and needles ranging from 21 to 26 G and length ranging from 16 to 40 mm were used. Plunger-stopper breakloose force, maximum force (Fmax), and dynamic glide force were measured by a texture analyzer at the crosshead speed of 1 mm/s. Testing was carried out into air or human subcutaneous tissue. The manual injectability of the highest viscosity product was assessed by ten evaluators. The comparison of the panel test score and the quantitative measurements of the forces permitted to score a given needle-syringe-formulation system keeping also in consideration the pressure created in the subcutaneous space and muscles at the injection site. In particular, the following relationship was drawn: at the Fmax up to 250 mPa, the injection was practically impossible; at Fmax ranging from 160 to 250 mPa, the injection was very difficult; at Fmax in the 125–160 mPa range, the injection was feasible, though with some difficulty; when the values of Fmax were lower 125 mPa, the injection went smoothly. On the basis of these preliminary data, a system of scoring the needle-syringe-formulation system is proposed to rationalize and support the selection of the optimal diameter and length of needles, keeping also in consideration the pressure created in the subcutaneous space and muscles at the injection site.
KEY WORDS: injectability, panel test, texture analyzer
Introduction
Syringeability and injectability are key-product performance parameters of any parenteral dosage form. The former refers to the ability of an injectable therapeutic to pass easily through a hypodermic needle on transfer from a vial prior to an injection, while the latter refers to the performance of the formulation during injection (1). Syringeability includes such factors as ease of withdrawal, clogging and foaming tendencies, and accuracy of dose measurements. Injectability includes pressure or force required for injection, evenness of flow, and freedom from clogging (i.e., no blockage of the syringe needle). Syringeability and injectability concepts are of particular significance for specialized dosage forms, such as injectable emulsions, suspensions, liposomes, microemulsions, and microspheres. Over the last 15–20 years, these systems have become increasingly important in order to overcome issues specifically related to the drug solubility and stability, and achieve the desired rate of release (e.g., prolonged release after intramuscular or subcutaneous injection). Viscosity, density, flow are of paramount importance when considering such non-conventional formulations (2,3).
Syringeability and injectability can be affected by the needle geometry, i.e. inner diameter, length, shape of the opening, as well as the surface finish of the syringe (4). This is of particular significance for self-injection devices, such as pens and auto-injectors, which are equipped with very thin needles. Indeed, patients can use pen injectors which employ 29–31-G needles. As far as pre-filled syringes are concerned, common needle configurations for subcutaneous dosing are 27 G and 25 G (4,5). While reducing the pain of injection, fine needles require an increased force to inject the drug.
It is clear that both the ease of withdrawal of a product from a container (syringeability) and its subsequent injection into the intended administration site (injectability) must be determined for the finished drug product. Both parameters should be understood and characterized during product development.
According to the ICH Q6A Note for Guidance, parenteral formulations packaged in pre-filled syringes or auto-injector cartridges should have test procedures and acceptance criteria related to the functionality of the delivery system (6). Moreover, in the FDA Guidance for Industry on container-closure systems for packaging human drugs and biologics, the evaluation of syringe's performance is required (7). This should be addressed by establishing the force to initiate and maintain plunger movement down the barrel, and the capability of the syringe to deliver the labeled amount of drug product.
In spite of these regulatory requirements, no compendial testing procedures are specified in Pharmacopoeias. If difficulties in syringeability can be easily solved varying the needle size used in the withdraw procedure, in the meantime issues related to injectability can have a big impact on patient's adherence and, therefore, such parameter should be investigated.
In 1979, Ritschel and Suzuki (8) proposed a method to determine injectability of parenterals by determining the time required to smoothly inject a solution, or suspension, into a meat sample under the specified pressure for a given syringe-needle system. In order to measure the force required to inject a liquid through a needle, a dynamometer (9,10) or a micro-capillary rheometer connected to a dynamometer (11,12) were also used. Eventually, the instrument developed by Chien et al. (13) was based on a constant nitrogen pressure, which moved a metallic punch, which was connected to the syringe plunger. These studies reported that injectability was related to both injection speed and product viscosity.
The current work aimed to propose a system of scoring the needle-syringe-formulation system in order to rationalize and support the selection of the optimal diameter and length of needles. Since measurement of injection force while the needle tip is exposed to air cannot sufficiently indicate the formulation's injectability in vivo, the extrusion testing was also carried out by inserting the needle directly in a human subcutaneous tissue model.
Materials and Methods
Materials
In order to evaluate the performances of injectable therapeutics at different viscosity, the following formulations were selected: high viscosity lipid-based systems (R&D Department of Italfarmaco, I, Formulation 1); aqueous suspension (Celestone® Cronodose®, Schering-Plough S.p.A., I, Formulation 2); W/O emulsion (Diprivan®, AstraZeneca, I, Formulation 3) and low-viscosity lipid-based systems (R&D Department of Italfarmaco, I, Formulation 4). A Luer Lock glass syringe (BD Hypak, USA), 0.6 mm inner diameter, was filled with 1 mL tested formulation. Needles of gauge size ranging from 21 G to 26 G and length ranging from 16 to 40 mm (Terumo Europe, B) were attached to the syringe tip.
Viscosity Measurement
The rheological properties of the four formulations were measured using an Ubbelohde capillary viscometer at a temperature of 20 ± 1°C maintained with a thermostatic bath. Values were expressed as average of three determinations (kinematic viscosity, cSt ± standard deviation).
Determination of Injectability
Panel Test
The injectability of the formulation at highest viscosity was assessed by 10 subjects who received different needle-syringe systems (Table I) filled with an aliquot of 1 mL of Formulation 1. Before injecting, the participants were appropriately trained. The participants were asked to evaluate the injectability in terms of the ease of injection and the formulation flow through the needle, using an arbitrary score from 1 to 4. In particular, the arbitrary score for both parameters was defined as following:
score 1 = injection: not possible or very difficult; flow: no flow or drop wise;
score 2 = injection: difficult; flow: initially drop wise, then continuous;
score 3 = injection: moderate; flow: continuous;
score 4 = injection: easy; flow: continuous.
Table I.
Score of Manual Injectability of Formulation 1. Injectability for a Given Needle-syringe Systems Filled with Aliquots of 1 mL of the Highest Viscosity Formulation was Considered Acceptable When the Total Score was up to 30, i.e. The Steady Flow of the Tested Formulation was Obtained with Moderate Difficulty During its Injection
| Needle size | Individual score | Total score | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gauge (G) | Length (mm) | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | |
| 22 | 40 | 4 | 4 | 4 | 3 | 3 | 4 | 4 | 3 | 3 | 4 | 36 |
| 23 | 16 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 38 |
| 30 | 3 | 3 | 1 | 1 | 2 | 4 | 4 | 2 | 3 | 2 | 25 | |
| 24 | 25 | 3 | 2 | 1 | 1 | 2 | 2 | 3 | 1 | 3 | 2 | 20 |
| 25 | 16 | 3 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 3 | 2 | 17 |
| 25 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 | |
| 26 | 12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 10 |
Injectability for a given needle-syringe systems was considered acceptable when the total score was up to 30, i.e. all volunteers were able to inject the tested formulation with moderate difficulty obtaining steady flow. The time required to empty the syringe was also measured.
Quantitative Determination
The measurement of the injection force was performed in compression mode by using a software controlled texture analyzer (Acquati, I). The syringe was positioned in the dynamometer holder, downward needle. The plunger end of the syringe was placed in contact with a 5-N loading cell. Testing was carried out at the crosshead speed of 1 mm/s, representative of manual syringe delivery to patient. The loading force required to displace the plunger was measured (N) as a function of plunger displacement (mm) at a frequency of 50 Hz.
The following parameters were also determined from the force-displacement plot (4):
plunger-stopper breakloose force (or “initial glide force”; PBF): the force required to initiate the movement of the plunger;
maximum force (Fmax): the highest force measured before the plunger finishes its course at the front end of the syringe;
dynamic glide force (DGF): the force required to sustain the movement of the plunger to expel the content of the syringe.
The registered force values were normalized by dividing them for the cross sectional area of the cylindrical plunger and therefore expressed in mPa. The experiments were performed in triplicate.
In order to evaluate the resistance of subcutaneous tissue towards injection, the force required to inject both Formulation 1 and Formulation 4 via 25 G, 16 mm and 24 G, 25 mm needles into human subcutaneous tissue was also assessed. The abdominal skin was obtained from a donor (Eurasian female) who underwent cosmetic surgery. The needle was manually inserted 1 in. underneath the skin; afterwards, the measurement of the injection force was carried out in compression mode as described above.
Statistical Analysis
Tests for significant differences and multi-regression analysis were performed by using the software Origin® 8.5 (Origin Lab., USA). Differences were considered significant at the p < 0.05 level.
Results and Discussion
The kinematic viscosity of the tested formulations increased in the following order: Formulation 2 (1.12 ± 0.00 cSt) < Formulation 3 (1.64 ± 0.00 cSt) < Formulation 4 (18.66 ± 0.02 cSt) < < Formulation 1 (101.23 ± 0.30 cSt).
Qualitative Determination of Injectability
Since it is well recognized that kinematic viscosity deeply affects the ejection of a formulation from the syringe via a needle to the injection site, the injectability of the highest viscosity product, namely Formulation 1, was manually assessed by the panel test. As the needle size might influence patient's comfort and compliance, in this study needles consistent with intramuscularly and subcutaneously injections were investigated.
All subjects were able to inject Formulation 1 into air, independently of needle diameter or length (Table I). The ease of injection into air was acceptable only for Formulation 1 via needle 22 G, 40 mm and 23 G, 16 mm (Table I). Since Formulation 1 was manually extruded by both needles at the average rate of approximately 1 mm/s, the measurements of injection force were carried out with the same crosshead speed.
Determination of Injectability by Texture Analyzer
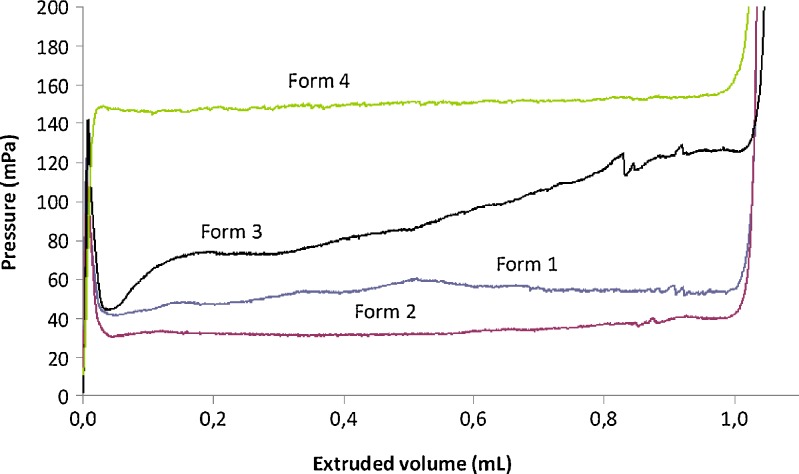
The force applied to a syringe plunger during the injection of a formulation via a needle is dissipated in three ways: (a) overcoming the resistance force of the syringe plunger; (b) imparting kinetic energy to the liquid; and (c) forcing the liquid through the needle (12). Additional force is also required to overcome the pressure resistance when the vehicle is administered to subcutaneous tissue. The prevalence of one or more events determines the profile of loading force versus plunger displacement graph. The patterns obtained by extruding four formulations through the needle 22 G, 40 mm of length are exemplified in Fig. 1.
Fig. 1.
Pressure required to expel the fluid (mPa) as a function of extruded volume (mL) at the crosshead speed of 1 mm/s. Testing was carried out on high viscosity lipid-based systems (Formulation 1), an aqueous suspension (Formulation 2); W/O emulsion (Formulation 3) and low-viscosity lipid-based systems (Formulation 4) via a 22 G, 40 mm needle into air
In the force vs. displacement plot of low-viscosity formulations, three different portions can be identified: the former is related to the force required to displace the plunger, namely plunger-stopper breakloose force (PBF). This maximum value is followed by a plateau (second portion) indicating the streamline of the formulation through the needle occurs with a constant force. In this portion the average load required to sustain the movement of the plunger to expel the content of the syringe is calculated and reported as dynamic glide force (DGF). During the third portion, the force rapidly increases because of the compression of syringe plunger against the end of syringe body. This trend was recorded in the case of Formulation 2 and Formulation 3 (Fig. 1). For both formulations (Table II and Table III), PBF overlapped the maximum force (Fmax) independently of the needle size, suggesting that the highest value of force was required to promote the plunger motion; afterwards, the formulation could freely flow through the needle. Also the force required to inject both formulations ranged from 95 mPa to 110 mPa independently of the needle diameter or length.
Table II.
Parameters of Injectability, Plunger-stopper Break Loose Force (PBF), Maximum Force (F max), and Dynamic Glide force (DGF), for Formulation 1 Injected by a Given Needle-syringe Systems, as Determined from the Force-displacement Plot. The Results are Expressed as the mean of Three Determinations ± Standard Deviation
| Needle | PBF (mPa) | F max (mPa) | DGF (mPa) | |
|---|---|---|---|---|
| Gauge (G) | Length (mm) | |||
| 21 | 40 | 95.96 ± 2.39 | 95.96 ± 2.39 | 42.86 ± 4.39 |
| 22 | 40 | 105.25 ± 16.50 | 105.25 ± 16.50 | 54.14 ± 3.46 |
| 23 | 30 | 95.82 ± 4.86 | 95.82 ± 4.86 | 49.71 ± 4.61 |
Table III.
Injectability Data, i.e.Plunger-stopper Break Loose Force (PBF), Maximum Force (F max), and Dynamic Glide Force (DGF), for Formulation 2 in air by texture analyser. The Results are Expressed as the Mean of Three Determinations ± Standard Deviation
| Needle | PBF (mPa) | F max (mPa) | DGF (mPa) | |
|---|---|---|---|---|
| Gauge (G) | Length (mm) | |||
| 22 | 40 | 107.98 ± 8.34 | 107.98 ± 8.34 | 36.86 ± 6.11 |
| 23 | 16 | 104.76 ± 11.89 | 104.76 ± 11.89 | 51.93 ± 3.57 |
| 24 | 25 | 101.55 ± 6.98 | 101.55 ± 6.98 | 36.75 ± 6.14 |
| 25 | 25 | 110.36 ± 3.62 | 110.36 ± 3.62 | 47.04 ± 9.96 |
| 26 | 12 | 111.67 ± 7.88 | 111.67 ± 7.88 | 43.71 ± 5.79 |
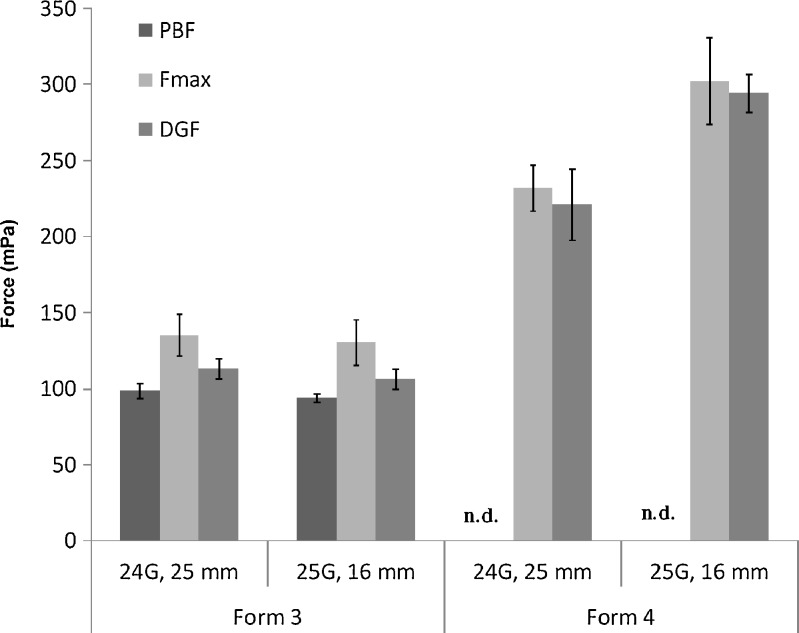
To get a continuous flow of Formulation 4, the maximum force was higher than PBF and DGF (Table IV). Moreover, it can be noticed in Fig. 1 that DGF increased linearly during the plunger displacement.
Table IV.
Injectability Data, i.e.Plunger-stopper Break Loose Force (PBF), Maximum Force (F max), and Dynamic Glide Force (DGF), for Formulation 3 in air by Texture Analyser. The results are Expressed as the Mean of Three Determinations ± Standard Deviation
| Needle | PBF (mPa) | F max (mPa) | DGF (mPa) | |
|---|---|---|---|---|
| Gauge (G) | Length (mm) | |||
| 22 | 30 | 67.14 ± 6.07 | 91.07 ± 10.00 | 71.79 ± 6.07 |
| 40 | 70.36 ± 3.93 | 107.14 ± 18.21 | 83.57 ± 9.64 | |
| 50 | 77.14 ± 10.00 | 113.57 ± 16.79 | 93.21 ± 14.64 | |
| 23 | 16 | 72.50 ± 3.93 | 91.79 ± 12.86 | 72.86 ± 6.43 |
| 25 | 86.43 ± 3.57 | 114.64 ± 16.07 | 90.00 ± 10.36 | |
| 30 | 91.07 ± 5.71 | 126.79 ± 20.36 | 100.36 ± 11.07 | |
| 24 | 25 | 98.57 ± 3.57 | 135.00 ± 13.93 | 113.21 ± 11.43 |
| 25 | 16 | 93.93 ± 5.00 | 130.36 ± 19.64 | 106.07 ± 11.07 |
| 25 | 104.29 ± 0.18 | 156.07 ± 12.50 | 127.50 ± 5.00 | |
| 26 | 12 | 121.07 ± 4.64 | 170.71 ± 11.43 | 142.50 ± 5.36 |
The lipid-based formulation at highest viscosity, namely Formulation 1, evidenced a different pattern (Fig. 1). Once Formulation 1 started to flow through the needle, the force remained almost constant in the second portion of the plot until the compression of plunger to the syringe's body was measured. Thus, PBF could not be determined (Table V). It can be assumed that the limit factor to get a steady streamline is the passage through the needle due to the viscosity of the formulation. Generally speaking, the kinematic viscosity (ν) of the formulation and DGF required to extrude the formulation through the needle were related by a semilogarithmic power law: using the 22 G, 40 mm needle the following relationship was found: DGF = 39 log (ν) + 39 (F = 23.07; R2 = 0.9079).
Table V.
Injectability Data, i.e. Plunger-stopper Break Loose Force (PBF), Maximum Force (F max), and Dynamic Glide Force (DGF), for Formulation 4 in air by Texture Analyzer. The results are Expressed as the Mean of Three Determinations ± Standard Deviation
| Needle size | PBF (mPa) | F max (mPa) | DGF (mPa) | |
|---|---|---|---|---|
| Gauge (G) | Length (mm) | |||
| 22 | 30 | --* | 126.43 ± 8.93 | 115.00 ± 10.00 |
| 40 | --* | 128.21 ± 6.07 | 122.86 ± 7.14 | |
| 50 | --* | 250.36 ± 28.21 | 237.50 ± 16.43 | |
| 23 | 16 | 78.21 ± 19.64 | 139.29 ± 18.93 | 129.29 ± 13.93 |
| 25 | --* | 161.79 ± 6.43 | 156.79 ± 6.43 | |
| 30 | --* | 172.14 ± 3.57 | 166.79 ± 5.36 | |
| 24 | 25 | --* | 275.36 ± 16.07 | 227.50 ± 8.93 |
| 25 | 16 | --* | 231.79 ± 5.00 | 221.07 ± 2.86 |
| 25 | --* | 302.14 ± 9.29 | 294.29 ± 10.00 | |
| 26 | 12 | --* | 373.57 ± 18.57 | 365.71 ± 18.93 |
* not detectable
In the case of lipid-based formulations, it can be noticed that the thinner the needle diameter, the higher the DGF and Fmax values. As an example, when the needle length was kept constant at 25 mm, linear relationships between the needle inner diameter expressed in mm (di) and the DGF as well as the Fmax were found:
Formulation 1, having higher viscosity than Formulation 4 also demonstrated higher slope value.
A linear trend was also observed keeping constant the needle inner diameter. As an example when the needle inner diameter was set at 22 G or 23 G, the DGF proportionally increased with respect to the needle length for Formulation 4 (R2 > 0.9984). Even if such correlations cannot have a general relevance, they allowed us to qualitatively highline the dependence of the injectability on kinematic differences of formulations. A full evaluation of the dependence of Fmax on the needle gauge and length and the formulation viscosity was also carried out by a multivariate regression analysis combining all 28 performed measurements. A poor sound of correlation was found:
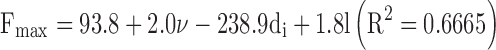 |
where l is the needle length. Moreover, the only significant parameter influencing the extrusion of formulation through a given needle-syringe systems appeared to be the nominal inner diameter of the needle (p < 0.02).
Measurements of injection force while the needle tip is exposed to air cannot indicate the formulation's injectability in vivo since subcutaneous tissues have limited physiological space and provide resistance toward injection. Hence, further experiments were carried out by using a subcutaneous human tissue model to determine injectability. For ethical concerns, the use of subcutaneous tissue was limited to investigate the performances of Formulation 1 and Formulation 4 via needles 24 G, 25 mm and 25 G, 26 mm. The measured texture profiles overlapped those recorded when injected towards air (data not shown) and the quantitative values are summarized in Fig. 2. As expected, the values of Fmax and DGF were higher because of the resistance opposed by the subcutaneous tissues.
Fig. 2.
Injectability paramenters (PBF: plunger-stopper break loose force, F max: maximum force, DGF: dynamic glide force) for Formulation 1 and Formulation 4 injected in subcutaneous tissue by texture analyzer. The results are expressed as the mean of three determinations ± standard deviation
In all cases, the ratios between the force values obtained in the different experimental set-ups were almost constant to 1.1. Being independent of needle size, these ratios were mainly related to the increase of force required to overcome the tissue resistance. Hence, the force values obtained injecting formulations towards air should be rectified by this ratio in order to obtain more comprehensive information supporting the selection of the needle/syringe system.
The comparison of the manual injections (Table I) and the in vitro normalized values for the highest viscosity formulation (Table II) led us to draw a relationship between the arbitrary score and the values of force measured by texture test, namely:
at the Fmax up to 250 mPa, the injection was practically impossible and it corresponds to the total score from 0 to 15;
at Fmax ranging from 160 to 250 mPa, the injection was very difficult, corresponding to the total score from 16 to 25;
at Fmax in the 125–160 mPa range, the injection was feasible, though with some difficulty, corresponding to the total score to the total score from 26 to 35;
when the Fmax were lower 125 mPa, the injection went smoothly and it corresponds to total score from 36 to 40.
Conclusion
At high viscosity value, the flow of the product through the needle was the most critical step, rather than the initial plunger displacement. To select the needle-syringe systems, the back pressure created in the subcutaneous space at the injection site should be always carefully taken in consideration because it might influence the force required to displace the plunger.
The preliminary results reported in this study allowed us to establish a scoring system to rationalize and support the selection of the optimal the needle-syringe-formulation system.
References
- 1.Groves MJ. Parenteral Technology Manual. Interpharm Press. 1988;9:99–100. [Google Scholar]
- 2.Veldhuis G, Gironès M, Bingham D. Monodisperse microspheres for parenteral drug delivery. Drug Deliv Tech. 2009;9(1):24–31. [Google Scholar]
- 3.Kumar R, Palmieri MJ. Points to consider when establishing drug product specifications for parenteral microspheres. AAPS J. 2010;12:27–32. doi: 10.1208/s12248-009-9156-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Overcashier DE, Chan EK, Hsu CC. Technical considerations in the development of prefilled syringes for protein products. Am Pharm Rev. 2006;9(7):77–83. [Google Scholar]
- 5.Jaber A, Bozzato GB, Vedrine L, Prais WA, Berube J, Laurent PE. A novel needle for subcutaneous injection of interferon beta-1a: effect on pain in volunteers and satisfaction in patients with multiple sclerosis. BMC Neurol. 2008;8:38. doi: 10.1186/1471-2377-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EMEA, ICH Q6A, Note for Guidance Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances (CPMP/ICH/367/96), May 2000. [PubMed]
- 7.FDA, CDER, Guidance for Industry Container Closure System for Packaging Human Drugs and Biologics, May 1999.
- 8.Ritschel WA, Suzuki K. In vitro testing of injectability. Pharm Ind. 1979;41:468–475. [Google Scholar]
- 9.Capes DF, Herring D, Sunderland VB, McMillan D, McDonald C. The effect on syringe performance of fluid storage and repeated use: implications for syringe pumps. PDA J Pharm Sci Technol. 1996;50(1):40–50. [PubMed] [Google Scholar]
- 10.Dexter MB, Schott MJ. The evaluation of the force to expel oily injection vehicles from syringes. J Pharm Pharmacol. 1979;31:497–500. doi: 10.1111/j.2042-7158.1979.tb13570.x. [DOI] [PubMed] [Google Scholar]
- 11.Allahham A, Stewart P, Marriott J, Mainwaring DE. Flow and injection characteristics of pharmaceutical parenteral formulations using a micro-capillary rheometer. Int J Pharm. 2004;270:139–148. doi: 10.1016/j.ijpharm.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Allahham A, Mainwaring DE, Stewart P, Marriott J. Development and application of a micro-capillary rheometer for in-vitro evaluation of parenteral injectability. J Pharm Pharmacol. 2004;56:709–716. doi: 10.1211/0022357023457. [DOI] [PubMed] [Google Scholar]
- 13.Chien YW, Przybyszewski P, Shami EG. Syringeability of nonaqueous parenteral formulations – development and evaluation of a testing apparatus. J Parenter Sci Technol. 1981;35(6):281–284. [PubMed] [Google Scholar]