Abstract
The influence of experimental temperature on the permeability of model diffusants across porcine buccal mucosa was investigated in vitro. The permeability increased significantly as the experimental temperature was increased in increments of approximately 7°C. It was observed that the apparent permeability and temperature were related by an exponential relationship that conformed to the Arrhenius equation. Diffusants with higher lipophilicities—buspirone and bupivacaine—had lower activation energies for diffusion when compared to hydrophilic diffusants—antipyrine and caffeine. The activation energy for diffusion of the model diffusants decreased linearly with increasing distribution coefficients across porcine buccal mucosa. The results suggested that the buccal mucosa acts as a stronger barrier to the diffusion of hydrophilic diffusants than the lipophilic ones. The log-linear relationship between permeability and temperature indicates that temperature should be carefully controlled in diffusion experiments. These results also point to the possibility of developing heat-generating buccal delivery devices, especially for hydrophobic diffusants.
KEY WORDS: activation energy, permeability enhancement, porcine buccal mucosa, temperature
INTRODUCTION
The peroral route is the most commonly employed route for the administration of medications. Due to the limitations associated with peroral route such as extensive first-pass metabolism and hydrolysis of acid-labile drugs, the potential use of other routes of drug administration, such as the buccal route, is being investigated. Drug-containing dosage forms like tablets, gels, solutions, and patches are placed in the buccal pouch. If the drug has the appropriate physicochemical properties, or advantageous modifications can be made to the membrane permeability or to the local environment, absorption of the active drug into the blood circulation is expected to occur. Drug delivery via the buccal mucosa possesses many advantages over the other routes and is rapidly emerging as an alternate route of delivery for certain drugs, such as those that undergo extensive first-pass metabolism. The buccal mucosa appears to be better in terms of permeability, surface area, patient compliance, etc., when compared to the other mucosal and transdermal routes of delivery (1). Frequent exposure to food materials and rapid cell turnover make the buccal mucosa more resistant to tissue damage and irritation compared to other mucosal routes of administration. Hence, the buccal route of diffusant delivery is a logical alternative delivery route for diffusants which undergo extensive degradation in the stomach and the liver (2–7).
The assessment of the permeability of diffusants across the buccal mucosa is usually conducted in vitro using porcine buccal tissue and a diffusion assembly. Porcine buccal mucosa is closest to that of human in terms of structure, composition, and permeability than any other animal (8–10). It is widely recognized that temperature must be controlled during in vitro buccal permeation studies (11). However, individual laboratories have used different controlled temperatures, such as room temperature (25°C) (10,12–14), 30°C (1), 34°C (15–20), or physiological temperature (37°C) (21–30). In the literature, the use of ambient or room temperature (25°C) for permeation studies has been justified by stating that diffusant permeation is not significantly different at body and ambient temperatures (14). It is also stated that since diffusant permeation occurs by simple diffusion across the oral mucosa, it is not affected by metabolic inhibitors (14).
Many investigators use Franz diffusion cells to carry out buccal permeation studies. The receiver chambers of these diffusion cells are typically maintained at 37°C while the donor chamber is kept at ambient temperature, typically 22–24°C (31–43). Such an experimental setup for in vitro permeation studies across the buccal mucosa does not mimic the in vivo condition. Both the donor and receiver chamber must be maintained at a constant temperature of 37°C to mimic the in vivo environment. Maintaining temperatures other than 37°C could potentially lead to significant differences in permeability, making it impractical to compare results from different studies and also difficult to correlate in vitro and in vivo permeability data. Hence, the present authors considered it important to investigate the effect of experimental temperature on diffusant permeability.
One may, in general, anticipate an increase in diffusant permeability with an increase in experimental temperature, but the nature of the kinetic relationship is hard to predict. For example, does permeability increases linearly or exponentially with temperature? The mechanism whereby permeability increases with temperature is also unclear, i.e., does permeability increase due to a change in the barrier structure (for example subtle changes in the chemical nature, but not the physical integrity, of the barrier) or to an overt change in the physical integrity of the buccal mucosa?
Apart from the effect of temperature on the results of experimental permeation studies, there is also the potential for heat-enhanced drug delivery via the buccal route; using suitable temperature modulated drug delivery devices. Over the past few years, attention has been focused on overcoming the problems associated with buccal drug delivery. One of the prime limitations facing buccal delivery is poor absorption when compared to the sublingual route of drug delivery. The success of a buccal drug delivery system depends on the ability of the drug to permeate the mucosal barrier at a concentration high enough to achieve its desired therapeutic effect. The buccal mucosa acts as a barrier to the permeation of exogenous material across the tissue. Transport across the buccal epithelium is via passive diffusion; active transport is rare, vitamin B12 being a notable exception. Various approaches including chemically assisted methods (e.g., penetration enhancers and supersaturated systems) or physically assisted techniques (e.g., ultrasound, iontophoresis, and microneedles) have been studied to overcome the barrier properties and to increase the rate and extent of diffusant absorption across the buccal mucosa.
Chemical penetration enhancers are being extensively studied to improve the delivery of diffusants across the buccal mucosa. However, the major limitation of these efficient permeation enhancers is the toxicity associated with their use. Hence, alternative methods of enhancing permeation, which are safe as well as effective, need to be investigated. One possible means to achieve this enhancement is to apply heat locally to increase the temperature in the buccal region.
The enhancing effect of heat on transdermal and transvaginal absorption has been well documented (44–52), but its effect on the buccal mucosa has not been fully explored. Some investigators have shown an approximate doubling of transdermal flux with each 6–8°C increase in temperature from 10°C to 60°C (46,53). Another investigation studied the effect of increase in temperature on the permeability of selected B-agonist across primary hamster cheek pouch cultures (54). A similar effect on diffusant transport across the buccal mucosa has not been investigated, so in this study, tranbuccal permeability of model diffusants were also studied at 7°C increase. If the permeability of a diffusant across the buccal mucosa is significantly enhanced with a relatively small increase in temperature, the principle of permeation enhancement with elevated temperature can be used to develop practical buccal drug delivery systems. Permeation enhancement with heat may be safer than the use of hazardous and toxic penetration enhancers.
Before it can be used as a means of permeation enhancement, however, studies should be performed to characterize the effects of temperature on both the penetrant and the tissues to which it is applied. For example, while it may be acceptable to use higher temperatures (≥45°C) in the in vitro studies, the prolonged use of such temperatures in drug delivery devices may cause patient discomfort. It has been shown in the case of certain transdermal formulations that burns (scalds) have occurred when subjects were exposed to temperatures in excess of 60°C for a short period (55).
The main objective of this work was to evaluate the influence of temperature on porcine buccal mucosal permeation of model diffusants of differing lipophilicities. The studied diffusants, with their log D (distribution coefficient) values at pH 6.8, are: buspirone (2.8), bupivacaine (2.5), antipyrine (0.39), and caffeine (−0.07). Such studies may provide a basis for designing buccal drug delivery systems that utilize transient temperature increases as the mechanism of permeation enhancement.
Theory
It is most likely that the effect of temperature on permeation across the buccal mucosa follows a relationship similar to the Arrhenius equation as given below (56):
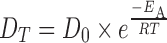 |
1 |
where
- DT
the diffusion coefficient at a certain temperature (square centimeters per second)
- D0
the theoretical maximum diffusion coefficient at infinite temperature (kelvin) (arbitrary value, preexponential factor)
- EA
activation energy of diffusion (joules per mole)
- R
the universal gas constant (83144 joules per mole kelvin)
- T
the temperature of interest (kelvin)
The activation energy can be defined as the energy used to counter the cohesive forces of the membrane in order to facilitate the diffusion process.
In this case, the permeability of a diffusant may be expressed as follows:
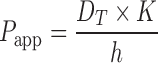 |
2 |
where
- Papp
apparent permeability of the diffusant at the temperature of interest
- K
partition coefficient of the diffusant
- h
thickness of the membrane
Combining Eqs. 1 and 2, we get
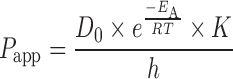 |
3 |
Assuming that the partition coefficient and the thickness of the membrane remain constant over the range of temperature studied, the following relationship can be derived from Eq. 3:
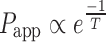 |
4 |
This suggests that the apparent permeability of the diffusant increases exponentially with temperature. A plot of log of apparent permeability versus absolute temperature will yield a linear relationship. The energy necessary for the permeant to break the restraining bonds and to diffuse, similar to energy of activation (EA), can be determined from the slope of this plot. The activation energy provides a measure of the resistance of the buccal mucosa to diffusion of the permeant. In general, the value of the activation energy is a function of both the diffusing permeant and the diffusion pathway (57).
The enhancement in permeability with increase in temperature is given by a ratio known as the enhancement factor (EF). It is calculated using the equation:
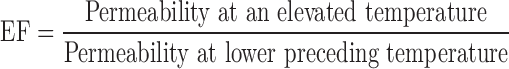 |
5 |
MATERIALS AND METHODS
Materials
Buspirone, bupivacaine, antipyrine, and caffeine were obtained from Sigma-Aldrich (St. Louis, MO, USA). Acetonitrile and methanol were obtained from VWR and were HPLC grade (West Chester, PA, USA).
Tissue Preparation
Buccal mucosa was obtained from freshly sacrificed pigs at a local ranch (Long Ranch Inc., Manteca, CA, USA). The mucosa was transported to the laboratory in an isotonic phosphate buffer (pH 7.4) and used within 2 h of animal sacrifice. The majority of the underlying connective tissue was removed with the help of a scalpel blade and then the remaining buccal mucosa was carefully trimmed with surgical scissors to a proximately uniform thickness of about 500 μm. The use of a dermatome for slicing the tissue was avoided since the process requires prior freezing of the tissue. Freezing may compromise the barrier properties of the epithelium as well as the connective tissue region of the buccal mucosa. The thickness of the tissue was measured with the help of a digital screw gauge and recorded. This preparation procedure helped to minimize thickness variations between tissue specimens (58).
Permeation Studies
In vitro permeation studies were conducted using horizontal, water-jacketed, side-by-side diffusion cells (PermeGear, Inc., Riegelsville, PA). The side-by-side diffusion cells had a cross-sectional area of 0.68 cm2. The buccal mucosa was mounted between the donor and receiver chambers for permeation studies, where the epithelium faced the donor chamber and the other (inner) side of the mucosal membrane faced the receiver chamber. The permeation studies were conducted while temperature control was maintained by water jackets surrounding both the chambers. Five different temperatures, viz., 23°C, 30°C, 37°C, 45°C, and 52°C, were employed in individual permeation experiments. The in vitro conditions may not mimic the in vivo conditions in the experiments involving temperatures other than 37°C, as the receiver compartment is still at 37°C, resulting in temperature gradient. The tissues were allowed to equilibrate with buffer for 15 min before commencement of each permeability experiment. The permeability experiments were carried out using phosphate-buffered saline (PBS) with pH 7.4 in the receiver chamber and known concentrations of diffusant solutions at pH 6.8 in the donor chamber to mimic the in vivo physiological pH conditions (22,58). The receiver chamber was filled with 3.5 mL of PBS while 3.5 mL of the diffusant solution was charged into the donor chamber. The receiver and the donor chambers were stirred with Teflon® coated magnetic stirring bars to minimize the unstirred water layers in the vicinity of the mucosal barrier. Permeation studies were carried out for up to 4 h, and 1-mL samples were withdrawn from the receiver chamber every 30 min. Bupivacaine shows a high lag time of 4 h, and in order to achieve a steady-state flux, its permeation was determined over 8 h. At each sampling point, 1 mL of PBS that was prewarmed to the respective experimental temperature was added to the receiver chamber to compensate for the volume of solution removed. This ensured sink conditions in the receiver chamber solution at all times. All the experiments were conducted in triplicate.
Quantification of Model Diffusants
Using PBS at pH 6.8, solutions of the model diffusants were prepared at known concentrations (antipyrine, 50 mg/mL; buspirone, 0.5 mg/mL; bupivacaine, 1 mg/mL; and caffeine, 20 mg/mL). The solutions were used as donor solutions for the respective experiments. Concentrations of the model diffusants in the permeation samples were quantified using high performance liquid chromatography (HPLC). For each experiment, the cumulative amount permeated (micrograms) was plotted against time (hours). The steady-state flux (micrograms per hour per square centimeter) of each model diffusant across the buccal mucosa was calculated from the slope of the linear portion of the plot. The apparent permeability, Papp (centimeters per second), was calculated from the permeation studies using the following equation:
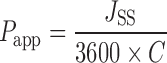 |
where JSS is the steady-state flux (micrograms per hour per square centimeter) of diffusant across buccal mucosa and C is the initial donor concentration of the diffusant (Table I).
Table I.
HPLC Conditions for the Diffusant Molecules
| HPLC conditions | Buspirone | Bupivacaine | Antipyrine | Caffeine |
|---|---|---|---|---|
| Mobile phase | Phosphate buffer and acetonitrile (60:40) | Phosphate buffer, acetonitrile, and methanol (50:25:25) | Phosphate buffer and acetonitrile (70:30) | Phosphate buffer and methanol (60:40) |
| Flow rate (mL/min) | 1 | 1 | 1 | 1 |
| UV detection (nm) | 238 | 220 | 254 | 273 |
The HPLC consisted of a Waters 510 pump, a Waters 717 plus autosampler (Milford, MA, USA), a Shimadzu SPD-10A UV–vis detector (Kyoto, Japan), and an EZ chromatography software. A reverse phase C18-column of 250 mm in length and 5 μm in internal diameter (Waters, Milford) was used to elute the diffusants. The HPLC conditions were developed and optimized for quantitative analysis of each individual diffusant as shown in Table II (3). The assay range for the four diffusants was established on the basis of relationship between the peak areas and the diffusant concentrations. The assay range bracketed the concentration of all permeation samples. Excellent correlation was seen (R2 = 0.99) between the peak area and drug concentrations in the four diffusants.
Table II.
Permeability of Model Diffusants at Different Experimental Temperatures
| Temperature (°C) | Bupivacaine (n = 3) | Buspirone (n = 3) | Antipyrine (n = 3) | Caffeine (n = 3) |
|---|---|---|---|---|
| 23 | (4.81 ± 0.04) × 10−6* | (6.43 ± 0.77) × 10−6* | (7.20 ± 1.15) × 10−7* | (1.73 ± 0.47) × 10−6* |
| 30 | (7.22 ± 1.56) × 10−6* | (1.00 ± 0.12) × 10−5* | (1.24 ± 0.30) × 10−6* | (3.35 ± 0.16) × 10−6* |
| 37 | (1.59 ± 0.35) × 10−5* | (1.74 ± 0.04) × 10−5* | (2.12 ± 0.01) × 10−6* | (8.14 ± 0.73) × 10−6* |
| 45 | (2.33 ± 0.35) × 10−5* | (3.18 ± 0.17) × 10−5* | (5.06 ± 0.64) × 10−6* | (1.58 ± 0.09) × 10−5* |
| 52 | (4.04 ± 0.49) × 10−5* | (4.44 ± 0.03) × 10−5* | (1.12 ± 0.08) × 10−5* | (2.36 ± 0.44) × 10−5* |
*p < 0.05
Data Analysis
The transbuccal permeability data were presented as a mean or mean and standard deviation (1) for each diffusant (n = 3 replicates per temperature condition) and the corresponding temperature. For statistical analysis, the software Excel 2002® (Microsoft Co., Redmond, WA) was used. Statistical differences of permeability values for different experimental temperatures were determined using the analysis of variance (one-way ANOVA). p < 0.05 was considered as the level of significance.
RESULTS
Effect of Temperature on the Permeation of Model Diffusants
The permeability of the model diffusants increased significantly with each incremental rise in the experimental temperature of approximately 7°C. The mean permeability values of three replicate tissue samples are presented with their standard deviation values (mean ± SD) and are shown in Table III.
Table III.
Enhancement Factor of Model Diffusants
| Temperature higher/lower (°C) | EF bupivacaine | EF buspirone | EF antipyrine | EF caffeine |
|---|---|---|---|---|
| 30/23 | 1.5 | 1.56 | 1.72 | 1.94 |
| 37/30 | 2.2 | 1.74 | 1.71 | 2.42 |
| 45/37 | 1.47 | 1.83 | 2.39 | 1.94 |
| 52/45 | 1.73 | 1.4 | 2.2 | 1.49 |
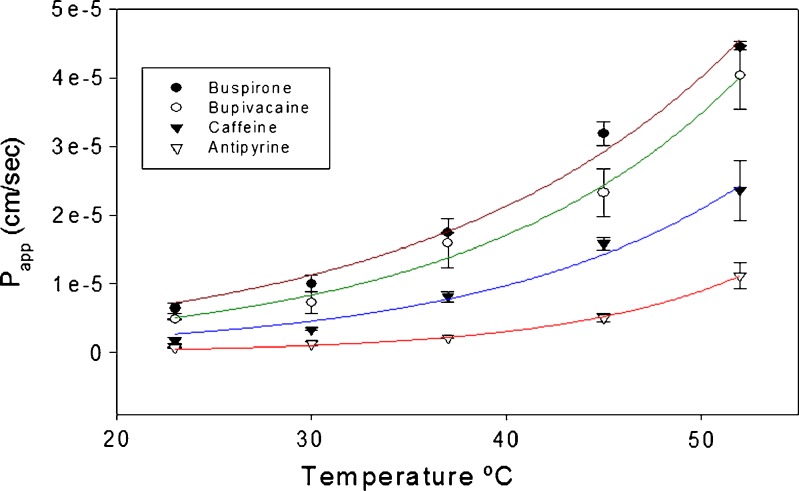
The permeation of all diffusants was found to increase by a factor of about 1.4 to 2.4 times for each incremental rise in experimental temperature of approximately 7°C (Table IV). An exponential relationship was observed between the experimental temperature and the permeability of each of the four model diffusants as shown in Fig. 1.
Table IV.
Physicochemical Properties of Model Compounds
| Physical properties | Buspirone (15, 55) | Bupivacaine (56, 57) | Antipyrine (51, 58) | Caffeine (38, 59) |
|---|---|---|---|---|
| Molecular weight | 385.5 | 288 | 188.23 | 194.19 |
| Aqueous solubility | 0.5 mg/mL | 1.16 mg/mL | 1,100 mg/mL | 21 mg/mL |
| pKa | 4.12, 7.32 | 8.1 | 1.45 | 10.4 |
| log P | 2.78 | 3.70 | 0.39 | −0.07 |
| log D | 2.80 | 2.48 | 0.39 | −0.07 |
Fig. 1.
Effect of experimental temperature on the permeability of model compounds
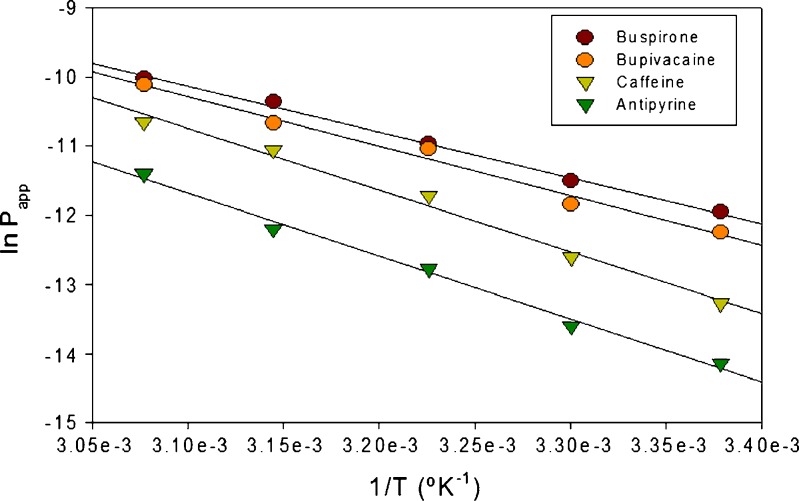
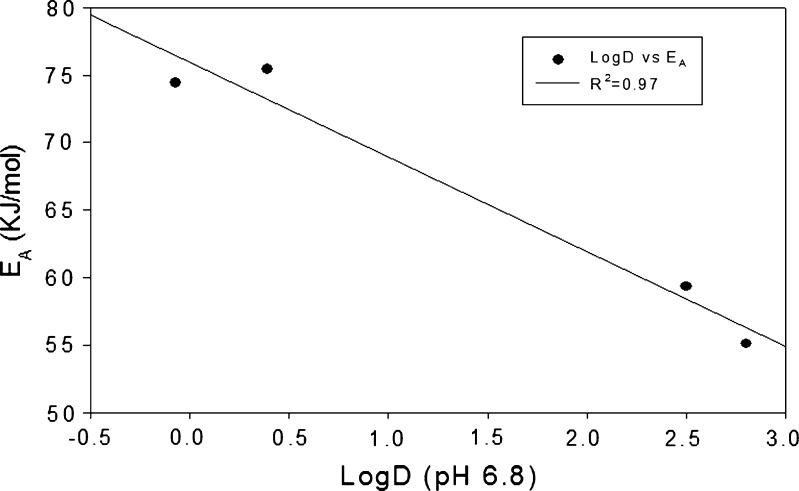
This relationship is more evident in Fig. 2, where the natural log of permeability was plotted as a function of the inverse of absolute temperature (kelvin). This yielded a linear relationship as defined in Eq. 3, and the activation energy of diffusion was calculated from the slope of these plots using Eq. 4. The data show that the lipophilic diffusants required lower energies for diffusion when compared to the relatively more hydrophilic diffusants (Fig. 3).
Fig. 2.
Plot of ln P(app) versus 1/T
Fig. 3.
Relationship between lipophilicity and activation energy
DISCUSSION
The influence of temperature on the permeability of diffusants was investigated in this study. The permeation experiments were conducted at five different temperatures individually ranging from 23°C to 52°C. The use of high temperatures, such as 45°C and 52°C, may raise concerns about lipid transitions. However, it should be noted that at these temperatures, lipid transitions occur only in the stratum corneum (59). The literature suggests that irreversible effects, in the buccal mucosa, only occur above 68°C (60). Hence, the temperature range used in the present study can be considered appropriate and safe.
Four model diffusants, namely, buspirone, bupivacaine, antipyrine, and caffeine, were chosen for in vitro transbuccal permeability studies based on the varying range of lipophilicities as well as other physicochemical properties (Table I). Buspirone has a low aqueous solubility and is a highly lipophilic diffusant (21,61,62). Bupivacaine is a moderately lipophilic diffusant with a log D value of 2.48 at a pH of 6.8 (63,64). These lipophilic diffusants preferentially transverse across the lipophilic cell membrane, and the route is called the transcellular route (trans—across). This route has a greater surface area, and the path length for transcellular route is relatively short; hence, the lipophilic diffusants traversing through this route show a high permeability with little or no lag time (65). Antipyrine and caffeine are moderately hydrophilic diffusants (44,66–69). Caffeine is used as a model hydrophilic marker in buccal permeation experiments (68). These hydrophilic diffusants transport across the buccal mucosa preferentially via the hydrophilic intercellular space and is called the paracellular (para—in between) route. It is difficult for a hydrophilic diffusant to penetrate into the lipophilic cell membrane, and hence, the intercellular space is the preferred route for such diffusants. The main limitations of paracellular pathways are limited surface area and tortuous pathways. Hence, the permeability of such diffusants is relatively low with presence of a lag time (65).
As shown in Table IV, with every rise in temperature of approximately 7°C, there was a significant increase (1.4–2.4 times) in the permeability of the four model diffusants, irrespective of their lipophilicities. This clearly shows that temperature has a significant effect on the permeability of a diffusant. In the case of buspirone and antipyrine, maximum enhancement was observed when the temperature was increased from 37°C to 45°C, whereas in the case of bupivacaine and caffeine, maximum enhancement occurred when the temperature was increased from 30°C to 37°C. It may be possible to exploit heat-aided enhancement in drug permeation for the commercial development of buccal delivery products. Zarr Pharma has utilized this approach and developed a “Controlled Heat-Assisted Diffusant Delivery System” (CHADD™) for transdermal delivery. CHADD™ is a transdermal patch which enhances the delivery of diffusants by raising the temperature of the skin via an oxidation reaction generated by a proprietary chemical mixture contained in the patch (70).
As shown in Fig. 2, an exponential relationship exists between the permeability of a diffusant and the inverse of the experimental temperature. This relationship is described by Eqs. 1–4 and is of the Arrhenius type. As shown in Fig. 2, the plot of natural log of permeability versus the inverse of temperature shows greater slopes for the lipophilic diffusants—buspirone and bupivacaine—than for the relatively hydrophilic diffusants—antipyrine and caffeine. Hence, these studies show that elevated temperature affects the permeation of lipophilic as well as hydrophilic diffusants. Such temperature dependence of permeation across porcine buccal mucosa has not been reported before. Due to the complex nature of the buccal mucosa, it is difficult to discern the mechanism(s) or pathways responsible for this temperature effect. However, the activation energies of diffusion for these four diffusants were calculated. As shown in Fig. 3, a strong linear correlation was observed between the diffusant’s lipophilicity and its activation energy. Diffusants with higher lipophilicities, such as buspirone and bupivacaine, had lower activation energies of diffusion when compared to hydrophilic diffusants, such as antipyrine and caffeine.
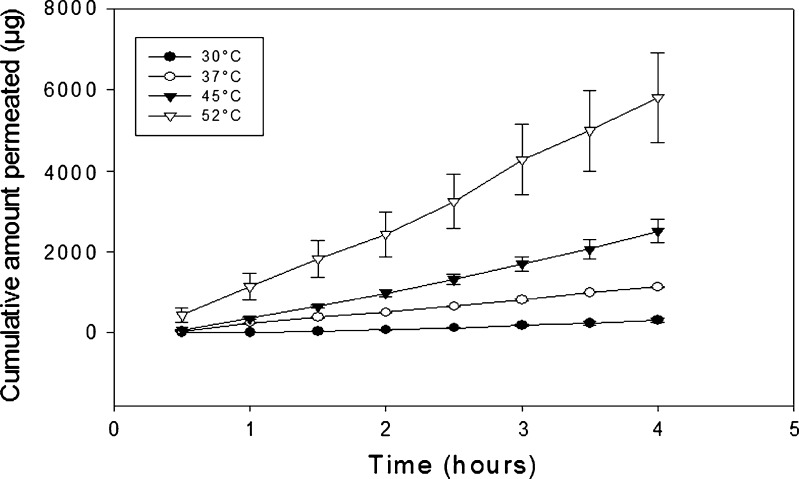
The permeability barrier of porcine buccal mucosa is attributed to the presence of various lipids in the bilayered cell membrane or in membrane-coating granules. These lipids may undergo gel to liquid-crystalline transition, as was reported to occur in the skin’s stratum corneum (58). In another reported study, no irreversible effects were found to occur in the lipid structure and function of canine buccal mucosa in the temperature range studied (59). In the present study, the plots of cumulative amount released versus time revealed that steady-state flux was obtained even at a temperature of 52°C for all four diffusants. A representative figure of cumulative amount permeated versus time profile for antipyrine at all temperatures is shown (Fig. 4). This observation suggests that no apparent damage to the permeability barrier occurred. The plot of log of apparent permeability versus 1/T was found to be linear in the temperature range employed, suggesting the absence of any structural alteration or phase transition within the buccal mucosa. Since irreversible lipid transitions in buccal mucosa have been reported to only occur at temperatures ≥68°C (58), it is proposed that the increase in apparent permeability, observed between 23°C and 52°C in this study, was primarily due to increases in the diffusion coefficients of the diffusants in the vehicle. The apparent permeability values also show that exposing the mucosal surface to high temperatures (52°C in this study) does not completely compromise its barrier properties since diffusion remained the rate-limiting process. At lower temperatures, the lipids are more solid and less liquid, thus hindering the permeation of diffusants across the buccal mucosa. It is recognized that these results do not rule out the possibility that a reversible increase in the fluidity of the mucosal lipids occurred at the highest temperatures studied, namely, 45°C and 52°C. Additionally, the fact that the temperature effect is consistent over the range of temperatures studied (i.e., the activation energy is essentially the same) is good evidence that temperature produced no significant mucosal damage.
Fig. 4.
Cumulative amount permeated versus time profile for antipyrine at different temperatures
The slope of the plot, which is proportional to the activation energy (EA), gives an indication of the energy necessary for the diffusant to break restraining bonds and diffuse, i.e., it provides a measure of the resistance of the penetrant to diffusion across the epidermis. In general, the value of the activation energy is a function of both the diffusing diffusant and the diffusion pathway (56). Due to the lipophilic nature of the buccal mucosa, the relatively hydrophilic diffusants, such as antipyrine and caffeine, encounter greater energy barriers and therefore have higher activation energies (approximately 75 kJ/mol) compared to the more lipophilic diffusants such as buspirone and bupivacaine (approximately 55 kJ/mol). The lower activation energies of lipophilic diffusants suggest that these diffusants permeate easily and fairly rapidly across the bilayered lipid pathways. The magnitude of the activation energy, therefore, takes into account the distribution coefficients of the diffusants.
It is surprising, however, that caffeine was found to have slightly smaller activation energy than antipyrine. This result suggests that caffeine, even though is a stronger hydrophilic diffusant, permeated the buccal mucosa more readily than antipyrine. The lower activation energy of caffeine correlated well with the actual values of permeability. The permeability of caffeine, in some studies, suggests that it may traverse the membrane via the lipoidal pathway (71). This observation may help to explain the anomaly observed in the present work.
The observations from these experiments will help to explain the mechanisms by which elevated temperature increases in vitro transbuccal permeation. It will also help in predicting the extent of increased permeation of model diffusants at elevated temperatures. This knowledge may be utilized and exploited in the development of buccal drug delivery systems based on a local increase in the temperature.
CONCLUSION
The permeability of model diffusants increased exponentially with temperature. Diffusants with higher lipophilicities such as buspirone and bupivacaine had lower activation energies of diffusion when compared to hydrophilic diffusants such as antipyrine and caffeine. The activation energy of diffusion of the model diffusants decreased linearly with increasing distribution coefficients across porcine buccal mucosa. The exponential nature of the relationship between permeability and temperature dictates that in vitro experiments utilizing buccal membranes should be carefully controlled for temperature to minimize variability in the results. Temperature should be standardized to allow meaningful comparisons between laboratories. These results also suggest that temperature may usefully be employed in drug delivery devices as a means to enhance the absorption of drugs across the buccal mucosa.
ACKNOWLEDGMENTS
The authors wish to thank Long Ranch, Inc. (Manteca, CA, USA) for providing fresh porcine buccal tissues.
REFERENCES
- 1.Galey WR, Lonsdale HK, Nacht S. The in vitro permeability of skin and buccal mucosa to selected drugs and tritiated water. J Invest Dermatol. 1976;67(6):713–717. doi: 10.1111/1523-1747.ep12598596. [DOI] [PubMed] [Google Scholar]
- 2.Shojaei AH. Buccal mucosa as a route for systemic drug delivery: a review. J Pharm Sci. 1998;1(1):15–30. [PubMed] [Google Scholar]
- 3.Kulkarni U, Mahalingam R, Pather SI, Li X, Jasti B. Porcine buccal mucosa as an in vitro model: relative contribution of epithelium and connective tissue as permeability barriers. J Pharm Sci. 2009;98(2):471–483. doi: 10.1002/jps.21436. [DOI] [PubMed] [Google Scholar]
- 4.Birudaraj R, Mahalingam R, Li X, Jasti BR. Advances in buccal drug delivery. Crit Rev Ther Drug Carrier Syst. 2005;22(3):295–330. doi: 10.1615/CritRevTherDrugCarrierSyst.v22.i3.20. [DOI] [PubMed] [Google Scholar]
- 5.Pather SI, Rathbone MJ, Senel S. Current status and the future of buccal drug delivery systems. Expert Opin Drug Deliv. 2008;5(5):531–542. doi: 10.1517/17425247.5.5.531. [DOI] [PubMed] [Google Scholar]
- 6.Smart JD. Buccal drug delivery. Expert Opin Drug Deliv. 2005;2(3):507–517. doi: 10.1517/17425247.2.3.507. [DOI] [PubMed] [Google Scholar]
- 7.Sudhakar Y, Kuotsu K, Bandyopadhyay AK. Buccal bioadhesive drug delivery—a promising option for orally less efficient drugs. J Control Release. 2006;114(1):15–40. doi: 10.1016/j.jconrel.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 8.Wertz PW, Squier CA. Cellular and molecular basis of barrier function in oral epithelium. Crit Rev Ther Drug Carr Syst. 1991;8:237–269. [PubMed] [Google Scholar]
- 9.de Vries ME, Bodde HE, Verhoef JC, Ponec M, Craane HM, Junginger HE. Localization of the permeability barrier inside porcine buccal mucosa: a combined in vitro study of drug permeability, electrical resistance and tissue morphology. Int J Pharm. 1991;76:25–35. doi: 10.1016/0378-5173(91)90340-T. [DOI] [Google Scholar]
- 10.Squier CA, Cox P, Wertz PW. Lipid content and water permeability of skin and oral mucosa. J Invest Dermatol. 1991;96(1):123–126. doi: 10.1111/1523-1747.ep12515931. [DOI] [PubMed] [Google Scholar]
- 11.Kulkarni U, Mahalingam R, Pather I, Li X, Jasti B. Porcine buccal mucosa as in vitro model: effect of biological and experimental variables. J Pharm Sci. 2010;99(3):1265–1277. doi: 10.1002/jps.21907. [DOI] [PubMed] [Google Scholar]
- 12.Manganaro AM, Wertz PW. The effects of permeabilizers on the in vitro penetration of propranolol through porcine buccal epithelium. Mil Med. 1996;161(11):669–672. [PubMed] [Google Scholar]
- 13.Mashru RC, Sutariya VB, Sankalia MG, Sankalia JM. Effect of pH on in vitro permeation of ondansetron hydrochloride across porcine buccal mucosa. Pharm Dev Technol. 2005;10(2):241–247. doi: 10.1081/pdt-54437. [DOI] [PubMed] [Google Scholar]
- 14.Lesch CA, Squier CA, Cruchley A, Williams DM, Speight P. The permeability of human oral mucosa and skin to water. J Dent Res. 1989;68(9):1345–1349. doi: 10.1177/00220345890680091101. [DOI] [PubMed] [Google Scholar]
- 15.Artusi M, Santi P, Colombo P, Junginger HE. Buccal delivery of thiocolchicoside: in vitro and in vivo permeation studies. Int J Pharm. 2003;250(1):203–213. doi: 10.1016/S0378-5173(02)00545-8. [DOI] [PubMed] [Google Scholar]
- 16.Hoogstraate AJ, Senel S, Cullander C, Verhoef JC, Junginger HE, Bodde HE. Effects of bile salts on transport rates and routes of FITC-labelled compounds across porcine buccal epithelium in vitro. J Control Release. 1996;40:211–221. doi: 10.1016/0168-3659(95)00187-5. [DOI] [Google Scholar]
- 17.Senel S, Hoogstraate AJ, Spies F, Verhoef JC, Bos-van Geest A, Junginger HE, et al. Enhancement of in vitro permeability of porcine buccal mucosa by bile salts: kinetic and histological studies. J Control Release. 1994;32(1):45–56. doi: 10.1016/0168-3659(94)90224-0. [DOI] [Google Scholar]
- 18.Senel S, Capan Y, Sargon MF, Ikinci G, Solpan D, Güven O, et al. Enhancement of transbuccal permeation of morphine sulfate by sodium glycodeoxycholate in vitro. J Control Release. 1997;45(2):153–162. doi: 10.1016/S0168-3659(96)01568-4. [DOI] [Google Scholar]
- 19.Hoogstraate AJ, Cullander C, Nagelkerke JF, Senel S, Verhoef JC, Junginger HE. Diffusion rates and transport pathways of fluorescein isothiocyanate (FITC)-labeled model compounds through buccal epithelium. Pharm Res. 1994;11(1):83–89. doi: 10.1023/A:1018949828548. [DOI] [PubMed] [Google Scholar]
- 20.Junginger HE, Hoogstraate AJ, Verhoef JC. Recent advances in buccal drug delivery and absorption—in vitro and in vivo studies. J Control Release. 1999;62(1–2):149–159. doi: 10.1016/S0168-3659(99)00032-2. [DOI] [PubMed] [Google Scholar]
- 21.Birudaraj R, Berner B, Shen S, Li X. Buccal permeation of buspirone: mechanistic studies on transport pathways. J Pharm Sci. 2005;94(1):70–78. doi: 10.1002/jps.20208. [DOI] [PubMed] [Google Scholar]
- 22.LeBrun PP, Fox PL, de Vries ME, Bodde HE. In vitro penetration of some ß-adrenoreceptor blocking drugs through porcine buccal mucosa. Int J Pharm. 1989;49:141–145. doi: 10.1016/0378-5173(89)90113-0. [DOI] [Google Scholar]
- 23.Nielsen HM, Rassing MR. Nicotine permeability across the buccal TR146 cell culture model and porcine buccal mucosa in vitro: effect of pH and concentration. Eur J Pharm Sci. 2002;16(3):151–157. doi: 10.1016/S0928-0987(02)00083-0. [DOI] [PubMed] [Google Scholar]
- 24.Shojaei AH, Zhuo SL, Li X. Transbuccal delivery of acyclovir (II): feasibility, system design, and in vitro permeation studies. J Pharm Sci. 1998;1(2):66–73. [PubMed] [Google Scholar]
- 25.Shojaei AH, Khan M, Lim G, Khosravan R. Transbuccal permeation of a nucleoside analog, dideoxycytidine: effects of menthol as a permeation enhancer. Int J Pharm. 1999;192(2):139–146. doi: 10.1016/S0378-5173(99)00301-4. [DOI] [PubMed] [Google Scholar]
- 26.Dowty ME, Knuth KE, Irons BK, Robinson JR. Transport of thyrotropin releasing hormone in rabbit buccal mucosa in vitro. Pharm Res. 1992;9(9):1113–1122. doi: 10.1023/A:1015883217858. [DOI] [PubMed] [Google Scholar]
- 27.Liu C, Xu HN, Li XL. In vitro permeation of tetramethylpyrazine across porcine buccal mucosa. Acta Pharmacol Sin. 2002;23(9):792–796. [PubMed] [Google Scholar]
- 28.Xiang J, Fang X, Li X. Transbuccal delivery of 2′,3′-dideoxycytidine: in vitro permeation study and histological investigation. Int J Pharm. 2002;231(1):57–66. doi: 10.1016/S0378-5173(01)00865-1. [DOI] [PubMed] [Google Scholar]
- 29.Hansen LB, Christrup LL, Bundgaard H. Enhanced delivery of ketobemidone through porcine buccal mucosa in vitro via more lipophilic ester prodrugs. Int J Pharm. 1992;88:237–242. doi: 10.1016/0378-5173(92)90321-R. [DOI] [Google Scholar]
- 30.Quadros E, Cassidy J, Gniecko K, LeRoy S. Buccal and colonic absorption of CGS 16617, a novel ACE inhibitor. J Control Release. 1991;19:77–86. doi: 10.1016/0168-3659(92)90066-Z. [DOI] [Google Scholar]
- 31.Sandri G, Rossi S, Ferrari F, Bonferoni MC, Muzzarelli C, Caramella C. Assessment of chitosan derivatives as buccal and vaginal penetration enhancers. Eur J Pharm Sci. 2004;21(2–3):351–359. doi: 10.1016/j.ejps.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 32.Kitano M, Maitani Y, Takayama K, Nagai T. Buccal absorption through golden hamster cheek pouch in vitro and in vivo of 17ß-estradiol from hydrogels containing three types of absorption enhancers. Int J Pharm. 1998;174:19–28. doi: 10.1016/S0378-5173(98)00234-8. [DOI] [Google Scholar]
- 33.Sandri G, Rossi S, Bonferoni MC, Ferrari F, Zambito Y, Di Colo G. Buccal penetration enhancement properties of N-trimethyl chitosan: influence of quaternization degree on absorption of a high molecular weight molecule. Int J Pharm. 2005;297(1–2):146–155. doi: 10.1016/j.ijpharm.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Consuelo ID, Jacques Y, Pizzolato GP, Guy RH, Falson F. Comparison of the lipid composition of porcine buccal and esophageal permeability barriers. Arch Oral Biol. 2005;50(12):981–987. doi: 10.1016/j.archoralbio.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Consuelo ID, Pizzolato GP, Falson F, Guy RH, Jacques Y. Evaluation of pig esophageal mucosa as a permeability barrier model for buccal tissue. J Pharm Sci. 2005;94(12):2777–2788. doi: 10.1002/jps.20409. [DOI] [PubMed] [Google Scholar]
- 36.Ceschel GC, Maffei P, Sforzini A, Borgia SL, Yasin A, Ronchi C. In vitro permeation through porcine buccal mucosa of caffeic acid phenetyl ester (CAPE) from a topical mucoadhesive gel containing propolis. Fitoterapia. 2002;73(Suppl 1):S44–S52. doi: 10.1016/S0367-326X(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 37.Ceschel GC, Maffei P, Moretti MD, Demontis S, Peana AT. In vitro permeation through porcine buccal mucosa of Salvia desoleana Atzei & Picci essential oil from topical formulations. Int J Pharm. 2000;195(1–2):171–177. doi: 10.1016/S0378-5173(99)00381-6. [DOI] [PubMed] [Google Scholar]
- 38.Senel S, Duchene D, Hincal AA, Capan Y, Ponchel G. In vitro studies on enhancing effect of sodium glycocholate on transbuccal permeation of morphine hydrochloride. J Control Release. 1998;51(2–3):107–113. doi: 10.1016/S0168-3659(97)00099-0. [DOI] [PubMed] [Google Scholar]
- 39.Mashru R, Sutariya V, Sankalia M, Sankalia J. Transbuccal delivery of lamotrigine across porcine buccal mucosa: in vitro determination of routes of buccal transport. J Pharm Sci. 2005;8(1):54–62. [PubMed] [Google Scholar]
- 40.Attia MA, El-Gibaly I, Shaltout SE, Fetih GN. Transbuccal permeation, anti-inflammatory activity and clinical efficacy of piroxicam formulated in different gels. Int J Pharm. 2004;276(1–2):11–28. doi: 10.1016/j.ijpharm.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 41.Veuillez F, Rieg FF, Guy RH, Deshusses J, Buri P. Permeation of a myristoylated dipeptide across the buccal mucosa: topological distribution and evaluation of tissue integrity. Int J Pharm. 2002;231(1):1–9. doi: 10.1016/S0378-5173(01)00850-X. [DOI] [PubMed] [Google Scholar]
- 42.Veuillez F, Ganem-Quintanar A, Deshusses J, Falson-Rieg F, Buri P. Comparison of the ex-vivo oral mucosal permeation of tryptophan-leucine (Trp-Leu) and its myristoyl derivative. Int J Pharm. 1998;170(1):85–91. doi: 10.1016/S0378-5173(98)00134-3. [DOI] [Google Scholar]
- 43.Jacobsen J. Buccal iontophoretic delivery of atenolol.HCl employing a new in vitro three-chamber permeation cell. J Control Release. 2001;70(1–2):83–95. doi: 10.1016/S0168-3659(00)00328-X. [DOI] [PubMed] [Google Scholar]
- 44.Akomeah F, Nazir T, Martin GP, Brown MB. Effect of heat on the percutaneous absorption and skin retention of three model penetrants. Eur J Pharm Sci. 2004;21(2–3):337–345. doi: 10.1016/j.ejps.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 45.Ohara N, Takayama K, Nagai T. Combined effect of d-limonene pretreatment and temperature on the rat skin permeation of lipophilic and hydrophilic drugs. Biol Pharm Bull. 1995;18(3):439–442. doi: 10.1248/bpb.18.439. [DOI] [PubMed] [Google Scholar]
- 46.Ogiso T, Hirota T, Iwaki M, Hino T, Tanino T. Effect of temperature on percutaneous absorption of terodiline, and relationship between penetration and fluidity of the stratum corneum lipids. Int J Pharm. 1998;176(1):63–72. doi: 10.1016/S0378-5173(98)00309-3. [DOI] [Google Scholar]
- 47.Clarys P, Alewaeters K, Jadoul A, Barel A, Manadas RO, Preat V. In vitro percutaneous penetration through hairless rat skin: influence of temperature, vehicle and penetration enhancers. Eur J Pharm Biopharm. 1998;46(3):279–283. doi: 10.1016/S0939-6411(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 48.Vanakoski J, Seppala T, Sievi E, Lunell E. Exposure to high ambient temperature increases absorption and plasma concentrations of transdermal nicotine. Clin Pharmacol Ther. 1996;60(3):308–315. doi: 10.1016/S0009-9236(96)90057-0. [DOI] [PubMed] [Google Scholar]
- 49.Ohara N, Takayama K, Nagai T. Influence of temperature on the percutaneous absorption for lipophilic and hydrophilic drugs across the rat skin pretreated with oleic acid. Int J Pharm. 1995;123:281–284. doi: 10.1016/0378-5173(95)00089-2. [DOI] [Google Scholar]
- 50.van der Bijl P, Venter A, van Eyk AD, Thompson IO. Effect of temperature on permeability of mucosa to water. SADJ. 1998;53(11):504–507. [PubMed] [Google Scholar]
- 51.van der Bijl P, van Eyk AD, Kriel J. Enhancing effect of temperature on the transmucosal penetration kinetics of 17 beta-estradiol. SADJ. 2003;58(3):95–101. [PubMed] [Google Scholar]
- 52.van der Bijl P, de Blois AM, van Eyk AD, Thompson IO. Permeability of vaginal mucosa to water at normal and elevated temperatures. SADJ. 2000;55(4):206–210. [PubMed] [Google Scholar]
- 53.Knutson K, Potts RO, Guzek DB, Golden GM, McKie JE, Lambert WJ. Macro-and molecular physical-chemical considerations in understanding drug transport in the stratum corneum. J Control Release. 1985;2:67–87. doi: 10.1016/0168-3659(85)90034-3. [DOI] [Google Scholar]
- 54.Tavakoli-Saberi MR, Audus KL. Physicochemical factors affecting β-adrenergic antagonist permeation across cultured hamster pouch buccal epithelium. Int J Pharm. 1989;56(2):135–142. doi: 10.1016/0378-5173(89)90006-9. [DOI] [Google Scholar]
- 55.Behl CR, Flynn GL, Kurihara T, Smith W, Gatmaitan O, Higuchi WI. Permeability of thermally damaged skin: I. Immediate influences of 60 degrees C scalding on hairless mouse skin. J Invest Dermatol. 1980;75(4):340–345. doi: 10.1111/1523-1747.ep12531096. [DOI] [PubMed] [Google Scholar]
- 56.Vieth WR. Diffusion in and through polymers. New York: Hanser Publishers; 1990. [Google Scholar]
- 57.Blank IH, Scheuplein RJ, MacFarlane DJ. Mechanism of percutaneous absorption. 3. The effect of temperature on the transport of non-electrolytes across the skin. J Invest Dermatol. 1967;49(6):582–589. [PubMed] [Google Scholar]
- 58.Kulkarni U, Mahalingam R, Pather SI, Li X, Jasti B. Porcine buccal mucosa as an in vitro model: relative contribution of epithelium and connective tissue as permeability barriers. J Pharm Sci. 2008;98(2):471–483. doi: 10.1002/jps.21436. [DOI] [PubMed] [Google Scholar]
- 59.Golden GM, Guzek DB, Harris RR, McKie JE, Potts RO. Lipid thermotropic transitions in human stratum corneum. J Invest Dermatol. 1986;86(3):255–259. doi: 10.1111/1523-1747.ep12285373. [DOI] [PubMed] [Google Scholar]
- 60.Harrison DJ, Knutson K. Thermal analysis of canine buccal epithelium by DSC and FTIR. Pharm Res. 1997;S660(Suppl):660. [Google Scholar]
- 61.Takács-Novák K, Avdeef A. Interlaboratory study of log P determination by shake-flask and potentiometric methods. J Pharm Biomed Anal. 1996;14(11):1405–1413. doi: 10.1016/0731-7085(96)01773-6. [DOI] [PubMed] [Google Scholar]
- 62.Bergstrom CA, Luthman K, Artursson P. Accuracy of calculated pH-dependent aqueous drug solubility. Eur J Pharm Sci. 2004;22(5):387–398. doi: 10.1016/j.ejps.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 63.Berggren S, Hoogstraate J, Fagerholm U, Lennernas H. Characterization of jejunal absorption and apical efflux of ropivacaine, lidocaine and bupivacaine in the rat using in situ and in vitro absorption models. Eur J Pharm Sci. 2004;21(4):553–560. doi: 10.1016/j.ejps.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Higaki K, Asai M, Suyama T, Nakayama K, Ogawara K, Kimura T. Estimation of intradermal disposition kinetics of drugs: II. Factors determining penetration of drugs from viable skin to muscular layer. Int J Pharm. 2002;239(1–2):129–141. doi: 10.1016/S0378-5173(02)00084-4. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Robinson JR. Routes of drug transport across oral mucosa. In: Rathbone MJ, editor. Oral mucosal drug delivery. New York: Mercel Dekker; 1996. pp. 51–64. [Google Scholar]
- 66.Avram MJ, Krejcie TC, Henthorn TK. The concordance of early antipyrine and thiopental distribution kinetics. J Pharmacol Exp Ther. 2002;302(2):594–600. doi: 10.1124/jpet.102.034611. [DOI] [PubMed] [Google Scholar]
- 67.Nicolazzo JA, Reed BL, Finnin BC. Assessment of the effects of sodium dodecyl sulfate on the buccal permeability of caffeine and estradiol. J Pharm Sci. 2004;93(2):431–440. doi: 10.1002/jps.10559. [DOI] [PubMed] [Google Scholar]
- 68.Nicolazzo JA, Reed BL, Finnin BC. The effect of various in vitro conditions on the permeability characteristics of the buccal mucosa. J Pharm Sci. 2003;92(12):2399–2410. doi: 10.1002/jps.10505. [DOI] [PubMed] [Google Scholar]
- 69.Nicolazzo JA, Reed BL, Finnin BC. Modification of buccal drug delivery following pretreatment with skin penetration enhancers. J Pharm Sci. 2004;93(8):2054–2063. doi: 10.1002/jps.20113. [DOI] [PubMed] [Google Scholar]
- 70.Shomaker TS, Zhang J, Love G, Basta S, Ashburn MA. Evaluating skin anesthesia after administration of a local anesthetic system consisting of an S-Caine patch and a controlled heat-aided drug delivery (CHADD) patch in volunteers. Clin J Pain. 2000;16(3):200–204. doi: 10.1097/00002508-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Dias M, Farinha A, Faustino E, Hadgraft J, Pais J, Toscano C. Topical delivery of caffeine from some commercial formulations. Int J Pharm. 1999;182(1):41–47. doi: 10.1016/S0378-5173(99)00067-8. [DOI] [PubMed] [Google Scholar]