Abstract
The permeability of five benzimidazole derivates with potential cannabinoid activity was determined in two models of membranes, parallel artificial membrane permeability assay (PAMPA) and skin, in order to study the relationship of the physicochemical properties of the molecules and characteristics of the membranes with the permeability defined by the Biopharmaceutics Classification System. It was established that the PAMPA intestinal absorption method is a good predictor for classifying these molecules as very permeable, independent of their thermodynamic solubility, if and only if these have a Log Poct value <3.0. In contrast, transdermal permeability is conditioned on the solubility of the molecule so that it can only serve as a model for classifying the permeability of molecules that possess high solubility (class I: high solubility, high permeability; class III: high solubility, low permeability).
Key words: BCS, benzimidazole, PAMPA, transdermal penetration
INTRODUCTION
Biopharmaceutical Drug Classification (BCS) arose in 1995 and established in which cases an in vivo bioavailability study can be replaced by an in vitro dissolution study (1). It is determined that this correlation depends basically on two parameters of the molecule: solubility in water and permeability. Thus, molecules can be classified into four classes: class I (high solubility, high permeability); class II (low solubility, high permeability); class III (high solubility, low permeability); and class IV (low solubility, low permeability) (1,2).
A provisional classification of the solubility of molecules can be done using values described in the bibliography (e.g., Merck Index, USP, etc.) (3). However, BCS expresses solubility by means of the dimensionless dose figure, Do. This Do corresponds to the ratio of drug concentration in the administrated volume (250 mL) to the saturation solubility of the drug in water [Do = (dose/250)/solubility] (1,4). In general, drugs with Do ≤ 1 were classified as high-solubility drugs, and drugs with Do > 1 were classified as low-solubility drugs (4). Ideally, in order to calculate the permeability of a molecule, experimental studies should be performed that allow relating data between the permeability of the jejunum and a reference intravenous dosage. However, transitory permeability can be calculated through Log Poct, which is defined as the ratio between the equilibrium concentrations of a substance dissolved in a system of two immiscible solvents: n-octanol and water (5,6). Poct is a dimensionless figure usually expressed as a base 10 logarithm (Log Poct) (7). Some authors sustain that an ideal Log Poct value is within 1 and 4 because in this range, the molecule has enough lipid affinity to cross membranes and enough water affinity to diffuse and dissolve in body fluids (6,8–10). The value of Log Poct is only an estimate of the permeability since the said parameter does not consider absorption through transporters, which is an important absorption mechanism of drugs (3).
One of the in vitro methods that can be used is the “parallel artificial membrane permeability assay” (PAMPA). This method consists of a hydrophobic filter material that is coated with a mixture of lecithin and inert organic solvent creating an artificial lipid membrane (11–15). The extent of permeation through the membrane is measured and compared with a known degree of drug absorption in humans. An excellent correlation was demonstrated between the flux across PAMPA systems and the extent of absorption of a diverse set of well-characterized drugs in humans (16–21). No good correlation is observed when the drug is transported by active transport mechanisms.
It would be interesting to study the potential of other membranes with characteristics that, albeit different from the intestinal epithelium (i.e., the skin), could serve as a model to classify the permeability of compounds as defined by the BCS.
In this paper, we study a series of benzimidazoles synthesized in our laboratories as modulators of CB1 cannabinoid receptors. We analyzed their permeability in two models of membranes—PAMPA and skin—in order to study the relationship of the physicochemical properties of the molecules and characteristics of the membrane with the permeability defined by the BCS.
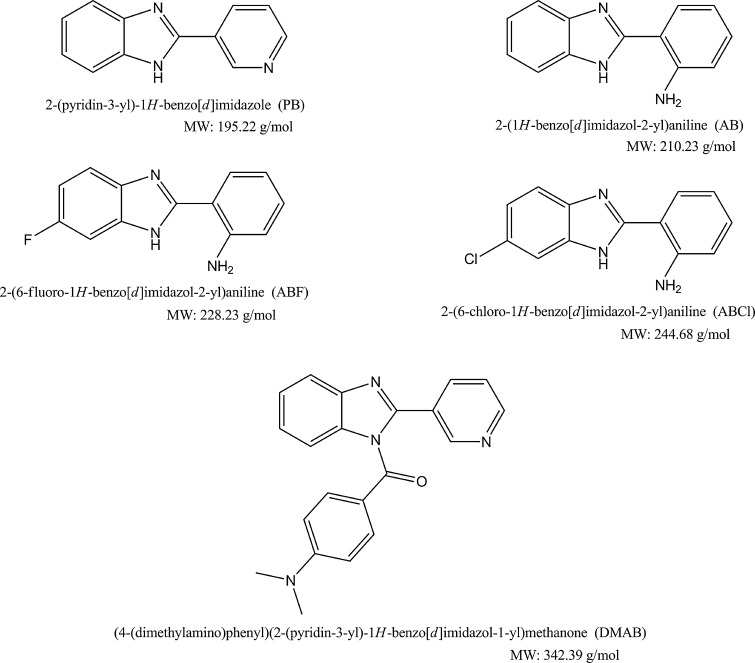
MATERIALS AND METHODS
Reagents
Acetonitrile and methanol (high-resolution liquid chromatography (HPLC) quality) were bought from Merck S.A. Lecithin from egg yolk, verapamil, and dodecane were bought from Sigma Aldrich. All the other reagents used (Na2HPO4, aniline, benzene, toluene, bromobenzene, naphthalene, etc.) were of analytic grade. The molecules 2-pyridin-3-yl-1H-benzimidazole (PB), 2-(1H-benzimidazole- 2-yl)aniline (AB), 2-(6 fluor-1H-benzimidazole-2-yl) aniline (ABF), 2-(6-cloro-1H-benzimidazole-2-yl)aniline (ABCl), and N,N-dimetyl-4-((2-fenyl-1H-benzoimidazole-1-yl) carbonyl) aniline (DMAB) were synthesized in the synthesis laboratory headed by Dr. David Pessoa-Mahana (Pontificia Universidad Católica de Chile, Faculty of Chemistry, Pharmacy Department). The filters for the PAMPA were Millipore MultiScreen® IP 0.45 μm hydrophob.
Determination of Log Poct of Benzimidazole Compounds
High-resolution liquid chromatography was used (22–24). To do so, a Shimadzu C-R6A liquid chromatograph was employed, with a model SPD-6A UV detector, a Shimadzu model LC-9A pump, and a Shimadzu C-R6A integrator. The analysis was done under the following conditions: LiChospher® 100 RP-18 (5 μm) column; mobile phase: For molecules found in the neutral state at pH 5.5, a mixture of methanol/water in a proportion of 65:35 was used, while for the determination of the Log Poct of molecules that present an ionized form at this pH, the pH was adjusted to 7.0; modifying the mobile phase to methanol/buffer Na2HPO4 0.02 M, pH 7.0, in equal proportion; flow, 0.8 mL/min. The reference compounds used were aniline, benzene, toluene, bromobenzene, naphthalene, and bencilic alcohol. For the quantification of each molecule, their maximum longitude of absorption was used (24). In this way, the correlation graph of Log k vs. Log Poct was obtained, k being the capacity factor.
Gastrointestinal Permeability Studies (PAMPA)
The permeability method used in these studies was carried out in a 96-well format. A 96-well microtiter plate and a 96-well filter plate (Millipore MultiScreen® IP 0.45 μm hydrophob, USA) were assembled into a “sandwich” such that each composite well was separated by a 125-μm microfilter disc. The hydrophobic filter was coated with 4 μL of 2% lecithin dissolved in dodecane. Subsequently, the filter plate was placed on the microtiter plate containing 330 μL of the compound in the range of concentration 20–200 μM dissolved in buffer KH2PO4 0.2 M, pH 7.4, with no more than 1% of DMSO. This constituted the donor solution. The acceptor wells (in the top of the wells) of the sandwich were hydrated with 330 μL of buffer KH2PO4 0.2 M, pH 7.4. The system was incubated for 4 h at 37°C and stirred at 200–300 rpm in an orbital well plate agitator throughout this time (Thermo Micromixer Mxi4t, Finepcr). To prevent loss by evaporation, the system was first covered with a wet paper and then with a plastic film.
Quantification of Benzimidazole Compounds in the PAMPA Studies
The receptor solutions were diluted in such a way as to allow quantification by fluorescence (Fluorimeter Perkin Elmer LS 55) using a calibrated line that was built for each compound under study by the corresponding longitude of absorption and excitation. The apparent permeability (P) was estimated using the equation (Eq. 1) (17):
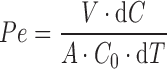 |
1 |
where P is the permeability in centimeters per second, V is the volume of the receiver compartment, A is the surface area (0.3 cm2), C0 is the starting concentration in the donor compartment in micromolar or nanograms per milliliter, and dC/dT is the rate of change of compound concentration, in micromolar per second or nanograms per milliliter, in the receiver compartment at 4 h.
Data Analysis by Permeability Studies (PAMPA)
Three replicates of each experiment were used. Results are presented in the text as the means + SDs. The data were analyzed by variance analysis and Dunn’s tests or Student–Newman–Keuls tests for comparisons of multiple means. Statistical significance was fixed at P < 0.05 (24,25).
Skin Absorption of Benzimidazole Compounds
The skin used was from 2- to 3-day-old pigs (25,26). This skin was frozen (−20°C) before being used. Around 1 h before each experiment, the skin was thawed, shaved, and cut to an adequate size. The epidermis was put into contact with the donor solution. Vertical diffusion cells of 4.15 cm2 were used (Laboratory Glass Apparatus Inc., USA) and thermostatized to 37°C using a water bath. The donor solution contained the benzimidazole compounds in a concentration of 0.5 mg/mL dissolved in PEG 20 oleyl ether (6%, v/v) (27). For each cell, 3 mL of each of the donor solutions was used. The receptor solution corresponded to PEG 20 oleyl ether (6%, v/v) using around 5 mL of these solutions for each cell.
Quantification of Benzimidazole Compounds in the Transdermal Studies
Quantification of benzimidazole compounds in receptor solutions was as follows: First, all of the receptor solution was evaporated to dryness in an oven (Fisher Isotemp Oven Senior Model) at 50°C. The dry residue was then dissolved in 0.8 mL of the HPLC mobile phase (see below), and the solution was then centrifuged (Universal 32R Hettich Zentrifugen) for 15 min at 11,000 rpm. The supernatant was filtered (Sartorious AG-37070, Germany, 0.45 μm) and the compounds quantified in the filtrate by HPLC, as below.
The compounds were quantified by HPLC (mobile phase: acetonitrile/buffer Na2HPO4 0.05 M, pH 6.5; 50:50; LiChospher®100 RP, 18.5-μm column; and flow rate 0.8 mL/min) using a calibrated line that was built for each compound under study by the corresponding maximum longitude of absorption.
Data Analysis by Transdermal Experiments
At least five or six replicates of each experiment were used. Results are presented in the text as the means + SDs. The data were analyzed by Dunn’s tests for comparisons of multiple means. Statistical significance was fixed at P < 0.05 (24,25).
RESULTS AND DISCUSSION
Determination of Log Poct of Benzimidazole Compounds
The calibrated line equation obtained with the reference compounds was Log K = −0.78678 + 0.50661 Log Poct (R2 = 0.99). The experimental Log Poct of the benzimidazole compounds were in the range of 1.8–3.5 (Table I). The theoretical method of the Log Poct determination considers 0.4 logarithmic units as the margin of error for low-molecular-weight and structurally non-complex molecules. Therefore, the values obtained experimentally are considered reliable (see Table I).
Table I.
Log P oct and Solubility of the Benzimidazole Compounds
| Compounds | Log P oct (experimental) | Log P oct (theoretical)a | Solubility (mg/mL)b |
|---|---|---|---|
| PB | 1.84 | 1.53 | 0.2730 |
| AB | 1.86 | 2.06 | 0.0970 |
| ABF | 2.18 | 2.22 | 0.0076 |
| ABCl | 2.81 | 2.62 | 0.0063 |
| DMAB | 3.54 | 3.66 | 0.0094 |
aUsing Chem Draw. computational program
bTheoretical value calculation by ADME-Tox. computational program
Gastrointestinal Permeability Studies (PAMPA)
Initially, we study the absorption of verapamil (50 mM) by PAMPA. The verapamil permeability described in the literature is about 14.0 (10−6 cm/s) (28,29); meanwhile, the experimental permeability determined in our studies was 11.5 ± 3.21 (10−6 cm/s), so it can be assumed that the conditions of the PAMPA experiment are correct. The five compounds studied are shown in Fig. 1. All of them are compounds derived from benzimidazole, as well as omeprazole, and have potential cannabinoid biological activity. Due to their physical chemical properties (Log Poct and solubility in water; Table I), PB and AB belong to class I according to biopharmaceutic classification: drugs with high solubility and high permeability. Likewise, the ABF, ABCl, and DMAB molecules would belong to class II according to this classification: drugs with low solubility and high permeability (1). Since our purpose was to provisionally classify according to the BCS a set of investigational compounds with unknown clinical potency (dose), we were unable to calculate the dose number as in BCS. However, we found it convenient to use the classification strategy by Dahan et al. (3). This strategy considered the analysis of a large number of drugs and concluded that in 65–68% of cases, solubility of <0.1 mg/mL implies that the drug may be classified as high solubility. Drugs that cannot be categorized in this way are high potency, such as digoxin (1,4). On the other hand, we will use the coefficient of partition as a criterion of permeability. Molecules exhibiting n-octanol/water partition coefficient value greater than metoprolol (Log Poct = 1.72) were categorized as high permeability since metoprolol is known to be absorbed from the GI and hence may be used as a reference standard for low/high class boundary (3). The fundamental difference between class I and class II drugs is the feasibility of establishing correlations among the dissolution studies in vitro and the absorption studies in vivo. However, all of them, once soluble, should pass through membranes without difficulty (2). Consequently, in vitro intestinal absorption was studied for these five compounds that theoretically possess high permeability and a varied range of solubility in order to establish whether the PAMPA method is adequate for predicting the permeability of all these molecules since it is known that Log Poct is only one of the parameters that determine absorption through membranes. The results of the different PAMPA studies are shown in Table II, where the influence of the concentration of each compound in the donor solution was studied. Not all studies were performed with the same range of concentrations, basically due to the different solubilities the compounds have. Although in all cases a higher concentration of each compound in the donor implied a greater absorption of the respective compound, there were no statistically significant differences among the different concentrations used in each compound. Consequently, in order to compare permeabilities among the compounds under study, the average value among the permeabilities found for each compound was used (Fig. 2).
Fig. 1.
Chemical structures of the benzimidazole compounds
Table II.
Gastrointestinal Permeability of the Benzimidazole Compounds Using PAMPA
| Compound | Concentration (μM) | Permeability (10−6 cm/s) |
|---|---|---|
| PB | 100 | 2.568 ± 0.244 |
| 200 | 2.768 ± 0.297 | |
| 250 | 2.862 ± 0.410 | |
| AB | 50 | 15.063 ± 0.763 |
| 75 | 20.088 ± 0.799 | |
| 90 | 21.940 ± 2.274 | |
| ABF | 50 | 11.398 ± 1.328 |
| 75 | 12.872 ± 1.125 | |
| 90 | 14.048 ± 0.120 | |
| ABCl | 50 | 4.874 ± 1.069 |
| 75 | 5.049 ± 0.941 | |
| 90 | 6.533 ± 0.881 | |
| DMAB | 60 | –a |
| 50 | –a | |
| 20 | –a |
Each data point represent the mean ± SD of three repeats. No significantly different between the different concentrations studied (Student–Newman–Keuls test, p < 0.05)
aCould not be quantified
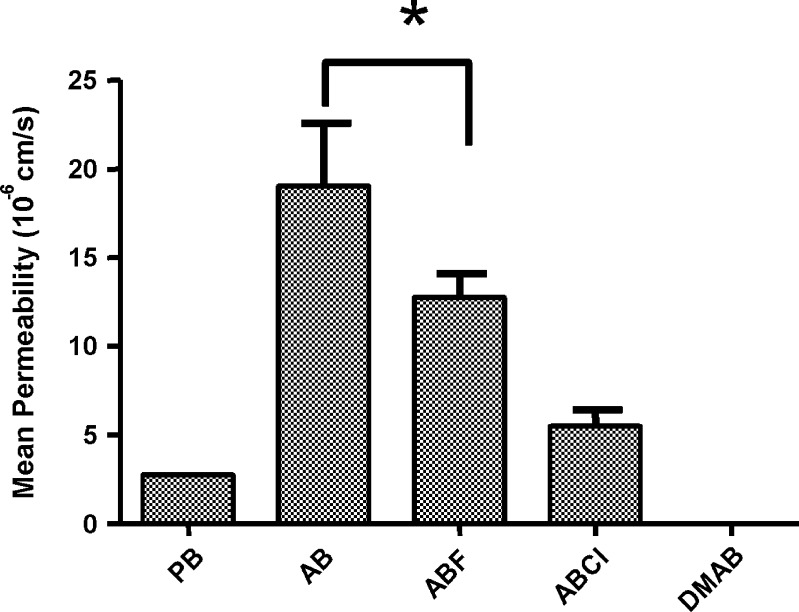
Fig. 2.
Gastrointestinal mean permeability of benzimidazole compounds using PAMPA. Each data point represents the mean ± SD of three repeats. *No significantly different (Dunn’s test, p < 0.05)
The compound that shows the greatest permeability is AB (19.03 ± 3.56 (10−6 cm/s)), followed by ABF (12.77 ± 1.33 (10−6 cm/s)), then ABCl (5.49 ± 0.91 (10−6 cm/s)), and finally, the last that can be calculated is PB (2.73 ± 0.15 (10−6 cm/s)). The compound DMAB was not quantified as a receptor because its permeability is considered null in these conditions.
In the analysis of the class I compounds AB and PB, the permeability values that are apparently very different are observed. However, both present a Pe > 1 (10−6 cm/s) (30). That is to say, the Log Poct values as well as the Pe values classify them as very permeable drugs, such that it is expected that if they were administered orally they would have an absorption >50%.
In the analysis of the class II compounds ABF, ABC1, and DMAB, we observe that the first two show a permeability value that classifies them as very permeable. In contrast, DMAB is not absorbed. It should be noted that the BCS does not establish upper limits on the Log Poct value to classify a drug as very permeable. However, it is known that the optimal Log Poct value to cross membranes is between 1 and 4 (5,7–9), such that the null permeability observed for DMAB could be attributed to its elevated Log Poct (3.7). The high lipophilia of DMAB makes one think that it was not absorbed because it possibly was retained in the membrane, a situation which is described in a PAMPA study using compounds with a similar structure by Brain-Isasi et al. (31).
Therefore, it can be noted that the PAMPA method of intestinal absorption is a good predictor for classifying molecules derived from benzimidazoles, independent of their degree of solubility, as long as they have a Log Poct < 3.0.
Skin Absorption of Benzimidazole Compounds
Transdermal analyses of the five compounds under study were performed with the aim of determining their respective permeability in this membrane (Fig. 3). It is known that optimal Log Poct values for transdermal absorption are between 2 and 3 (32), which are values a little higher than the ideal for oral absorption, the ideal molecular weight being under 500 g/mol (33,34).
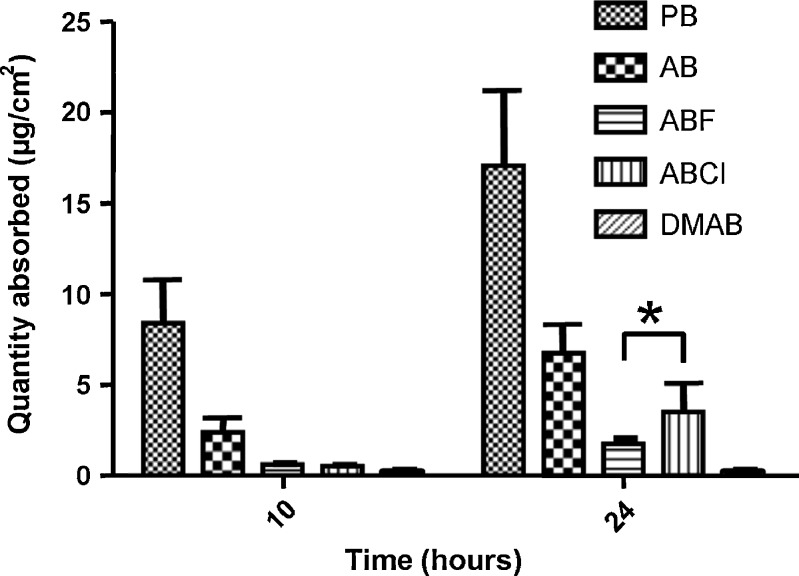
Fig. 3.
Transdermal absorption of benzimidazole compounds. Each data point represents the mean ± SD of five or six repeats. *No significantly different (Dunn’s test, p < 0.05)
As is well known, the skin is not a reference membrane to oral absorption, and there is not a model drug for transdermal permeability equivalent to metoprolol for the oral route (3). In addition, the permeability and potency of the drug must be known for using transdermal administration. There is general agreement that the maximum daily dose that can be absorbed is no more than 10 mg (35,36). Analyzing the results of this study, it can be affirmed that all the compounds are absorbed through skin.
The compound that is most absorbed is PB (17.05 ± 4.26 μg/cm2 at 24 h), followed by the compound AB (6.74 ± 1.07 μg/cm2 at 24 h), with the transdermal absorption of PB 2.5 times greater than that of AB, with both values presenting a statistically significant difference at 24 h of study. Both compounds also have practically equal lipophilia (Log Poct of 1.8 and 1.9, respectively). However, the solubility of PB is around three times greater than that of AB (see Fig. 1), although both compounds are classified as very soluble according to the BCS. This latter parameter can be attributed to the difference in transdermal absorption that was found. Nevertheless, as the ideal value of permeability is unknown, it cannot be concluded that they would be classified as very permeable. It can only be affirmed that the two are the most permeable of the series.
For the other compounds under study, ABF, ABCl, and DMAB, statistically significant differences are only found between ABF and ABCL at 24 h of study (1.73 ± 0.30 and 3.50 ± 1.58 μg/cm2, respectively). This is fundamentally attributable to the reliability of the quantification technique for finding low transdermal absorption values since both compounds have very similar solubility and Log Poct values. This low permeability of ABF and ABCl is in comparison to PB and AB, but does not necessarily imply that they should be categorized as low permeable in a model that uses skin as a membrane. The compound that was least absorbed, DMAB (0.22 ± 0.08 μg/cm2 at 24 h), which also was not possible to quantify between 10 and 24 h of study, possesses the greatest lipophilia, with a Log Poct value greater than the ideal described for the skin. It is possible that DMAB gets trapped in the skin, the same as that observed in previous transdermal studies performed by our research group in which microemulsions were used as the administration vehicle (24). It would be inappropriate to assume this low permeability as a consequence of its higher molecular weight because it is under 500 g/mol (see Fig. 1).
It could be inferred then that for benzimidazole derivates that have low solubility in water (<0.001 mg/mL), what determines their higher or lower transdermal permeability is their lipophilia, with the optimal Log Poct range for these types of compounds being 2.2–2.8. Log Poct values greater than this make the adequate penetration of the molecule difficult (31).
The transdermal permeation results for benzimidazole derivates suggest that the high aqueous solubility enhances the experimental precision of the permeability measure, thus increasing the reliability of the skin model in classifying derivatives as per BCS. This could be explained by the tortuous path a water-soluble compound must diffuse through when crossing the stratum corneum, the rate-limiting step for transdermal absorption. On the other hand, models of oral absorption such as PAMPA can be visualized as two aqueous compartments separated by a lipid barrier, the latter being the rate-limiting step for mass transport.
CONCLUSIONS
It was established that the PAMPA intestinal absorption method is a good predictor for classifying benzimidazole derivates as very permeable, independent of their degree of thermodynamic solubility, if and only if these have a Log Poct value < 3.0. However, skin can only serve as a model for classifying benzimidazole derivates with high solubility (class I: high solubility, high permeability; class III: high solubility, low permeability). The main difference between these two studied models resides on the hydrophilicity requirements for compounds to be delivered by each administration route. In the case of the transdermal delivery, the molecule must cross hydrophilic regions, so water solubility takes more relevance as a physicochemical property.
Acknowledgments
This work was supported by the Faculty of Chemistry of the Pontificia Universidad Católica de Chile and FONDECYT (grant 1100493).
References
- 1.Amidon GL, Lennernas H, Shah VP, John RC. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo biovailability. Pharm Res. 1995;12(3):413–20. doi: 10.1023/A:1016212804288. [DOI] [PubMed] [Google Scholar]
- 2.Dressmann JB, Amidon GL, Reppas C, Shah VP. Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm Res. 1998;15(1):11–22. doi: 10.1023/A:1011984216775. [DOI] [PubMed] [Google Scholar]
- 3.Dahan A, Jonathan M, Miller JM, Amidon GL. Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS J. 2009;11(4):740–6. doi: 10.1208/s12248-009-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kasim NA, Whitehouse M, Ramachandran C, Bermejo M, Lennernais H, Hussain AS, Juginger HE, Stavchansky SA, Midha K, Shah VP, Amidon GL. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol Pharm. 2004;1(1):85–96. doi: 10.1021/mp034006h. [DOI] [PubMed] [Google Scholar]
- 5.Avendaño C. Introducción a la Química Farmacéutica. 2a. Spain: McGraw Hill Interamericana; 2001. [Google Scholar]
- 6.Graham LP. An introduction to medicinal chemistry. 3a. Oxford: Oxford University Press; 2005. [Google Scholar]
- 7.Hansch C, Leo A, Mekapati SC, Kurup A. QSAR and ADME. Bioorg Med Chem. 2004;12:391–340. doi: 10.1016/j.bmc.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 8.Costas G, Tsantili-Kakoulidou A. Alternative measures of lipophilicity: from octanol–water partitioning to IAM retention. J Pharm Sci. 2008;97(8):2984–3004. doi: 10.1002/jps.21244. [DOI] [PubMed] [Google Scholar]
- 9.Poole SK, Poole CF. Separation methods for estimating octanol–water partition coefficients. J Chromatogr B. 2003;797:3–19. doi: 10.1016/j.jchromb.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Liu R, Zhou D. Using molecular fingerprint as descriptors in the QSPR study of lipophilicity. J Chem Inf. 2008;48:542–9. doi: 10.1021/ci700372s. [DOI] [PubMed] [Google Scholar]
- 11.Fujikawa M, Ano K, Nakao K, Shimizu R, Akamatsu M. Relationships between structure and high throughput screening permeability of diverse drugs with artificial membranes: application to prediction of Caco-2 cell permeability. Bioorg Med Chem. 2005;13:4721–32. doi: 10.1016/j.bmc.2005.04.076. [DOI] [PubMed] [Google Scholar]
- 12.Fujikawa M, Nakao K, Shimizu R, Akamatsu M. QSAR study on permeability of hydrophobic compounds with artificial membranes. Bioorg Med Chem. 2007;15:3756–67. doi: 10.1016/j.bmc.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 13.Kansy M, Senner F, Gubernator K. Physicochemical high throughput screening: parallel artificial membrane permeation assay in the description of passive absorption processes. J Med Chem. 1998;41(7):1007–10. doi: 10.1021/jm970530e. [DOI] [PubMed] [Google Scholar]
- 14.Sugano K, Nabuchi Y, Machida M, Aso Y. Prediction of human intestinal permeability using artificial membrane permeability. Int J Pharm. 2003;257:245–51. doi: 10.1016/S0378-5173(03)00161-3. [DOI] [PubMed] [Google Scholar]
- 15.Wohnsland F, Faller B. Throughput permeability ph profile and high throughput alkane/water log P with artificial membranes. J Med Chem. 2001;44:923–30. doi: 10.1021/jm001020e. [DOI] [PubMed] [Google Scholar]
- 16.Avdeef A, Bendels S, Di L, Faller B, Kansy M, Sugano K, Yamauchi Y. PAMPA—critical factors for better predictions of absorption. J Pharm Sci. 2007;96(11):2893–909. doi: 10.1002/jps.21068. [DOI] [PubMed] [Google Scholar]
- 17.Balimane PV, Pace E, Chong S, Zhu M, Jemal M, Pelt CK. A novel high-throughput automated chip-based nanoelectrospray tandem mass spectrometric method for PAMPA sample analysis. J Pharm Biomed Anal. 2005;39(1–2):8–16. doi: 10.1016/j.jpba.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 18.Bermejo M, Avdeef A, Ruiz A, Nalda R, Ruell JA, Tsinman O, González I, Fernández C, Sánchez G, Garrigues TM, Merino V. PAMPA—a drug absorption in vitro model 7. Comparing rat in situ, Caco-2, and PAMPA permeability of fluoroquinolones. Eur J Pharm Sci. 2004;21:429–41. doi: 10.1016/j.ejps.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Corti G, Maestrelli F, Cirri M, Zerrouk N, Mura P. Development and evaluation of an in vitro method for prediction of human drug absorption II. Demonstration of the method suitability. Eur J Pharm Sci. 2006;27:354–62. doi: 10.1016/j.ejps.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Kansy M, Avdeef A, Fischer H. Advances in screening for membrane permeability: high-resolution PAMPA for medicinal chemists. Drug Discov Today Technol. 2004;1(4):349–55. doi: 10.1016/j.ddtec.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Kerns EH, Di L, Petusky S, Farris M, Ley R, Jupp P. Combined application of parallel artificial membrane permeability assay and Caco-2 permeability assays in drug discovery. J Pharm Sci. 2004;93(6):1440–53. doi: 10.1002/jps.20075. [DOI] [PubMed] [Google Scholar]
- 22.Yamagami C, Kawase K, Iwaki K. Hydrophobicity parameters determined by reverse-phase liquid chromatography. XV: optimal conditions for prediction of log Poct by using RP-HPLC procedures. Chem Pharm Bull. 2002;50(12):1578–83. doi: 10.1248/cpb.50.1578. [DOI] [PubMed] [Google Scholar]
- 23.Dias NC, Nawas MI, Poole CF. Evaluation of a reversed-phase column (Supelcosil LC-ABZ) under isocratic and gradient elution conditions for estimating octanol–water partition coefficients. Analyst. 2003;128:427–33. doi: 10.1039/b300574g. [DOI] [PubMed] [Google Scholar]
- 24.Alvarez-Figueroa MJ, Pessoa-Mahana CD, González-Bustamante DA. Influence of lipophilia and of the vehicle used in the transdermal absorption of novel benzimidazole compounds with possible anti-HIV activity. Pharm Dev Technol. 2008;13:127–33. doi: 10.1080/10837450701831138. [DOI] [PubMed] [Google Scholar]
- 25.Alvarez-Figueroa MJ, Araya-Silva I, Díaz-Tobar C. Iontophoretic transdermal delivery of haloperidol. Pharm Dev Technol. 2006;11:371–5. doi: 10.1080/10837450600770148. [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Figueroa MJ, Blanco-Méndez J. Transdermal delivery of methotrexate: iontophoretic delivery from hydrogels and passive delivery from microemulsions. Int J Pharmacogn. 2001;215:57–65. doi: 10.1016/S0378-5173(00)00674-8. [DOI] [PubMed] [Google Scholar]
- 27.Bronaugh RL, Stewart RF. Methods for in vitro percutaneous absorption studies III: hydrophobic compounds. J Pharm Sci. 1984;73(9):1255–8. doi: 10.1002/jps.2600730916. [DOI] [PubMed] [Google Scholar]
- 28.Kerns EH, S Petusky, Farris M, Ley R, Jupp P. Combined application of parallel artificial membrane permeability assay and Caco-2 permeability assays in drug discovery. J Pharm Sci. 2004;93(6):1440–53. doi: 10.1002/jps.20075. [DOI] [PubMed] [Google Scholar]
- 29.Ruell JA, Tsinman KL, Avdeef A. PAMPA—a drug absorption in vitro model 5. Unstirred water layer in iso-pH mapping assays and pKaflux-optimized design (pOD-PAMPA) Eur J Pharm Sci. 2003;20:393–402. doi: 10.1016/j.ejps.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 30.Fischer H, Kansy M, Avdeef A, Senner F. Permeation of permanently positive charged molecules through artificial membranes—influence of physico-chemical properties. Eur J Pharm Sci. 2007;31:32–42. doi: 10.1016/j.ejps.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 31.Brain-Isasi S, Quezada C, Pessoa H, Morello A, Kogan MJ, Álvarez-Lueje A. Determination and characterization of new benzimidazoles with activity against Trypanosoma cruzi by UV spectroscopy and HPLC. Bioorg Med Chem. 2008;16:7622–30. doi: 10.1016/j.bmc.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 32.Zheng XS, Duan CZ, Xiao ZD, Yao BA. Transdermal delivery of praziquantel: effects of solvents on permeation across rabbit skin. Biol Pharm Bull. 2008;31(5):1045–8. doi: 10.1248/bpb.31.1045. [DOI] [PubMed] [Google Scholar]
- 33.Subedi RK, Oh SY, Chun M-K, Choi H-K. Recent advances in transdermal drug delivery. Arch Pharm Res. 2010;33(3):339–51. doi: 10.1007/s12272-010-0301-7. [DOI] [PubMed] [Google Scholar]
- 34.Barry B. Farmacia, la ciencia del diseño de las formas farmacéuticas. Chapter 33: Administración de fármacos por vía transdérmica. M.E. Aulton (ed). Elsevier España S.A., 2004 second edition. p. 499–533.
- 35.Guy RH. Transdermal drug delivery. Hand Exp Pharmacol. 2010;197:399–410. doi: 10.1007/978-3-642-00477-3_13. [DOI] [PubMed] [Google Scholar]
- 36.Barry BW. Is the transdermal drug delivery research still important today? DDT. 2001;6(19):967–71. doi: 10.1016/s1359-6446(01)01938-9. [DOI] [PubMed] [Google Scholar]