Abstract
Many nasally applied compounds gain access to the brain and the central nervous system (CNS) with varying degree. Direct nose-to-brain access is believed to be achieved through nervous connections which travel from the CNS across the cribriform plate into the olfactory region of the nasal cavity. However, current delivery strategies are not targeted to preferentially deposit drugs to the olfactory at cribriform. Therefore, we have developed a pressurized olfactory delivery (POD) device which consistently and non-invasively deposited a majority of drug to the olfactory region of the nasal cavity in rats. Using both a hydrophobic drug, mannitol (log P = −3.1), and a hydrophobic drug, nelfinavir (log P = 6.0), and POD device, we compared brain and blood levels after nasal deposition primarily on the olfactory region with POD or nose drops which deposited primarily on the respiratory region in rats. POD administration of mannitol in rats provided a 3.6-fold (p < 0.05) increase in cortex-to-blood ratio, compared to respiratory epithelium deposition with nose drop. Administration of nelfinavir provided a 13.6-fold (p < 0.05) advantage in cortex-to-blood ratio with POD administration, compared to nose drops. These results suggest that increasing the fraction of drug deposited on the olfactory region of the nasal cavity will result in increased direct nose-to-brain transport.
Key words: intranasal drug delivery, mannitol, nelfinavir, nose-to-brain, olfactory
Introduction
It is generally accepted that once drug molecules are applied to the nasal cavity and deposited on the nasal epithelium, they either act locally, or diffuse through the epithelium into the lamina propria where they are absorbed into the capillaries of the nasal cavity and distribute to the rest of the body through the blood stream (1). In this case, drugs are first absorbed from the nose to the blood before reaching the central nervous system (CNS). Instead, direct nose-to-brain delivery describes the phenomenon where drugs applied to the nasal cavity penetrate directly from the nasal cavity to the brain and cerebral spinal fluid (CSF) thus bypassing the blood in their first passage. Some possible advantages that can be gained through this nose-to-brain route are rapid distribution to the brain, lower systemic concentrations, and CNS delivery of drugs that do not cross the blood–brain barrier (BBB) (2). While the exact mechanisms of direct nose-to-brain transport are not completely understood, growing anatomical and experimental evidence suggests that drugs primarily utilize the nervous connections in the olfactory region of the nasal cavity in order to directly distribute to the CNS (3).
The olfactory neurons in the olfactory epithelium have dendrites permeating the olfactory epithelium while projecting a single axon past the porous cribriform plate onto the olfactory bulb. These neurons represent the only primary neurons that are directly exposed to the outside environment (4). These olfactory neurons and their glial support cells extend the subarachnoid matter, which contains CSF, through the cribriform plate and into the lamina propria. These subarachnoid extensions surrounding the axon create a low-resistance fluid pathway between the olfactory mucosa and the CSF in the subarachnoid space (5). Many nose-to-brain drug delivery studies have described high concentrations of drug appearing in and around the olfactory bulb within minutes after drug administration (6–8). In addition, several drugs have been visualized crossing these fluid pathways in the cribriform plate region from the nasal cavity to the CNS (9–11).
Despite the potential therapeutic use of the nose-to-brain delivery route, some studies have found very limited nose-to-brain transport (12) and others have found no evidence of direct transport from the nasal cavity to the brain (13) after nasal administration. Inconsistent deposition of drug on the olfactory region of the nasal cavity could be an important experimental factor limiting the nose-to-brain distribution route. The olfactory epithelium makes up only 3–10% of the surface area of the nasal cavity in humans (14) and its position in the nasal cavity makes it a challenge to consistently deliver drug to the olfactory epithelium lining between nasal cavity and the brain. The olfactory region is located in the upper back quartile of the nasal cavity in a narrow slit approximately 1–2 mm wide. Traditional nasal pump devices used in most nasal drug delivery studies in humans are estimated to only deposit ≤5% of a typical dose in the olfactory region (15). Typical nasal pumps tend to have a wide plume angle that mainly deposit on the respiratory epithelium which would lead primarily to uptake in the blood stream and limit the percentage of drug directly distributed to the brain.
In addition, although much of the experimental evidence for nose-to-brain distribution comes from rodent studies (16), very few studies have investigated the localized distribution of drug within the nasal cavity. Although the olfactory region in rodents makes up 50% of the total surface area of the nasal cavity (17), it is also located in a part of the nasal cavity which is difficult to apply drug. A variety of nasal delivery methods are typically used in studies investigating nose-to-brain delivery in rodents, but very few studies specifically and consistently deposit drug on the olfactory epithelium. The most commonly used nasal administration modes are nose drops (18–21), in which the animal is placed on its back in a supine position while liquid drops are snorted into the nasal cavity, or inserting a soft catheter connected to a microsyringe into the nasal cavity and applying a volume of drug a set distance into the nasal cavity from the naris (11,22). These methods may effectively deliver drug to the nasal cavity; however, they tend to involve a large dose volume resulting in saturation of both the respiratory and olfactory epithelium. In addition, very few studies have investigated the drug localization within the nasal cavity and the percentage of drug deposited on the olfactory region could vary significantly.
Charlton et al., as part of a study of intranasal delivery of an angiotensin antagonist, delivered drug with a catheter at a distance of either 7 or 15 mm into the nasal cavity (23), primarily targeting the respiratory or olfactory epithelium, respectively. Delivering the drug with the 15-mm catheter resulted in significantly higher drug concentrations in the olfactory bulbs with both a standard solution and a mucoadhesive formulation. Significantly less angiotensin was also found in the blood after delivery deeper into the nasal cavity. Despite using a small sample number (N = 3), this data suggest that variation in drug deposition localization within the nasal cavity can impact the brain/blood concentration ratios and possibly the fraction of drug directly delivered to the brain. However, nasal catheter may not be practical or convenient for general human applications.
Thus, the goal of this study is determine whether enhanced drug deposition on the olfactory epithelium will translate into corresponding improvements in the brain. To do so, we have evaluated a hydrophilic marker, mannitol, and a hydrophobic drug, nelfinavir, as two model compounds for direct nose-to-brain drug delivery. Concentrations of these drugs in various regions of the brain, blood stream, and nasal cavity were determined at 30 min. We discovered that preferential drug deposition on the olfactory region leads to increased drug distribution from the nasal cavity to the brain for both hydrophilic and hydrophobic compounds.
Materials and Methods
Materials
Coomassie blue (Sigma-Aldrich, St. Louis, MO) dye was used to determine nasal cavity deposition and aerosol patterns. Mannitol was 14C labeled with a specific activity of 0.1 mCi/ml and purchased from Moravek Biochemicals (Brea, CA). Unlabeled mannitol was purchased from Sigma-Aldrich (St. Louis, MO). Nelfinavir was 14C labeled with a specific activity of 1.0 mCi/ml and purchased from Amersham Biosciences (Piscataway, NJ). Unlabeled nelfinavir was gratefully donated by the NIH AIDS Research and Reference Reagent Program (Germantown, MD). Biosol and Bioscint were purchased from National Diagnostics (Atlanta, GA). Propylene glycol was purchased from Sigma (St. Louis, MO). Ethanol was purchased from Fisher Scientific (Fair Lawn, NJ). Medical grade nitrogen gas was purchased from Airgas Nor Pac (Vancouver, WA). The 0.9% saline solution was purchased from Hospira Inc (Lake Forest, IL). Nembutal was purchased from Abbott Laboratories (North Chicago, IL). Nembutal was purchased from Abbot Laboratories (North Chicago, IL). Biosol and Bioscint were purchased from National Diagnostics (Atlanta, GA). All other materials were reagent grade.
Drug Formulation
Mannitol doses used in the histopathology study consisted of 0.2 mg mannitol dissolved in 0.9% saline solution, pH 7.4. Radiolabeled mannitol dose solutions used in the distribution studies consisted of 0.2 mg unlabeled mannitol and 2.0 μCi 14C mannitol in a solution of 98% H2O and 2% EtOH, pH 7.4. The total volume of each nasal mannitol dose was 20 μl. Nelfinavir dose solutions consisted of 0.12 mg unlabeled nelfinavir and 2.0 μCi 14C nelfinavir in a solution of 75% propylene glycol and 25% EtOH. The total volume of each nasal nelfinavir dose was 20 μl. All drug solutions were mixed on the day of drug administration.
Animals
Adult male Sprague–Dawley rats (200–300 g; Harlan, Indianapolis, IN) were housed under a 12 h light/dark cycle with food and water provided ad libitum. Animals were cared for in accordance with institutional guidelines, and all experiments were performed with an approved protocol from the University of Washington Institutional Animal Care and Use Committee.
Pressurized Olfactory Delivery Device Construction
The overall construction of the pressurized olfactory delivery (POD) nasal aerosol device is illustrated in Fig. 1. A pressurized nitrogen gas supply was connected to a standard two-valve pressure regulator. Plastic tubing with a 200-psi pressure rating connected the pressure regulator to the inflow connection of a pneumatic solenoid (Cramer Decker Industries, Santa Ana, CA). The solenoid was regulated with a foot pedal actuated Gralab 555 digital timer (Gralab Corporation, Centerville, OH). On the outflow connection of the solenoid was a 3-ml cylinder, which made up the device body and fit securely to the solenoid. On the end of the device body was a custom fit aerosol nozzle with a 0.8-mm outside diameter. The nozzle was fitted with an aerosol insert composed of a small length (2.0 mm) of metal cylinder, which had two spiral fluid passages in which the fluid/gas mixture traveled. This served to mix the nitrogen and liquid drug to create an aerosol output to enhance penetration into the nasal cavity towards the cribriform plate area while minimizing pressure on the nasal epithelia. Finally, a fluorinated ethylene–propylene liner was placed over the outside of the metal tip in order to protect the nasal epithelia from being damaged by the nozzle during use.
Fig. 1.
Schematic presentation of the pressurized olfactory delivery device. The pressurized nitrogen is controlled by a pneumatic solenoid which releases the gas in increments of 0.1 s. The gas enters the nozzle outlet and mixes with the liquid dose, which flows through the outlet producing a narrow flow with rotational velocity
The basic operation of the POD device in rats was as follows: the animal was anesthetized with pentobarbital, the dose was loaded into the device and the nozzle was carefully placed 8.0 mm into the rat nasal cavity and pointed in the direction of the cribriform plate. Then the foot pedal switch was pressed to actuate the solenoid for 0.1 s to discharge the aerosol dose.
POD Device Characterization
A fluorescence assay was used to determine if the total desired volume was dispensed from the device with each actuation. A solution of 50 μg/ml fluorescein was dispensed from the device into a well in a 24-well plate that was prefilled with 1 ml deionized H2O. After collection of the aerosol, the solution was mixed by pipetting up and down several times. Three volumes 5, 10, and 25 μl which could be used for rat nasal delivery were tested at two different pressures, 20 and 30 psi. The fluorescence of the solution was measured on a PerkinElmer 1420 multivariable fluorescence plate reader (PerkinElmer, Waltham, MA). The fluorescence signal of the collected volume was compared with the expected signal to calculate the percent of drug expelled from the device with each actuation.
The aerosol droplet size distributions of the POD device were determined by a Phase Doppler Particle Analyzer (TSI, Shoreview, MN) using a 200-mW argon laser emitting beams of 488 and 514.5 nm wavelength (Ion Laser Technology, model # 5500A-00). Initially, the measurement volume was moved across the aerosol stream to determine the edges of the spray. Then, sizing measurements were determined at 1-mm intervals across the width of the spray, taking 30,000 measurements at each interval. Sizing data is presented as a volume weighted mean and span, defined in Eq. 1 where Dv is droplet frequency distribution.
 |
1 |
Nasal Cavity Dye Deposition Testing
Deposition of a coomassie blue dye was tested after intranasal administration using a variety of administration techniques. Under pentobarbital anesthesia, the rats (N = 6) received dye solution as a nasal instillation of a bolus of 10 μl dye solution via a catheter, which was either 15 or 7 mm long, while in the supine position. The animals receiving dye solution via the POD device were treated as described above as the standard use of the POD device. The animals receiving dye solution via nose drops were placed on their back and a 10-μl drop of dye solution was placed near the right naris with a pipette and allowed to be snorted into the nasal cavity. After 2 min, the process was repeated in opposite naris until the desired volume was reached. Shortly after administration was complete (<5 min), the animals were overdosed with 250 mg/kg pentobarbital. The nasal cavity was then bisected at the septum, the septum was removed, and the tissues were examined for dye localization.
Drug Administration and Tissue Collection
Animals were anesthetized with pentobarbital and were placed on their backs on a rodent heat pad (Harvard Apparatus, Holliston, Massachusetts) to maintain body temperature. A group of animals were given 5 μl of mannitol as nose drops every minute in alternating naris for a total of 20 μl volume. In another group, mannitol was given using the POD aerosol device. These rats were first given a dose in the left naris; followed after 2 min with a second 10 μl dose in the right naris for a total volume of 20 μl. Nelfinavir nose drop and POD doses were administered with identical methods and volumes as described for the mannitol. At 30 min after radiolabeled drug dosing the animals were euthanized. Blood was collected and the brain was removed. The brain was dissected into the olfactory bulbs, cortex, diencephalon, brainstem, and cerebellum. The olfactory bulbs were the last tissues to be removed from the skull. Cervical spinal cord from C1 to C5 was removed from the body as well. The nasal cavity was also carefully opened and the olfactory and respiratory epithelia were also removed for deposition quantification.
Sample Processing
Tissue samples were weighed and placed in 4-ml polypropylene scintillation vials with 400 μl of Biosol. Each blood sample was placed into 400 μl of Biosol immediately after collection. All samples were placed in a water bath at 55°C overnight to digest the tissues. The tissues were allowed to cool to room temperature and the vials were filled with Bioscint scintillation fluid. A volume of 40 μl of 30% hydrogen peroxide was added to each of the tissue sample with Bioscint before scintillation counting to determine radioactivity of the drugs. The samples were kept at room temperature for 2 days before determination of radioactivity.
Radiolabeled Sample Analysis
Radioactivity in each sample was analyzed with a Packard Tricarb 1600 TR liquid scintillation counter (Packard Instruments, Meriden, CT). With each sample set analysis, standard curve and control samples were run with the unknown samples.
Data Analysis
Brain and blood concentrations were determined with standard curves constructed with blank tissue or blood that was spiked with radiolabeled drug and processed according to the methods described for the radiolabeled samples. An unpaired t test was used to compare drug distribution in the brain after nose drops or POD delivery with mannitol and nelfinavir.
Histopathologic Examination of Rat Nasal Cavity
For histopathologic analysis, rats that received POD drug administration were anesthetized with pentobarbital delivered via IP injection. These animals (N = 6) were placed in the supine position and they received a 10-μl dose of 0.1 mg mannitol dissolved in 0.9% NaCl with the POD device in each naris according to the method described above. Nasal tissues from two untreated control rats were processed in the same manner as the treated tissues. Three driving pressures (10, 20, and 30 psi) of the POD device were tested. Within 5 min of dosing, the animal was euthanized. Histopathologic analysis of the POD nasal device was conducted according to the method of Young (24). In brief, the head was removed and the brain and jaw were removed from the head along with any other listed tissues. The nasal cavity was initially fixed in a solution of 10% formalin and then decalcified in a solution of 10% EDTA. The tissue was then placed in 70% ethanol before being embedded in paraffin, sectioned, and stained with hematoxylin and eosin stain.
Results
POD Device Characterization
The POD device produced consistent volume and aerosol size suitable for targeting the dispensed aerosol to olfactory produce the following characteristics: the volumetric mean aerosol diameter of the solution using the POD aerosol device was 29.18 μm with a span of 9.5 μm using a device input pressure of 20 psi. The output pressure appeared to be lower than 5 psi at a 3-mm distance. At 10 μl dose, the device dispensed the set volume with 99.3 ± 2.6% accuracy. This device under these conditions was used for the subsequent experiments to characterize intranasal and olfactory deposition and drug delivery studies.
Nasal Cavity Deposition Testing
Dye deposition within the rat nasal cavity was determined after delivery with either the POD device or nose drops or nasal catheter. All of the panels in Fig. 2 show a sagittal view of the rat nasal cavity. When using 10 μl of dye, the POD administration resulted in deposition primarily on the olfactory epithelium area of the nasal cavity (Fig. 2, left panels). The dye was found primarily on the posterior two-thirds of the olfactory turbinates and within the folds of the turbinates as well as deeper within the nasal cavity along the cribriform plate region of the nasal cavity. In addition, the dye could be visualized on the cribriform plate when looking from both the nasal cavity and brain cavity. When using 30 μl of dye administered with the POD device, the localization within the nasal cavity was similar to that after a 10-μl POD dose except that the dye localized more broadly on the olfactory turbinate structures including the front third and there was minor deposition on the respiratory epithelium near the center of the nasal cavity.
Fig. 2.
Dye deposition of POD or nose drops within rat nasal cavity. Ten microliters (upper panels) or 30 μl (lower panels) of blue dye was administered to the rat nasal cavity using a single dose from the POD device (left panels) or nose drops administered in 5 μl drops every minute (right panels). The blue circle indicates the olfactory epithelium within the rat nasal cavity, while the green circle outlines the respiratory epithelium. The white line indicates the cribriform plate, which is the interface between the nasal cavity and the olfactory bulb area of the brain
Administering the dye by nose drops (Fig. 2, right panels) resulted in deposition primarily on the respiratory epithelium. Administering 10 μl of dye per naris as nose drops resulted in the dye being localized completely to the respiratory epithelium. No noticeable dye staining was apparent in the olfactory region, or in the trachea or esophagus. The nose drop administration of 30 μl of dye per naris resulted in saturation of the entire nasal cavity and thus, deposition throughout the respiratory epithelium and possibly partially on the olfactory epithelium. In contrast to the deposition of dye after the POD delivery, the 10 μl nose drops per naris only led to minor if any deposition on the olfactory epithelium which was limited to the very anterior portion of the epithelium.
In the radiolabeled drug experiments we quantified the relative amount of drug on the respiratory and olfactory epithelia 30 min after delivery to get a quantitative indication of the relative deposition on the respiratory and olfactory epithelia after each nasal drug delivery method. Nose drop administration led to 89.3 ± 6.5% of drug on the respiratory epithelium and 10.7 ± 2.1% on the olfactory epithelium at 30 min after drug administration. After POD administration, 68.3 ± 7.1% of drug in the nasal cavity was found on the olfactory epithelium and 31.7 ± 6.8% was found on the respiratory epithelium 30 min after drug administration.
Effects of POD Delivery on Mannitol and Nelfinavir Distribution in the Brain and Blood
To evaluate the effects of the POD device, which provides preferential deposition of aerosolized compound to olfactory region, on enhancement in the brain drug exposure, we tested the POD for dispensing a hydrophilic compound mannitol (log P = −3.9) and a hydrophobic anti-HIV drug nelfinavir (log P = 6.0). Mannitol and nelfinavir were chosen based on each drug having very poor penetration into the brain, due to limited membrane permeability and efflux transport, respectively. In addition, using these two drugs allowed for a better understanding of the impact of hydrophobicity on transport from the nasal cavity to the brain. Rats administered with these two compounds in the POD device or nose drop were evaluated for the drug concentrations in the brain at 30 min to determine the differential effects.
As shown in Fig. 3, compared to animals treated with nose drops (which deposit the dose primarily on the respiratory region of the nasal cavity; Fig. 2), those that received mannitol with the POD device resulted in significantly higher brain concentrations in the olfactory bulbs, cortex, diencephalon, and cerebellum. The animals dosed with mannitol via the POD device exhibited 1.86 ± 0.10 nmol/g (mean ± SD) in the olfactory bulbs and 0.15 ± 0.10 nmol/g in the cortex at 30 min. These tissues after POD administration were 25.6-fold and 11.7-fold higher respectively than after nose drop administration. Due to the variability, the brainstem and spinal cord, which exhibited higher concentrations with POD treatment, did not reach statistically significant difference, when compared with nasal drops. There was also no significant difference in blood mannitol concentration between the POD and nose drop administration. In comparison, animals receiving mannitol by nose drops exhibited less than 0.1 nmol/g in the olfactory bulbs, and less than 0.02 nmol/g in the brain’s cortex, brain stem, and spine at an identical time point. The diencephalon and cerebellum were also significantly different when dosed via POD compared to nose drop administration. These data were analyzed further by normalization with mannitol concentrations in blood and are presented in Fig. 4. Again, the largest difference observed was in the olfactory bulbs. The administration of mannitol with the POD device resulted in a 9.64-fold increased concentration compared to nose drops. The cortex also had a large concentration difference with POD administration of mannitol resulting in 3.56-fold increase in concentration compared to nose drop.
Fig. 3.
Brain concentrations of mannitol 30 min after a 0.2-mg dose. The brain concentrations in the olfactory bulbs, cortex, diencephalon, and cerebellum were significantly greater when delivered near the cribriform plate with the POD device than when delivered to the respiratory region with nose drops (*p < 0.05)
Fig. 4.
Blood-normalized brain concentrations of mannitol 30 min after a 0.2-mg dose. The blood-normalized brain concentrations after nose drop were significantly higher than after IV administration in all brain regions. The blood-normalized olfactory bulb and cortex mannitol concentrations after POD administration were significantly higher than after nose drop (*p < 0.05 compared with nose drop administration)
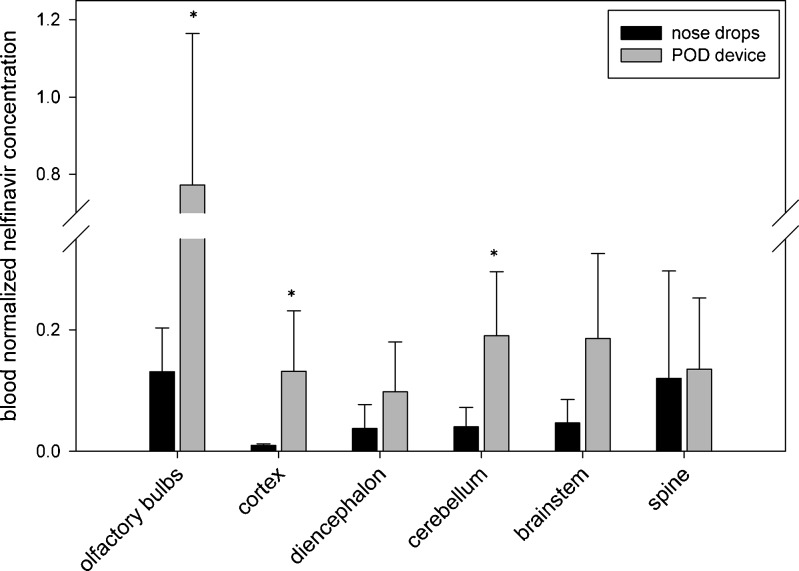
We next investigated a lipophilic (log P = 6) anti-HIV drug nelfinavir. Despite the lipophilicity of nelfinavir, we found a lower fraction of drug accumulated in the olfactory bulb than that observed for mannitol. Nevertheless, compared to animals treated with nelfinavir in nose drops, those receiving nelfinavir via the POD device exhibited higher drug concentrations in the olfactory epithelium of the nasal cavity and significantly higher brain concentrations (P < 0.05; Fig. 5). At 30 min, the olfactory bulb and the cortex nelfinavir concentrations in animals treated with the POD device were significantly higher than after nose drop administration. These tissues had concentrations of 0.77 ± 0.39 and 0.132 ± 0.10 nmol/g, respectively, whereas those treated with nose drops had concentrations of 0.13 ± 0.07 and 0.01 ± 0.003 nmol/g, respectively. Interestingly, the blood concentration of nelfinavir was 3.2 times higher (P < 0.05) after nose drop administration than after POD administration. In addition the blood concentration of nelfinavir after POD administration was significantly lower than after nose drops (Fig. 6). When comparing the blood-normalized nelfinavir concentrations in the brain, the olfactory bulbs, cortex, and cerebellum all displayed significantly higher values compared to after nose drop administration.
Fig. 5.
Brain concentrations of nelfinavir. The brain concentrations 30 min after a 0.14-mg dose of nelfinavir were significantly greater when delivered near the cribriform plate via POD spray than when delivered to the respiratory region with nose drops (*p < 0.05)
Fig. 6.
Blood-normalized brain concentrations of nelfinavir. The blood-normalized brain concentrations 30 min after a 0.14-mg dose of nelfinavir were significantly greater when delivered near the cribriform plate via POD than when delivered to the respiratory region with nose drops (*p < 0.05)
Some of the variability in brain concentrations we saw with both drugs was probably due to regional differences in brain distribution. In order to better understand the overall nose-to-brain differences between POD administration and nose drops all of the brain regions except for the olfactory bulbs were combined and then averaged across animals for both mannitol and nelfinavir. When looking at the blood-normalized concentrations of the olfactory bulb and the remainder of the brain it is clear that POD delivery led to enhanced brain delivery that cannot be accounted for by blood uptake (Fig. 7).
Fig. 7.
Blood-normalized drug concentrations 30 min after delivery. The blood-normalized olfactory bulb and brain concentrations of nelfinavir and mannitol were significantly higher after delivery to the olfactory region with the POD device compared to the nose drop treatments (*p < 0.05)
Histopathologic Examination of Rat Nasal Cavity
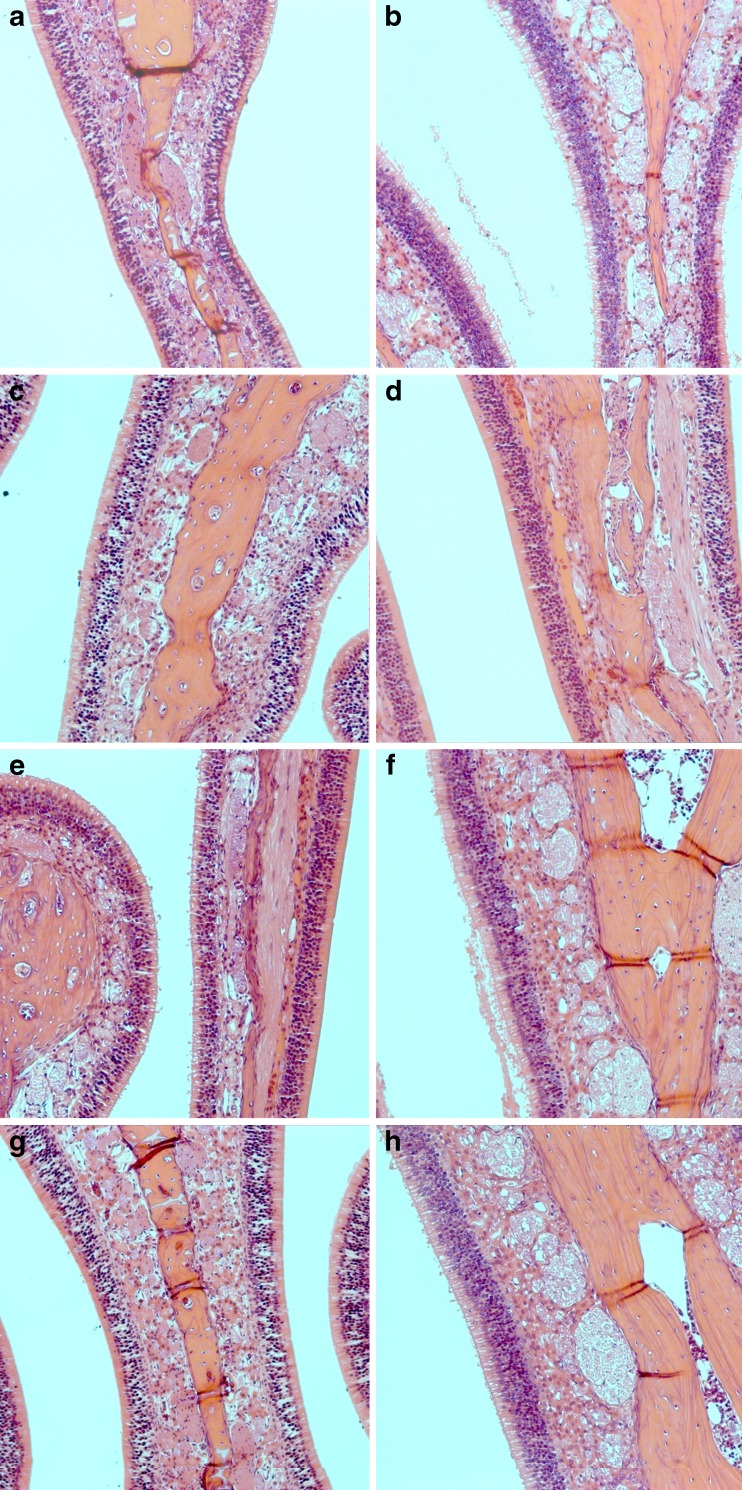
To determine whether the impact of aerosol delivered by POD device on rat nasal tissue, we performed a histopathology analysis on the nasal tissues after exposing the rat with aerosol generated by POD device with increasing pressure. Analysis of the tissues collected from rats exposed to aerosol generated by various pressures did not appear to be different from the control rats (Fig. 8). The septum area of the nasal cavity was closely examined because the method of administration of the POD device included placing the nozzle approximately 8 mm into the rat naris primarily traveling along the septum. The histopathology sections presented in Fig. 8 show the same location of the septum which would have been in immediate contact with the POD aerosol, in which there is no discernable damage in the POD-administered animals compared to untreated control animals. There was also no detectable difference in the olfactory region after administration of POD with increasing pressures tested.
Fig. 8.
Histopathology images after POD spray. All images are from septum of nasal cavity in the olfactory region of rat nasal cavity. a, b shows control animals which received no POD spray, c, d received POD spray with a driving pressure of 10 psi, e, f with a driving pressure of 20 psi, and g, h with a driving pressure of 30 psi. No histological damage was observed from the POD spray
Discussion
While the direct access of drug and xenobiotics to the brain has been described, varying degree of success and replication of the reported results continue to challenge the field. One of the key limitations is a device or approach that can provide consistent deposition of the drug to the site of entry to the brain, i.e., olfactory epithelium located at nose to brain interface supported by the cribriform plate. We have successfully designed and used a pressurized olfactory delivery device to deposit aerosol drug to the olfactory epithelium, instead of the respiratory epithelium, within the nasal cavity. The dye deposition experiments in rats demonstrated that the POD administration deposited the dye preferentially at the olfactory region with no noticeable damage to the nasal mucosa. In contrast, the nose drops at the volumes used in this study (<30 μl) deposited at of the respiratory region. Our preliminary studies with 7- and 15-mm catheters for deposition to the olfactory region also showed much lower fraction of drug deposited at the olfactory region [even though this method has been used in many studies with the objective of targeting the olfactory region (11,25–27)]. With catheter administration, the dye was localized to the distance that the catheter was inserted, but the dye did not penetrate the tight spaces between the nasal septum and the turbinates of the olfactory region well (data not shown). In addition, the rats exhibited a head shaking reaction when the 15-mm catheter was placed in the nasal cavity, which was not observed with POD administration. Based on the results of these dye deposition studies, the nose drop and POD methods of nasal administration were used in the radiolabeled drug distribution studies. These data highlighted the need to monitor the intranasal deposition to measure drug delivery to the brain and CNS by the nasal route.
Our study with POD drug delivery to the posterior olfactory region of the nasal cavity resulted in preferential brain distribution compared with drug delivery to the anterior respiratory region in rats by typical nasal drop administration. Also, the POD device mediated direct brain delivery was observed for both a hydrophilic and hydrophobic small molecule drug, albeit to different degrees. Administering mannitol to the olfactory region with POD led to dramatically increased concentrations in olfactory bulbs, with a 25.6-fold increased concentration compared to nose drops. The cortex, diencephalon, and cerebellum within the brain also exhibited significantly increased concentrations after delivery to the olfactory region. In addition, delivery of the lipophilic drug nelfinavir to the olfactory region of the nasal cavity resulted in higher concentrations in the brain, with 5.8-fold higher concentrations in the olfactory bulbs and 13.8-fold higher concentrations in the cortex, and lower concentrations in the blood stream compared to drug delivery to the respiratory region of the nasal cavity.
Mannitol is a hydrophilic small molecule drug (log P = −3.4) with properties that make it an ideal substrate to investigate direct transport from the nasal cavity to the brain. It is metabolically inert and eliminated exclusively by the kidneys, and has a half life of 100 min (28). Mannitol also does not interact with any known receptors and has an extremely low permeability across biologic membranes (29). Mannitol has long been used as a diuretic and as a marker to test renal function (30). It is also used as a non-absorbable marker for intestinal drug absorption studies. In our studies, we found that this non-absorbable maker, mannitol was able to penetrate and appear in the brain when administered with POD. There was significantly enhanced brain exposure at 30 min after mannitol delivery with POD. After IV administration the brain exposure of mannitol is very low. This observation is consistent with well-documented non-absorbable property of mannitol which could not penetrate easily through blood–brain barrier within the brain capillaries. Once mannitol is in the blood stream it would have to cross the BBB by non-saturable passive diffusion in order to penetrate the brain parenchyma. A study investigating mannitol distribution into the CNS spaces found that after a 10-min IV infusion, brain concentrations reached a very low level, 1.2% of blood concentration (31).
It is interesting to note that mannitol to the olfactory region of the nasal cavity by POD resulted in higher blood concentrations compared to nose drop delivery. This is most likely due to the mannitol being able to more easily penetrate the olfactory epithelia and gain access to the lamina propria. Jansson et al. showed that hydrophilic dextran could not penetrate the tight junctions of the respiratory epithelium but could penetrate beyond the olfactory epithelium to the lamina propria within 5 min of administration (10). Similarly, mannitol appears to have more easily penetrated the olfactory epithelium and gained greater access to the lamina propria and the blood.
Despite the higher blood concentrations of mannitol, the blood-normalized mannitol concentrations after POD administration were significantly higher than nose drops in the olfactory bulbs and the cortex, and supports the conclusion that POD delivery to the olfactory region resulted in a greater fraction of drug directly delivered to the brain. This data suggests that the POD administration enabled a greater fraction of the mannitol to travel directly from the nasal cavity to the brain. Our results are consistent with the hypothesis that the olfactory nerves in the nasal cavity create fluid-filled pathways in which molecules can undergo a non-receptor-mediated transport to the olfactory bulbs and other brain regions.
POD delivery of the hydrophobic anti-HIV drug nelfinavir also resulted in higher concentrations in brain compared to nose drop delivery (Fig. 5). Nelfinavir was chosen as a model lipophilic drug for this study because it exhibits low brain penetration despite a log P value of 6.0. Nelfinavir is a substrate of multidrug-resistant transporter or P-glycoprotein (P-gp) which is expressed at the blood–brain barrier and removes nelfinavir from the brain (32). As a result, like other oral anti-HIV drugs it does not reach the brain in effective concentrations after reaching therapeutic drug levels in the blood. As a consequence, this process may lead to neurological complications in HIV patients (33). Some have suggested that intranasal delivery of these compounds that bypass blood–brain barriers could lead to enhanced brain distribution for neurological complications of HIV (34).
After POD distribution to the olfactory region, nelfinavir concentrations in the olfactory bulbs and cortex were significantly increased compared to nose drop delivery to the respiratory region. In addition, the blood concentrations were significantly lower than after nose drops administration. It is possible that systemically administered nelfinavir does not readily appear in the brain is due to the efflux pump P-glycoprotein which is highly expressed at the BBB (35). This protein is also highly expressed within the olfactory epithelium of the nasal cavity and much less so at the respiratory region (36). Due to its lipophilicity (log P = 6.0), it is likely that nelfinavir can penetrate the respiratory epithelium after nose drop delivery and quickly enters the capillary system in the lamina propria. This would result in the higher blood concentrations and lower brain concentrations observed after nose drop delivery to the respiratory epithelium. These and other possibilities remain to be investigated.
Due to P-glycoprotein that is present at the olfactory epithelium (37), nelfinavir that penetrates across the olfactory epithelium may be effluxed by P-gp back into the nasal cavity. This process will likely limit nelfinavir uptake into the blood and the brain from the lamina propria of the olfactory region of the nasal cavity. As a result, the overall amount of drug directly transported into the brain via POD delivery may be underestimated at 30 min time point, although the exact influence of P-gp in this case would require further studies. This hypothesis is consistent with our observation that POD-mediated mannitol delivery to the brain achieved higher overall brain exposure than that for nelfinavir (Figs. 3 and 5). However, nelfinavir may still have direct access to the brain and CNS through the olfactory nerves connecting the olfactory bulb and the brain. This drug penetration process was demonstrated with morphine (11). Several other studies have shown that intranasally delivered P-glycoprotein substrates GF120918, rifampin, and verapamil can still result in improved brain concentrations (12,38). It is likely that most of the enhanced brain distribution of nelfinavir, and possibly other lipophilic P-gp substrates, could be due to direct nose-to-brain transport from the drug deposited at the olfactory region.
In summary, we have demonstrated for the first time with a novel POD device which preferentially deposits drug molecules at the olfactory that there is a significant difference in CNS distribution after depositing drug on the olfactory region vs. the respiratory region. In addition, this data indicates that depositing drug specifically on the olfactory region results in a higher fraction of direct distribution from the nasal cavity to the CNS. This confirms the hypothesis that direct transport from the nasal cavity to the CNS is primarily due to the direct fluid pathways between the olfactory epithelium and the subarachnoid space surrounding the olfactory bulb (16).
Conclusions
In summary, using a novel method of nasal drug administration in rats, we show for the first time that depositing a majority of drug on the olfactory region in the nasal cavity can result in enhanced brain-to-blood ratios. This enhanced drug delivery to the brain is likely due to drug molecules following anatomical connections in the olfactory region of the nasal cavity that bypass the blood stream and distribute directly from the nasal cavity to the brain. Localizing the drug to the respiratory epithelium could be desirable when trying to avoid distribution in the brain. However, depositing a majority of drug on the olfactory region could lead to enhanced effective brain concentrations for many drugs with limited ability to penetrate or cross the BBB.
Acknowledgments
Supported in part by NIH grants AI 077390 and MH086351, and University of Washington Technology Innovation grant TGIF-1001. RJYH is also supported by Milo Gibaldi Endowment.
References
- 1.Newman SP, Pitcairn GR, Dalby RN. Drug delivery to the nasal cavity: in vitro and in vivo assessment. Crit Rev Ther Drug Carrier Syst. 2004;21(1):21–66. doi: 10.1615/CritRevTherDrugCarrierSyst.v21.i1.20. [DOI] [PubMed] [Google Scholar]
- 2.Dhuria SV, Hanson LR, Frey WH., 2nd Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654–1673. doi: 10.1002/jps.21924. [DOI] [PubMed] [Google Scholar]
- 3.Illum L. Is nose-to-brain transport of drugs in man a reality? J Pharm Pharmacol. 2004;56(1):3–17. doi: 10.1211/0022357022539. [DOI] [PubMed] [Google Scholar]
- 4.Hilger P. Applied anatomy and physiology of the nose. In: Adams GL, LRBaPAH, editors. Boies’s fundamentals of otolaryngology. Philadelphia: W.B. Saunders; 1989. pp. 177–145. [Google Scholar]
- 5.Jackson RT, Tigges J, Arnold W. Subarachnoid space of the CNS, nasal mucosa, and lymphatic system. Arch Otolaryngol. 1979;105(4):180–184. doi: 10.1001/archotol.1979.00790160014003. [DOI] [PubMed] [Google Scholar]
- 6.Thorne RG, Frey WH., 2nd Delivery of neurotrophic factors to the central nervous system: pharmacokinetic considerations. Clin Pharmacokinet. 2001;40(12):907–946. doi: 10.2165/00003088-200140120-00003. [DOI] [PubMed] [Google Scholar]
- 7.Ross TM, Martinez PM, Renner JC, Thorne RG, Hanson LR, Frey WH., 2nd Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: a non-invasive treatment strategy for multiple sclerosis. J Neuroimmunol. 2004;151(1–2):66–77. doi: 10.1016/j.jneuroim.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Dahlin M, Jansson B, Bjork E. Levels of dopamine in blood and brain following nasal administration to rats. Eur J Pharm Sci. 2001;14(1):75–80. doi: 10.1016/S0928-0987(01)00151-8. [DOI] [PubMed] [Google Scholar]
- 9.Dahlin M, Bergman U, Jansson B, Bjork E, Brittebo E. Transfer of dopamine in the olfactory pathway following nasal administration in mice. Pharm Res. 2000;17(6):737–742. doi: 10.1023/A:1007542618378. [DOI] [PubMed] [Google Scholar]
- 10.Jansson B, Bjork E. Visualization of in vivo olfactory uptake and transfer using fluorescein dextran. J Drug Target. 2002;10(5):379–386. doi: 10.1080/1061186021000001823. [DOI] [PubMed] [Google Scholar]
- 11.Westin U, Piras E, Jansson B, Bergstrom U, Dahlin M, Brittebo E, et al. Transfer of morphine along the olfactory pathway to the central nervous system after nasal administration to rodents. Eur J Pharm Sci. 2005;24(5):565–573. doi: 10.1016/j.ejps.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Padowski JM, Pollack GM. Examination of the ability of the nasal administration route to confer a brain exposure advantage for three chemical inhibitors of P-glycoprotein. J Pharm Sci. 2010;99(7):3226–3233. doi: 10.1002/jps.22070. [DOI] [PubMed] [Google Scholar]
- 13.Merkus FW, van den Berg MP. Can nasal drug delivery bypass the blood–brain barrier?: questioning the direct transport theory. Drugs R D. 2007;8(3):133–144. doi: 10.2165/00126839-200708030-00001. [DOI] [PubMed] [Google Scholar]
- 14.Morrison EE, Costanzo RM. Morphology of the human olfactory epithelium. J Comp Neurol. 1990;297(1):1–13. doi: 10.1002/cne.902970102. [DOI] [PubMed] [Google Scholar]
- 15.Foo MY, Cheng YS, Su WC, Donovan MD. The influence of spray properties on intranasal deposition. J Aerosol Med. 2007;20(4):495–508. doi: 10.1089/jam.2007.0638. [DOI] [PubMed] [Google Scholar]
- 16.Illum L. Transport of drugs from the nasal cavity to the central nervous system. Eur J Pharm Sci. 2000;11(1):1–18. doi: 10.1016/S0928-0987(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 17.Gross EA, Swenberg JA, Fields S, Popp JA. Comparative morphometry of the nasal cavity in rats and mice. J Anat. 1982;135(Pt 1):83–88. [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne RG, Emory CR, Ala TA, Frey WH., 2nd Quantitative analysis of the olfactory pathway for drug delivery to the brain. Brain Res. 1995;692(1–2):278–282. doi: 10.1016/0006-8993(95)00637-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu XF, Fawcett JR, Thorne RG, DeFor TA, Frey WH., 2nd Intranasal administration of insulin-like growth factor-I bypasses the blood–brain barrier and protects against focal cerebral ischemic damage. J Neurol Sci. 2001;187(1–2):91–97. doi: 10.1016/S0022-510X(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 20.Hanson LR, Roeytenberg A, Martinez PM, Coppes VG, Sweet DC, Rao RJ, et al. Intranasal deferoxamine provides increased brain exposure and significant protection in rat ischemic stroke. J Pharmacol Exp Ther. 2009;330(3):679–686. doi: 10.1124/jpet.108.149807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127(2):481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Van den Berg MP, Merkus P, Romeijn SG, Verhoef JC, Merkus FW. Hydroxocobalamin uptake into the cerebrospinal fluid after nasal and intravenous delivery in rats and humans. J Drug Target. 2003;11(6):325–331. doi: 10.1080/10611860310001640075. [DOI] [PubMed] [Google Scholar]
- 23.Charlton ST, Davis SS, Illum L. Nasal administration of an angiotensin antagonist in the rat model: effect of bioadhesive formulations on the distribution of drugs to the systemic and central nervous systems. Int J Pharm. 2007;338(1–2):94–103. doi: 10.1016/j.ijpharm.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 24.Young JT. Histopathologic examination of the rat nasal cavity. Fundam Appl Toxicol. 1981;1(4):309–312. doi: 10.1016/S0272-0590(81)80037-1. [DOI] [PubMed] [Google Scholar]
- 25.Chow HS, Chen Z, Matsuura GT. Direct transport of cocaine from the nasal cavity to the brain following intranasal cocaine administration in rats. J Pharm Sci. 1999;88(8):754–758. doi: 10.1021/js9900295. [DOI] [PubMed] [Google Scholar]
- 26.Chow HH, Anavy N, Villalobos A. Direct nose-brain transport of benzoylecgonine following intranasal administration in rats. J Pharm Sci. 2001;90(11):1729–1735. doi: 10.1002/jps.1122. [DOI] [PubMed] [Google Scholar]
- 27.Charlton ST, Whetstone J, Fayinka ST, Read KD, Illum L, Davis SS. Evaluation of direct transport pathways of glycine receptor antagonists and an angiotensin antagonist from the nasal cavity to the central nervous system in the rat model. Pharm Res. 2008;25(7):1531–1543. doi: 10.1007/s11095-008-9550-2. [DOI] [PubMed] [Google Scholar]
- 28.Cloyd JC, Snyder BD, Cleeremans B, Bundlie SR, Blomquist CH, Lakatua DJ. Mannitol pharmacokinetics and serum osmolality in dogs and humans. J Pharmacol Exp Ther. 1986;236(2):301–306. [PubMed] [Google Scholar]
- 29.Miki K, Butler R, Moore D, Davidson G. Rapid and simultaneous quantification of rhamnose, mannitol, and lactulose in urine by HPLC for estimating intestinal permeability in pediatric practice. Clin Chem. 1996;42(1):71–75. [PubMed] [Google Scholar]
- 30.Williams TF, Hollander W, Jr, Strauss MB, Rossmeisl EC, Mc LR. Mechanism of increased renal sodium excretion following mannitol infusion in man. J Clin Invest. 1955;34(4):595–601. doi: 10.1172/JCI103108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sisson WB, Oldendorf WH. Brain distribution spaces of mannitol-3H, inulin-14C, and dextran-14C in the rat. Am J Physiol. 1971;221(1):214–217. doi: 10.1152/ajplegacy.1971.221.1.214. [DOI] [PubMed] [Google Scholar]
- 32.Kaddoumi A, Choi SU, Kinman L, Whittington D, Tsai CC, Ho RJ, et al. Inhibition of P-glycoprotein activity at the primate blood–brain barrier increases the distribution of nelfinavir into the brain but not into the cerebrospinal fluid. Drug Metab Dispos. 2007;35(9):1459–1462. doi: 10.1124/dmd.107.016220. [DOI] [PubMed] [Google Scholar]
- 33.Minagar A, Commins D, Alexander JS, Hoque R, Chiappelli F, Singer EJ, et al. NeuroAIDS: characteristics and diagnosis of the neurological complications of AIDS. Mol Diagn Ther. 2008;12(1):25–43. doi: 10.1007/BF03256266. [DOI] [PubMed] [Google Scholar]
- 34.Hanson LR, Frey WH., 2nd Strategies for intranasal delivery of therapeutics for the prevention and treatment of neuroAIDS. J Neuroimmune Pharmacol. 2007;2(1):81–86. doi: 10.1007/s11481-006-9039-x. [DOI] [PubMed] [Google Scholar]
- 35.Jarvis B, Faulds D, Nelfinavir A review of its therapeutic efficacy in HIV infection. Drugs. 1998;56(1):147–167. doi: 10.2165/00003495-199856010-00013. [DOI] [PubMed] [Google Scholar]
- 36.Kandimalla KK, Donovan MD. Localization and differential activity of P-glycoprotein in the bovine olfactory and nasal respiratory mucosae. Pharm Res. 2005;22(7):1121–1128. doi: 10.1007/s11095-005-5420-3. [DOI] [PubMed] [Google Scholar]
- 37.Graff CL, Pollack GM. P-Glycoprotein attenuates brain uptake of substrates after nasal instillation. Pharm Res. 2003;20(8):1225–1230. doi: 10.1023/A:1025053115583. [DOI] [PubMed] [Google Scholar]
- 38.Graff CL, Pollack GM. Functional evidence for P-glycoprotein at the nose–brain barrier. Pharm Res. 2005;22(1):86–93. doi: 10.1007/s11095-004-9013-3. [DOI] [PubMed] [Google Scholar]