The authors conclude that robotic technology can facilitate the performance of robotic tubal anastomosis.
Keywords: Robotics, Tubal anastomosis, Pregnancy
Abstract
Objectives:
To describe the surgical technique of robotic tubal anastomosis.
Methods:
Retrospective chart and video review of the instrumentation and methodology used for robotically assisted tubal anastomosis.
Results:
All tubal anastomoses were performed with the use of 3 or 4 robotic arms, 3 or 4 robotic instruments, and 1 assistant trocar.
Conclusions:
Robotic technology facilitates the performance of robotic tubal anastomosis.
INTRODUCTION
Sterilization is considered the most common method of birth control accounting for approximately 39% of contraceptive methods used by reproductive age women (15 years to 44 years) and their partners in the United States. Of those, 28% have tubal sterilization.1 Following tubal ligation, the most common reason for regret is the desire for more children. Young age at sterilization is associated with a higher possibility of regret and the desire of having more children. It has been estimated that women sterilized before age 25 years are 18 times more likely to request reversal over the course of follow-up than women older than 30 years at the time of sterilization.2 Although most tubal surgery has been abandoned for in vitro fertilization (IVF), there may be a continuing role for tubal reversal surgery given the trend towards transferring fewer embryos to avoid multiple births. This trend towards the transfer of fewer embryos has resulted in a decreased per cycle pregnancy rate that makes the success rate of tubal reversal more appealing.
A wide variety of surgical options is available for sterilization reversal, including laparotomy, laparoscopy, and more recently robotically assisted tubal anastomosis. Robotic technology is being increasingly adopted in complex benign and cancer-related gynecologic surgeries. It has also been shown to have a relatively fast learning curve for the performance of hysterectomy for benign indications with a learning curve of about 20 cases.3 The first robotic tubal reversal reported was completed with different robotic systems.4–6
The goal of this article is to describe our robotic approach for the performance of tubal anastomosis using the most up-to-date robotic system. Our robotic approach for tubal anastomosis has evolved from the incorporation of new and different robotic instruments, different trocar placement, assistant's role, and surgeons' experience in the performance of tubal anastomosis. The results of the surgical outcomes have been reported previously.7
MATERIALS AND METHODS
A retrospective video review of patients undergoing a robotic tubal anastomosis for tubal sterilization reversal using the technique outlined below was used.
Operative and Patient Setup
Patients are placed in the modified-lithotomy position using Allen stirrups (Allen Medical, Acton, MA) with the arms tucked to each side. Adequate padding is used to protect both arms and legs. To avoid sliding while in the Trendelenburg position, the patient lies on her bare back directly on an antiskid bean bag (Tyco/Kendall, Mansfield, MA). The bean bag is secured to the operating table. The da Vinci column is positioned between the patient's legs to allow ample access to the vagina. Standard draping of the operative field as for a conventional laparoscopic procedure is used.
A 12-mm trocar is always placed at the umbilicus for introduction of the laparoscope, unless a myomectomy is also performed in which case this port is placed 20cm to 25cm above the pubic symphysis. The umbilical port is at a sufficient distance from the operative field, because the laparoscope is often very close to the operative field so the magnification can be used to the maximum. Two specially designed 8-mm robotic trocars are placed 8cm to 10cm at 15 degrees to the right and left of the umbilicus, to be fastened to the right and left robotic arms, respectively. More recently 5-mm EndoWrist robotic ports and instruments have been used to allow smaller instrument introduction.
A 5-mm or 10-mm accessory trocar is placed in the left or right lower quadrant to facilitate introduction of needles. Contrary to standard robotic technique where the accessory trocar is placed high in the abdomen for microsurgery, it should be placed low. This is to allow direct visualization for the introduction of the very small needles used. If the needle for these small sutures were to be lost on introduction, the possibility of finding it would be very small. A third robotic arm trocar is used only in a few patients with adhesions or obesity, and placed 8cm lateral and caudal respectively to the right robotic trocar. The operating table is placed in the Trendelenburg position until the small bowel and sigmoid are displaced out of the pelvis before docking. A uterine manipulator (Rumi Colpo-occluder, Cooper Medical, Trumbull, CT) with a side channel for injection of indigo carmine is used in all patients. If needed, an active smoke evacuator (Skytron Medical, Grand Rapids, MI) is also used.
Docking and Instrumentation
The robotic tower is moved to the operating table and positioned between the patient's feet in preparation for docking. Abdominal ports of the robot (da Vinci ports, Intutitive Surgical, Inc.) are the 12-mm umbilical port for the camera and two 8-mm side ports lateral and at 15 degrees below the umbilical one, placed in the midaxillary line. We use a 5-mm to 10-mm accessory fourth port placed on the left side or right side between the umbilicus and the anterior superior iliac spine (ASIS). This port is used for suction irrigation and introduction or removal of sutures and needles.
The 2 robotic arms are attached to the lateral right and left trocars. Occasionally, the fourth arm is also used. Subsequently, the robotic instruments are introduced. An EndoWrist PK grasper (Intuitive Inc., Sunnyvale, CA) is loaded in the left robotic arm. An EndoWrist monopolar hook (Intuitive Inc., Sunnyvale, CA) is used in the right robotic arm. That is replaced with an EndoWrist monopolar scissors (Intuitive Inc., Sunnyvale, CA) when needed. An EndoWrist Prograsper (Intuitive Inc., Sunnyvale, CA) is placed in the fourth robotic arm whenever used. For tubal anastomosis, the monopolar hook is replaced by an EndoWrist needle holder to facilitate intracorporeal stitching (Intuitive Inc, Sunnyvale, CA). No cautery is used on the muscularis-mucosal layer. The assistant helps with suction and irrigation, tissue retraction, and introduction and retrieval of sutures for tubal anastomosis via the accessory port. The scrub nurse helps with cleaning the lens of the laparoscope and switching of the instruments.
Upon completion of the docking process, manipulation of the robotic handles is transmitted to a computer that filters, scales, and then translates the surgeon's movements to the robotic arms. The EndoWrist instruments attached to the robotic arms are modeled to simulate the human wrist with full range of motion. Movement transmission is attained via a high-strength cable system transposing fingers to instrument tips with 7 degrees of freedom. Movements are scaled as being transmitted from the surgical console to the robotic EndoWrist instrument. For example, a scaling ratio of 10 to 1 means that for every 1cm the surgeon moves the handles at the console, the robotic instrument will move 1mm only. Overall, the setup is similar to that of traditional laparoscopy, because the patient is placed in a modified dorsal lithotomy position then kept in the Trendelenburg position. The uterus is manipulated through an intrauterine canula.
Surgical Technique
Development of the Proximal and Distal Ends of the Fallopian Tube
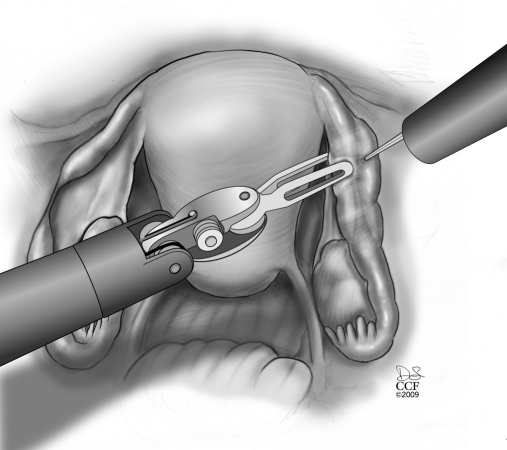
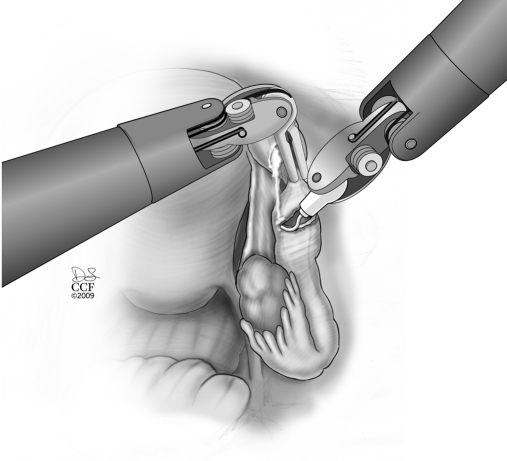
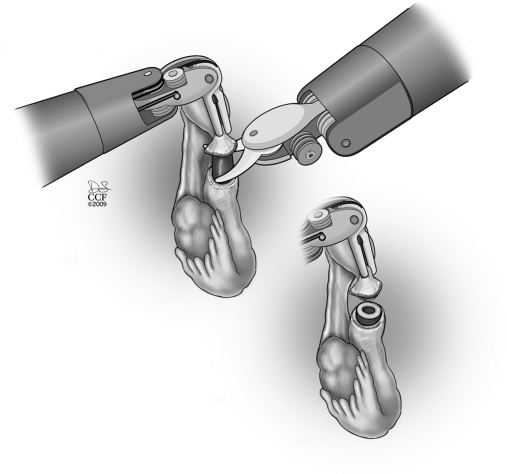
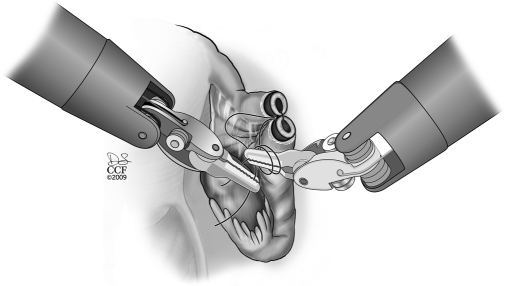
The procedure starts with the subserosal injection of vasopressin diluted 20U in 200mL of normal saline in both proximal and distal ends and in the mesosalpinx to facilitate subsequent dissection of both ends and hemostasis (Figure 1). Injection of indigo carmine dye transcervically allows the proximal tubal portion to be identified and proximal tubal obstruction excluded. A probe (Karl Storz Inc, Germany) can placed through the fimbriated end of the distal portion and indigo carmine injected to identify the distal anastomosis site. Using the monopolar cautery, the serosal covering of the proximal and distal anastomosis site is incised (Figure 2 and 3). Subsequently, microscissors are used to cut the muscularis-mucosal portion of the tube to open the proximal and distal ends of the tube (Figure 4).
Figure 1.
Subserosal injection of vasopressin diluted 20U in 200mL of normal saline in both proximal and distal ends and in the mesosalpinx to facilitate subsequent dissection of both ends and hemostasis.
Figure 2.
Excision of the serosal covering of the distal tubal cut end by using monopolar cautery.
Figure 3.
Excision of the proximal tubal end serosal covering with monopolar cautery.
Figure 4.
Microscissors used to cut the blind tubal ends creating a lumen in both proximal and distal ends.
Reconstruction of the Mesosalpinx
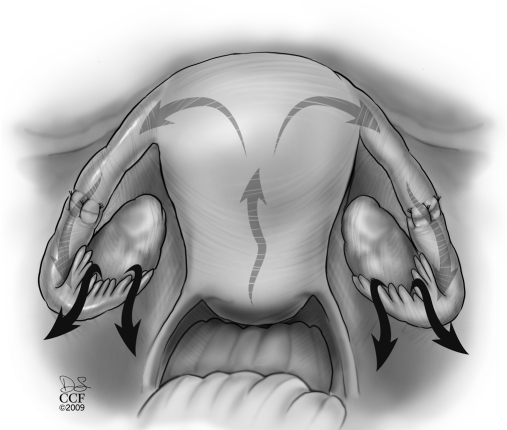
The mesosalpinx is then approximated using a series of 6-0 Vicryl stitches to bridge the gap between the 2 ends of the fallopian tube and to facilitate subsequent suturing with fine suture (Figure 5 and 6). This step brings the tubal segments close together to prevent tension on the anastomosis site. Sometimes when there is a major discrepancy in size between the proximal and distal anastomosis site, a stent is placed to facilitate the suturing of the 2 ends. Typically, we use the inner plastic component of the Novy cannulae (COOK Medical Inc., Bloomington, IN, USA) that is used to cannulate the tube hysteroscopically. A 9-cm length of this small diameter flexible tube is cut and introduced through a port. It is inserted through each anastomosis site.
Figure 5.
Suturing of the mesosalpinx with 5-0 Vicryl sutures to approximate both tubal ends for subsequent anastomosis.
Figure 6.
Three to 4 interrupted 8-0 Vicryl sutures used for suturing both tubal mucosal and muscular layers.
Tubal Reanastomosis
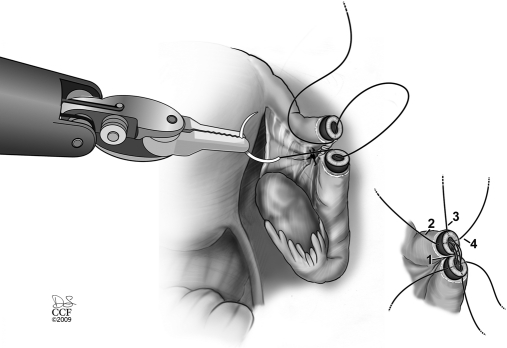
The mucosal and muscular layers of the tubal segments are sutured with 4 interrupted 8-0 Vicryl sutures. This step requires a lot of training to focus on moving both hands simultaneously while tying the knot. Excessive force can easily avulse the needle or damage the suture. Visual cues need to be used for this step.
The first suture is placed at 6 o'clock. The suture is placed in such a way that the knot lies on the outside of the lumen. The suture is tied with the intracorporeal knot tying technique (Figure 7). The second and third sutures are placed at 3 o'clock and 9 o'clock but not tied (Figure 6). The fourth stitch is placed at 12 o'clock and then the 3 and 9 o'clock stitches are tied. Attention to proper suturing will avoid misalignment or rotation of the distal tubal segment along its longitudinal axis. The serosa is then closed separately with running 7-0 Vicryl sutures. The immediate success of the procedure is evaluated by chromotubation to document tubal patency (Figure 8). In a few cases, we have used the NOVY catheter (Cook, Bloomington, IN, USA) to act as a stent, but because the magnification is so great, we do not use it most of the time.
Figure 7.
Bringing both tubal ends together so no tension applied on the anastomosis sutures.
Figure 8.
Using chromotubation for assurance of tubal patency after successful anastomosis.
Postoperative Course
The Foley catheter is removed at the end of the procedure. Oral intake, including oral medications, is started on the same day of the operation, and patients are allowed to ambulate as soon as possible. A final postoperative visit is performed 6 weeks after the surgery.
RESULTS
All cases have been completed robotically without conversion to laparotomy or complications. Tubal anastomoses have been performed with 3 or 4 robotic arms, 3 or 4 robotic instruments, and 1 assistant trocar. Instrument articulation facilitates the procedure. The short- and the long-term outcomes in a series of patients undergoing the above-described technique of tubal anastomosis has been published by our group elsewhere.4–7 The mean operating time was 279.68 minutes. Patients were uniformly sent home the same day, typically within 4 hours. The mean blood loss was 54.7mL, and all patients went home on the same day of surgery.
DISCUSSION
Sterilization reversal has been performed via minilaparotomy.8 With the advent of minimally invasive surgery, laparoscopic tubal anastomosis has been successfully attempted.9,10 More recently, surgical robots have been implemented to facilitate laparoscopic tubal anastomosis.11 Evidence has been accumulating about the unique properties of the surgical robot, including 3D visualization, tremor filtration, motion downscaling, and the EndoWrist instrument. These properties indeed have facilitated the performance of complex surgeries, such as tubal anastomosis in a minimally invasive fashion.5,12,13 In particular, we noted advantages for the dissection of the cut ends of the fallopian tube and precise placement of the anastomotic stitches. In our experience, the robotic technique for tubal anastomosis required significantly prolonged surgical and anesthesia times over outpatient minilaparotomy.7 However, there was no significant difference in pregnancy outcomes between the robotic and the laparotomy technique. Costs were higher with the robotic technique. On the other hand, return to normal activity was shorter with the robotic technique, and this may diminish overall costs.7 The robot offered a more comfortable position for the surgeon sitting at the console and more relaxing ergonomics with the surgeon's arms resting on the bars, reducing hand fatigue.14
CONCLUSION
Previous robotic experience with less complex pelvic procedures is necessary to achieve adequate surgical skill sets necessary for the performance of robotic tubal anastomosis. Robotic technology is an alternative approach for the performance of tubal anastomosis in spite of the extensive experience with minilaparotomy and traditional laparoscopic tubal anastomosis.
Contributor Information
Mohamed A. Bedaiwy, Department of Obstetrics and Gynecology, University Hospitals Case Medical Center, Case Western Reserve University, Cleveland, Ohio, USA..
Ehab M. Barakat, Obstetrics-Gynecology and Women's Health Institute, Cleveland Clinic, Cleveland, Ohio, USA..
Tommaso Falcone, Obstetrics-Gynecology and Women's Health Institute, Cleveland Clinic, Cleveland, Ohio, USA..
References:
- 1. Piccinino LJ, Mosher WD. Trends in contraceptive use in the United States: 1982-1995. Fam Plann Perspect. 1998;30:4–10, 46 [PubMed] [Google Scholar]
- 2. Hardy E, Bahamondes L, Osis MJ, et al. Risk factors for tubal sterilization regret, detectable before surgery. Contraception. 1996;54:159–162 [DOI] [PubMed] [Google Scholar]
- 3. Kho RM, Hilger WS, Hentz JG, et al. Robotic hysterectomy: technique and initial outcomes. Am J Obstet Gynecol. 2007;197:113 e111–114 [DOI] [PubMed] [Google Scholar]
- 4. Falcone T, Goldberg J, Garcia-Ruiz A, et al. Full robotic assistance for laparoscopic tubal anastomosis: a case report. J Laparoendosc Adv Surg Tech A. 1999;9:107–113 [DOI] [PubMed] [Google Scholar]
- 5. Falcone T, Goldberg JM, Margossian H, et al. Robotic-assisted laparoscopic microsurgical tubal anastomosis: a human pilot study. Fertil Steril. 2000;73:1040–1042 [DOI] [PubMed] [Google Scholar]
- 6. Goldberg JM, Falcone T. Laparoscopic microsurgical tubal anastomosis with and without robotic assistance. Hum Reprod. 2003;18:145–147 [DOI] [PubMed] [Google Scholar]
- 7. Rodgers AK, Goldberg JM, Hammel JP, et al. Tubal anastomosis by robotic compared with outpatient minilaparotomy. Obstet Gynecol. 2007;109:1375–1380 [DOI] [PubMed] [Google Scholar]
- 8. Slowey MJ, Coddington CC. Microsurgical tubal anastomoses performed as an outpatient procedure by minilaparotomy are less expensive and as safe as those performed as an inpatient procedure. Fertil Steril. 1998;69:492–495 [DOI] [PubMed] [Google Scholar]
- 9. Barjot PJ, Marie G, Von Theobald P. Laparoscopic tubal anastomosis and reversal of sterilization. Hum Reprod. 1999;14:1222–1225 [DOI] [PubMed] [Google Scholar]
- 10. Yoon TK, Sung HR, Cha SH, et al. Fertility outcome after laparoscopic microsurgical tubal anastomosis. Fertil Steril. 1997;67:18–22 [DOI] [PubMed] [Google Scholar]
- 11. Degueldre M, Vandromme J, Huong PT, et al. Robotically assisted laparoscopic microsurgical tubal reanastomosis: a feasibility study. Fertil Steril. 2000;74:1020–1023 [DOI] [PubMed] [Google Scholar]
- 12. Dharia Patel SP, Steinkampf MP, Whitten SJ, et al. Robotic tubal anastomosis: surgical technique and cost effectiveness. Fertil Steril. 2008;90:1175–1179 [DOI] [PubMed] [Google Scholar]
- 13. Falcone T, Goldberg JM. Robotics in gynecology. Surg Clin North Am. 2003;83:1483–1489, xii [DOI] [PubMed] [Google Scholar]
- 14. Magrina JF, Kho RM, Weaver AL, et al. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008;109:86–91 [DOI] [PubMed] [Google Scholar]