Abstract
The effects of spray-drying process and acidic solvent system on physicochemical properties of chitosan salts were investigated. Chitosan used in spray dryings was obtained by deacetylation of chitin from lobster (Panulirus argus) origin. The chitosan acid salts were prepared in a laboratory-scale spray drier, and organic acetic acid, lactic acid, and citric acid were used as solvents in the process. The physicochemical properties of chitosan salts were investigated by means of solid-state CP-MAS 13C nuclear magnetic resonance (NMR), X-ray powder diffraction (XRPD), differential scanning calorimetry, and Fourier transform infrared spectrometry (FTIR) and near-infrared spectroscopy. The morphology of spray-dried chitosan acid salts showed tendency toward higher sphericity when higher temperatures in a spray-drying process were applied. Analysis by XRPD indicated that all chitosan acid salts studied were amorphous solids. Solid-state 13C NMR spectra revealed the evidence of the partial conversion of chitosan acetate to chitin and also conversion to acetyl amide form which appears to be dependent on the spray-drying process. The FTIR spectra suggested that the organic acids applied in spray drying may interact with chitosan at the position of amino groups to form chitosan salts. With all three chitosan acid salts, the FTIR bands at 1,597 and 1,615 cm−1 were diminished suggesting that –NH groups are protonated. The FTIR spectra of all chitosan acid salts exhibited ammonium and carboxylate bands at 1,630 and 1,556 cm−1, respectively. In conclusion, spray drying is a potential method of preparing acid salts from chitosan obtained by deacetylation of chitin from lobster (P. argus) origin.
KEY WORDS: chitin, chitosan salt, lobster (Panulirus argus), physicochemical properties, spray drying
INTRODUCTION
Chitosan (CH) has gained increasing interest as a safe excipient and functional material in many fields including pharmaceutical, food, cosmetics, biomedical, agricultural, paper, textile, and water treatment industries (1–3). Chitosan is a basic polymer of helix structure with reactive amine groups, which gives a lot of possibilities for modification and ionic interactions (4). It is a hydrophilic biopolymer usually obtained from chitin through chemical deacetylation. Chitin is one of the most abundant natural amino polysaccharides obtained from crustacean source such as shrimp, crab, or lobster (shells or squid).
Water-soluble chitosan acid salts are perhaps the most frequently used derivatives of chitosan in drug delivery today. Chitosan forms water-soluble salts with both inorganic acids and organic acids such as hydrochloric acid, formic acid, glutamic acid, lactic acid, citric acid, acetic acid, and ascorbic acid (5–7). The reactive amino groups present in chitosan chains can be protonated (NH+3OCOR−) by the abovementioned acids, and the resultant water-soluble polysaccharide is positively charged (5). There are a number of recent examples in the literature on applications of chitosan salts in pharmaceutical drug delivery systems. Chitosan acid salts such as glutamate, aspartate, hydrochloride, and acetate were used for colon-specific drug delivery and to enhance the delivery of therapeutic peptide across intestinal epithelia (8–12). Chitosan and some chitosan acid salts were reported to be carriers for transfection of DNA (13). More recently, Weecharangsan et al. evaluated different chitosan acid salts as a DNA complexing agent (14) and as non-viral gene vectors in CHO-K1 cells (15). Chitosan ascorbate salt showed good penetration enhancement properties toward both buccal mucosa and Caco-2 cell monolayer (16). Lipid-based dried powders as transfection competent carriers for respiratory gene delivery prepared by cationic and chitosan loaded liposomes have been reported (17).
Spray drying is a well-known continuous manufacturing process that is used to produce pharmaceutical dry powders, microspheres, granules, or agglomerates from drug–excipient solutions and suspensions (18). Spray drying can be regarded as the only process that directly converts a liquid into a dry solid in a single step, and consequently, it is especially well suited for, e.g., heat-sensitive products. To date, several research articles have been published on microspheres prepared by spray-drying solution of chitosan using organic acids as solvents (10,12,19–22). A chitosan derivative as an acetate salt was successfully prepared by using a spray-drying technique and characterized as a binder for sustained release tablets (10). Rege et al. reported that drying method (spray drying versus tray drying) affects physicochemical and micromeritic properties of water-soluble chitosan salts (21). Cerchiara et al. evaluated the influence of chitosan acid salts on the release behavior of vancomycin hydrochloride from the spray-dried and freeze-dried systems (22,23). In vitro release of vancomycin was retarded by chitosan hydrochloride salt (22). More recently, Adamiec and Modrzejewska described the possibility of applying spray drying in the formation of chitosan microgranules (24). Spray-dried microspheres of hydroxypropyl methylcellulose, chitosan, and carbopol were successfully prepared to deliver drug through nasal cell monolayer (25).
To date, virtually all chitosan acid salt solutions applied in spray drying have been prepared from chitosan of shrimp and crab shells origin. Chitosan can be obtained also by deacetylation of chitin originated from lobster (Panulirus argus) shells. It is evident that chemical, physical, and biological properties of chitosan as well as chitosan acid salts are very much dependent on the source of chitin (starting material of chitosan) since it affects the degree of deacetylation and the molecular weight of the polysaccharide.
The aim of the present study was to investigate the effects of spray drying and form of organic acid (solvent) on the physicochemical properties of three chitosan acid salts. The chitosan acid salts studied were prepared by using a laboratory-scale spray drier, and organic acetic acid, lactic acid, and citric acid were applied as solvents. Chitosan was obtained by deacetylation of chitin derived from lobster (P. argus) shells. Physicochemical properties of the spray-dried chitosan acid salts were thoroughly evaluated.
MATERIALS AND METHODS
Materials
CH was obtained by N-deacetylation of chitin from lobster (P. argus) origin in accordance with the procedure described in our earlier paper (26). Molecular weight and degree of deacetylation were 309,000 g/mol and 83%, respectively. Lactic acid (BDH, England), citric acid (Proquibasa, Spain), and acetic acid (Merck, Germany) used were of analytical grade.
Spray Drying of Chitosan Acid Salts
The 4% (w/w) chitosan dispersions were prepared by dissolving chitosan powder in 10% (w/w) aqueous solutions of lactic acid, citric acid, and acetic acid. The samples were mixed for 24 h at room temperature with constant stirring until complete dissolution. The ratio of chitosan/organic acid used for salt preparations was 1:24. Finally, the chitosan solutions were filtered and subsequently spray-dried.
Spray drying was performed using a laboratory-scale spray drier (Mini Spray Dryer Büchi B-191, Switzerland) equipped with a standard 0.7-mm nozzle. The spray flow rate used was of 588 ml/h. Compressed air flow rate was 600 l/h, the air flow rate was 60 m3/h, and air pressure was 42 mbar. The effects of temperature on physicochemical properties of the spray-dried powders were studied by setting the inlet/outlet temperatures at 120/80°C, 140/90°C, and 160/100°C, respectively. In this study, the inlet temperature levels studied in a spray-drying process were selected based on earlier related studies on spray-dried chitosan salts (10,19,21).
Characterization of Chitosan Acid Salts
Particle Size, Shape, and Surface Morphology
Particle size, shape, surface morphology, and microstructure of chitosan flakes and chitosan acid salts were studied by using a scanning electron microscope (Zeizz, DSM 962, Germany). Scanning electron micrographs of the platinum-coated samples were taken at appropriate magnification.
Viscosity, Water Content, and Water Activity
Viscosities of chitosan acid salt solutions were measured before spray drying at 20 ± 0.1°C by using a Haake RV-20 (Germany) viscometer at a rotating speed of 0–500 l/s. Moisture content of chitosan acid salts was determined in triplicate by using a Karl Fischer method (Mettler DL35, Switzerland). The water activity measurements were carried out with an AquaLab (Series 3TE, Sweden) water activity meter. The measurements were carried out in triplicate.
X-ray Powder Diffraction
X-ray powder diffraction (XRPD) patterns were obtained by using a variable temperature X-ray diffractometer (D8 Advance Bruker AXS GmbH, Karlsruhe, Germany; VT-XRPD). The VT-XRPD experiments were performed in symmetrical reflection mode with Cu Ka radiation (1.54 Å) using Göbel Mirror bent gradient multiplayer optics. The scattered intensities were measured with a scintillation counter. The angular range was from 5° to 40° with steps of 0.2°, and the measuring time was 3 s/step.
Solid-State CP-MAS 13C NMR
The 13C nuclear magnetic resonance (NMR) analyses were performed with a Varian Unity Inova spectrometer operating at 300 MHz for 1 H frequency, using the combined techniques of proton dipolar decoupling, magic angle spinning (MAS), and cross-polarization (CP). The contact time was 1 ms, the acquisition time was 50 ms, and the recycle delay was 4 s. The proton pulse width was 6 μs and 18-kHz spectral window was used. A typical number of 2,000 scans were acquired for each spectrum. The chemical shifts were externally referenced by setting the methyl resonance of hexamethylbenzene to 17.3 ppm. The samples were contained in a SiN4 cylindrical rotor, which was spun at 5 kHz during measurements.
Fourier Transform Infrared Spectrometry
The Fourier transform infrared spectrometry (FTIR) spectra were recorded by using an IR spectrometer (FT/IR Jasco 460-plus, Japan). The samples were prepared by processing compressed KBr disks.
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) thermograms of chitosan and chitosan acid salts were obtained by using a differential scanning calorimeter (DSC 823e, Mettler Toledo, Greifensee, Switzerland). Samples were accurately weighed into aluminum pans and sealed. In this method, a small hole was made at the top of the pan in order to allow the release of moisture. A nitrogen purge with a flow rate of 50 ml/min was used in the furnace. The measurements were performed at a heating rate of 10°C/min from 25°C to 220°C to chitosan lactate and from 25°C to 300°C to the rest of the samples.
Thermogravimetric Analysis
Thermogravimetric analyses (TGA) of chitosan acid salts were performed by using a thermogravimetric analyzer (TGA 850, Mettler Toledo, Switzerland). A nitrogen purge of 50 ml/min was used in the furnace, and sample sizes of 5 mg were used for all experiments. The measurements were carried out at 25–250°C at a heating rate of 10°C/min.
Near-Infrared Spectroscopy
The near-infrared (NIR) spectroscopy analyses were performed with a FT-NIR spectrometer (Nirvis, Bühler, Switzerland). The spectra were measured through the bottom of the glass vial containing the sample. The measurements were carried out in triplicate. The spectra were recorded over a range of 10,000–4,000 cm−1 with a resolution of 16 cm−1 and averaged over 32 scans. Second derivative transformations of absorbance, log (1/R), were performed with 11-point Savitzky and Golay smoothing (27) using a Matlab software (v. 5.3, MathWorks Inc., Natick, MA, USA).
RESULTS AND DISCUSSION
Particle Size, Shape, and Surface Morphology
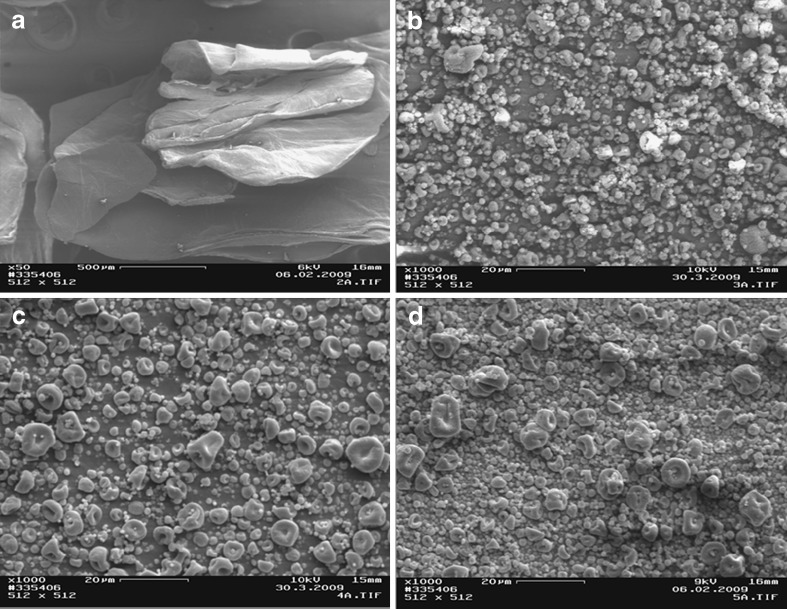
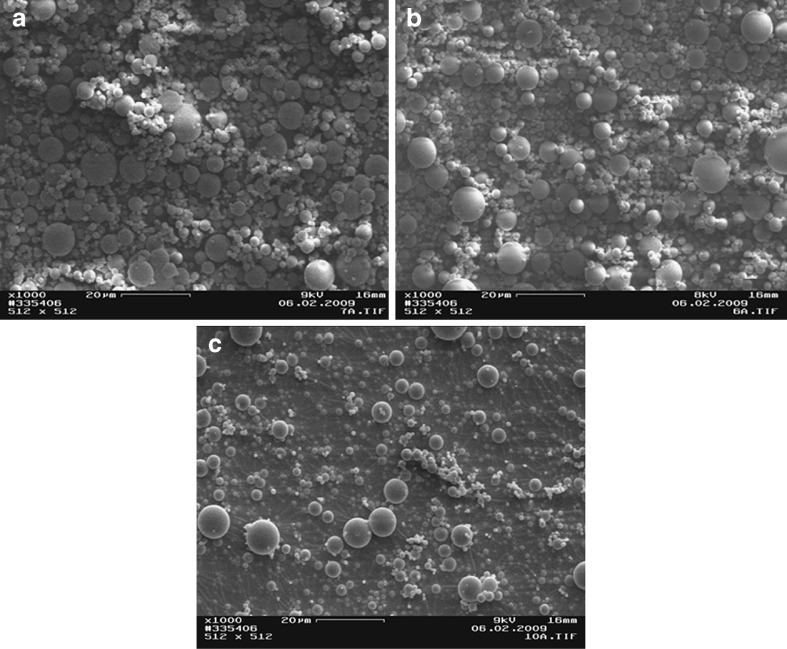
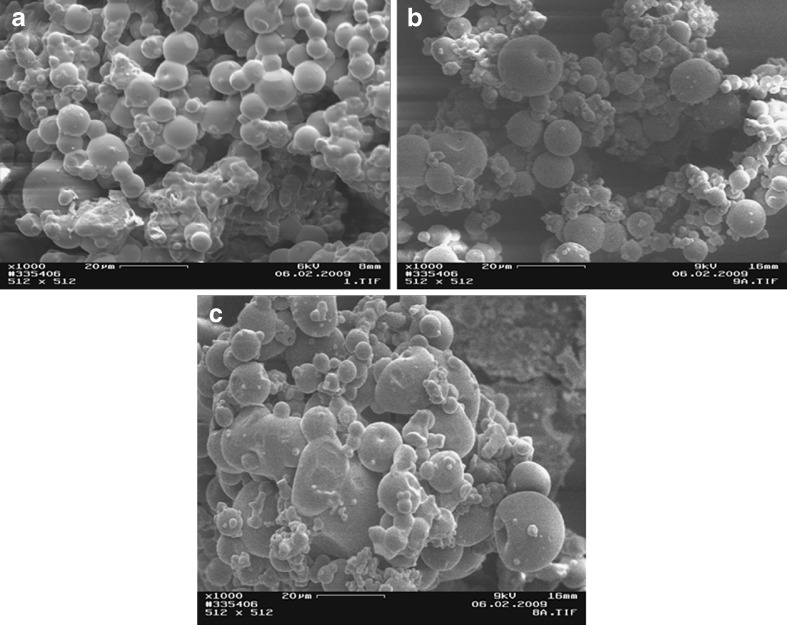
The scanning electron micrographs of chitosan and chitosan acid salts are illustrated in Figs. 1, 2, and 3. The chitosan originally derived from lobster chitin consisted of particles (flakes) of rather irregular size and shape (Fig. 1a). The temperature used in a spray-drying process greatly affected the particle size and shape of chitosan acid salts (Figs. 1, 2, and 3). In general, with the increase of process temperature, chitosan acid salts exhibited more spherical particles. Interestingly, with all chitosan salts, small particles around larger particles were observed. In particular, chitosan citrate obtained by spray drying at low temperatures exhibited irregular and sticky aggregated particles. Similar results have been reported also in the earlier studies on chitosan citrates and lactates prepared by spray drying (12).
Fig. 1.
Scanning electron micrographs of a chitosan flakes, b chitosan acetate spray-dried at 120/80°C, c chitosan acetate spray-dried at 140/90°C, and d chitosan acetate spray-dried at 160/100°C
Fig. 2.
Scanning electron micrographs of a chitosan lactate spray-dried at 120/80°C, b chitosan lactate spray-dried at 140/90°C, and c chitosan lactate spray-dried at 160/100°C
Fig. 3.
Scanning electron micrographs of a chitosan citrate spray-dried at 120/80°C, b chitosan citrate spray-dried at 140/90°C, and c chitosan citrate spray-dried at 160/100°C
As seen in Figs. 1 and 2, spray-dried chitosan lactate powder was composed of particles with more spherical shape in comparison to spray-dried chitosan acetate powder which showed more agglutination of the particles. Chitosan citrate powder particles were greatly affected by the temperature used in a spray-drying process: The particles were irregular in shape, sticky aggregated, and the color became more evident as the salt solution was sprayed at a higher temperature (Fig. 3). The present phenomena may be attributed to an increase in the humidification of the sample which causes agglomeration of the particles.
Viscosity of Solutions
The viscosity of chitosan acid salt solutions measured before spray drying at 20 ± 0.1°C, decreased in the following order: CH acetic (273.6 mPas) > CH lactic (249.0 mPas) > CH citric (220.9 mPas). This can be attributed to the ability of different acids to form a salt with the amino group of chitosan. All chitosan acid salt solutions used in this study were easily atomized.
Moisture Content and Water Activity
Chitosan powder is readily soluble in acetic, citric, and lactic acid solutions because chitosan is a weak polyelectrolyte. Acetic acid having the lowest molecular mass (acetic acid 60.06; lactic acid 90.08, and citric acid 192.12) and the lowest boiling point (acetic acid 118.2°C and lactic acid 122°C) (28) is the most volatile acid among the acids studied here.
The moisture content and the water activity (aw) of spray-dried chitosan acid salts are shown in Table I. All chitosan salt samples contained water since the acidic solvent used in the sample preparation was not totally evaporated during spray drying. As seen in Table I, chitosan acetate exhibited higher moisture content than chitosan lactate and citrate. As the inlet temperature in a spray-drying process was increased, the moisture content of chitosan acid salts was slightly decreased. It can be assumed that an increase in energy among adsorbed water molecules makes it possible for these molecules to leave “active places” of the chitosan salts, and consequently, sorption of water is decreased.
Table I.
Moisture Content, Water Activity (a w), and TGA Mass Loss of Chitosan Acid Salt Samples (n = 3)
| Samples | Moisture content (%) | a w (%) | TGA mass loss (%) |
|---|---|---|---|
| CHacet 160/100 | 8.089 ± 0.284 | 0.267 ± 0.002 | 7.075 |
| CHacet 140/90 | 7.988 ± 0.269 | 0.273 ± 0.000 | 7.131 |
| CHacet 120/80 | 9.088 ± 0.108 | 0.248 ± 0.003 | 7.562 |
| CHlact 160/100 | 3.140 ± 0.032 | 0.352 ± 0.007 | 3.063 |
| CHlact 140/90 | 3.801 ± 0.248 | 0.364 ± 0.017 | 2.236 |
| CHlact 120/80 | 3.112 ± 0.439 | 0.380 ± 0.004 | 1.394 |
| CHcitr 160/100 | 4.623 ± 0.023 | 0.241 ± 0.004 | 4.492 |
| CHcitr 140/90 | 4.643 ± 0.130 | 0.250 ± 0.008 | 4.530 |
| CHcitr 120/80 | 4.808 ± 0.051 | 0.308 ± 0.003 | 4.688 |
Water activity (aw) reflects the active part of moisture content or the part which, under normal circumstances, can be exchanged between the product and its environment (29). Like moisture content, also the water activity (aw) of chitosan acid salts was dependent on the inlet temperature used in a spray-drying process. The values for water activity were higher with chitosan lactate salt compared to the respective values obtained with chitosan acetate and citrate.
Citrate and lactate ions have a potential to compete with water molecules in binding on the surface of powder particles thus rendering their dehydration capacity. However, citric acid has the highest salting out effect in the Hofmeister’s series. Watthanaphanit et al. suggested that the chitosan molecule and citrate ion have electrostatic interaction which could reduce the hydrophilicity of chitosan (30). Therefore, in our study, the moisture content of chitosan citrate and lactate salt samples was found to be smaller compared to that of chitosan acetate salt (Table I). In a spray-drying process, this salting out effect of chitosan citrate could be even accelerated.
X-ray Powder Diffraction
The rigid crystalline structure of pure chitosan is stabilized mainly by intra- and intermolecular hydrogen bonds (31). When glucosamine units in chitosan are protonated, hydrogen bonding involving the NH2 groups is disrupted, and consequently, the rigid crystalline structure weakens.
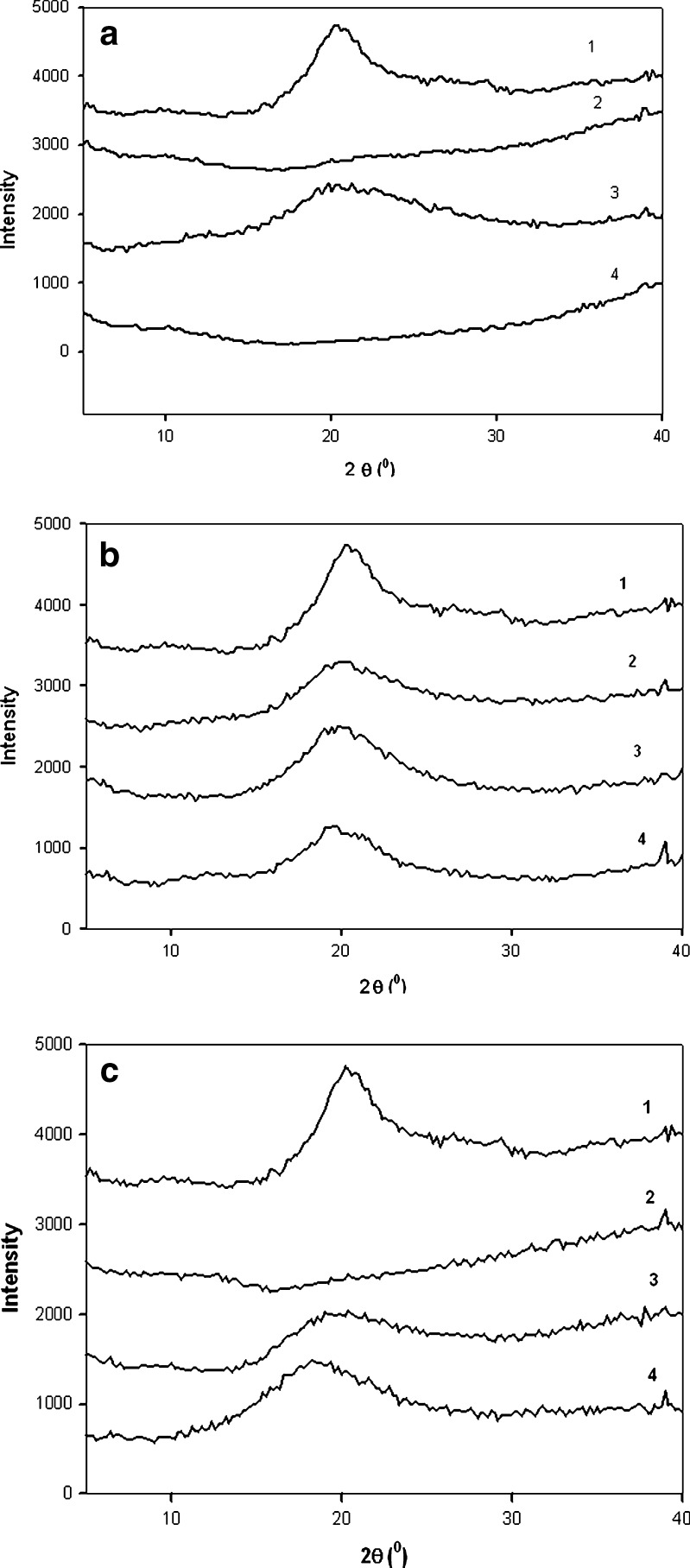
Figure 4a–c shows the XRPD patterns of three spray-dried chitosan acid salts and chitosan powder. The XRPD patterns of chitosan acid salts and chitosan exhibited a broad peak at around 20° (2θ) which is characteristic peak for crustacean chitosans (32). The XRPD pattern of spray-dried chitosan acetate salt showed even absence of such characteristic peak at 20° (2θ; Fig. 4a). It is evident that spray drying affected crystallinity of chitosan. With all spray-dried chitosan acid salts, the characteristic peak at 20° (2θ) was lower than the respective peak observed with a reference chitosan powder. Halo diffraction patterns of all three chitosan salts studied indicated amorphous state. The appearance of a broad peak at around 9–10° (2θ) would be indicative for a hydrated polymorph of chitosan (33), but no peak at this position could be observed in the XRPD patterns. The present results are in agreement with those reported by Nunthanid et al. (10), Ritthidej et al. (34), and recently Tanigawa et al. (35).
Fig. 4.
X-ray powder diffraction patterns of chitosan powder (1) and spray-dried a chitosan acetate, b chitosan lactate, and c chitosan citrate samples at (2) 160/100°C, (3) 140/90°C, and (4) 120/80°C
As seen in Fig. 4b, the XRPD pattern of chitosan lactate exhibited also clearly lower peak at 20° (2θ) than the diffraction pattern of chitosan reference powder. As lactic acid reacts with chitosan, it will take place at random along the chitosan chain giving rise to a random copolymer. According to Yao et al., this will destroy regular packing of the original chitosan chains resulting in formation of almost amorphous copolymer (36). After spray drying, halo diffraction patterns of chitosan lactate salt evidenced amorphous state. As regards with the spray-drying process, inlet/outlet temperature appears to have a slight influence on the exhibition of a characteristic peak (Fig. 4b). Diffraction patterns of chitosan lactate salts showed a broad reflection with low intensity at 20° (2θ; 160/100°C), at 19.8° (2θ; 140/90°C), and at 19.6° (2θ; 120/80°C).
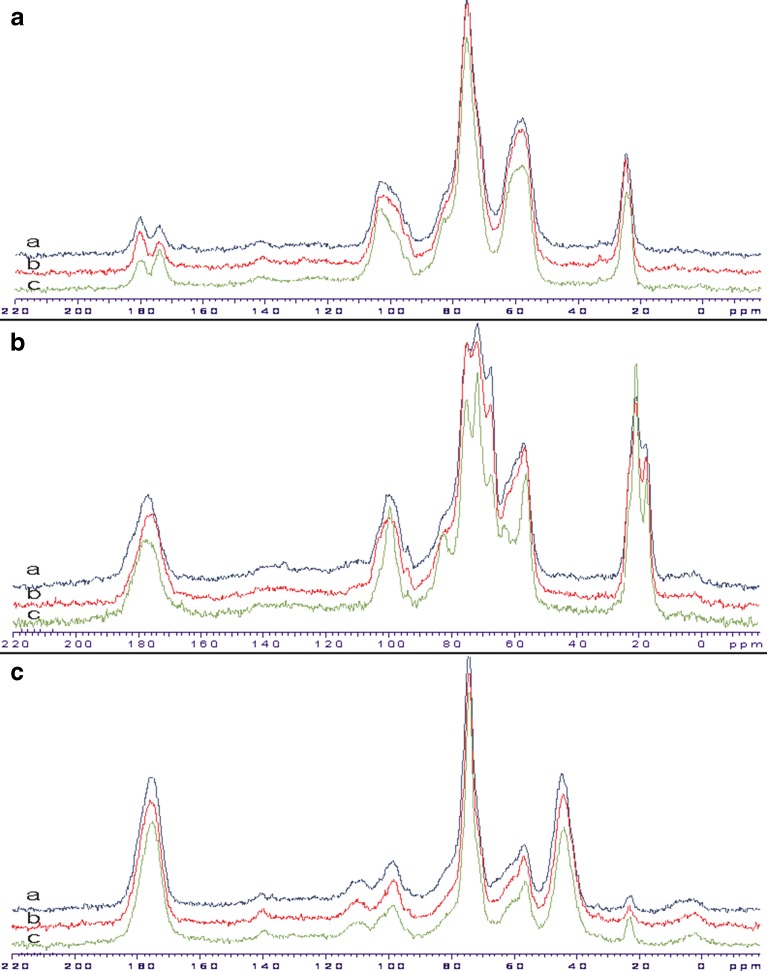
Solid-State NMR Spectroscopy
Solid-state NMR spectroscopy is an emerging technique for the analysis of any changes in the form of solid material that may have occurred during processing. Figure 5 shows the CP-MAS 13C NMR spectra of spray-dried chitosan salt samples. Corresponding chemical shifts values are summarized in Table II. The sugar resonances at 50–110 ppm assigned to methine/methylene carbon which indicates the presence of glucosamine units. The methyl and carbonyl signals associated with the monomeric form of chitin were detectable in the polymeric chain of all chitosan salts. This suggests incomplete deacetylation of the original chitin. The methyl has a chemical shift of 22 ppm in the solid-state NMR spectra of chitosan citrate while the respective shift is overlapped in the spectra of the other chitosan acid salts studied. The amide carbonyl has a chemical shift at 174 ppm in the solid-state NMR spectra of chitosan acetate, but in the spectra of the other chitosan salts, this signal is overlapped.
Fig. 5.
CP-MAS 13C NMR spectra of a chitosan acetate, b chitosan lactate, and c chitosan citrate samples spray-dried at a 160/100°C, b 140/90°C, and c 120/80°C
Table II.
13C NMR Chemical Shift Values for Chitosan Acid Salts
| Nucleus | Values (ppm) | |||
|---|---|---|---|---|
| Chitosan | Chitosan acetate | Chitosan lactate | Chitosan citrate | |
| C=O | 173.65 | 174.16, 180.39 | 176.60 | 175.53 |
| C1 | 105.00 | 103.67 | 99.78 | 98.36 |
| C4 | 82.52 | 82.60 | 82.83 | 81.52 |
| C3/C5 | 75.39 | 75.72 | 79.65 | 74.13 |
| C6 | 60.91 | 57.63 | 63.00 | 59.89 |
| C2 | 57.97 | 57.63 | 56.26 | 56.52 |
| CH3 | 23.75 | 24.48 | 22.85 | |
| CH2 (citric acid) | – | – | – | 44.03 |
Figure 5a shows the CP-MAS 13C NMR spectra of spray-dried chitosan acetate. An additional resonance at 180 ppm assigned to carbonyl group indicates the presence of acetate functional group. This suggests that chitosan acetate is properly formed as a result of spray drying. The CP-MAS 13C NMR spectra of chitosan acetate salt (spray-dried at 120/80°C), however, showed the additional peak with a chemical shift of around 174 ppm. This is obviously due to the presence of acetyl amide functional group (that has a chemical shift at 174 ppm). It is evident that conversion of chitosan acetate to acetyl amide form is dependent on the spray-drying process. The conversion of chitosan acetate molecular structure to N-acetylglucosamine at higher temperature has been reported by Nunthanid et al. (10).
The CP-MAS 13C NMR spectra of chitosan lactate exhibited sharp signals at 21 ppm and at 75 ppm (Fig. 5b), thus differing from solid-state NMR spectra of the other two chitosan salts. In addition, the broad lines in chitosan lactate salt indicate that the spray-dried salt is in amorphous state. The present broad lines in the solid-state NMR are characteristic to amorphous polymers. As seen in Fig. 5c, the CP-MAS 13C NMR spectra of chitosan citrate exhibited the methyl signal from chitosan as a small line at 22 ppm. When compared this signal to the one obtained with chitosan acetate and chitosan lactate, the resonance at 180 ppm was much stronger due to (in a lesser extent) carbonyl groups of chitosan and COOH− groups of citric acid signals that contribute to the same region. In the solid-state NMR spectra of chitosan citrate (Fig. 5c), the intense peak between 40 and 50 ppm can be observed, and this peak cannot be found in the solid-state NMR spectrum of chitosan. This signal is attributed to the presence of CH2 groups from citric acid. While the broad shape of this signal may indicate close association of citric acid with the chitosan backbone, it cannot be taken as a direct proof of complex formation between chitosan and citric acid. In case of chitosan acetate, a strong evidence of an amidation process exists as the signal area of amide carbonyl at 174 ppm increases due to prolonged reaction time at 120°C. With chitosan lactate and citrate, no separate signal for amide carbon can be seen, and therefore, no conclusions of such process can be made.
The solid-state NMR spectrum revealed evidence of partial conversion of chitosan acetate structure to chitin. However, such conversion of chitosan lactate and citrate salts was difficult to detect due to the interfering of the key signals in the NMR spectrum. This amidation may be the reason for the higher decomposition temperatures observed with chitosan acetate and citrate.
In this study, solid-state NMR spectra revealed the functional groups of chitosan acetate salt in its molecular structure. The signals of carboxylic acids were found in both spray-dried chitosan citrate and chitosan lactate powders since the intensities are increased in comparison to the solid-state NMR spectrum of pure chitosan.
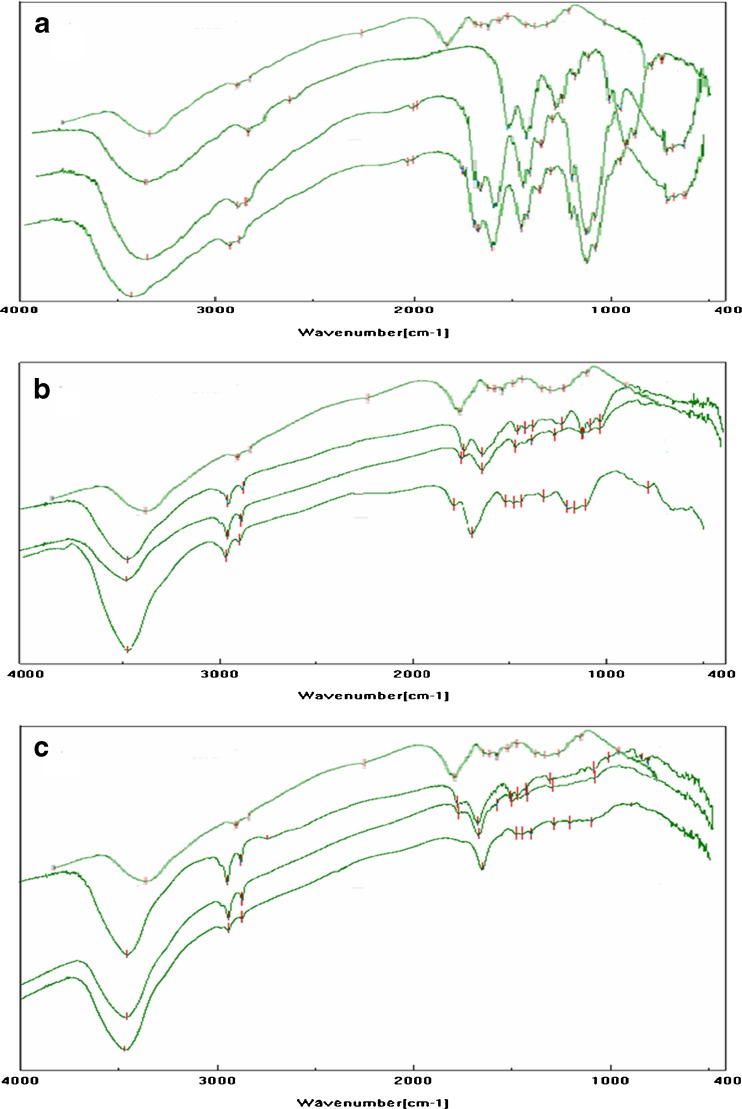
Fourier Transform Infrared Spectroscopy
The FTIR spectra of spray-dried chitosan acid salts are illustrated in Fig. 6. The FTIR spectra exhibited broad bands in the range of 3,450–3,400 cm−1 (which are assigned to on OH stretching) indicating intermolecular hydrogen bonding. The NH stretching also could overlap in the same region of the spectra. The amine group has a characteristic absorption bands at 1,597 and 1,615 cm−1 in the FTIR spectrum of chitosan. With all three chitosan salts, these bands at 1,597 and 1,615 cm−1 are diminished suggesting that –NH groups are protonated. The carboxylate band of –COO− at 1,556 cm−1 appeared in all chitosan acid salts. Consequently, it is reasonable to assume that there is an ionic interaction between chitosan and acids.
Fig. 6.
FTIR spectra of a chitosan acetate, b chitosan lactate, and c chitosan citrate spray-dried at 120/80°C (the second curve from the top), 140/90°C (the third curve), and 160/100°C (the fourth curve). In each figure, a–c, the first curve (from the top) represents the FTIR spectrum of chitosan powder as a reference
The intense peaks at 1,550–1,600 cm−1 and the weak peaks near 1,400 cm−1 (attributed to carboxylate anion stretching, respectively) were observed in the FTIR spectra of chitosan acetate (Fig. 6). This suggests that chitosan acetate is properly formed as a result of spray drying. However, the incidence of partial conversion of chitosan acetate to chitin is observed in the FTIR spectrum of chitosan acetate salt spray-dried at 120/80°C. This is in good agreement with earlier solid-state NMR results of chitosan acetate. The FTIR spectra of chitosan lactate reveal some spectral changes compared to the chitosan acetate. As seen in Fig. 6, the FTIR spectrum of spray-dried chitosan lactate shows a large band –NH2 at 1,630 cm−1. The shift of this vibration to higher wave-numbers compared with the usual wave-numbers of the amine group proves the formation of a carboxylate between the –COO− groups of the acid and the –NH3+ groups of chitosan (8).
Carbonyl absorption bands at 1,700 cm−1 or higher (indicating presence of carboxylic acids) can be seen in the FTIR spectra of chitosan citrate and lactate. The band around 1,730 cm−1 corresponds to the ester of carboxylic group of oligo(lactic acid) existing as free or side chain (37). This suggests the presence of free lactic acid in the spray-dried chitosan lactate salt. This finding is also in agreement with earlier works (36). The FTIR spectra of chitosan citrate show bands at 1,630 and 1,400 cm−1 assigned to –NH3+ and –COO−, respectively. In addition, the peaks at 1,730 cm−1 can be seen suggesting the presence of free –COOH groups. These results suggest that chitosan citrate is formed between chitosan and citric acid molecules, and many carboxyl group of citric acid are present without contributing salt formation. The present results suggest that the organic acids applied in spray drying may interact with chitosan at the position of amino groups to form chitosan salts. The FTIR spectra of all chitosan acid salts exhibited ammonium and carboxylate bands. However, unreact acidic carboxyl groups in chitosan citrate and lactate were shown in the FTIR spectra at the wavelength higher than 1,700 cm−1.
Thermal Properties of Chitosan Salts
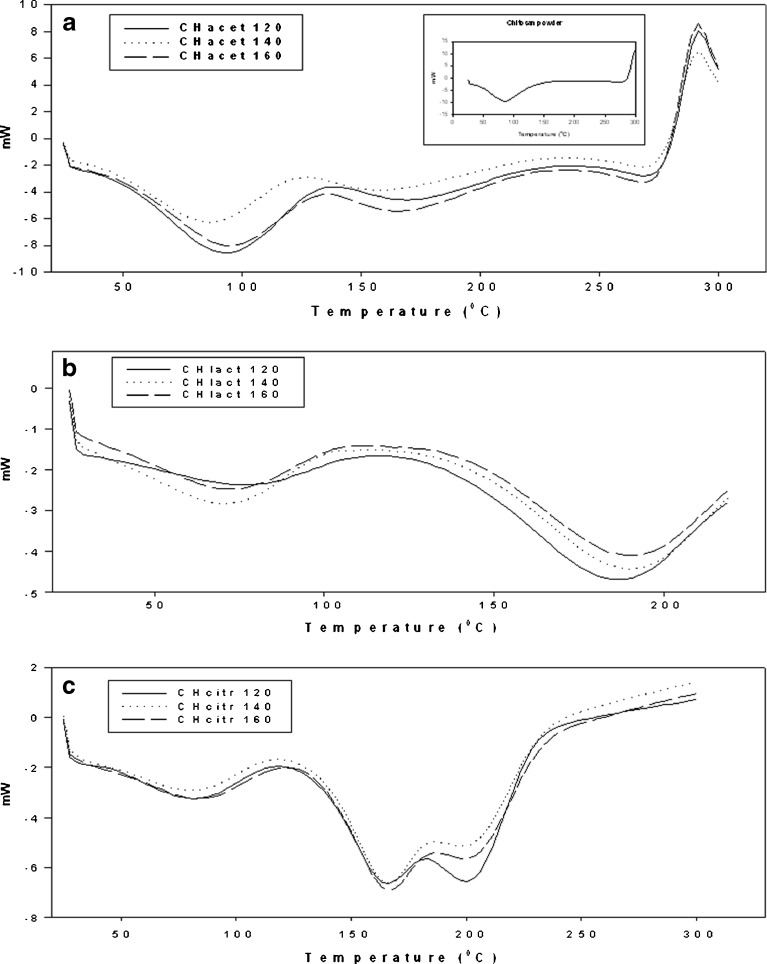
Figure 7 shows the DSC curves of the spray-dried chitosan acid salts studied. The DSC curves of all samples are characterized by broad endothermic peaks in the temperature range 70–120°C. Chitosan lactate and citrate salts exhibited a broad second endothermic peak at around 150–200°C, which is attributed to a loss of crystallization water and the melting point of the mixtures. Similar behavior was previously reported to chitosan lactate and citrate microcapsules (38) and films (34). On the other hand, Phaechamud et al. suggested that the second endotherm with the chitosan citrate films is obviously related to transition of chitosan citrate from crystalline to amorphous form (39).
Fig. 7.
DSC thermograms of spray-dried a chitosan acetate, b chitosan lactate, and c chitosan citrate. In a, the DSC thermogram of chitosan powder is also shown as a reference (small figure)
As seen in Fig. 7, the spray-dried chitosan acetate also showed an additional endothermic peak in the temperature range of 150–200°C. According to the literature, endothermic peaks of chitosan acetate are related to the weight loss of the salt (10). The fact that the values for ΔH increased with the moisture content of the samples may indicate that the chitosan salts studied here differ from each other with respect to their water–polymer interaction and their water holding capacity. The ΔH values for chitosan acetate salt that was spray-dried at 120/80°C, 140/90°C, and 160/100°C were 152.1, 122.2, and 105.2 J g−1, respectively. The ΔH values for chitosan lactate spray-dried at 120/80°C, 140/90°C, and 160/100°C were 28.5, 51.9, and 45.8 J g−1, respectively. With chitosan citrate, the values for ΔH were 53.4 J g−1 (120/80°C), 53.5 J g−1 (140/90°C), and 50.7 J g−1 (160/100°C).
Thermal decomposition of chitosan is an exothermic process where crystal structure of the material is contracted, and this process starts after the dehydration (40). In this study, the exothermic peaks of chitosan salts were observed at about 290°C (Fig. 7). Chitosan citrate exhibited the highest exothermic peak temperature (>350°C) compared with the other two chitosan salts studied. Chitosan lactate was found to be less stable than chitosan, chitosan acetate, and chitosan citrate due to lower degradation temperature. After heating above 220°C, an irreversible change in the solid-state salt structure was observed with chitosan lactate. As regards with the melting point of the acids used in the salt formation, lactic acid has the lowest melting point of 17°C, and citric acid has a melting point of 153°C.
The present results suggest greater thermal stability of chitosan acetate and chitosan citrate compared to chitosan lactate. In the case of chitosan citrate (and use of citric acid), this finding can be attributed to the higher number of carboxylic groups in the molecule of citric acid in relation to the molecule of the other two acids employed, thus allowing a greater ionic interaction with chitosan (28,38). At elevated temperatures, the carboxylic acids may protonate and slowly react with the amine to form an amide (34,36). This amidation decreases the amount of hydrophilic groups and result in lower water sorption (34). In this study, chitosan citrate and chitosan lactate samples were found to have lower moisture content compared with chitosan acetate (Table I). This is possibly due to the intramolecular and intermolecular condensations of carboxylic acid and chitosan (41).
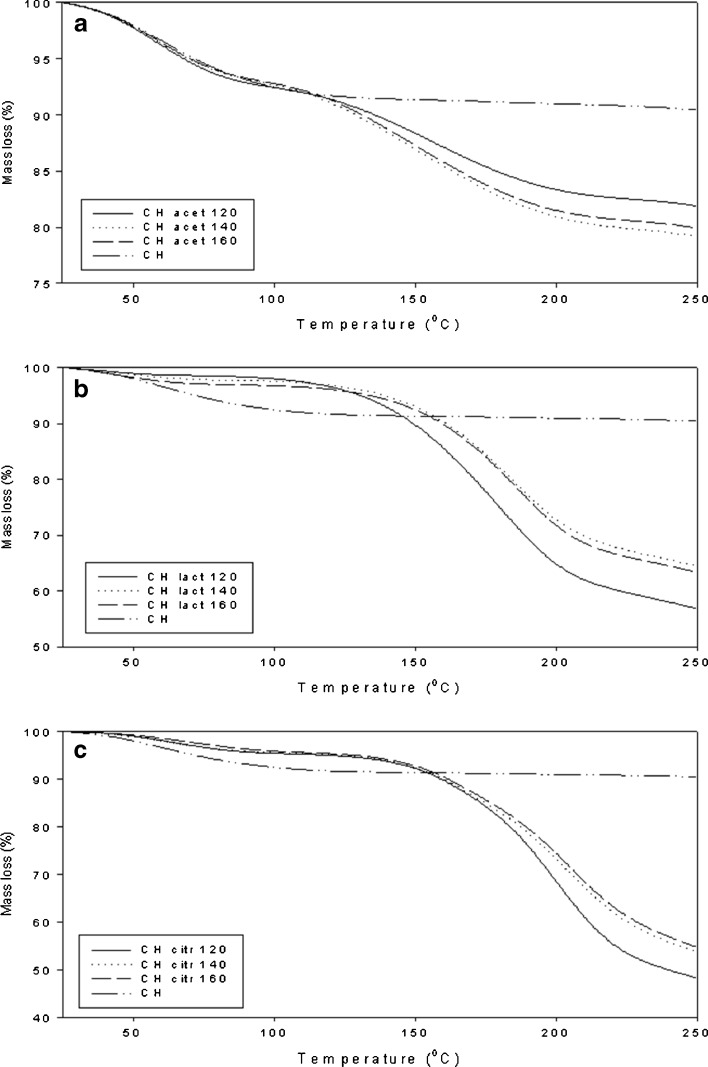
Figure 8 shows the values for weight loss of chitosan acid salts at heating. According to TGA analyses, weight loss is associated with the endothermic changes observed in DSC. Weight loss of the chitosan salts at lower temperatures (25–100°C) was in good agreement with the water contents measured by a Karl Fisher method (Table I). Chitosan acetate exhibited higher moisture content than chitosan citrate and lactate. The results on the weight loss at higher temperatures (at around 150–200°C) were in accordance with earlier findings with chitosan salts (34,38). Based on the weight loss, the chitosan acid salts studied can be ranked in the following descending order: chitosan citrate > chitosan lactate > chitosan acetate. The weight loss change was not observed with chitosan powder.
Fig. 8.
TGA thermograms of chitosan and spray-dried a chitosan acetate, b chitosan lactate, and c chitosan citrate
Near-Infrared Spectroscopy
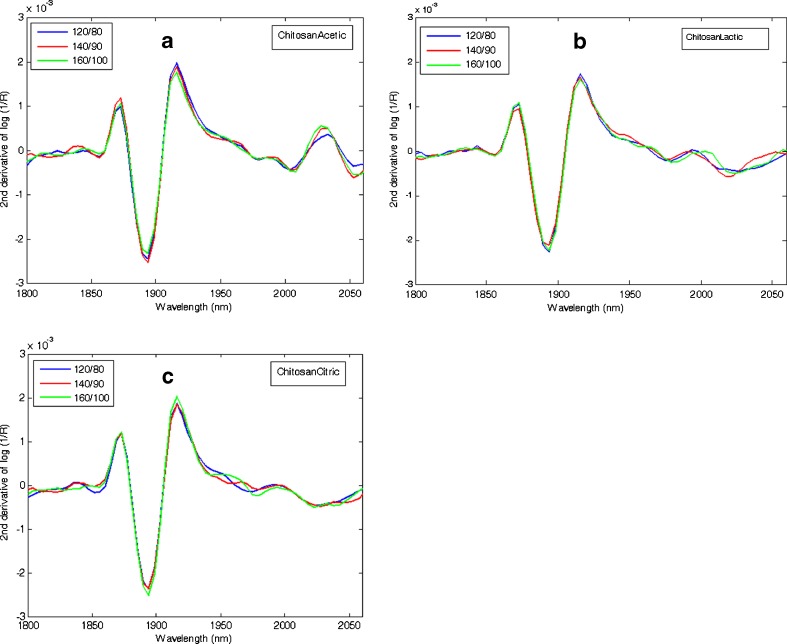
After spray-drying process, the NIR spectra of the chitosan acid salts were comparable and not dependent on the inlet/outlet temperature used in the process (Fig. 9). As seen in the NIR spectra, all three chitosan salts had free moisture due to the aqueous solvent used in the sample preparation. This free water was not totally evaporated during spray drying. According to literature, chitosan has three predominant absorption sites: the hydroxyl group, the amino group, and the polymer chain end (37). In chitosan, the water is bound to the hydroxyl group more strongly than to the amine group. The internal water of amorphous solids (such as chitosan salts) transfers slowly because the remainder water diffuses out through the solid. The same water bands at around 1,900 nm were identified with NIR spectroscopy from all three amorphous chitosan salts (Fig. 9).
Fig. 9.
Near-infrared reflectance spectra of spray-dried a chitosan acetate, b chitosan lactate, and c chitosan citrate. The second derivative of absorbance, log (1/R), at 1,800–2,060 nm
CONCLUSIONS
This study demonstrates that chitosan derived from lobster (P. argus) chitin can be converted as a salt form with acetic, lactic, and citric acids by means of spray drying. The chitosan acetate, lactate, and citrate salts prepared by spray drying are amorphous solids. The particle shape of all chitosan acid salts studied here show tendency toward higher sphericity when spray-dried at higher temperatures. The chitosan acetate salts are characterized by higher moisture content compared with chitosan lactate and citrate salts. Chitosan citrate and acetate salts are more stable with higher exothermic temperature than chitosan lactate salt. Partial conversion of chitosan acetate structure to chitin is evident due to the effect of high temperature involved in the spray-drying process. Further studies on applicability of the present chitosan acid salts as excipients in pharmaceutical applications as well as on stability of the salts are suggested.
ACKNOWLEDGEMENTS
The authors wish to thank the Division of Pharmaceutical Technology, Faculty of Pharmacy, University of Helsinki, Finland for financial support of the present study. This research was also supported by European Social Fund’s Doctoral Studies and Internationalisation Programme DoRa. We gratefully acknowledge Sari Airaksinen, Ph.D. (Pharm.) for her suggestions related to NIR spectroscopy, and Simo Siiriä, M.Sc.(Physics) for his assistance in X-ray powder diffraction measurements.
REFERENCES
- 1.Dutta PK, Ravikumar MNV, Dutta J. Chitin and chitosan for versatile applications. J Macromolecular Sci, Part-C Polymer Rev. 2002;C42(3):307–354. doi: 10.1081/MC-120006451. [DOI] [Google Scholar]
- 2.Mourya VK, Inamdar NN. Chitosan-modifications and applications: opportunities galore. React Funct Polym. 2008;68:1013–1051. doi: 10.1016/j.reactfunctpolym.2008.03.002. [DOI] [Google Scholar]
- 3.Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Shamov MV, Bratskaya SY, Avramenko VA. Interaction of carboxylic acids with chitosan: effect of pK and hydrocarbon chain length. J Colloid Interface Sci. 2002;249:316–321. doi: 10.1006/jcis.2002.8248. [DOI] [PubMed] [Google Scholar]
- 5.Illum L. Chitosan and its use as a pharmaceutical excipient. Pharm Res. 1998;15:1326–1331. doi: 10.1023/A:1011929016601. [DOI] [PubMed] [Google Scholar]
- 6.Kumar MN, Muzzarelli RAA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem Rev. 2004;104:6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 7.Vinsova J, Vavrikova E. Recent advances in drugs and prodrugs design of chitosan. Curr Pharm Des. 2008;14(13):1311–1326. doi: 10.2174/138161208799316410. [DOI] [PubMed] [Google Scholar]
- 8.Orienti I, Cerchiara T, Luppi B, Bigucci F, Zuccari G, Zecchi V. Influence of different chitosan salts on the release of sodium diclofenac in colon-specific delivery. Int J Pharm. 2002;238:51–59. doi: 10.1016/S0378-5173(02)00060-1. [DOI] [PubMed] [Google Scholar]
- 9.Zambito Y, Di Colo G. Preparation and in vitro evaluation of chitosan matrices for colonic controlled drug delivery. Pharm Pharmaceut Sci. 2003;6(2):274–281. [PubMed] [Google Scholar]
- 10.Nunthanid J, Laungtana-anan M, Sriamornsak P, Limmatvapirat S, Puttipipatkhachorn S, Lim L, et al. Characterization of chitosan acetate as a binder for sustained release tablets. J Control Release. 2004;9(9):15–26. doi: 10.1016/j.jconrel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Rai G, Jain SK, Agrawal S, Bhadra S, Pancholi SS, Agrawal GP. Chitosan hydrochloride based microspheres of albendazole for colonic drug delivery. Pharmazie. 2005;60(2):131–134. [PubMed] [Google Scholar]
- 12.Nunthanid J, Huanbutta K, Luangtana-anan M, Sriamornsak P, Limmatvapirat S, Puttipipatkhachorn S. Development of time-, pH-, and enzyme-controlled colonic drug delivery using spray-dried chitosan acetate and hydroxypropyl methylcellulose. Eur J Pharm Biopharm. 2008;68:253–259. doi: 10.1016/j.ejpb.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Corsi K, Chellat F, Yahia L, Fernandes JC. Mesenchymal stem cells, MG63 and HEK293 transfection using chitosan–DNA nanoparticles. Biomaterials. 2003;24:1255–1264. doi: 10.1016/S0142-9612(02)00507-0. [DOI] [PubMed] [Google Scholar]
- 14.Weecharangsan W, Opanasopit P, Ngawhirunpat T, Rojanarata T, Apirakaramwong A. Chitosan lactate as a nonviral gene delivery vector in COS-1 cells. AAPS PharmSciTech. 2006;7(3):E74–E79. doi: 10.1208/pt070366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weecharangsan W, Opanasopit P, Ngawhirunpat T, Apirakaramwong A, Rojanarata T, Ruktanonchai U, et al. Evaluation of chitosan salts as non-viral gene vectors in CHO-K1 cells. Int J Pharm. 2008;348:161–168. doi: 10.1016/j.ijpharm.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Rossi S, Marciello M, Sandri G, Bonferoni MC, Ferrari F, Caramella C. Chitosan ascorbate: a chitosan salt with improved penetration enhancement properties. Pharm Dev Technol. 2008;13(6):513–521. doi: 10.1080/10837450802288865. [DOI] [PubMed] [Google Scholar]
- 17.Colonna C, Conti B, Genta I, Alpar OH. Non-viral dried powders for respiratory gene delivery prepared by cationic and chitosan loaded liposomes. Int J Pharm. 2008;364:108–118. doi: 10.1016/j.ijpharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Ameril M, Maa Y-F. Spray drying of biopharmaceuticals: stability and process considerations. Drying Technol. 2006;24:763–768. doi: 10.1080/03602550600685275. [DOI] [Google Scholar]
- 19.He P, Davis SS, Illum L. Chitosan microspheres prepared by spray drying. Int J Pharm. 1999;187:53–65. doi: 10.1016/S0378-5173(99)00125-8. [DOI] [PubMed] [Google Scholar]
- 20.Muzzarelli C, Tosi G, Francescangeli O, Muzzarelli RAA. Alkaline chitosan solutions. Carbohydr Res. 2003;338:2247–2255. doi: 10.1016/S0008-6215(03)00373-2. [DOI] [PubMed] [Google Scholar]
- 21.Rege PR, Garmise RJ, Block LH. Spray-dried chitinosans. Part I: preparation and characterization. Int J Pharm. 2003;252:41–45. doi: 10.1016/S0378-5173(02)00604-X. [DOI] [PubMed] [Google Scholar]
- 22.Cerchiara T, Bigucci F, Zecchi V. Chitosan salts as nasal sustained delivery systems for peptidic drugs. J Pharm Pharmacol. 2003;55(12):1623–1627. doi: 10.1211/0022357022322. [DOI] [PubMed] [Google Scholar]
- 23.Cerchiara T, Luppi B, Bigucci F, Petrachi M, Orienti I, Zecchi V. Controlled release of vancomycin from freeze-dried chitosan salts coated with different fatty acids by spray-drying. J Microencapsul. 2003;20(4):473–478. doi: 10.1080/0265204031000094329. [DOI] [PubMed] [Google Scholar]
- 24.Adamiec J, Modrzejewska Z. Some structural properties of spray-dried chitosan microgranules. Drying Technol. 2005;23:1601–1611. doi: 10.1081/DRT-200064989. [DOI] [Google Scholar]
- 25.Harikarnpakdee S, Lipipun V, Sutanthavibul N, Ritthidej GC. Spray dried mucoadhesive microspheres: preparation and transport through nasal cell monolayer. AAPS PharmSciTech. 2006;7(1):E79–E88. doi: 10.1208/pt070112. [DOI] [PubMed] [Google Scholar]
- 26.Fernández Cervera M, Heinämäki J, Räsänen M, Maunu SL, Karjalainen M, Nieto OM, et al. Solid-state characterization of chitosans derived from lobster chitin. Carbohydr Polym. 2004;58:401–408. doi: 10.1016/j.carbpol.2004.08.017. [DOI] [Google Scholar]
- 27.Savitzky A, Golay M. Smoothing and differentiation of data by simplified least square procedures. Anal Chem. 1964;36:1627–1639. doi: 10.1021/ac60214a047. [DOI] [Google Scholar]
- 28.Demarger-Andre S, Domard A. Chitosan carboxylic acid salts in solution and the solid state. Carbohydr Polym. 1994;23:211–219. doi: 10.1016/0144-8617(94)90104-X. [DOI] [Google Scholar]
- 29.Snider B, Liang P, Pearson N. Implementation of water activity testing to replace Karl Fischer water testing for solid oral-dosage forms. Pharm Technol. 2007;31(2):56–71. [Google Scholar]
- 30.Watthanaphanit A, Supaphol P, Furuike T, Tokura S, Tamura H, Rujiravanit R. Novel chitosan-spotted alginate fibers from wet-spinning of alginate solutions containing emulsified chitosan-citrate complex and their characterization. Biomacromolecules. 2009;10:320–327. doi: 10.1021/bm801043d. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa K. Conformational diversity of chitosan. J Met Mater Miner. 2005;15(1):1–5. [Google Scholar]
- 32.Muzzarelli RAA, Terbojevich M, Muzzarelli C, Francescangeli O. Chitosans depolymerized with the aid of papain and stabilized as glycosylamines. Carbohydr Polym. 2002;50:69–78. doi: 10.1016/S0144-8617(01)00378-2. [DOI] [Google Scholar]
- 33.Prashanth KVH, Kittur FS, Tharanathan RN. Solid state of chitosan prepared under different N-acetylating conditions. Carbohydr Polym. 2002;50:27–33.
- 34.Ritthidej GC, Phaechamud T, Koizumi T. Moist heat treatment on physicochemical change of chitosan salt films. Int J Pharm. 2002;232:11–22. doi: 10.1016/S0378-5173(01)00894-8. [DOI] [PubMed] [Google Scholar]
- 35.Tanigawa J, Miyoshi N, Sakurai K. Characterization of chitosan/citrate and chitosan/acetate films and applications for wound healing. J Appl Polym Sci. 2008;110:608–615. doi: 10.1002/app.28688. [DOI] [Google Scholar]
- 36.Yao F, Chen W, Wang H, Liu H, Yao K, Sun P, et al. A study on cytocompatible poly(chitosan-g-L-lactic acid) Polymer. 2003;44:6435–6441. doi: 10.1016/S0032-3861(03)00676-1. [DOI] [Google Scholar]
- 37.Gocho H, Shimizu H, Tanioka A, Chou TJ, Nakajima T. Effect of polymer chain end on sorption isotherm of water by chitosan. Carbohydr Polym. 2001;41:87–90. doi: 10.1016/S0144-8617(99)00113-7. [DOI] [Google Scholar]
- 38.Parize AL, Rozone de Souza TC, Costa Brighente IM, de Fávere VT, Laranjeira MC, Spinelli A, et al. Microencapsulation of the natural urucum pigment with chitosan by spray drying in different solvents. Afr J Biotechnol. 2008;7(17):3107–3114. [Google Scholar]
- 39.Phaechamud T, Koizumi T, Ritthidej GC. Chitosan citrate as film former: compatibility with water-soluble anionic dyes and drug dissolution from coated tablet. Int J Pharm. 2000;198:97–111. doi: 10.1016/S0378-5173(99)00460-3. [DOI] [PubMed] [Google Scholar]
- 40.Drebushchak VA, Shakhtshneider TP, Apenina SA, Drebushchak TN, Medvedeva AS, Safronova LP, et al. Thermoanalytical investigation of drug-excipient interaction Part I: piroxicam, cellulose and chitosan as starting materials. J Therm Anal Cal. 2006;84:643–649. doi: 10.1007/s10973-005-9990-4. [DOI] [Google Scholar]
- 41.Roth HJ, Eger K, Troschutz R, editors. Pharmaceutical chemistry. New York: Ellis Horwood; 1988. [Google Scholar]