INTRODUCTION
Assessment of the disintegration, dissolution and drug release profiles of oral drug formulations are routinely performed by subjecting them to standard pharmacopeial test methods. However, the unpredictable failure of dosage forms in vivo has drawn attention to the need for more biorelevant test methods. There are several factors which affect the disintegration and dissolution of a formulation within the gastrointestinal (GI) tract, including the physiology of the GI tract in terms of composition of the secretions and motility (shear forces and turbulent flow), and other factors such as the presence of food or alcohol (1,2).
The dilution and digestion of the ingested bolus in the stomach occurs not only as a consequence of the addition of gastric acid and enzyme secretions, but also is largely influenced by the grinding forces applied by contraction waves as they move down from the body of the stomach into the antrum. The mechanical processing of the gastric bolus reduces the size of solid particles. Sieving solid materials as a function of their particle size occurs prior to emptying via the pylorus into the duodenum. This means that initially liquid and small particulates tend to be emptied preferentially into the duodenum (3). This effect is very important in the case of tablets, and several studies have shown that the passage through the pylorus of an ingested tablet (and capsules) is related not only to their dimensions and density (4–8), but also to the position of the administered tablet within the stomach (9). Thus, the mechanical forces exerted by the stomach and the shear forces generated within its content can influence the mixing, disintegration and erosion of administered tablets, resulting in inhomogeneous mixing and in some cases an undesirable and unpredictable release of the active ingredient from the formulation. This functional failure is particularly well documented for diclofenac extended release tablets for which the presence of two peaks on the plasma profile has been found to be formulation dependent and due to dose dumping induced by the peristaltic forces applied by the stomach (10).
Several studies (11,12) have investigated the flow pattern of systems such as the Dissolution Apparatus USP-I (basket) and USP-II (paddle) at various speeds by using Computational Fluid Dynamics. However, the hydrodynamics of these systems are far from that calculated for the human stomach (13). In fact, the drug dissolution from a solid formulation is greatly influenced by fluid flow and mechanical forces, and this must be taken into account when designing an in vitro method which aims to predict the in vivo behaviour of a formulation. Thus, a more comprehensive simulation of the gastric digestion should not only mimic the biochemistry of the process but also its mechanical forces, since only a combined approach of the two will result in a close simulation of the in vivo scenario.
More physiologically relevant media have been designed in order to simulate the composition of the stomach contents in both fasted and fed states (14,15), along with apparatuses which attempt to replicate the complexity of the stresses applied to an ingested formulation to recreate gastric pressure, shear and flows (10,16), the physical mechanical stress encountered by the tablet once in the GI tract (17) or the hydrodynamics of the stomach (18). However, these models are not able to quantitatively recreate the grinding forces produced in vivo by the human stomach.
In this study, the grinding forces of a recently developed computer-controlled, real-time physical simulator of gastric processing, the Dynamic Gastric Model (DGM) (19,20) and a Dissolution Apparatus USP-II, operated at two rotational speeds (50 and 100 rpm), were measured using the breakdown of agar gel beads of various fracture strengths in high- and low-viscosity meals. The DGM was designed to replicate the real-time changes in pH, enzyme addition, shearing, mixing, and retention time of an adult human stomach. The model can be fed ‘meals’ ranging from a glass of water to high fat meals (i.e. the FDA high fat American breakfast) and deliver samples from its ‘antrum’ in the same processed form and at the same rate as seen in vivo. The data used to program the DGM were derived from echo-planar imaging studies (21,22) and from published references detailing physiological ranges for the rate of production of gastric secretions (23). The results obtained from the DGM and the USP II were compared to those previously observed in human volunteers (24).
MATERIALS AND METHODS
Materials
Locust bean gum (LBG) and agar were both purchased from Sigma (Poole, Dorset, UK). For the DGM reagents egg l-α-phosphatidylcholine (lecithin, grade 1, 99% purity) was obtained from Lipid Products (South Nutfield, Surrey, UK), porcine gastric mucosa pepsin (activity 3,300 units/mg of protein calculated using haemoglobin as substrate) was obtained from Sigma (Poole, Dorset, UK) and a gastric lipase analogue derived from Rhizopus orizae (Lipase F-AP15) was purchased from Amano Enzyme Inc. (Nagoya, Japan). All other salts and chemicals used were of analytical grade and purchased from Sigma.
Preparation of the Test Meals and Agar Beads
LBG test meals of low viscosity (LV; 0.06 Pa s) and high viscosity (HV; 30 Pa s) were prepared by adding 10.5 and 40.0 g, respectively, of gum powder to 1 L of ultrapure water (MilliQ grade 18 mΩ, SG Labostar, SG Water, Germany). In order to prepare the low-viscosity meal, the LBG powder was left to hydrate while vigorously stirring overnight. The high-viscosity meal was produced by autoclaving the LBG with water (121°C for 1 h) and left to cool slowly overnight at room temperature. The consistency of the LV LBG meal was similar to water, whereas the HV LBG meal was barely pourable. Viscosity of the meals was measured using a TA Instruments AR2000 controlled stress rheometer (TA, Crawley, Surrey, UK) in continuous rotation. The measuring geometry was an acrylic cone (diameter 60 mm, cone angle 2º) and plate held at 37ºC. Measurements were taken over a 2-min period.
Agar beads of varying fracture strengths were prepared as previously described (24). Briefly, spherical agar gel beads of 1.27 cm diameter were cast at four linearly increasing fracture strengths (0.53, 0.65, 0.78 and 0.90 N) by autoclaving (121°C for 1h) food-grade agar in water (1.51%, 1.89%, 2.39%, and 3.0% agar in water respectively for the four fracture strengths). The liquid hot agar gel was injected into aluminium moulds, which were then left to slowly cool down overnight at room temperature. The rheological behaviour of the agar gel beads has been previously assessed (24). After casting, the beads were dyed with bromophenol blue for easy visualisation and identification of the beads’ fragment in the two in vitro systems.
Dynamic Gastric Model
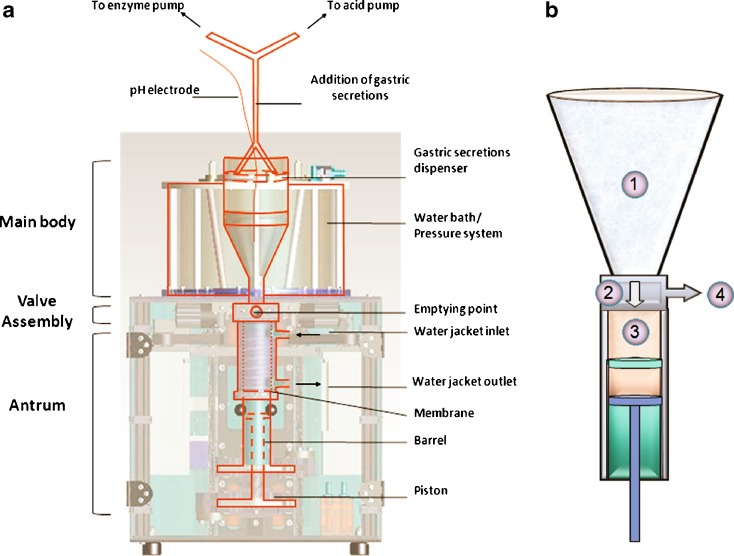
The DGM is able to process complex foods through mechanical and enzymatic digestion as the human stomach. It is composed of three parts, the main body (fundus), the antrum and the valve assembly (Fig. 1a). The main body of the DGM can accommodate up to 800 mL, equivalent to a large meal (e.g. the FDA high fat American breakfast). The system is thermostated at 37°C. In Fig. 1b are depicted the four steps of the processing mechanism of the DGM. In the main body of the DGM (Fig. 1b (1)) the inhomogeneous mixing of the stomach is reproduced by gentle contractions, three per minute, induced by computer-controlled changes in the applied water pressure in the thermostated water bath surrounding the main body. Gastric acid and enzymes are added from a dispenser that is floating on the top of the main body contents. The dispenser is designed in such a way as to deliver the enzyme and acid evenly from the sides of the main body, replicating the human gastric secretions originating from the walls of the stomach. The rate of addition of both enzymatic and acid secretions is computer-controlled and regulated by two peristaltic pumps (323 Du/D, Watson Marlow, Cornwall, UK). In the case of the enzymatic additions, the rate is calculated as a function of the volume of the food content, while the acid additions are pH-dependent. The pH that controls the acid addition is recorded through a pH catheter (Albyn Medical Ltd, Ross-shire, UK) directly immersed in the main body of the DGM. The feedback mechanisms that control enzyme and acid additions are designed so that the secretions are added at physiological rates (23). Since the meal consisted of a non-digestible matrix, the addition of gastric secretions in this study served to reproduce the dilution effect during gastric processing, as in vivo. The food material is allowed to move from the main body into the antrum, and vice versa (Fig. 1b (2)). The DGM antrum (Fig. 1b (3)) was designed to simulate the strong shear forces of the human antrum and to reproduce the breakdown of the food particles and the preferential sieving observed in vivo (25). The mechanical processing of the food within the antrum is achieved by the sliding of a piston within a barrel, which forces the material through an elastic annulus where selective sieving takes place. Once ready, the processed bolus is ejected through the valve assembly (Fig. 1b (4)) and can be collected for further analysis.
Fig. 1.
Schematic representations of the Dynamic Gastric Model. a depicts the main components of the DGM (adapted from (19)), while b illustrates the mechanism of the mechanical digestion: (1) The meal/gastric content in the main body is inhomogeneously mixed with the gastric secretions through application of pulsatiles contractions. (2) The content is allowed to go into the antrum through the valve assembly. The inlet valve is open during the process allowing reflux and mixing between the main body and the antrum. (3) The chyme is processed mechanically by the movement of the piston and barrel, and it is forced through an annular membrane. (4) The chyme is emptied from the antrum and collected for analysis
The DGM was calibrated prior to each experiment by measuring the addition rate of the acid and enzyme additions and by calibrating the pH electrode using standard pH buffers 4.0 and 2.0. For both LV and HV meals, the empty DGM was brought to 37oC and primed with 20 mL of gastric acid secretions (0.01 M hydrochloric acid and salts without gastric enzymes) to simulate the mean residual gastric fluid volume in the fasted stomach. One bead (n = 5) of each strength was tested with each meal. When processing the LV LBG meal, the bead was poured into the main body of the DGM together with the meal; while with the HV meal, because of its very thick consistency, the meal was poured first and the bead was manually placed approximately in the middle of the meal to simulate ingestion of the bead with the meal.
The DGM was set to ‘digest’ 800 and 500 mL of the LV and the HV LBG meals respectively. The processed antral volume was 70 mL in total from which 60 mL were emptied at 10 min intervals. The digestion process was continued until there was evidence of the beads being broken. For the purpose of this study, the DGM emptying times (10 min intervals) were not reflective of the in vivo gastric emptying times but were chosen in order to offer a more accurate evaluation of the beads’ breaking times. Each sample ejected was collected in a beaker and sieved (No. 30, 600 μm mesh size) in order to retain any bead fragments contained in the sample.
As the samples were emptied from the DGM every 10 min, each bead’s Mean Breaking Time (MBT) was determined as the mid time point between the time of collection of the last sample with no bead fragments (T1) and the time at which the first sample containing bead fragments is collected (T2):
 |
1 |
This was done in order to compare the data obtained in vitro with those previously published by Marciani et al. (24).
Calculation of MBT from In Vivo Data
Data
In vivo MBT data were obtained from Marciani’s half residence time  (24) by considering that the number of intact beads in the stomach with time followed an exponential decay. For such a function, with a decay constant λ:
(24) by considering that the number of intact beads in the stomach with time followed an exponential decay. For such a function, with a decay constant λ:
 |
2 |
and
 |
3 |
Therefore,
 |
4 |
Paddle Dissolution Apparatus
The breaking forces of the agar beads in a paddle Dissolution Apparatus (USP II) were measured using a Caleva 8ST (UK) paddle dissolution bath at the temperature of 37°C and paddle speeds of 50 and 100 rpm. For each experiment, 20 mL of acid solution (0.01 M hydrochloric acid and salts without gastric enzymes) were added to 500 mL of LV or HV meals and seven beads of one strength were added. In the case of the LV meal, all seven beads and the liquid meal were poured simultaneously into the vessel of the apparatus; the beads spontaneously sank to the bottom of the vessel. For the HV meal, the beads were manually inserted within the meal, to simulate the ingestion of the beads while eating the HV meal and to avoid them floating on top of the meal. The appearance of the beads was checked every 15 min for 2 h. All experiments were made in duplicate.
Statistical Analysis
GraphPad Prism (version 5.01, GraphPad Software, Inc.) was used to analyse the MBT of intact beads, estimated for the different bead strengths. The data were compared for statistical significance by one-way analysis of variance (ANOVA) followed by pair wise comparisons adjusted for multiplicity by the Tukey method. A p value < 0.05 was considered significant.
SigmaPlot 11.0 (built 11.2.0.5 Systat Software Inc. 2008) was used to perform the linear regression of the in vitro–in vivo data. All results are expressed as means ± their standard error (±SE).
RESULTS
Dynamic Gastric Model
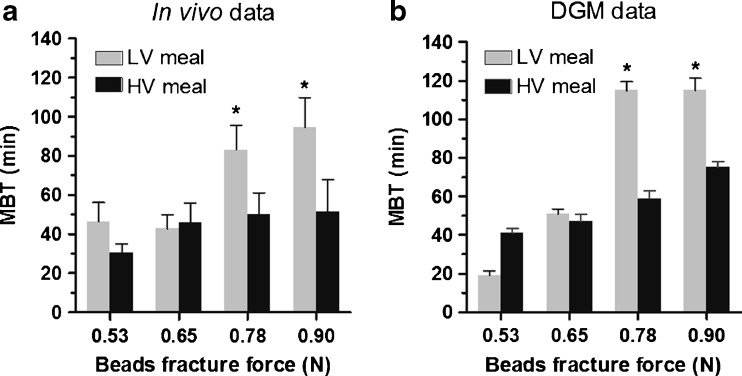
All beads tested in the DGM broke after a certain amount of gastric processing. The results expressed as MBT obtained for the beads at the four strengths administered in LV LBG and HV LBG meals are summarised in Table I. A graphic representation of the data is also shown in Fig. 2b in order to facilitate a direct visual comparison with the in vivo data, Fig. 2a. The gastric processing of the DGM showed that the MBT of intact beads increased linearly with increasing strength when they were administered with a HV meal, while similar MBT values were found for the beads at 0.78 and 0.90 N when administered with the LV meal, Fig. 2b.
Table I.
Mean Breaking Time (MBT) ± Standard Error (SE) of Agar Beads Digested with Low- (LV) and High-Viscosity (HV) Locust Bean Gum (LBG); n = Number of Beads Tested
| Beads fracture force (N) | LV LBG meal | HV LBG meal | ||
|---|---|---|---|---|
| MBT ± SE (min) | N | MBT ± SE (min) | n | |
| 0.53 | 19 ± 2.4 | 5 | 41 ± 2.4 | 5 |
| 0.65 | 51 ± 2.4 | 5 | 47 ± 3.7 | 5 |
| 0.78 | 115 ± 4.5 | 5 | 59 ± 4.0 | 5 |
| 0.90 | 115 ± 6.3 | 5 | 75 ± 3.2 | 5 |
Fig. 2.
Mean Breaking Time (MBT) of agar gel beads for four bead breakdown forces for both low-viscosity (LV—grey bars) and high-viscosity (HV—black bars) meals. Panel a from Marciani (24); n = 9 for the LV and n = 8 for the HV for each bead strength. Panel b in the DGM; n = 5 for each bead strength. *p < 0.05 vs. each of the two lower beads strengths for the LV meal
Paddle Dissolution Apparatus
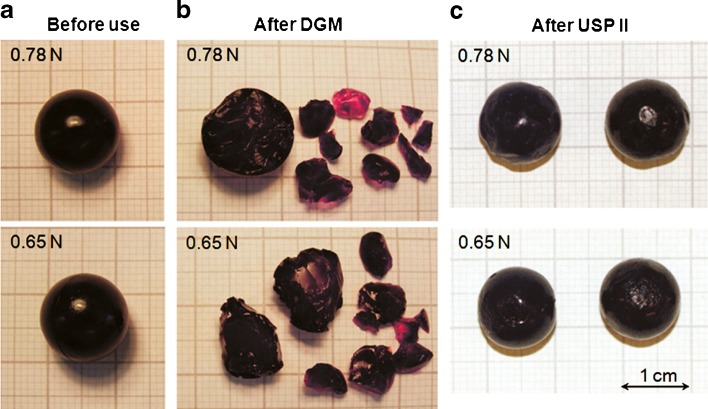
In the USP II, only three beads were found broken in total. Two of them were of strength 0.53 N and one of them was of strength 0.65 N. All three beads broke while they were being processed at 100 rpm with the HV meal and their breaking was observed after 30 min (0.53 N bead), 2 h (0.53 N bead) and 1 h (0.65 N bead), respectively. The rest of the beads were all found intact after 2 h of processing in the vessel of the dissolution apparatus, showing only an etched surface which was more evident after dispersion in the HV meal (Fig. 3). There was no correlation between bead strength or meal viscosity and the breaking of the three beads and therefore these events are likely to be caused by the random impact of the beads with the paddle of the dissolution apparatus.
Fig. 3.
Example of the appearance of the beads at 0.78 and 0.65 N: a before processing; b after digestion in the DGM fed with LV meal; c after 2 h processing in the USP II (100 rpm) containing LV meal
DISCUSSION
Where it is desirable for a drug formulation to dissolve or disintegrate in the gastric environment, antral shearing and mixing can effectively disperse the dose. However, conversely, where it is essential for the formulation to remain intact, antral shearing may result in breakage or erosion and compromise functionality. Often, there is a fairly fine dividing line between the constraints of manufacture and handling and the need to ensure in vivo performance under the range of conditions likely to be imposed by end users. Although mechanical behaviour may be well understood in manufacture, the one area where there is a lack of understanding is the extent of physical shearing in the antrum under fasting and fed conditions.
Previous in vivo studies of ingested agar beads of different strengths have shown that the shear forces exerted by the antral walls of the human stomach during processing are between 0.53 and 0.78 N, as derived by the fact that a threshold at 0.65 N was observed after which the beads’ emptying times were significantly slower (24); also, increasing the viscosity of the meal reduced the survival time of the harder beads, Fig. 2b. This is to be expected, because the higher the viscosity, the more the shear force will be transmitted to the beads. The reduced survival time of beads fed with a viscous meal could also have a contribution from an increase in the frequency of contractions, rate of propagation of the contractions or degree of occlusion of the antrum, but this does not appear to be the case (22). These findings are of fundamental importance because they permit the construction of a model in which the shear rate (shear pattern) is a constant but the energy input is variable and viscosity dependent. This is the basis of the design of the antral compartment in the Dynamic Gastric Model used in this study. In order to establish that the shear developed in the DGM is the same as that measured in vivo, the performance of the model was tested with agar beads as used by Marciani and co-workers (24). These authors observed that whole beads were only infrequently seen to be ejected from the antrum in vivo (four occasions with the harder beads) so that the loss of identifiable intact beads in the antrum was essentially due to bead breakage. The number of intact beads remaining in the stomach at each time point, and thus the half residence time of intact beads in the stomach, could easily be calculated from the MRI images. In a similar way, the antral compartment of the DGM did not eject intact beads but because it was not possible to determine the condition of the beads in the model directly, the model was set such that the preferential sieving allowed bead fragments to be ejected and the first time at which this happened was taken as the time of disintegration. With the USP II, practically no beads were broken after a 2-h processing irrespective of bead strength or meal viscosity.
Although the hydrodynamic flow pattern of the fluid and the shear rates of the USP II, even at 50 rpm (11), are much greater than that found in vivo, the shear created is not sufficient to break the agar beads, rather they are simply picked up in the flow and remain suspended in the moving fluid even in the high-viscosity medium. In fact, the USP II is a dissolution apparatus designed to maximise the rate of dissolution or disintegration by minimising confounding factors such as diffusion and mixing. As such, it maximises the rate of mixing by using high hydrodynamic flow rates (high shear rates) in a large volume whilst reducing the shearing forces. This model is the opposite of that found in vivo where the shear rates are relatively low but the shear forces are greater (26). Despite the greater work input required to maintain rotational speed with the HV medium, this had no effect on the survival of the beads, presumably because the shear forces did not reach that required to break even the softest beads. The three beads that were recovered broken from the USP II showed signs of impact damage presumably through being hit by the stirrer paddle. As an example, in Fig. 3 are shown beads of 0.78 and 0.65 N before use (panel a) and after processing within the DGM (panel b) and the USP II dissolution apparatus (panel c).
Furthermore, the variation of the medium viscosity did not have any impact on the breaking times of the agar beads when using the USP II, as no difference was found between the LV and HV meals. The behaviour in the USP II is in contrast with the in vivo finding which has shown that in the fed state greater shear forces are generated by the gastric muscles in response to the presence of the HV meal (24). Abrahamsson and co-workers (1) reported that the presence of food delayed tablet disintegration and dissolution in dogs but that this was due to the precipitation of a film around the dosage form rather than the ability of the dosage form to resist shearing. Clearly then there are two issues. Dosage forms designed to disintegrate and dissolve in the gastric compartment become well distributed when the ‘meal’ is fluid and can mix easily through the action of the gastric contractions but may remain much more localised in the fed state where mixing is reduced. Conversely, dosage forms designed to resist gastric conditions may perform well in the fasted state or in the presence of fluid gastric contents but may fail in the presence of a more viscous gastric bolus because of increased shear in the antrum.
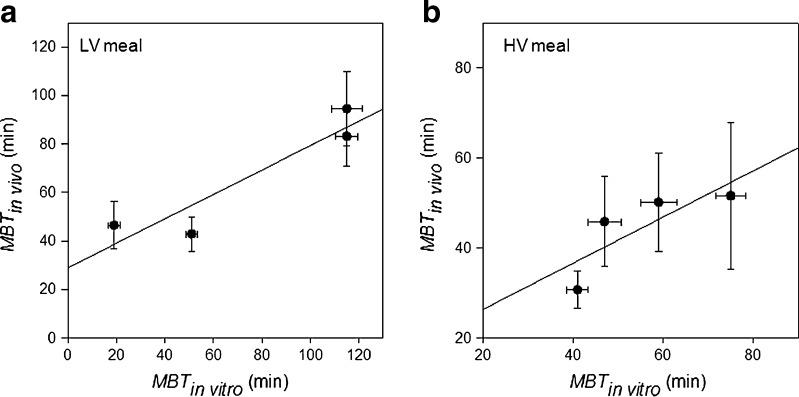
The interrelationship between the in vitro data obtained with the DGM and those observed in vivo (24) is clearly visible from the graphs depicted in Fig. 4. The regression equations of the data obtained for the LV (Fig. 4a) and HV (Fig. 4b) meals were, respectively:
 |
5 |
 |
6 |
Fig. 4.
Correlation of the in vitro MBT (DGM) and in vivo MBT for the beads at the different breaking forces. Panel a LV meal. Panel b HV meal. Straight lines are the regression lines (refer to text for fitting Eqs. 5 and 6). Bars represent SE
Even though the R2 in Eqs. 5 and 6 are not very high, there is a clear correlation between the in vitro (DGM) and the in vivo (24) data. One-way ANOVA analysis showed that no statistical difference exists between any of the data collected from the DGM and those found in human. This indicates that the forces produced during the DGM processing are within the range of forces exerted by the human gastric compartment in vivo.
Furthermore, the DGM showed to be able to discriminate between the two meals, similarly to the finding of Marciani and co-workers (24). The different behaviour observed for the beads in the LV and HV meals is of special interest particularly when considering the effect that it may have on dosage forms for which the drug release is greatly susceptible to the shear forces applied to its surface, as in the case of erodible matrixes.
CONCLUSIONS
The results obtained for the DGM confirmed the claim that a biorelevant simulation of the gastric processing forces found in vivo can be mimicked, while the Dissolution Apparatus II failed to produce any breaking forces, despite the high turbulent flow generated by the paddle at 100 rpm. Thus, the DGM does not only allow for an imitation of the biochemical conditions found in the human stomach from a compositional and temporal perspective, but it is also able to simulate the grinding forces as found in vivo. Because of these unique features, the DGM appears to be a more attractive in vitro model than the ones currently in use, and it could be utilized to predict the behaviour of drug delivery systems within the gastric compartment.
Acknowledgments
The authors would like to thank Dr. Luca Marciani from the University of Nottingham from providing us with the original data from their in vivo study (24) and Dr. Gillian Rich for her useful suggestions on the calculations of the in vivo MBT values.
References
- 1.Abrahamsson B, Albery T, Eriksson A, Gustafsson I, Sjöberg M. Food effects on tablet disintegration. Eur J Pharm Sci. 2004;22(2–3):165–172. doi: 10.1016/j.ejps.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Lennernäs H. Ethanol-drug absorption interaction: potential for a significant effect on the plasma pharmacokinetics of ethanol vulnerable formulations. Mol Pharm. 2009;6(5):1429–1440. doi: 10.1021/mp9000876. [DOI] [PubMed] [Google Scholar]
- 3.Collins PJ, Horowitz M, Cook DJ, Harding PE, Shearman DJC. Gastric emptying in normal subjects—a reproducible technique using a single scintillation camera and computer system. Gut. 1983;24:1117–1125. doi: 10.1136/gut.24.12.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis SS, Hardy JG, Taylor MJ, Whalley DR, Wilson CG. A comparative study of the gastrointestinal transit of a pellet and tablet formulation. Int J Pharm. 1984;21(2):167–177. doi: 10.1016/0378-5173(84)90091-7. [DOI] [Google Scholar]
- 5.Podczeck F, Course N, Newton JM, Short MB. Gastrointestinal transit of model mini-tablet controlled release oral dosage forms in fasted human volunteers. J Pharm Pharmacol. 2007;59(7):941–945. doi: 10.1211/jpp.59.7.0005. [DOI] [PubMed] [Google Scholar]
- 6.Podczeck F, Course NC, Newton JM, Short MB. The influence of non-disintegrating tablet dimensions and density on their gastric emptying in fasted volunteers. J Pharm Pharmacol. 2007;59(1):23–27. doi: 10.1211/jpp.59.1.0004. [DOI] [PubMed] [Google Scholar]
- 7.Podczeck F, Mitchell CL, Newton JM, Evans D, Short MB. The gastric emptying of food as measured by gamma-scintigraphy and electrical impedance tomography (EIT) and its influence on the gastric emptying of tablets of different dimensions. J Pharm Pharmacol. 2007;59(11):1527–1536. doi: 10.1211/jpp.59.11.0010. [DOI] [PubMed] [Google Scholar]
- 8.Podczeck F, Mitchell CL, Newton JM, Evans D, Short MB. The gastric emptying of food as measured by gamma-scintigraphy and electrical impedance tomography (EIT) and its influence on the gastric emptying of tablets of different dimensions (Journal of Pharmacy and Pharmacology (2007) 59, (1527–1536)) J Pharm Pharmacol. 2008;60(4):533. doi: 10.1111/j.2042-7158.2008.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 9.Weitschies W, Wedemeyer RS, Kosch O, Fach K, Nagel S, Söderlind E, et al. Impact of the intragastric location of extended release tablets on food interactions. J Control Release. 2005;108(2–3):375–385. doi: 10.1016/j.jconrel.2005.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Garbacz G, Wedemeyer R-S, Nagel S, Giessmann T, Mönnikes H, Wilson CG, et al. Irregular absorption profiles observed from diclofenac extended release tablets can be predicted using a dissolution test apparatus that mimics in vivo physical stresses. Eur J Pharm Biopharm. 2008;70(2):421–428. doi: 10.1016/j.ejpb.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 11.D’Arcy DM, Corrigan OI, Healy AM. Evaluation of hydrodynamics in the basket dissolution apparatus using computational fluid dynamics-dissolution rate implications. Eur J Pharm Sci. 2006;27(2–3):259–267. doi: 10.1016/j.ejps.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 12.McCarthy LG, Kosiol C, Healy AM, Bradley G, Sexton JC, Corrigan OI. Simulating the hydrodynamic conditions in the United States pharmacopeia paddle dissolution apparatus. AAPS PharmSciTech. 2003;4(2) [DOI] [PMC free article] [PubMed]
- 13.Pal A, Indireshkumar K, Schwizer W, Abrahamsson B, Fried M, Brasseur JG. Gastric flow and mixing studied using computer simulation. P Roy Soc B Biol Sci. 2004;271(1557):2587–2594. doi: 10.1098/rspb.2004.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galia E, Nicolaides E, Hörter D, Löbenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharm Res. 1998;15(5):698–705. doi: 10.1023/A:1011910801212. [DOI] [PubMed] [Google Scholar]
- 15.Jantratid E, Janssen N, Reppas C, Dressman J. Dissolution media simulating conditions in the proximal human gastrointestinal tract: an update. Pharm Res. 2008;25(7):1663–1676. doi: 10.1007/s11095-008-9569-4. [DOI] [PubMed] [Google Scholar]
- 16.Garbacz G, Golke B, Wedemeyer R-S, Axell M, Söderlind E, Abrahamsson B, et al. Comparison of dissolution profiles obtained from nifedipine extended release once a day products using different dissolution test apparatuses. Eur J Pharm Sci. 2009;38(2):147–155. doi: 10.1016/j.ejps.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Aoki S, Ando H, Tatsuishi K, Uesugi K, Ozawa H. Determination of the mechanical impact force in the in vitro dissolution test and evaluation of the correlation between in vivo and in vitro release. Int J Pharm. 1993;95(1–3):67–75. doi: 10.1016/0378-5173(93)90391-R. [DOI] [Google Scholar]
- 18.Abrahamsson B, Pal A, Sjöberg M, Carlsson M, Laurell E, Brasseur JG. A novel in vitro and numerical analysis of shear-induced drug release from extended-release tablets in the fed stomach. Pharm Res. 2005;22(8):1215–1226. doi: 10.1007/s11095-005-5272-x. [DOI] [PubMed] [Google Scholar]
- 19.Mercuri A. A physico-chemical investigation into the factors affecting the behaviour of self-emulsifying drug delivery systems. Norwich: University of East Anglia; 2009. [Google Scholar]
- 20.Wickham M, Faulks R. Apparataus, system and method. In: Organization WIP, editor. EU, US, Canada, Australia and India2006
- 21.Marciani L, Faulks R, Wickham MSJ, Bush D, Pick B, Wright J, et al. Effect of intragastric acid stability of fat emulsions on gastric emptying, plasma lipid profile and postprandial satiety. Brit J Nutr. 2009;101(06):919–928. doi: 10.1017/S0007114508039986. [DOI] [PubMed] [Google Scholar]
- 22.Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, et al. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am J Physiol Gastrointest Liver Physiol. 2001;280(6):G1227–1233. doi: 10.1152/ajpgi.2001.280.6.G1227. [DOI] [PubMed] [Google Scholar]
- 23.Geigy Scientific Tables . Units of measurement, body fluids, composition of the body, nutrition. BasleSwitzerland: CIBA-GEIGY; 1981. [Google Scholar]
- 24.Marciani L, Gowland PA, Fillery-Travis A, Manoj P, Wright J, Smith A, et al. Assessment of antral grinding of a model solid meal with echo-planar imaging. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G844–849. doi: 10.1152/ajpgi.2001.280.5.G844. [DOI] [PubMed] [Google Scholar]
- 25.Marciani L, Young P, Wright J, Moore R, Coleman N, Gowland PA, et al. Antral motility measurements by magnetic resonance imaging. Neurogastroent Motil. 2001;13(5):8. doi: 10.1046/j.1365-2982.2001.00285.x. [DOI] [PubMed] [Google Scholar]
- 26.Katori N, Ma W-S, Aoyagi N, Kojima S. Effect of destruction force on drug release from multiple unit controlled release dosage forms in humans. Pharm Res. 1996;13(10):1541–1546. doi: 10.1023/A:1016087814824. [DOI] [PubMed] [Google Scholar]