Abstract
Ferulic acid (FA) is a natural product that occurs in seeds of many plants where it is generally located in the bran. This compound is a multifunctional ingredient endowed with antioxidative, radical scavenging, sunscreening and antibacterial actions. The aim of this study was to analyse the ferulic acid cutaneous permeation and distribution, through and into the skin layers, from different cosmetic vehicles, an O/W emulsion (pH 6.0) and two gel-type formulations at different pH levels (6.0 and 7.4), containing FA alone or an inclusion complex with α-cyclodextrin (CD-FA). In vitro permeation studies were performed in vertical diffusion cells using hairless rat excised skin. At appropriate intervals of time, the amount of permeated sunscreen/radical scavenger was evaluated by high-performance liquid chromatography (HPLC). At the end of experiments, treated skin samples were sectioned with a cryomicrotome and the FA content of the individual slices was analysed by HPLC. FA-containing formulations, O/W emulsion, gels A and B, originated FA fluxes of 8.48 ± 2.31, 8.38 ± 0.89 and 5.72 ± 0.50 μg/cm2 h, respectively, thus suggesting the pH influence on FA percutaneous permeation. The use of the inclusion complex, CD-FA, determined in all cases a decrease of FA transdermal permeation while no influence of pH was observed. Gel-type formulations containing FA ensured higher sunscreen storage in the superficial layers if compared with O/W emulsion. When FA was included in α-cyclodextrin, FA amount retained into skin layers decreased markedly.
KEY WORDS: α-cyclodextrin complex, ferulic acid, skin distribution, skin permeation, sunscreen/antioxidant agents
INTRODUCTION
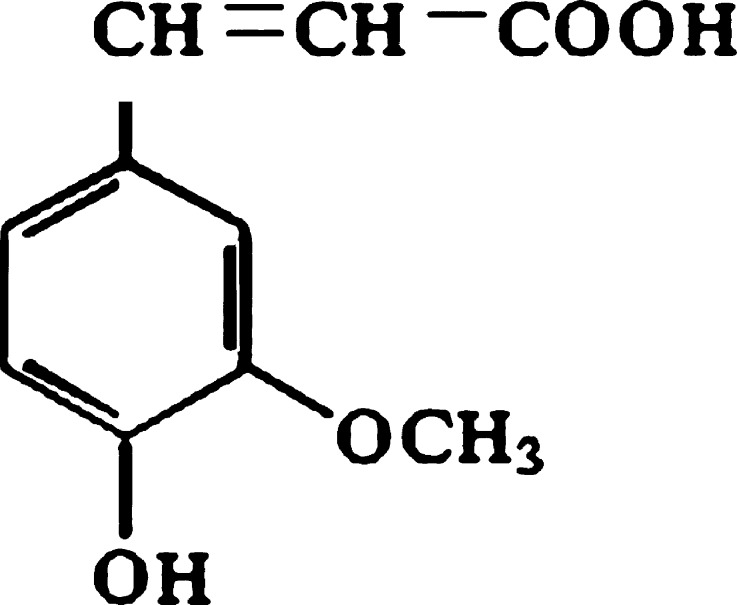
Ferulic acid (FA) or 4-hydroxy-3-methoxycinnamic acid (Fig. 1) exhibits a wide range of therapeutic effects against various pathologies like cancer, diabetes, cardiovascular and neurodegenerative diseases (1–3). It has received much attention in the study of Chinese medicine for the prevention of thrombosis and atherosclerosis and for a chemo-preventive action against colon and rectal cancer. A wide range of beneficial activity for human health can be attributed to this phenolic compound, most of which can be ascribed to its strong antioxidant activity. FA is an effective scavenger of free radicals and it has been approved in certain countries as food additive to prevent lipid peroxidation (4). Topically applied antioxidant can protect the skin if it reaches deeper cutaneous layers.
Fig. 1.
Structure of ferulic acid
Furthermore, ferulic acid is endowed with a high UV absorber property suggesting that it might help to protect skin from sun damage and in virtue of these promising properties it has been approved in Japan as a sunscreen (5). The exposure of the skin to UV radiation induces the generation of reactive oxygen/nitrogen species resulting in oxidative damage. The topical application of FA on the skin can be useful to prevent damages induced by UV light such as precancerous and cancerous skin lesions and premature skin ageing thus preserving physiological integrity of cells exposed to both air and UV radiation (6). This positive effect is limited by the poor stability of the molecule that under physical and thermal stresses can break down into inactive products. Anselmi et al. (7–9) tried to overcome this problem by the use of cyclodextrins (CDs), which in pharmaceutical field have been shown to form stable inclusion complexes through non-covalent interactions with various hydrophobic small molecules. The advantageous changes of guest molecular properties after formation of inclusion complexes have led to many applications of cyclodextrins in the industries related to food, pharmaceuticals, cosmetics, chemicals, agriculture, etc.
In the cosmetic industry, the formation of an inclusion complex between cyclodextrin and a broad variety of organic compounds increases the stability and solubility of cosmetic active ingredients and protect them from light- and oxidation-induced degradation (10).
Furthermore, cyclodextrins have been used as systems to achieve a reduction of the percutaneous absorption of common sunscreen agents (11–13). Since UV filters exert their effect on the skin surface, it is important that after topical application, the sunscreen agents accumulate in the outermost cutaneous layers with minimal permeation into deeper skin tissues.
However, few reports regarding FA skin permeation have been published (14,15) and information concerning its permeation from cyclodextrin inclusion complexes is not provided.
Since a previous study (9) assessed the increased stability of FA by the formation of a stable inclusion complex with α-cyclodextrin, the aim of this paper was to modulate the cutaneous permeation and distribution of FA, in view of its use as sunscreen and/or radical scavenger. To this purpose, FA or its α-cyclodextrin inclusion complex were included in different cosmetic formulations and in vitro studies through hairless rat skin were performed.
MATERIALS AND METHODS
Materials
Ferulic acid was kindly gifted by Tsuno Rice Fine Chemicals (Wakayama, Japan); α-cyclodextrin (α-CD), deuterium oxide (D2O) and sodium 2,2,3,3-2H4-trimethyl-silan-propionate for 1H NMR experiments were purchased from Aldrich (Aldrich, Milan, Italy). The excipients for the emulsion were obtained as follows: tri-C12-13 alkyl citrate (Cosmacol ECI) from Sasol Italy S.p.A. (Milan, Italy); cetearyl glucoside, cetearyl alcohol (Montanov 68) from Seppic S.A. (Paris, France); potassium cetyl phosphate (Amphysol K) from ResPharma (Trezzo sull’Adda, Milan, Italy); hydroxyethylcellulose (Natrosol HR) from Eigenmann & Veronelli SpA (Milan, Italy); disodium ethylenediaminotetra-acetic acid salt (disodium ethylenediaminetetraacetic acid (EDTA)) and triethanolamine (TEA) from BASF Italia Spa (Cesano Maderno, Milano, Italy); methylchloroisothiazolinone, methylisothiazolinone (Kathon CG) from ROHM and HAAS Italia Srl (Mozzate, Como, Italy); imidazolydinyl urea (Kemipur 100) from Acef SpA (Fiorenzuola d’Arda, Italy). Sodium dodecylsulfate (SDS) was purchased from Sigma-Aldrich (Sigma-Aldrich S.r.l., Milan, Italy). All other chemicals and solvents were of analytical grade.
Ferulic ACID-α-CD Complex Preparation and Characterization
The complex was prepared by mixing (at 1:1 M ratio) ferulic acid and α-CD according to the coprecipitation method as previously reported (8,9). Briefly, a methanol solution of FA was added to CD dissolved in water and stirred for 24 h in the dark and at room temperature. After reaction completeness, the precipitate was collected by filtration, dried in vacuum and washed with ether to remove residual FA.
α-CD inclusion complex obtained was characterized by X-ray diffraction and NMR analyses as previously described (9).
The inclusion percentage of FA was determined by HPLC analysis as previously reported (7,9). The chromatographic determination was performed using the conditions afterwards described. The ferulic acid association complex with α-CD was dissolved in dimethylformamide (1 mg/ml) and filtered through a 0.45 μm filter and the concentration of FA was determined by comparison with appropriate calibration curve. No interference from α-CD was observed.
Formulations
In vitro permeation and distribution studies of FA were performed using the following formulations, containing 3% w/w of FA alone or 20% w/w of α-cyclodextrin inclusion complex (CD/FA) that contained an equal amount of the compound included: O/W emulsions (pH 6) containing FA (O/W-FA) or CD/FA (O/W-CD/FA); pH 6 gel containing FA (gel A-FA) or CD/FA (gel A-CD/FA); pH 7.4 gel with FA (gel B-FA) or CD/FA (gel B-CD/FA). Their composition (% w/w) is reported in Table I.
Table I.
Composition (% w/w) of the Formulations Under Study
| Components | O/W-FA | O/W-CD/FA | Gel A-FA | Gel A-CD/FA | Gel B-FA | Gel B-CD/FA |
|---|---|---|---|---|---|---|
| FA | 3.0 | – | 3.0 | – | 3.0 | – |
| CD/FA | – | 20.05 | – | 20.87 | – | 19.10 |
| Montanov 68 | 5.0 | 5.0 | – | – | – | – |
| Cosmacol ECI | 15.0 | 15.0 | – | – | – | – |
| Amphisol K | 0.5 | 0.5 | – | – | – | – |
| Natrosol HR | – | – | 2.0 | 2.0 | 2.0 | 2.0 |
| Kathon CG | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 | 0.05 |
| Kemipur 100 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Disodium EDTA | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| TEA | 2.24 | to pH = 6 | to pH = 6 | to pH = 6 | to pH = 7.4 | to pH = 7.4 |
| Water | to 100.0 | to 100.0 | to 100.0 | to 100.0 | to 100.0 | to 100.0 |
To prepare the emulsions, cetearyl glucoside, cetearyl alcohol and Tri C12–13 alkyl citrate (phase A) were melt at 60–70°C and potassium cetyl phosphate was dissolved in water at 70°C (phase B). Then, phase A was added to phase B under continuous stirring. Finally, FA and TEA (to increase FA water solubility) or CD/FA, previously dissolved/dispersed in a little amount of water, were added at about 40°C under energetic stirring to obtain final emulsions. Gels were prepared by dissolving useful amount of TEA in water containing 0.3% w/w imidazolydinyl urea, 0.1% w/w disodium EDTA and 0.05% w/w methylchloroisothiazolinone, methylisothiazolinone, then adding FA (gel A-FA and gel B-FA) or the complex (gel A-CD/FA and gel B-CD/FA) and, under continuous stirring, 2% (w/w) hydroxyethylcellulose.
As reference, in order to investigate the influence of pH on FA permeation and distribution, two solutions containing 0.0075% of FA in isotonic phosphate buffer at pH 6 and 7.4 (SOL-1 and SOL-2, respectively) were prepared.
Skin Model
Rat skin was obtained from 6 to 8 weeks old hairless male animals (HsdHan™/RNU-Foxn1 rnu, Harlan Italy s.r.l., Correzzana, Italy). The animals were killed by cervical dislocation immediately before the experiments; the skin was carefully excised and the adhering fat and subcutaneous tissue were removed. The study performed in this section was approved by the Ethical Committee of the University of Pisa and the protocol was compliant with the European Union Directive 86/609/EEC for the use of experimental animals.
In vitro Permeation Studies
Permeation tests through excised rat skin were carried out as previously described (16) using Gummer-type diffusion cells with an available diffusion area of 1.23 cm2 and stratum corneum facing the donor compartment. One millilitre of each formulation was placed on the skin surface. The receiving phase (5 ml) was isotonic phosphate buffer saline (PBS, 66.7 mM, pH 7.4) containing 0.003% (w/v) sodium azide to prevent bacterial growth. The solubility of FA in the receiving phase was 5.42 mg/ml. Both donor and receiving solutions were stirred and thermostated at 37°C. At predetermined intervals of time, 5.0-ml samples of the receiving phase were withdrawn for HPLC analysis and replaced with the same volume of fresh fluid. All experiments lasted 24 h and were performed five times, and sink conditions were maintained throughout the entire study.
Skin Distribution Studies
At the end of the in vitro permeation experiments, the skin was removed from the diffusion cells, rinsed with distilled water to remove excess formulation from the skin surface and gently wiped with cotton wool tampons. The samples were then frozen and sliced horizontally with a cryomicrotome (MEV Cryostat, Slee-Technik GMBH, Mainz, Germany). The precision of the microtome gives reproducible sections of 0–60 μm in 1 μm step-up to 10 μm, 2 μm step-up to 20 μm and 5 μm step-up to 60 μm.
Although skin was flattened with a weight of 2 kg for 1 min before sectioning, the first slices were usually incomplete because of the irregular surface of the skin samples. The mean thickness of the incomplete slices and skin residues was calculated from their weight with reference to a standard slice of known weight and thickness (17–19).
The sunscreen was extracted from the skin slices by treatment with 1.0 ml of 2% SDS for 24 h. After treatment with methanol (2.0 ml) for 1 h, the mixture was centrifuged at 12,000 rpm for 15 min. One-hundred-fifty-microlitre aliquots of supernatant were dried in vacuo and subsequently dissolved in methanol for HPLC analysis.
For validation of the extraction procedure, a different series of 20 or 25-μm slices of blank skin was submitted to the assay and the retention time of endogenous compounds was compared with that of ferulic acid in order to verify that there were no interferences in analysing the molecule. Moreover, a known aliquot of FA was added to blank skin, and the extraction recovery was determined by computing the ratio of the amount of sunscreen extracted from the skin to the amount added. The percentage of recovery was 96.69 ± 3.09 [mean ± standard error (SE)].
HPLC Analysis
The concentration of FA in receiving fluid and in skin samples was selectively determined by HPLC (liquid chromatograph with LC-10AS pump and 20 μl Rheodyne injector, SPD-10AV detector and computer integrating system, Shimadzu Corp., Kyoto, Japan). The column (30 cm × 3.9 mm) was packed with Bondclone C18 (Phenomenex, Torrance, CA, USA). The mobile phase consisted of a mixture of 2% glacial acetic acid and acetonitrile (81:19; flow rate, 1.0 ml/min). The retention time and the detection wavelength were respectively 9.0 min and 290 nm. The limit of quantization of FA was 40.2 ng/ml in the skin samples and 14.3 ng/ml in the receiving fluid. The amount of sunscreen in the samples was determined by comparison with appropriate standard curves. In case of biological materials, a standard curve was obtained by adding increasing amounts of FA to blank biological samples. Any interference with FA from its metabolites or oxidized forms was found.
Data Analysis
Linear regression analysis of pseudo steady-state diffusion plots allowed calculation of the following parameters: steady-state flux (J), given by Q/A.t, where Q is the amount of permeant diffusing across the area A in time t; lag time, indicating the time taken by the drug to saturate the membrane and to reach the receiving phase, calculated from the x-axis intercept values of the regression lines; percent sunscreen permeated at the end of the experiment (Q%24 h). Moreover, the digestion procedure allowed calculation of the FA content (FAskin) as micrograms of sunscreen per milligram of skin retained in each skin layer.
Data are the average of five determinations ± SE for all the formulations tested except SOL-1 and SOL-2 which are the average of three determinations ± SE. Statistical differences between permeation parameters were assessed by GraphPad Prism software (GraphPad Software Inc., San Diego, CA). The evaluation included calculation of means and standard errors, and group comparisons using the Student’s two-tailed unpaired t test. Differences were considered statistically significant at p < 0.05.
RESULTS AND DISCUSSION
Ferulic Acid α-CD Inclusion Complex Characterization
Results obtained by XRD and NMR analyses have confirmed the formation of association complex. The X-ray diffractometric pattern of the inclusion complex CD-FA showed a completely different profile from that of physical mixture (data not shown) which indicates the formation of a new crystalline phase suggesting the association of ferulic acid with α-cyclodextrin.
Regarding NMR analysis we observed the same significant changes in resonance frequencies of both α-CD and FA protons that we previously reported (9).
These data suggest and confirm that the insertion of the FA into the lipophilic cavity of α-CD involves the α,β-unsaturated carboxyl moiety and part of the aromatic skeleton, while the phenol and methoxyl groups are projected outside the wider rim.
A quantitative analysis on the CD-FA complex showed that the percentage of the ferulic acid included is 14.97 ± 0.05 (mean±SD, n = 5, data not shown).
The increased stability of FA in this inclusion complex was investigated in a previous work (9). The CD/FA complex formulated in O/W emulsion showed the best photostability after irradiation at ten minimal erythemal dose (MED; 1 MED = 25 mJ/cm2 for skin phototype II) (20) in comparison to the emulsion containing free FA. In the HPLC profile of free FA, after irradiation, we observed a sharp peak due to its partial conversion to the cis-isomer.
By contrast, complexation with α-CD improves the photostability of FA, since no cis-isomer and other degradation products are formed after irradiation. Moreover, inclusion in other CDs (β or γ) does not ensure the same degree of FA protection (9).
Regarding antioxidant activity, the results previously reported (9) indicate that at all concentrations tested, the CD/FA complex had less antioxidant potency than the equimolecular mixture of FA and α-CD or FA alone. These results confirm that in the inclusion complex, FA is firmly encapsulated within the α-CD cavity, and that its phenol group is consequently less available for the interaction with peroxyl radicals.
Skin Permeation Studies
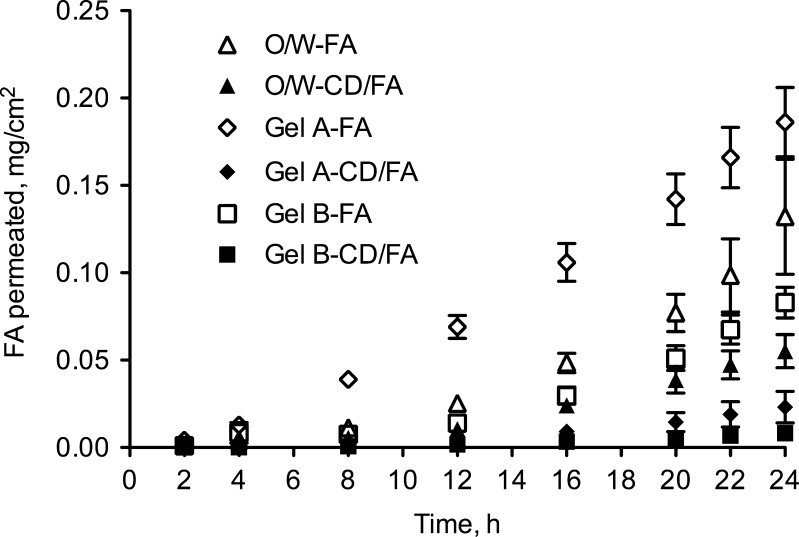
Results of FA permeation experiments are illustrated graphically in Fig. 2, while the relevant permeation parameters (flux, lag time and FA percent permeated after 24 h) are summarized in Table II.
Fig. 2.
Permeation profiles of ferulic acid alone (FA) or complexed with α-cyclodextrin (CD/FA) through excised hairless rat skin from different cosmetic vehicles: O/W emulsion and two gel-type formulations at different pH
Table II.
Permeation Parameters: Flux (J), Lag Time and Percent Sunscreen Permeated at the End of the Experiment (Q%24 h) from Different Formulations Containing the Compound Alone (FA) or its α-Cyclodextrin Complex (CD/FA)
| Formulations | J (μg/cm2.h) | Lag time (h) | Q%24 h |
|---|---|---|---|
| O/W-FA | 8.48 ± 2.31 | 8.79 ± 1.09 | 1.04 ± 0.20 |
| O/W-CD/FA | 4.76 ± 0.83 | 8.36 ± 0.38 | 0.51 ± 0.06* |
| Gel A-FA | 8.38 ± 0.89 | 2.64 ± 0.07 | 1.47 ± 0.15 |
| Gel A-CD/FA | 1.56 ± 0.62* | 7.87 ± 1.98* | 0.18 ± 0.07* |
| Gel B-FA | 5.72 ± 0.50 | 10.32 ± 0.51 | 0.65 ± 0.07 |
| Gel B-CD/FA | 0.54 ± 0.10* | 9.10 ± 0.15 | 0.07 ± 0.01* |
*Significantly different from the corresponding FA formulation, p < 0.05
FA-containing formulations (O/W, gels A and B) originated FA fluxes of 8.48 ± 2.31, 8.38 ± 0.89 and 5.72 ± 0.50 μg/cm2 h, respectively. Gel A (pH 6) produced FA permeation significantly higher than gel B (pH 7.4) thus suggesting pH influence on sunscreen/radical scavenger percutaneous permeation contrarily to what reported by Saija et al. (14) for saturated aqueous solutions. It is noteworthy that a dissociable substance permeates through the skin preferentially in the non-ionized form, with the ratio between the ionized and non-ionized forms being dependent on the pH of the solution and on the pKa of the compound (21–23). Since FA exhibits two pKa values of 4.58 and 9.88 (15), we suppose that both at pH 6 and pH 7.4 the phenolic moiety was non-ionized, while the carboxylic one was more in the ionized form at pH 7.4 than at pH 6. Taking into account that in this study we applied the infinite dose technique in order to obtain the maximal thermodynamic activity, the pH differences seems to be highlighted and ferulic acid appears more easily transported through the skin at pH 6 than at pH 7.4.
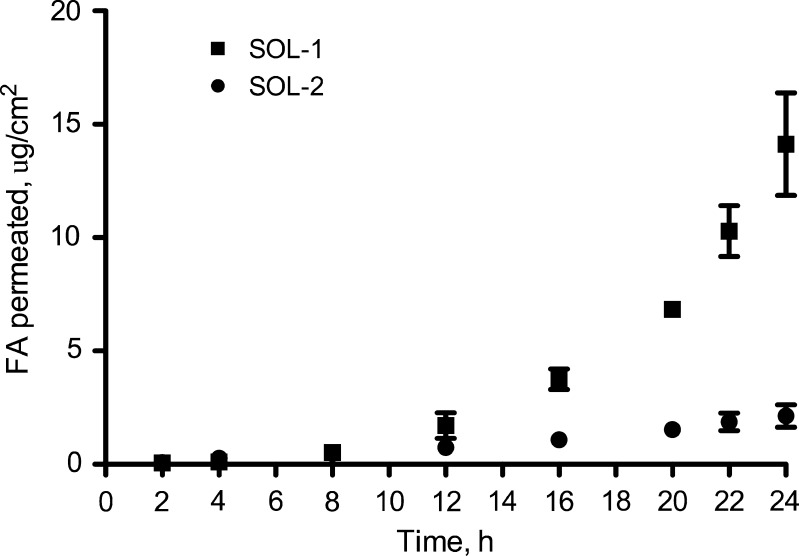
Moreover, the effect of pH on the permeation kinetic of FA was previously evaluated in our researches by performing permeation experiments with 0.0075% FA dissolved in phosphate buffer solutions at pH 6 (SOL-1) and 7.4 (SOL-2). Comparing the permeation parameters (Table III) of FA from the two different pH solutions evidenced that FA permeated the skin eightfold faster when applied at pH 6 than at pH 7.4. For more clarity the permeation profiles are illustrated in Fig. 3.
Table III.
Permeation Parameters of Ferulic Acid from Solutions at Different pH: SOL-1 pH 6.0 and SOL-2 pH 7.4
| Formulations | Flux (J) (μg/cm2.h) | Lag time (h) |
|---|---|---|
| SOL-1 | 0.81 ± 0.14 | 9.16 ± 0.97 |
| SOL-2 | 0.10 ± 0.026* | 4.06 ± 2.02 |
*Significantly different from SOL-1 (p < 0.01)
Fig. 3.
Permeation profiles through excised hairless rat skin of ferulic acid from SOL-1 and SOL-2
Somewhat different results were observed when the sunscreen/radical scavenger was included in the α-CD complex. In all cases the formation of the FA-CD complex determined a decrease of FA transdermal permeation with respect to the same formulations containing free FA. Any significant change in lag time was noted, except for formulation gel A, where the steady-state was reached in half the time in the presence of CD-FA with respect to the active ingredient alone.
Conflicting reports exist in literature regarding the relationship between complexation and transdermal kinetic of topically applied compounds. Some researchers assessed that CD functioned as penetration enhancers, while others reported any increase of the flux across the skin (24). It is noteworthy that CD may enhance transdermal permeation by increasing active ingredients solubility and facilitating partitioning towards the lipophilic surface of the skin (25). A theoretical model of the complexation and transdermal permeation of topically applied compounds included in CD has been developed from Godwin et al. (24), where they assessed that an excess of CD concentration shifts the equilibrium of a suspension of a drug towards the complexed form. Since only a free drug in a solution is capable of penetration into and through the skin, it can explain why in our study the complexation of FA with CD resulted in a decreased permeation flux in comparison with free FA.
As evidenced in Table II, gel A-CD/FA and gel B-CD/FA produced flux values not statistically different from each other (J = 1.56 ± 0.62 and 0.54 ± 0.10 μg/cm2 h, respectively; p = 0.1455), indicating that there was no influence of pH on the permeation of FA when the sunscreen was complexed with α-CD. Moreover, permeation from O/W-CD/FA was higher than the one from gel A-CD/FA (p = 0.0153) and gel B-CD/FA (p = 0.0010), producing a transdermal flux of 4.76 ± 0.83 μg/cm2 h.
Skin Distribution Studies
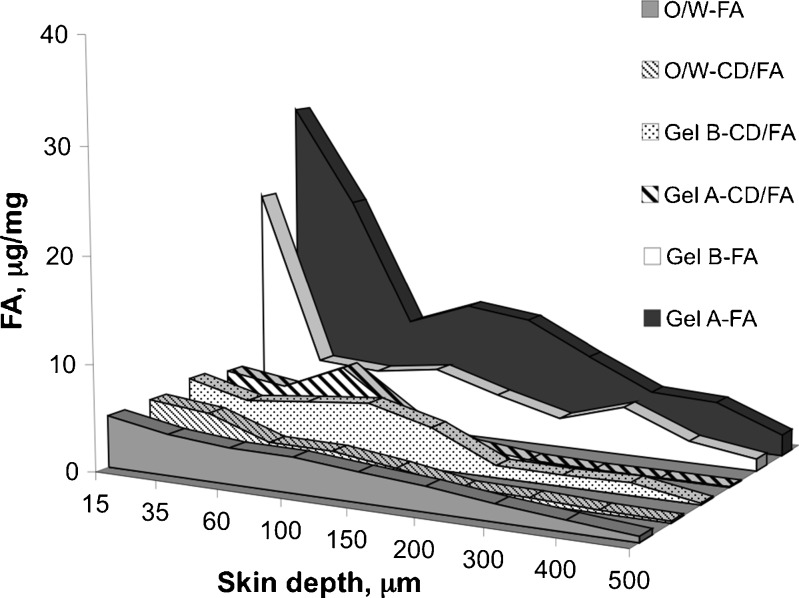
The in vitro penetration data are summarized in Table IV as micrograms of compound per milligram of skin retained in each skin layer. The corresponding concentration/depth profiles are shown in Fig. 4 up to the overall thickness of the skin.
Table IV.
Penetration Data of Ferulic Acid Free (FA) or Complexed with α-Cyclodextrin (CD/FA; μg/mg of Skin) from Different Cosmetic Vehicles: O/W Emulsion and Two Gel-Type Formulations at Different pH (6.0 and 7.4)
| Skin depth (μm) | FAskin (μg/mg ± ES) | |||||
|---|---|---|---|---|---|---|
| O/W-FA | O/W-CD/FA | Gel A-FA | Gel A-CD/FA | Gel B-FA | Gel B-CD/FA | |
| 15 | 4.87 ± 1.04 | 5.03 ± 1.21 | 30.15 ± 6.20 | 5.30 ± 2.01 | 21.99 ± 1.06 | 5.81 ± 1.30 |
| 35 | 3.85 ± 0.94 | 4.44 ± 1.87 | 21.01 ± 4.83 | 4.43 ± 2.30 | 5.82 ± 1.31 | 4.33 ± 1.83 |
| 60 | 3.30 ± 0.91 | 2.43 ± 0.96 | 9.24 ± 2.33 | 6.95 ± 4.21 | 5.39 ± 1.21 | 4.93 ± 2.03 |
| 100 | 3.28 ± 0.63 | 2.37 ± 0.69 | 11.41 ± 0.70 | 2.88 ± 1.65 | 6.19 ± 2.13 | 5.48 ± 2.83 |
| 150 | 2.75 ± 0.35 | 1.59 ± 0.33 | 10.60 ± 2.00 | 0.77 ± 0.08 | 4.35 ± 0.44 | 3.90 ± 1.94 |
| 200 | 2.29 ± 0.34 | 0.66 ± 0.20 | 7.55 ± 0.63 | 0.09 ± 0.02 | 2.75 ± 0.52 | 1.15 ± 0.21 |
| 300 | 1.59 ± 0.25 | 0.56 ± 0.10 | 4.72 ± 0.39 | 0.08 ± 0.02 | 4.56 ± 1.03 | 1.05 ± 0.64 |
| 400 | 1.05 ± 0.20 | 0.30 ± 0.05 | 4.41 ± 0.52 | 0.10 ± 0.01 | 2.06 ± 0.51 | 1.18 ± 0.70 |
| 500 | 0.55 ± 0.11 | 0.18 ± 0.01 | 1.96 ± 0.29 | 0.02 ± 0.01 | 1.14 ± 0.51 | 0.15 ± 0.06 |
Fig. 4.
Distribution profiles of ferulic acid alone (FA) or complexed with α-cyclodextrin (CD/FA) retained into skin layers after application of the formulations under study
For all the formulations containing free FA, the amount of sunscreen/radical scavenger accumulated was higher in the first layers of the skin. In particular, gel-type formulations containing FA ensured higher compound storage in the upper layers if compared with the O/W emulsion. At a depth of 15 μm, gel A-FA and gel B-FA showed a concentration peak of 30.15 ± 6.20 and 21.99 ± 1.06 μg/mg, respectively, both values considerably higher than that obtained from the O/W-FA (4.87 ± 1.04 μg/mg).
As evidenced from the graph in Fig. 4, gel A-FA allowed retaining in the skin more active ingredient than gel B-FA and O/W-FA. This could be explained accordingly to pH dependence of FA skin permeation/penetration. Table V clearly shows that the amount of FA retained in the skin was higher at pH 6 (SOL-1) than at pH 7.4 (SOL-2) indicating that the less ionized form penetrates the skin better than the ionized form.
Table V.
Penetration Data of FA (μg/mg of Skin) from Solutions at Different pH: SOL-1 pH 6.0 and SOL-2 pH 7.4
| Skin depth (μm) | FAskin (μg/mg ± ES) | |
|---|---|---|
| SOL-1 | SOL-2 | |
| 10 | 0.26 ± 0.08 | 0.005 ± 0.003 |
| 35 | 1.97 ± 0.45 | 0.02 ± 0.007 |
| 60 | 1.45 ± 0.29 | 0.21 ± 0.14 |
| 100 | 0.61 ± 0.35 | 0.32 ± 0.12 |
| 150 | 0.93 ± 0.46 | 0.13 ± 0.08 |
| 200 | 1.41 ± 0.35 | 0.05 ± 0.006 |
| 300 | 0.54 ± 0.08 | 0.16 ± 0.21 |
| 400 | 0.47 ± 0.15 | 0.19 ± 0.05 |
| 500 | 0.41 ± 0.01 | 0.07 ± 0.02 |
By analysing the penetration profiles of the formulations under study, we found that FA penetration from gel A-FA decreased remarkably in the depth range from 35 to 60 μm, passing from 21.01 ± 4.83 to 9.24 ± 2.33 μg/mg, while the amount of compound penetrated into the skin from gel B-FA started to decrease suddenly after the first slice, passing from 21.99 ± 1.06 to 5.82 ± 1.31 μg/mg at a depth of 15 and 35 μm, respectively. When compared with the values obtained with both gel formulations, penetration of FA from O/W-FA was minimal.
When FA was included in α-cyclodextrin (CD-FA), FA amount retained into the skin layers from gel-type formulations decreased markedly throughout the entire thickness of the skin. In particular, in the upper skin layers (15–100 μm) the amount of FA accumulated from gel A-FA was from 1.3- to 5.7-fold higher than that obtained with gel A-CD/FA and such a difference increased markedly going to further depths. On the contrary, gel B-FA and gel B-CD/FA produced different penetration values at a depth of 15 μm, then maintaining comparable values from 35 to 150 μm of depth and again resulting in higher penetration for gel B-FA up to 500 μm. For what concerns O/W emulsion, there were not differences in the level of FA accumulated within the horny layer (15–35 μm) between the preparations containing free and cyclodextrin complexed FA, thus concluding that the amount of FA penetrated in the epidermal region was not influenced by the complexation of the sunscreen with α-CD. When free FA is used in the emulsion at pH 6, the non-ionized form is distributed between the lipid phase of the vehicle and the stratum corneum, while in the case of O/W-CD/FA the complex remain in the aqueous phase and directly interact with the biological barrier, making the free FA available at the absorptive surface (26). Therefore, in both cases there is a limiting step for the diffusion of FA through the cutaneous barrier: in one hand the partition phenomenon between the emulsion phases (27) and in the other hand the availability from the complex.
CONCLUSIONS
The research highlighted very interesting points regarding the possible application of ferulic acid in topical products where radical scavenging or UV absorbing activities are requested.
We investigated several cosmetic and dermatologic formulations such as gels and emulsions. They were an interesting vehicle both for free FA and its α-CD complex.
Regarding free FA, in gel-type formulations, pH plays an important role to promote or reduce the permeation. Thus, in the case of molecules endowed with acid groups, it is very important to choose the right pH value with the aim to both avoid and increase storage of product into deeper skin layers and also the cutaneous permeation.
In this study, a reduction of the flux of about one third was observed when the pH of gels was changed from 6 to 7.4. Moreover, any differences in the flux values were observed when different formulations were compared: gel A and O/W-FA (both at pH 6) determined a FA permeation of 8.38 and 8.48 μg/cm2 h. Some important differences were found in the penetration, where both gel-type formulations produced 5–6-fold higher storage of FA in the first skin layer (15 μm) with respect to the FA-containing emulsion. As well as to assure sun protection, without negative effects, a sunscreen should accumulate preferentially in the stratum corneum not reaching biological fluids, the best formulation for free FA among those investigated in this study could be indicated as the gel B at physiological pH. On the other hand, if we focus the attention to the antioxidant activity, it is important that the molecule could reach the deeper cutaneous layers, even without permeation, as performed again, by gel B.
In the attempt to preserve FA properties during storage of formulation a complex with α-CD was prepared and formulated in the same formulations used for the free FA. The use of the inclusion complex CD-FA determined in all cases, gels A and B and emulsion, a decrease of FA transdermal permeation. Moreover, when FA was included in α-cyclodextrin, FA amount retained into the skin layers decreased markedly.
In all cases, the inclusion in the CD complex produced a minor skin permeation and penetration of the sunscreen, probably due to the slow release rate of the FA from the cyclodextrin cavity (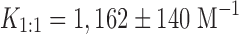 ), thus suggesting that CD-FA may be considered an effective reservoir/delivery system of sunscreen for cosmetic purpose by virtue of its slow release (8,9).
), thus suggesting that CD-FA may be considered an effective reservoir/delivery system of sunscreen for cosmetic purpose by virtue of its slow release (8,9).
When the FA-CD complex was used, the permeation/penetration was not affected by the pH of the formulations tested (see Table II). This is probably due to the fact that FA binds to α-cyclodextrin cavity with the aromatic ring, in particular the accommodation of the guest inside the cavity forces the α–β-unsaturated carboxyl moiety and part of the aromatic skeleton of FA in the α-cyclodextrin interior near the narrow rim, while the phenol and methoxyl groups are left outside the wider rim. Therefore, the carboxylic group (pKa1 = 4.58) was protected by the changes of the vehicle pH and the free phenolic group was mainly undissociated in these experimental conditions (9).
The results reported in a previous work (9) indicate that the CD-FA complex had less antioxidant potency than FA alone. However, the inclusion in CD protects the molecule against the photodegradation, allowing better photostability of FA and consequently assures a longer-lasting protection. In fact, there was neither formation of the less active cis-isomer of FA nor other degradation products. In these conditions, the FA activity should be preserved inside the formulation, ready to be explicated in contact with the skin, where the free FA could remain on the skin surface, to act as a sunscreen, or penetrate in the skin depth, to be used as a radical scavenger. To conclude, we can affirm that the best vehicle for the CD complex is, once again, the gel B, which assure storage of FA in both the skin surface and the deeper skin layers, reaching the receiving phase at low concentrations.
On the basis of these results, it seems that the physiological pH could be the preferred condition to the cutaneous administration of FA; for this reason, we cannot exclude that the emulsion pH 7.4 will show positive results. Further studies could be performed to verify this hypothesis.
ACKNOWLEDGEMENT
The technical support of Tsuno Rice Fine Chemicals is gratefully acknowledged.
REFERENCES
- 1.Maggi-Capeyron MF, Ceballos P, Cristol JP, Delbosc S, Le Doucen C, Pons M, et al. Wine phenolic antioxidants inhibit AP-1 transcriptional activity. J Agric Food Chem. 2001;49:5646–5652. doi: 10.1021/jf010595x. [DOI] [PubMed] [Google Scholar]
- 2.Wang BH, Ou-Yang JP. Pharmacological actions of sodium ferulate in cardiovascular system. Cardiovasc Drug Rev. 2005;23:161–172. doi: 10.1111/j.1527-3466.2005.tb00163.x. [DOI] [PubMed] [Google Scholar]
- 3.Han C, Ding H, Casto B, Stoner GD, D’Ambrosio SM. Inhibition of the growth of premalignant and malignant human oral cell lines by extracts and components of black raspberries. Nutr Cancer. 2005;51:207–217. doi: 10.1207/s15327914nc5102_11. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan M, Sudheer AR, Menon VP. Ferulic acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MHW Ministry of health and welfare, Notification No. 331/2000, Standards for cosmetics, 2000.
- 6.Graf E. Antioxidant potential of ferulic acid. Free Radic Biol Med. 1992;13:435–448. doi: 10.1016/0891-5849(92)90184-I. [DOI] [PubMed] [Google Scholar]
- 7.Anselmi C, Centini M, Ricci M, Buonocore A, Granata P, Tsuno T, et al. Analytical characterization of a ferulic acid/gamma-cyclodextrin inclusion complex. J Pharm Biomed Anal. 2006;40:875–881. doi: 10.1016/j.jpba.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Centini M, Maggiore M, Casolare M, Andreassi M, Maffei Facino R, Anselmi C. Cyclodextrins as cosmetic delivery systems. J Incl Phenom Macrocycl Chem. 2007;87:109–112. doi: 10.1007/s10847-006-9212-0. [DOI] [Google Scholar]
- 9.Anselmi C, Centini M, Maggiore M, Gaggelli N, Andreassi M, Buonocore A, et al. Non-covalent inclusion of ferulic acid with α-cyclodextrin improves photo-stability and delivery: NMR and modelling studies. J Pharm Biomed Anal. 2008;46:645–652. doi: 10.1016/j.jpba.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Li Z, Wang M, Wang F, Gu Z, Du G, Wu J, et al. γ-Cyclodextrin: a review on enzymatic production and applications. Appl Microbiol Biotechnol. 2007;77:245–255. doi: 10.1007/s00253-007-1166-7. [DOI] [PubMed] [Google Scholar]
- 11.Simeoni S, Scalia S, Benson HAE. Influence of cyclodextrins on in vitro human skin absorption of the sunscreen, butyl-methoxydibenzoylmethane. Int J Pharm. 2004;280:163–171. doi: 10.1016/j.ijpharm.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 12.Felton LA, Wiley CJ, Godwin DA. Influente of hydroxypropyl-beta-cyclodextrin on the transdermal permeation and skin accumulation of oxybenzone. Drug Dev Ind Pharm. 2002;28:1117–1124. doi: 10.1081/DDC-120014578. [DOI] [PubMed] [Google Scholar]
- 13.Scalia S, Tursilli R, Iannuccelli V. Complexation of the sunscreen agent, 4-methylbenzylidene camphor with cyclodextrins: effect on photostability and human stratum corneum penetration. J Pharm Biomed Anal. 2007;44:29–34. doi: 10.1016/j.jpba.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Saija A, Tomaino A, Trombetta D, De Pasquale A, Uccella N, Barbuzzi T, et al. In vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int J Pharm. 2000;199:39–47. doi: 10.1016/S0378-5173(00)00358-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhang LW, Al-Suwayeh SA, Hsieh PW, Fang JY. A comparison of skin delivery of ferulic acid and its derivatives: evaluation of their efficacy and safety. Int J Pharm. 2010;399(1–2):44–51. doi: 10.1016/j.ijpharm.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 16.Monti D, Saettone MF, Giannaccini B, Galli-Angeli D. Enhancement of transdermal penetration of dapiprazole through hairless mouse sin. J Control Release. 1995;33:71–77. doi: 10.1016/0168-3659(94)00079-A. [DOI] [Google Scholar]
- 17.Wagner H, Kostka KH, Lehr CM, Schaefer UF. Drug distribution in human skin using two different in vitro test systems: comparison with in vivo data. Pharm Res. 2000;17:1475–1481. doi: 10.1023/A:1007648807195. [DOI] [PubMed] [Google Scholar]
- 18.Wagner H, Kostka KH, Lehr CM, Schaefer UF. Human skin penetration of flufenamic acid: in vivo/in vitro correlation (deeper skin layers) for skin samples from the same subject. J Invest Dermatol. 2002;118:540–544. doi: 10.1046/j.0022-202x.2001.01688.x. [DOI] [PubMed] [Google Scholar]
- 19.Monti D, Brini I, Tampucci S, Chetoni P, Burgalassi S, Paganuzzi D, et al. Skin permeation and distribution of two sunscreens: a comparison between reconstituted human skin and hairless rat sin. Skin Pharmacol Physiol. 2008;21:318–325. doi: 10.1159/000154927. [DOI] [PubMed] [Google Scholar]
- 20.Pathak MA. In: Sunscreens development, evaluation and regulatory aspects. Lowe NJ, Shaath NA, Pathak MA, editors. New York: Marcel Dekker; 1997. pp. 59–79. [Google Scholar]
- 21.Casolaro M, Anselmi C, Picciocchi G. The protonation thermodynamics of ferulic acid/gamma-cyclodextrin inclusion compounds. Termochim Acta. 2005;425:143–147. doi: 10.1016/j.tca.2004.06.016. [DOI] [Google Scholar]
- 22.Maegawa Y, Sugino K, Sakurai H. Identification of free radical species derived from caffeic acid and related polyphenols. Free Radic Res. 2007;41:110–119. doi: 10.1080/10715760600943892. [DOI] [PubMed] [Google Scholar]
- 23.Poquet L, Clifford MN, Williamson G. Transport and metabolism of ferulic acid through the colonic epithelium. Drug Metab Dispos. 2008;36:190–197. doi: 10.1124/dmd.107.017558. [DOI] [PubMed] [Google Scholar]
- 24.Godwin DA, Wiley CJ, Felton LA. Using cyclodextrin complexation to enhance secondary photoprotection of topically applied ibuprofen. Eur J Pharm Biopharm. 2006;62:85–93. doi: 10.1016/j.ejpb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Iervolina M, Raghavan SL, Hadgraft J. Membrane penetration enhancement of ibuprofen using supersaturation. Int J Pharm. 2000;198:229–238. doi: 10.1016/S0378-5173(00)00346-X. [DOI] [PubMed] [Google Scholar]
- 26.Challa R, Ahuja A, Ali J, Khar R. Cyclodextrins in drug delivery: an updated review. AAPS PharmSciTech. 2005;6(2):E329–E357. doi: 10.1208/pt060243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pekkarinen SS, Stockmann H, Karin Schwarz, Heinonen IM, Hopia AI. Antioxidant activity and partitioning of phenolic acids in bulk and emulsified methyl linoleate. J Agric Food Chem. 1999;47:3036–3043. doi: 10.1021/jf9813236. [DOI] [PubMed] [Google Scholar]