Abstract
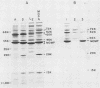
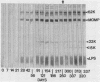
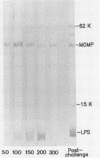
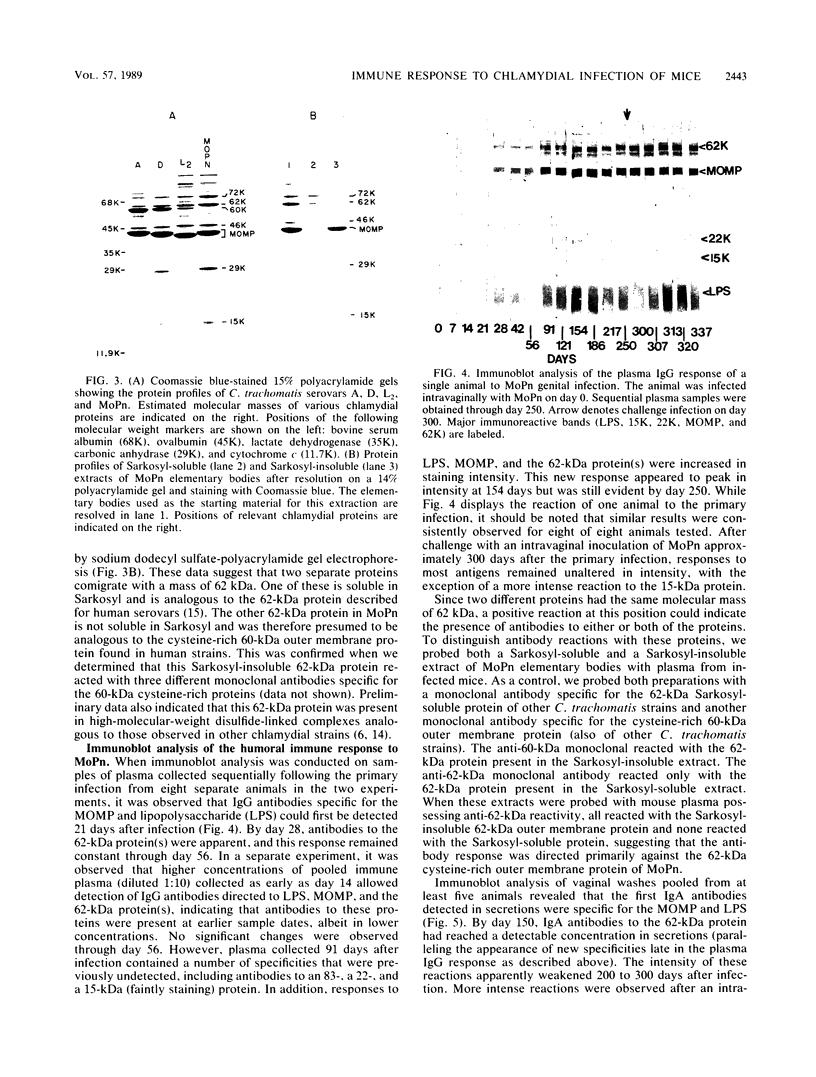
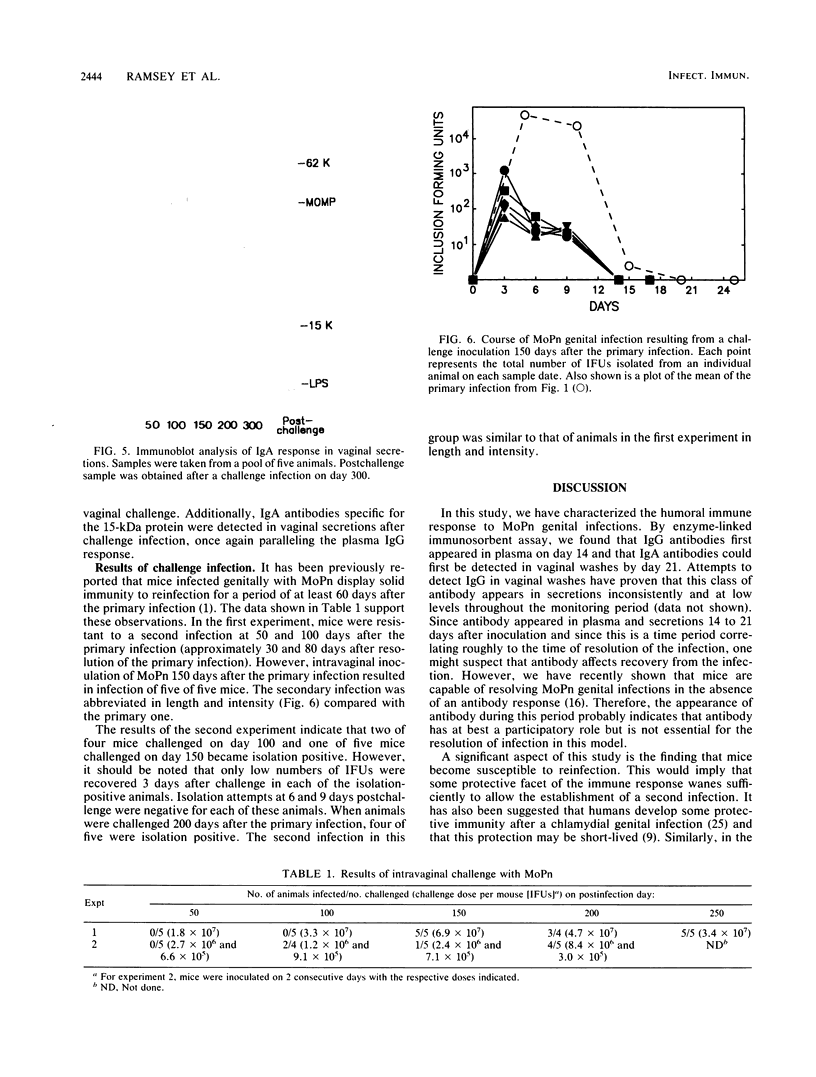
The objective of this study was to characterize the humoral immune response to chlamydial genital infection of mice with the mouse pneumonitis agent (MoPn). With an enzyme-linked immunoabsorbent assay, immunoglobulin G antibodies to MoPn were first detected in plasma by day 14. Peak plasma antibody concentrations were reached by day 49, and this response did not decline significantly throughout the 300-day monitoring period. Immunoglobulin A against MoPn could first be detected in pooled vaginal washes by day 21 after infection and had reached peak concentrations by day 28, but anti-MoPn immunoglobulin G was not consistently present in secretions. The antibody response in secretions had declined slightly by day 300. Immunoblot analysis revealed that the early phase of the plasma antibody response to MoPn as a result of genital infection was against lipopolysaccharide, the major outer membrane protein, and a 62-kilodalton (kDa) protein. In secretions, early-phase immunoglobulin A antibodies were directed to the major outer membrane protein and lipopolysaccharide. Late reactions to 15-, 22-, and 83-kDa proteins in plasma were noted. Late reactions to the 62-kDa protein in secretions were also noted. The cause of these late responses remains unexplained. When mice were challenged intravaginally with MoPn at 50-day intervals after the primary infection, it was found that mice inoculated on day 100 or after were susceptible to reinfection. Susceptibility could not be related to a decline in the antibody concentration in plasma or secretions or in the antibody response to specific components of MoPn as measured by immunoblot analysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barron A. L., Rank R. G., Moses E. B. Immune response in mice infected in the genital tract with mouse pneumonitis agent (Chlamydia trachomatis biovar). Infect Immun. 1984 Apr;44(1):82–85. doi: 10.1128/iai.44.1.82-85.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron A. L., White H. J., Rank R. G., Soloff B. L., Moses E. B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981 Jan;143(1):63–66. doi: 10.1093/infdis/143.1.63. [DOI] [PubMed] [Google Scholar]
- Batteiger B. E., Newhall W. J., 5th, Jones R. B. Differences in outer membrane proteins of the lymphogranuloma venereum and trachoma biovars of Chlamydia trachomatis. Infect Immun. 1985 Nov;50(2):488–494. doi: 10.1128/iai.50.2.488-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Rank R. G. Analysis of the humoral immune response to chlamydial genital infection in guinea pigs. Infect Immun. 1987 Aug;55(8):1767–1773. doi: 10.1128/iai.55.8.1767-1773.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T. Identification and properties of chlamydial polypeptides that bind eucaryotic cell surface components. J Bacteriol. 1986 Jan;165(1):13–20. doi: 10.1128/jb.165.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough A. J., Jr, Rank R. G. Induction of arthritis in C57B1/6 mice by chlamydial antigen. Effect of prior immunization or infection. Am J Pathol. 1988 Jan;130(1):163–172. [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Osborn M. F., Thomas B. J., Hetherington C. M., Taylor-Robinson D. Immunity to reinfection of the genital tract of marmosets with Chlamydia trachomatis. Br J Exp Pathol. 1981 Dec;62(6):606–613. [PMC free article] [PubMed] [Google Scholar]
- Katz B. P., Batteiger B. E., Jones R. B. Effect of prior sexually transmitted disease on the isolation of Chlamydia trachomatis. Sex Transm Dis. 1987 Jul-Sep;14(3):160–164. doi: 10.1097/00007435-198707000-00008. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. K., Moulder J. W. Persistent infection of mouse fibroblasts (McCoy cells) with a trachoma strain of Chlamydia trachomatis. Infect Immun. 1981 May;32(2):822–829. doi: 10.1128/iai.32.2.822-829.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W., Levy N. J., Schulman L. P. Persistent infection of mouse fibroblasts (L cells) with Chlamydia psittaci: evidence for a cryptic chlamydial form. Infect Immun. 1980 Dec;30(3):874–883. doi: 10.1128/iai.30.3.874-883.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller S. A., Borrebaeck C. A. A filter immuno-plaque assay for the detection of antibody-secreting cells in vitro. J Immunol Methods. 1985 May 23;79(2):195–204. doi: 10.1016/0022-1759(85)90099-7. [DOI] [PubMed] [Google Scholar]
- Newhall W. J., 5th Biosynthesis and disulfide cross-linking of outer membrane components during the growth cycle of Chlamydia trachomatis. Infect Immun. 1987 Jan;55(1):162–168. doi: 10.1128/iai.55.1.162-168.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey K. H., Soderberg L. S., Rank R. G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988 May;56(5):1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Effect of antithymocyte serum on the course of chlamydial genital infection in female guinea pigs. Infect Immun. 1983 Aug;41(2):876–879. doi: 10.1128/iai.41.2.876-879.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Barron A. L. Humoral immune response in acquired immunity to chlamydial genital infection of female guinea pigs. Infect Immun. 1983 Jan;39(1):463–465. doi: 10.1128/iai.39.1.463-465.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Batteiger B. E. Protective role of serum antibody in immunity to chlamydial genital infection. Infect Immun. 1989 Jan;57(1):299–301. doi: 10.1128/iai.57.1.299-301.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Batteiger B. E., Soderberg L. S. Susceptibility to reinfection after a primary chlamydial genital infection. Infect Immun. 1988 Sep;56(9):2243–2249. doi: 10.1128/iai.56.9.2243-2249.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Soderberg L. S., Barron A. L. Chronic chlamydial genital infection in congenitally athymic nude mice. Infect Immun. 1985 Jun;48(3):847–849. doi: 10.1128/iai.48.3.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., Soderberg L. S., Sanders M. M., Batteiger B. E. Role of cell-mediated immunity in the resolution of secondary chlamydial genital infection in guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1989 Mar;57(3):706–710. doi: 10.1128/iai.57.3.706-710.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rank R. G., White H. J., Barron A. L. Humoral immunity in the resolution of genital infection in female guinea pigs infected with the agent of guinea pig inclusion conjunctivitis. Infect Immun. 1979 Nov;26(2):573–579. doi: 10.1128/iai.26.2.573-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Cles L. D., Ray R. M., Hesse F. E. Is there immunity to chlamydial infections of the human genital tract? Sex Transm Dis. 1983 Jul-Sep;10(3):123–125. doi: 10.1097/00007435-198307000-00004. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenman W. M., Meuser R. U. Chlamydia trachomatis elementary bodies possess proteins which bind to eucaryotic cell membranes. J Bacteriol. 1986 Feb;165(2):602–607. doi: 10.1128/jb.165.2.602-607.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E., Taylor H. R. Immune mechanisms in chlamydial eye infection. Development of T suppressor cells. Invest Ophthalmol Vis Sci. 1986 Apr;27(4):615–619. [PubMed] [Google Scholar]