Abstract
Cardiomyocytes use glucose as well as fatty acids for ATP production. These substrates are transported into the cell by glucose transporter 4 (GLUT4) and the fatty acid transporter CD36. Besides being located at the sarcolemma, GLUT4 and CD36 are stored in intracellular compartments. Raised plasma insulin concentrations and increased cardiac work will stimulate GLUT4 as well as CD36 to translocate to the sarcolemma. As so far studied, signaling pathways that regulate GLUT4 translocation similarly affect CD36 translocation. During the development of insulin resistance and type 2 diabetes, CD36 becomes permanently localized at the sarcolemma, whereas GLUT4 internalizes. This juxtaposed positioning of GLUT4 and CD36 is important for aberrant substrate uptake in the diabetic heart: chronically increased fatty acid uptake at the expense of glucose. To explain the differences in subcellular localization of GLUT4 and CD36 in type 2 diabetes, recent research has focused on the role of proteins involved in trafficking of cargo between subcellular compartments. Several of these proteins appear to be similarly involved in both GLUT4 and CD36 translocation. Others, however, have different roles in either GLUT4 or CD36 translocation. These trafficking components, which are differently involved in GLUT4 or CD36 translocation, may be considered novel targets for the development of therapies to restore the imbalanced substrate utilization that occurs in obesity, insulin resistance and diabetic cardiomyopathy.
Keywords: Cardiac metabolism, Intracellular traffic, GLUT4, CD36, Insulin resistance, Diabetes
Introduction
We previously reported that known signaling mechanisms, such as insulin and contraction, similarly affect myocyte GLUT4 and CD36 trafficking [1]. However, in insulin resistance and type 2 diabetes CD36 permanently relocates to the sarcolemma, while GLUT4 internalizes [2, 3]. As a corollary there must be mechanisms which selectively recruit either GLUT4 or CD36. Therefore, we began to investigate distinct intracellular processes involved in vesicular transport to uncover mechanisms that are differently involved in GLUT4 and CD36 trafficking [4, 5]. We revealed coat-proteins, actin filaments, the cellular pH gradient and vesicle-associated membrane proteins (VAMPs) to be involved in GLUT4 and CD36 trafficking, and, importantly, indeed found mechanisms that are differently involved in glucose and fatty acid uptake in cardiomyocytes.
In this review, we first describe how cardiac glucose and fatty acid uptake are regulated and which alterations occur during insulin resistance and type 2 diabetes. Then, we address signaling pathways and subcellular trafficking components that are involved in GLUT4 and CD36 translocation. Finally, we focus on components that are differently involved in glucose and fatty acid uptake and describe novel targets to restore metabolic disturbances in type 2 diabetes.
Regulation of cardiac glucose and fatty acid uptake
Glucose and fatty acids are of fundamental importance for energy production in all eukaryotic cells. In cardiomyocytes, the continuous supply of both substrates is especially crucial to maintain contractile activity [6]. Cardiomyocytes are metabolically flexible, i.e., they preferably use fatty acids, but can also use glucose, ketone and lactate to produce ATP [7]. These different substrates cannot sufficiently enter cardiomyocytes by diffusion and thus have to be taken up by facilitated transport. Glucose uptake into cells involves a family of glucose transport proteins—called GLUTs—which shuttle sugars across plasmalemmal membranes through their aqueous pore [8]. The GLUT family consists of several members which are expressed by various cell types [9]. In cardiomyocytes, GLUT family members 1 and 4 fulfil this function. While GLUT1 is mainly involved in basal glucose uptake, GLUT4 translocates to the plasma membrane to enhance glucose uptake in response to extracellular stimuli like insulin or increased cardiac work. Cellular fatty acid uptake is facilitated by several membrane proteins with high-affinity binding sites for fatty acids [1], but the exact mechanisms by which these proteins mediate transmembrane passage of fatty acids is not known [10]. In cardiomyocytes, the most important fatty acid transporters are fatty acid translocase (FAT), also referred to as CD36 [10, 11], and two members of the family of 6 fatty acid transport proteins (FATP), i.e., FATP 1 [12] and 6 [13].
Both GLUT4 and CD36 are integral membrane proteins. GLUT4 consists of 12 transmembrane domains with both termini in the cytoplasm and one large intracellular and one large extracellular loop [14]. CD36 has a hairpin-like structure with two transmembrane regions and both the C-terminal and N-terminal tails in the cytoplasm [1]. The translocation of both proteins from intracellular storage compartments to the plasma membrane, and vice versa, relies on a complex trafficking system, schematically represented in Fig. 1 [15]. Immunoadsorption studies in rat cardiomyocytes showed that GLUT4 resides in two distinct pools, one of which is called GLUT4 storage vesicles (GSV) that does not contain CD36 and is sensitive to insulin [16]. On the other hand, CD36 appears to reside in one subcellular pool which does not contain GLUT4 [16], suggesting that GLUT4 and CD36 are stored separately and travel independently.
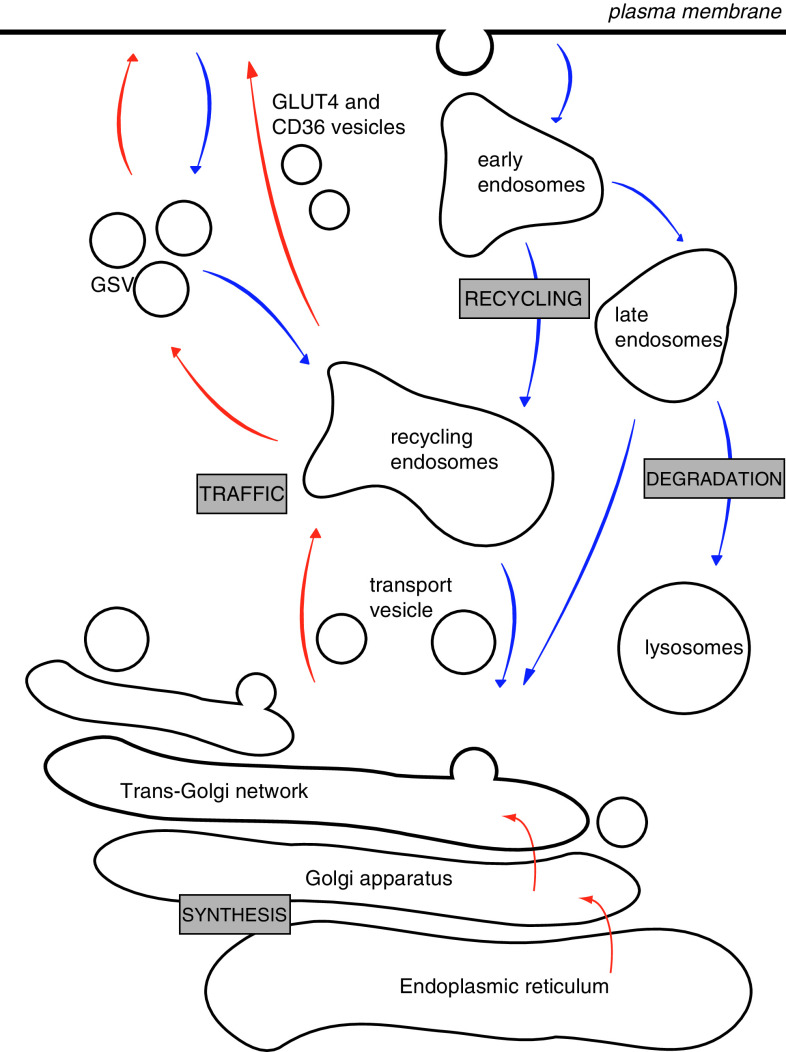
Fig. 1.
The endomembrane system of cardiomyocytes consists of several functionally different compartments. In the translocation process (red arrows) proteins are synthesized in the endoplasmic reticulum and further modified in the Golgi apparatus. There, the budding of transport vesicles occurs and vesicles are transported to the endosomal system. GLUT4 is further transported to specific insulin-responsive GLUT4 storage vesicles (GSV). GLUT4 and CD36 can be stimulated to translocate from their compartments to the sarcolemma. In the endocytosis process (blue arrows), sarcolemmal proteins are taken up into the cell again and transported through the early endosomes and directed towards lysosomes for protein breakdown or towards the recycling endosomes for renewed use at the sarcolemma
Physiological stimuli, with circulating plasma insulin concentrations and increased cardiac work being the most important, stimulate the heart to quickly alter cardiac substrate utilization via reversible translocation of GLUT4 and CD36 from intracellular membrane compartments to the sarcolemma (Fig. 2a) [1, 10]. In contrast to CD36, the other fatty acid transporters FATP1 and FATP6 do not traffic between intracellular storage compartments and the sarcolemma in cardiomyocytes [17–19]. Therefore, FATP1 and FATP6 do not contribute to inducible fatty acid uptake. In addition, other studies have disclosed that these transporters also have a minor contribution to basal fatty acid uptake. From experiments with cardiomyocytes from wild-type and CD36 null mice, it is known that the contribution of CD36 to fatty acid uptake is about 70% [17]. In addition, in cardiomyocytes treated with the specific CD36 inhibitor sulfo-N-succinimydyl-oleate (SSO), the insulin/contraction-mediated increase in fatty acid uptake was totally blocked, meaning that CD36 is irreplaceable in stimulus-induced fatty acid uptake [20]. The system of regulated substrate uptake is crucial during exercise to supply cardiomyocytes with a sufficient amount of substrates, or to quickly replenish intracellular substrate storage pools after a meal. A dysfunction in this system of regulated glucose and fatty acid uptake into muscle, adipose tissue, beta cells and heart contributes to the development and progression of type 2 diabetes [10, 21].
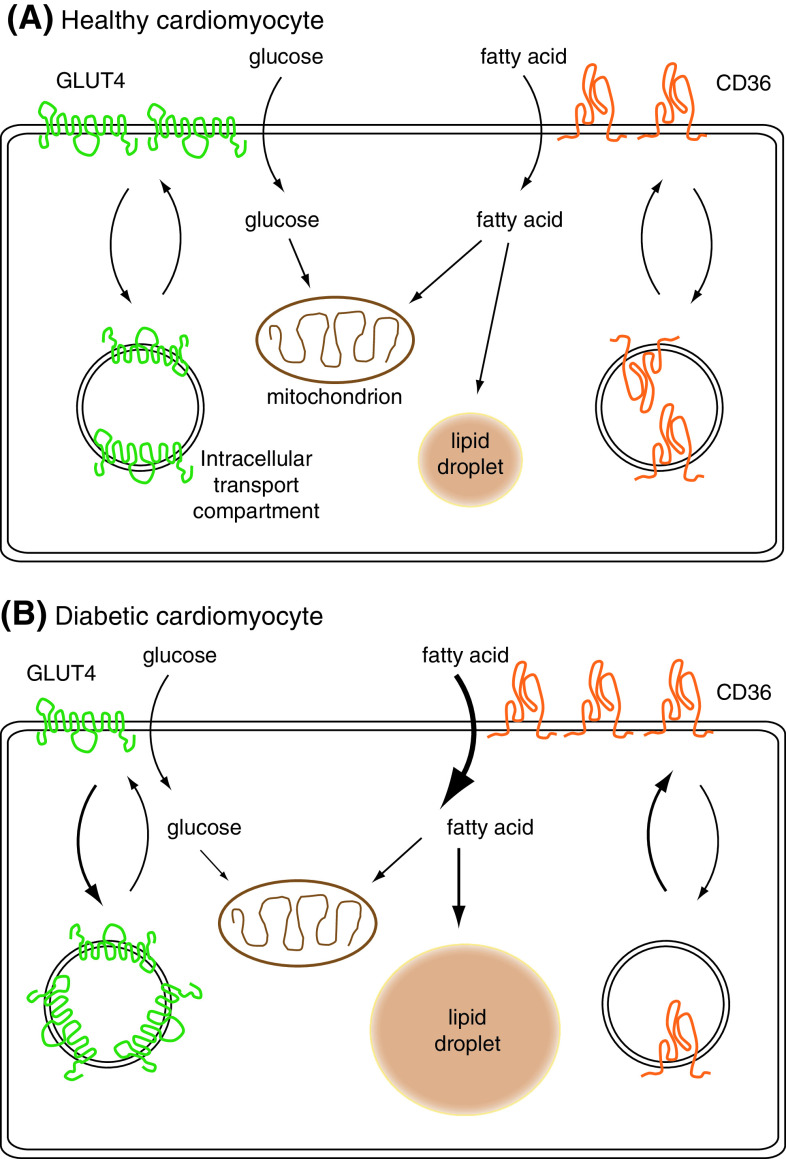
Fig. 2.
Alterations in transporter localisation in diabetic cardiomyocytes compared to healthy cardiomyocytes. a Substrate uptake in healthy cardiomyocytes. The entry of glucose and fatty acids is facilitated by glucose transporter 4 (GLUT4) and fatty acid transporter CD36. The heart can acutely react to a change in energy demand by reversible translocation of these transporters from intracellular storage compartments. Once these substrates have entered the cell they can be used for ATP production by mitochondrial oxidation or they can be stored as glycogen (not shown) or in lipid droplets. b Substrate uptake in diabetic cardiomyocytes: during the development of insulin resistance and type 2 diabetes, the localisation of GLUT4 and CD36 permanently alters. CD36 presence at the sarcolemma increases which associates with increased fatty acid uptake. In addition, fatty acid storage increases which is thought to interfere with insulin-stimulated glucose uptake
The amount of glucose and fatty acid transporters present at the sarcolemma is a major regulatory factor of cardiac glucose and fatty acid utilization [22]. This implicates that the metabolic machinery is more than adequately suited to process all incoming substrates. Accordingly, intracellular concentrations of fatty acids and glucose will remain low. This is also true for the diabetic heart in which intracellular concentrations of these substrates are not markedly increased [1, 23]. Hence, in both the healthy and the diabetic heart, the sarcolemmal presence of GLUT4 and CD36 determines cardiac substrate flux [24].
Alterations in transporter location in insulin resistance
Obesity, insulin resistance and type 2 diabetes show a strong association with changes in lipid metabolism [2, 25]. Permanent relocation of CD36 to the sarcolemma and increased fatty acid uptake were strongly linked in rodent models for insulin resistance [2, 26, 27] and obese humans [26, 28, 29]. Furthermore, the sarcolemmal content of CD36 correlated well with increased uptake of fatty acids, increased intramuscular triacylglycerol [28, 30, 31] and reduced insulin-stimulated GLUT4 translocation and glucose uptake [32]. Cardiac in vivo positron-emission tomography (PET) in humans with type 2 diabetes showed increased fatty acid uptake and oxidation, and reduced insulin-stimulated glucose uptake, paralleled by decreased diastolic function as compared to age-matched healthy controls [33].
The increase in intramyocardial lipid concentrations cannot just be attributed to a reduction in mitochondrial fatty acid oxidation, since studies have shown that cardiac fatty acid oxidation remained unchanged, slightly reduced, or even increased in several rodent models of obesity and insulin resistance [10]. Inhibition of CD36-mediated fatty acid uptake with SSO normalized fatty acid utilization in different rodent models of insulin resistance [26]. This indicates that the increase in intramyocardial lipid concentrations in insulin-resistant cardiomyocytes is caused by increased CD36-mediated fatty acid uptake. We and others showed that the surface presence of CD36 was increased in rodent models for insulin resistance and in skeletal muscle of obese humans [2, 3, 32]. The increase in surface presence of CD36 is not due to increased tissue expression, but instead to a permanent relocation from its intracellular storage compartment. This permanent CD36 relocation appears to be an early event in the sequence of maladaptive changes in the hearts of rodents with type 2 diabetes. We also obtained evidence that there is no decrease in cardiac mitochondrial function in this early pre-diabetic stage. However, it is very well possible that mitochondrial dysfunction develops at later stages which can lead to even more lipid storage. Moreover, it has been shown that relocation of CD36 was specific for this fatty acid transporter because such changes were not observed for plasmalemmal FABPpm, FATP1 or FATP4 [10]. Thus, permanent relocation of CD36 to the sarcolemma is important in the development toward diabetic cardiomyopathy.
It is becoming evident that the increased intramyocardial triacylglycerol content is not the main contributor to the development of insulin resistance and cardiac dysfunction. Other lipid metabolites, such as ceramides and diacylglycerols, appear to be the main contributors to reduced insulin sensitivity (see [34, 35] for excellent reviews on this topic).
Collectively, obesity, insulin resistance and type 2 diabetes impose metabolic stress on the heart [36–39], which may ultimately lead to cardiac metabolic inflexibility, lipotoxicity, and subsequent development of diabetic cardiomyopathy [3, 32] (Fig. 2b).
Signaling pathways involved in GLUT4 and CD36 translocation
As mentioned above, postprandial increases in circulating plasma insulin levels and an elevated cardiac work are the most important physiological stimuli to enhance cardiac glucose and fatty acid uptake via induction of GLUT4 and CD36 translocation, respectively [1]. However, the metabolic fates of glucose and fatty acids are quite different during insulin-stimulation versus contraction-stimulation. Both substrates are preferentially stored under insulin-stimulated conditions, and preferentially oxidized upon increased contraction [1]. Since translocation of GLUT4 and CD36 are similarly induced by insulin and contraction, these translocation processes do not contribute to the different metabolic fates of both substrates during insulin- versus contraction-stimulation. Rather, upon intracellular trapping of both substrates [glucose via hexokinase and fatty acids via acetyl-CoA synthase (ACS)], the metabolic fates of glucose and fatty acids are determined by the activation state of key metabolic enzymes. Specifically, glucose and fatty acids are directed towards storage via insulin-induced activation of glycogen synthase (GS) and glycerol-3-phosphate acyltransferase (GPAT), respectively [1]. In addition, the contraction-induced drop in the intracellular ATP concentrations triggers phosphofructokinase, pyruvate dehydrogenase and TCA cycle progression for acceleration of glucose oxidation, and also carnitine-palmitoyl transferase-1 for acceleration of fatty acid oxidation [1]. Despite differential effects of insulin and contraction on the metabolic machinery, both stimuli similarly induce translocation of GLUT4 and CD36 to the sarcolemma [1]. This similarity in responsiveness of both transporters to both physiological stimuli suggests that similar signaling mechanisms are involved in GLUT4 and CD36 translocation. In addition, it suggests that GLUT4 and CD36 migrate together to the sarcolemma in response to each of the stimuli. However, increasing numbers of studies report differences in the regulation of both substrate transporters, leading to a more differentiated understanding of GLUT4 and CD36 traffic.
Exactly how insulin and increased workload achieve increased abundance of GLUT4 and CD36 at the sarcolemma is unclear, but there are two primary candidates: increasing exocytosis or inhibiting endocytosis [40–43]. Insulin is proposed to stimulate fusion of intracellular GLUT4 containing-vesicles with the plasma membrane in cardiac and skeletal myocytes while it does not affect the rate of endocytosis [42, 44, 45]. In myocytes, fusion of intracellular membranes with the plasmalemmal membrane is considered to be inducible, while endocytosis of GLUT4 and CD36 is regarded a housekeeping process which cannot be regulated. However, it has been reported that decreased endocytosis in response to insulin regulates GLUT4 translocation in adipocytes [46]. Some studies found that, in line with the effect of insulin on GLUT4 translocation, contraction increases GLUT4 translocation [44, 45]. In contrast, another study found that GLUT4 endocytosis is inhibited [42]. In summary, the exact mechanism by which insulin and contraction increase presence of CD36 and GLUT4 at the plasma membrane requires further studies aiming at the rate of endocytosis and/or the rate of translocation.
Insulin-signaling
From all stimulus-induced transporter translocation processes, insulin-induced GLUT4 translocation is the most intensively studied [47]. Several of the kinases involved in insulin-induced GLUT4 translocation have been tested on their additional involvement in insulin-induced CD36 translocation. So far, these kinases seem to play a similar role in both translocation events, as detailed below. The insulin-signaling axis is initiated by the binding of insulin to its receptor and subsequent activation of insulin receptor substrate (IRS) 1 [48] and IRS2 [49] (Fig. 3a). These activate the regulatory subunit of phosphatidylinositol-3-kinase (PI3K), which consists of a catalytic p110 subunit and a regulatory p85 subunit [50]. Pharmacological inhibitors (most notably wortmannin) have greatly facilitated the investigation of the role of PI3K in GLUT4 and CD36 translocation, and have pinpointed this lipid kinase as a key component [51–53]. The main phosphatidylinositol (PI)-phosphate generated by PI3K during insulin action is PI-3,4,5-trisphosphate (PIP3) [54]. Generation of PIP3 at the plasma membrane directly drives the activation of a number of different protein kinases with lipid binding pleckstrin homology domains [55]. Three of these kinases play an essential role in insulin-induced glucose uptake: (1) Akt/protein kinase B (PKB)-isoform 2, (2) protein kinase C (PKC)-λ/ζ and (3) 3-phosphoinositide-dependent protein kinase (PDK) [56–59]. Akt/PKB-2 and PKC-λ/ζ have additionally been implicated in insulin-induced CD36 translocation [24, 59].
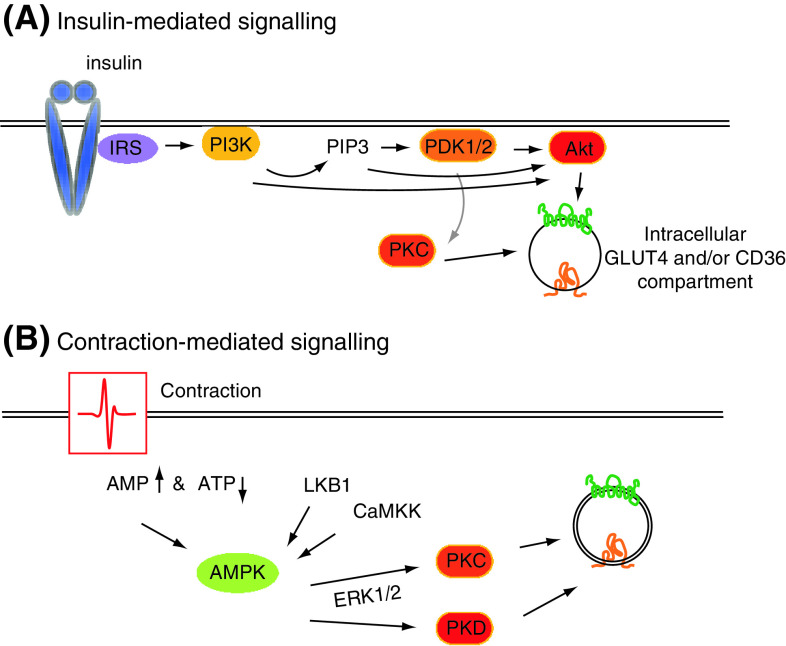
Fig. 3.
signaling proteins involved in insulin-stimulated (a) and contraction-stimulated (b) induction of GLUT4 and CD36 trafficking. a Upon binding of insulin to its receptor the downstream signaling axis (Akt axis) will be activated which induces the translocation of intracellular GLUT4 and CD36 vesicles. This signaling axis consists of insulin receptor substrate (IRS), phosphatidylinositol-3-kinase (PI3K), PI-3-phosphate (PIP3), 3-phosphoinositide-dependent protein kinase (PDK), protein kinase C (PKC) and Akt. b Cardiac work increases the AMP/ATP-ratio and, subsequently, AMP-activated protein kinase (AMPK) activity. For full activation the action of upstream kinases, LKB1 or CaMKK, are warranted. Downstream, and alongside, of AMPK, ERK1/2, PKC and PKD are other players in GLUT4 and CD36 translocation. For figure simplicity, GLUT4 and CD36 are drawn in one transport compartment while we know that they are present in several different compartments
Activation of Akt requires dual phosphorylation at Ser473 and Thr308 in addition to PI3K-mediated recruitment to the plasma membrane [1]. The Thr308 phosphorylation within the activation loop of Akt is mediated by PDK1 [60] and the Ser473 phosphorylation by a putative PDK2 [61]. Upon its activation, Akt phosphorylates TBC1D1 and TBC1D4, also referred to as AS160, as a final step to induce GLUT4 translocation [62, 63]. Next to Akt, PDK1 also activates PKC-ζ upon unfolding of its pseudosubstrate domain and exposure of the activation loop [64]. The simultaneous and combined activation of Akt and PKC-λ/ζ is necessary for insulin-induced GLUT4 translocation in both heart [65] and skeletal muscle [66], and likely also essential for insulin-induced CD36 translocation.
To date, GLUT4 and CD36 translocation seem identically regulated by insulin. However, it is possible to translocate GLUT4 from insulin-responsive intracellular compartments in cardiomyocytes without changes in subcellular CD36 localization using the thiol-modifying agent arsenite [67]. Hence, there are arsenite-sensitive proteins that contribute to insulin-induced GLUT4 translocation without affecting CD36 dynamics.
Contraction-signaling
Increased cardiac work results in a rapid rise of both glucose and fatty acid uptake in heart and muscle [1, 68]. Upon increased contraction, the intracellular concentration of adenosine-monophosphate (AMP), cyclic AMP, reactive oxygen species (ROS) and calcium increase. Together, these second messengers activate a complex signaling system that involves AMP-activated protein kinase (AMPK), protein kinase A (PKA), atypical PKCs, protein kinase D (PKD), calcium–calmodulin-dependent protein kinases (CaMK), and the extracellularly regulated protein kinases (ERK) 1 and 2 [68] (Fig. 3b). Of all of these, AMPK, PKD and CaMK have been studied for their effects on metabolic processes.
AMPK is a heterotrimer consisting of a catalytic α-domain, a glycogen-binding regulatory β-domain and an AMP-binding regulatory γ-domain. Binding of AMP to the regulatory γ-subunit of AMPK leads to a conformational change of the kinase, which makes it accessible for upstream AMPK kinases (AMPKK). These phosphorylate AMPK at threonine-172 and consequently activate this kinase. In the heart, activation of the α2 isoform of AMPK is essential for contraction-induced GLUT4 and CD36 translocation [69]. Kinases with suggested AMPKK activity include CaMK-kinase (CaMKK) and the tumor suppressor protein LKB1 [70]. In LKB1-null mice contraction-induced GLUT4 and CD36 translocation are completely abrogated, indicating that LKB1 is essential for both translocation processes [69]. Additionally, LKB1 is involved in hypoxia-induced AMPK-mediated cardiac GLUT4 translocation [71].
CaMK-kinase (CaMKK) is described as an alternative upstream kinase of AMPK [72]. However, evidence for the physiological importance of CaMKK in cardiomyocyte metabolism is scarce. One study reported basal activity and phosphorylation of AMPK in LKB1-deficient cells that could be further stimulated by calcium ionophores [72]. Another study, in which CaMKKβ was overexpressed or pharmacologically inhibited, showed that AMPK can also be activated by CaMKK [73]. In addition, it was shown that CaMKK is important for contraction-mediated GLUT4 and CD36 translocation in skeletal muscle [74]. However, the importance of CaMKK in cardiac GLUT4 and CD36 translocation remains to be established.
Downstream events of AMPK involve atypical PKCs, ERK1/2, TBC1D1 and TBC1D4. It was shown that exercise increases glucose transport in skeletal muscle via AMPK through atypical PKCs. Furthermore, these effects of atypical PKCs were dependent on the ERK1/2 pathway, activation of proline-rich tyrosine kinase-2 (PYK2), and phospholipase D (PLD) [75]. In a muscle-specific knockout mouse model for the atypical PKC PKCλ, glucose transport was impaired and mice developed the metabolic syndrome [76]. In rat skeletal muscle, ERK1/2 inhibitors decreased contraction-induced fatty acid uptake and CD36 translocation [77]. Hence, atypical PKCs and ERKs are involved in contraction-stimulated substrate uptake in skeletal muscle. However, their function in substrate uptake of the heart still needs to be disclosed.
We recently identified involvement of the contraction-activated protein PKD to be involved in contraction-induced GLUT4 translocation and glucose uptake [78]. Pharmacological inhibition of PKD1 in cardiomyocytes inhibited contraction-stimulated GLUT4 translocation and glucose uptake [78]. Furthermore, contraction-stimulated PKD1 activation was still able to induce GLUT4 translocation and glucose uptake in AMPKα2 knockout mice suggesting that PKD1 acts independently of AMPK [78]. These findings suggest that contraction-mediated translocation of GLUT4 is a dual input mechanism which needs both the input of AMPK and PKD. Future experiments should reveal the role of PKD in contraction-induced CD36 translocation and fatty acid uptake. Additionally, upstream and downstream proteins of PKD1 have not yet been elucidated.
Surprisingly, the two Akt substrates TBC1D1 and TBC1D4 are also substrates of activated AMPK and therefore also part of the contraction signaling cascade [66, 79]. Although these data suggest a complementary role of both TBC1D isoforms, the high expression of TBC1D1 in muscle compared to fat tissue indicates a pivotal role of this protein in contractile tissue [79]. Still, further research is needed to unravel (1) whether the two isoforms are physiologically relevant, and (2) whether they fulfil similar functions in CD36 translocation.
In summary, GLUT4 and CD36 translocation seem identically regulated by increased workload, but the roles of CaMKK and PKD in contraction-induced CD36 translocation still await exploration.
Trafficking machinery involved in GLUT4 and CD36 translocation
The trafficking machinery involved in GLUT4 translocation [45, 80–85], especially in adipocytes and muscle cells, has gained much more attention than the trafficking machinery involved in CD36 translocation [4, 5]. The isolation of GLUT4 vesicles revealed approximately 50 proteins that regulate vesicle fission, -transport and -fusion, as well as the specificity of GLUT4 transport [86]. CD36-containing vesicles have been isolated [16], but not so far extensively studied with a proteomics approach.
Cellular protein traffic generally involves three major steps. Firstly, vesicle fission: at the donor compartment, the membranes will be curved into a bud that will subsequently excise. This process is dependent on bilayer destabilizing proteins, coat proteins, Rab GTPases and a number of adaptor proteins forming a fission complex. Secondly, subcellular vesicle transport: these newly formed transport vesicles move along one of the cytoskeletal networks with the aid of motor proteins which are regulated by Rab GTPases. And thirdly, vesicle fusion: at the acceptor compartment, the vesicle membranes fuse with the acceptor lipid bilayer requiring the formation of a SNARE complex and is modulated by Rab GTPases. Both translocation and endocytotic routes between endosomes and the sarcolemma proceed accordingly (Figs. 1, 4).
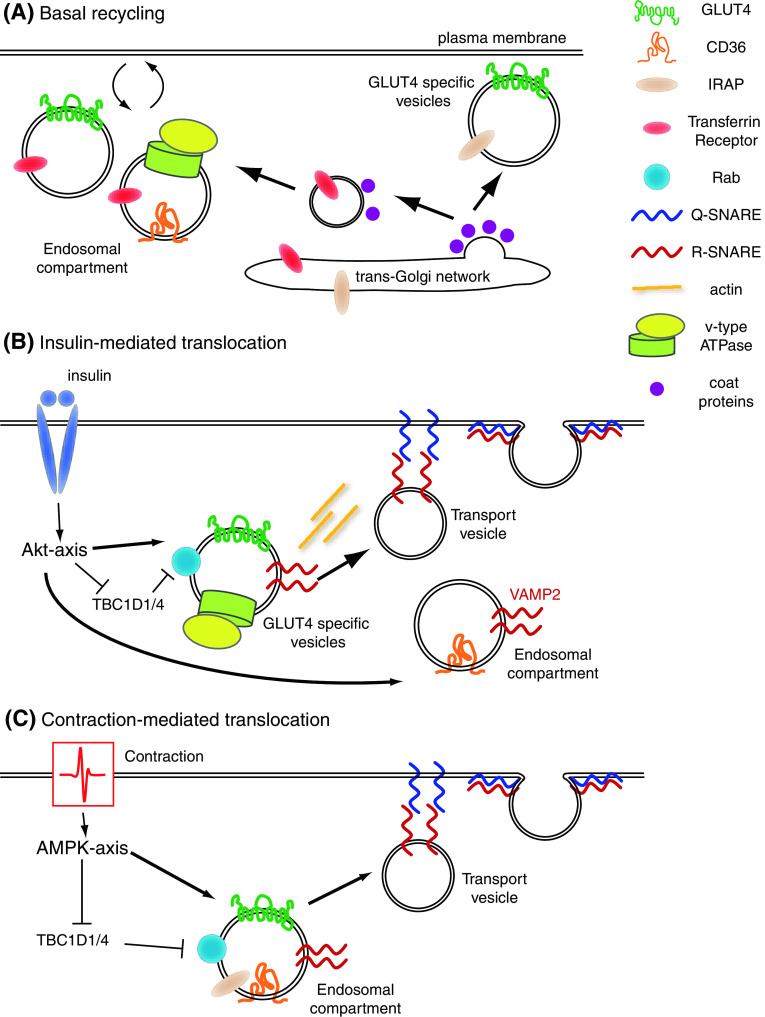
Fig. 4.
Signaling-pathways and subcellular trafficking components involved in GLUT4 and CD36 translocation in cardiomyocytes. a Basal recycling: during basal recycling of endosomal vesicles GLUT4 is directed towards an insulin-responsive GLUT4 specific vesicle compartment which also contains IRAP. Coat proteins and the transferrin receptor are found in GLUT4 and CD36 vesicles in the endosomal compartment. V-ATPase is only involved in basal recycling of CD36 containing vesicles. b insulin-mediated translocation (Akt-axis): insulin releases the vesicles from the inhibitory action of TBC1D1/4 and acts on VAMP2 containing GLUT4 and CD36 vesicles. V-ATPase is only involved in insulin-mediated GLUT4 translocation and c contraction-mediated translocation (AMPK-axis): contraction also affects functioning of TBC1D1/4. Furthermore, VAMP3 is involved in contraction-mediated translocation of GLUT4 and CD36
Although it is generally believed that GLUT4 and CD36 translocation are vesicle-mediated endosomal processes, GLUT4 localization overlaps only for 30–40% with markers of this endocytosis system, e.g., the transferrin receptor and Rabs. In addition, chemical ablation of endosomes does not fully block insulin-stimulated GLUT4 translocation in adipocytes [87].
Recently, posttranslational modification of GLUT4 by ubiquitination was found to be important for its intracellular sorting [88]. CD36 can also be ubiquitinated, but in this case ubiquitination affects protein expression rather than localization [89].
Recent data, which will be discussed below, have revealed the role of these trafficking components in GLUT4 and CD36 translocation.
Coat proteins
Coat proteins are essential players in vesicle fission and fusion. To inititate vesicle fission, these proteins are recruited to membrane spots where cargo is concentrated by adaptor proteins. There, coat proteins form a ‘bulb’ in the membrane and thus start budding of a vesicle. Once the vesicle is formed and detached from the organelle, the coat proteins are released. The following protein families are known to function as coat proteins: coat protein complex (COP), clathrin and caveolin, which reside in specific subcellular compartments [15, 90].
COPI and II are involved in transport from the ER to the Golgi apparatus, where sorting receptors couple specific cargo to COPI and COPII transport vesicles [15, 91]. Much of the knowledge on COPI vesicle formation is derived from pharmacological studies using brefeldin-A as a non-competitive inhibitor of Arf1 [92, 93], although it has also been reported that brefeldin-A affects clathrin functioning [94]. Treatment of cells with brefeldin-A results in rapid fusion of the Golgi apparatus with the ER, which suggests an important role of these coat proteins in the maintenance of the distinct organelles [92]. However, the use of brefeldin-A in rat adipocytes could not clearly prove an involvement of COPI in insulin-stimulated GLUT4 translocation to the plasma membrane [95–98]. Studies performed in cardiomyocytes show that COP proteins and/or clathrin function in stimulus-induced glucose and fatty acid uptake in cardiomyocytes [4]. Hence, GLUT4 and CD36 translocation in cardiomyocytes are both closely related vesicle-mediated processes (Figs. 1, 4).
Caveolins—caveolin-1, -2 and -3—reside in cholesterol-enriched lipid rafts of the plasma membrane called caveolae. Caveolins could play a role in plasmalemmal docking of GLUT4- or CD36-containing vesicles, and could be involved in GLUT4 or CD36 internalization [99, 100]. Caveolin-1 [101, 102] has been proposed to play a role in initiation of GLUT4 endocytosis in adipocytes, but in skeletal muscle, the muscle-specific isoform caveolin-3 does not colocalize with GLUT4 [103]. Hence, the role of caveolins in GLUT4 trafficking is unclear. Caveolin-1 has been shown to be involved in CD36 localization and function in smooth muscle cells [104] and fibroblast [105]. However, caveolin-3 does not seem to play a role in CD36 translocation in muscle cells because regulation of cardiac LCFA uptake was not altered in caveolin-3 knockout mice. In conclusion, there is evidence that caveolin-1 is involved in GLUT4 and CD36 translocation in non-muscle cells, but caveolin-3 most likely does not play a role in translocation of both transporters in (cardiac) myocytes.
Cytoskeletal filaments
It is well established that reorganization of filamentous actin beneath the plasma membrane plays a role in insulin-induced GLUT4 translocation [83, 106–108]. Insulin signaling bifurcates at the level of PI3K towards Akt and Rac. Insulin activates GTP loading of Rac within 5 min and then induces reorganization of actin. It has been shown that agents that disturb actin polymerization, e.g., latrunculin B or cytochalasin D, inhibit insulin-induced GLUT4 translocation in adipocytes, skeletal muscle cells and cardiomyocytes [4, 82, 83, 109]. Findings obtained by these pharmacological approaches were confirmed by overexpression of a dominant negative Rac mutant [110] or siRNA-mediated knockdown of Rac [111]. Recently, it was shown that Arp2/3 is a downstream effector of Rac and that cofilin regulates actin depolymerization which again proposes that active actin cycling is essential for insulin-stimulated GLUT4 translocation [83]. In contrast, latrunculin B did not inhibit stimulus-induced CD36 translocation in cardiomyocytes [4]. Hence, actin filaments are involved in GLUT4 translocation, but not in CD36 translocation in the heart.
Microtubule involvement in GLUT4 translocation has mainly been studied in adipocytes. Controversy exists about their role in stimulus-induced GLUT4 translocation. Some groups reported that microtubule-disrupting agents inhibited insulin-induced GLUT4 translocation in 3T3-adipocytes [81, 112], while others did not find any effect on this process [113]. However, microtubules do not seem to play a role in stimulus-induced GLUT4 or CD36 translocation in skeletal muscle and cardiomyocytes [4, 114].
Although the cytoskeleton is clearly involved in GLUT4 and CD36 translocation in cardiomyocytes, its role in the altered transporter localization in diabetes has not yet been studied. However, investigators in other fields of research have studied the cytoskeleton in the diabetic heart. For example, in ventricular cells from streptozotocin-induced diabetic rats, impairment of cytoskeletal function and structure—actin and microtubules—was found. In their study, the insulin-deficient conditions affected the cardiac potassium-current in a cytoskeleton-dependent manner [115], suggesting that this may also affect insulin-dependent GLUT4 translocation.
Endosomal pH
The acidity of intracellular compartments, such as endosomes, is essential for various cellular processes, including endosomal function and vesicular trafficking [116, 117]. Endosomal acidification is regulated by vacuolar-ATPase (v-ATPase or V1V0-ATPase), a large multisubunit complex that functions as an ATP-driven proton pump. It has a similar build-up as the F1F0-ATPase located at the mitochondrial membrane; however, it needs ATP to pump protons whereas the F1F0 needs protons to produce ATP [118].
Surprisingly, not much is known about the role of endosomal acidity in trafficking of GLUT4 or CD36. The role of v-ATPases in GLUT4 translocation has been studied in 3T3-L1 adipocytes [119], and in cardiomyocytes [120]. Upon inhibition of v-ATPase with bafilomycin A1 in 3T3-L1 adipocytes, insulin-stimulated glucose uptake was disrupted and GLUT4 accumulated in intracellular membranes, while Akt and IRS1 signaling were still intact. From these data, it was concluded that proper regulation of endosomal pH is important for the formation of small insulin-responsive vesicles [119]. This has also been studied in cardiomyocytes, and in these cells, v-ATPases in the GLUT4-containing vesicles may play a role in insulin-stimulated increase of GLUT4 translocation and glucose uptake [120, 121]. When examining GLUT1 in mouse mammary epithelial cells, it was found that endosomal acidification was important for directed trafficking of GLUT1 [122].
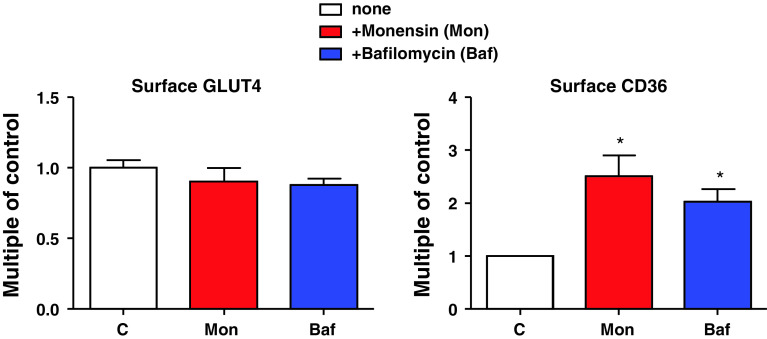
We recently used the specific v-ATPase inhibitor bafilomycin-A and the proton ionophore monensin to study the role of endosomal acidification in CD36 translocation. While reducing glucose uptake only in the acutely stimulated state, both compounds already increased basal CD36 translocation and subsequent fatty acid uptake [4]. Thus, v-ATPase seems to be involved in stimulus-induced, but not in basal, GLUT4 translocation. This seems to be opposite for CD36 translocation, where v-ATPase is involved in basal, but not in stimulus-induced, processes (Figs. 4 and 5). A novel function of v-ATPase is the modulation of protein traffic between early and late endosomes. Here, vATPase acts as a pH sensor interacting with proteins such as small GTPases of the Arf-family and their regulatory proteins [123]. Whether this function of the v-ATPases is involved in GLUT4 and CD36 translocation is not yet known. However, the proton pump function of the v-ATPases is clearly important since bafilomycin A and monensin similarly affect GLUT4 and CD36 localization.
Fig. 5.
Endosomal pH is important for cardiomyocyte transporter translocation. Isolated rat cardiomyocytes were treated with pH gradient disturbing agents, monensin (Mon) and bafilomycin A (Baf). Afterwards, GLUT4 and CD36 presence at the cell surface were assayed. During basal recycling, disturbance of the pH gradient only affects CD36 translocation [4]
Taken together, regulation of the endosomal pH seems an interesting target to restore the metabolic substrate balance in type 2 diabetes.
Rab proteins
Rab-GTPases are regulators of multiple steps of vesicular transport (e.g., vesicle formation, transport along the actin- and microtubule-based cytoskeleton and fusion with target membranes) by facilitating the formation of SNARE complexes [124, 125]. Therefore, Rab-GTPases are considered to play a key role in the control of GLUT4 and CD36 vesicle trafficking [126]. Adding to the complexity, 19 isoforms of the more than 60 currently known Rabs are present on GLUT4 vesicles [62]. Examples are Rab4 and Rab11a, which are involved in the intracellular GLUT4 sequestration and endocytosis of both GLUT4 and CD36, respectively [127–129]. Interestingly, Rab11a was activated and recruited to GLUT4 vesicles upon insulin stimulation [130, 131]. In this context, it was also observed that Rab11a shifted from microsomal fractions to the plasma membrane after insulin stimulation. In addition, Rab4 GTP-loading is also stimulated by insulin, and this complex is known to bind syntaxin4, which functions in the docking and fusion of vesicles with the plasma membrane [132, 133]. Two proteins interacting with Rab11a, i.e., FIP2 and Rip11, have been investigated for their role in GLUT4 and CD36 recycling. FIP2, which functions as an adaptor for interaction of Rab11 with the motor protein myosin-Vb [134], mediates endocytosis of both GLUT4 and CD36 in cardiac myoblast cultures [128]. In the same cell system, Rip11, which colocalizes with Rab11 in endosomal membranes [135], is involved in CD36 endocytosis, but does not influence GLUT4 dynamics [128]. However, in cultured adipocytes, Rip11 does influence GLUT4 traffic, indicating a cell type-specific function of this Rab11a regulator [136].
Rab-GTPase activating proteins (Rab-GAPs) silence Rab activity by keeping them in an inactive state [137]. TBC1D1 and TBC1D4, two proteins mentioned as Akt- and AMPK substrates in “Signalling pathways involved in GLUT4 and CD36 translocation”, are Rab-GAPs that silence Rab-function on GLUT4 translocation [62, 138]. By doing so, they restrain GLUT4 in its intracellular stores under basal conditions. Insulin releases this brake on GLUT4 translocation by stimulating Akt-mediated phosphorylation and inactivation of TBC1D1 and 4. This will promote the formation of GLUT4 transport vesicles and eventually their translocation to the plasma membrane [63, 139]. As detailed in “Contraction-signaling”, under conditions of increased contractions, activated AMPK acts analogous to Akt, indicating a common mechanism of GLUT4 mobilization that is shared by different signaling pathways [140]. Which Rab isoforms are regulated by TCB1D1 and TBC1D4, respectively? In vitro Rab-GAP assays revealed a strong activity of both GAPs against Rab2a, -8, -10 and -14, and no activity against the above-mentioned Rab4 and Rab11a [62, 63]. Still, identifying one Rab protein to be the crucial isoform for GLUT4 translocation remains challenging, as Rab8a, -10 and -13 all appear to be essential for complete insulin-dependent GLUT4 translocation to plasma membrane [141–143]. In addition, if and how TBC1D1, TBC1D4 and Rabs are involved in CD36 translocation needs further study.
SNARE proteins
Integration of vesicular GLUT4 and CD36 into the plasma membrane is regulated by soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), the mechanistic core complexes of membrane fusion [144]. Subtypes of the SNARE proteins have been classified depending on the amino acid residue within the SNARE motifs which is either a glutamine (Q-SNAREs) or an arginine (R-SNAREs) [145]. When the vesicle-associated R-SNAREs interact with a specific subset of Q-SNAREs at the target membrane, a hetero-oligomeric SNARE complex is formed which catalyzes the fusion of the vesicle with the target membrane [145].
It is well accepted that insulin-stimulated translocation of GLUT4 in adipocytes, skeletal- and cardiac myocytes involves the R-SNARE VAMP2 [146], which interacts with the Q-SNAREs syntaxin4 and SNAP23 at the plasma membrane (Fig. 4) [147, 148]. We have recently shown that insulin-stimulated CD36 translocation is also dependent on VAMP2 [5]. Another VAMP isoform, VAMP3, links contraction signaling to GLUT4 and CD36 translocation, disclosing a strong resemblance of the mechanisms of GLUT4 and CD36 trafficking [5]. Although present on GLUT4 vesicles, VAMP3 does not translocate to the plasma membrane upon increased contraction, pointing to a rather unclear function of this VAMP isoform in GLUT4 translocation [149]. However, other VAMP isoforms, like VAMP4 and VAMP7, are differentially involved in the regulation of GLUT4 and CD36 traffic and could be the basis for selective regulation of transporter distribution [5]. Still, their physiological function needs to be disclosed.
Concluding remarks
A complex interplay between signaling pathways and trafficking components is involved in the regulation of GLUT4 and CD36 translocation (Fig. 4). The signaling pathways appear to similarly affect GLUT4 and CD36 translocation and thus are unsuitable targets for restoring the improper subcellular localization of both substrate transporters in the diabetic heart, for example by bringing GLUT4 to the cell surface and internalizing CD36. However, the subcellular trafficking machinery is able to discriminate between regulation of GLUT4 and CD36 translocation, and therefore could form the basis of a novel approach to restore cardiac substrate preference in metabolic diseases with altered substrate utilization. Coat proteins are similarly involved in GLUT4 and CD36 translocation. Other trafficking components are differentially involved in both processes. In detail, actin organisation and v-ATPase are specifically involved in GLUT4 translocation, while v-ATPase is specifically involved in CD36 endocytosis. Future experiments should explore the possible disturbance of these subcellular trafficking components in animal models and in biopsies from patients with type 2 diabetes.
Importantly, all these trafficking components that differentiate between GLUT4 and CD36 translocation are novel targets for the development of therapies to restore the metabolic balance in cardiomyocytes during disease characterized by unbalanced substrate usage (e.g., insulin resistance, diabetic cardiomyopathy and heart failure). Another advantage in therapeutically targeting the GLUT4 and CD36 trafficking machineries is that this can be achieved in a tissue-specific manner because many members of the major trafficking protein families display a tissue-specific distribution pattern [144, 150]. Future research will undoubtedly unmask more GLUT4 and CD36-dedicated trafficking proteins that could be added to the list of novel anti-diabetic targets.
Acknowledgments
This work was supported by the Dutch Diabetes Research Foundation (Grant: 2006.00.044) and the EU European Cooperation in the field of Scientific and Technical Research (COST) Action BM0602 (Adipose tissue: a key target for prevention of the metabolic syndrome).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Glatz JF, Luiken JJ, Bonen A. Membrane fatty acid transporters as regulators of lipid metabolism: implications for metabolic disease. Physiol Rev. 2010;90(1):367–417. doi: 10.1152/physrev.00003.2009. [DOI] [PubMed] [Google Scholar]
- 2.Ouwens DM, Diamant M, Fodor M, Habets DD, Pelsers MM, El Hasnaoui M, Dang ZC, van den Brom CE, Vlasblom R, Rietdijk A, Boer C, Coort SL, Glatz JF, Luiken JJ. Cardiac contractile dysfunction in insulin-resistant rats fed a high-fat diet is associated with elevated cd36-mediated fatty acid uptake and esterification. Diabetologia. 2007;50(9):1938–1948. doi: 10.1007/s00125-007-0735-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Brom CE, Huisman MC, Vlasblom R, Boontje NM, Duijst S, Lubberink M, Molthoff CF, Lammertsma AA, van der Velden J, Boer C, Ouwens DM, Diamant M. Altered myocardial substrate metabolism is associated with myocardial dysfunction in early diabetic cardiomyopathy in rats: studies using positron emission tomography. Cardiovasc Diabetol. 2009;8:39. doi: 10.1186/1475-2840-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinbusch LK, Wijnen W, Schwenk RW, Coumans WA, Hoebers NT, Ouwens DM, Diamant M, Bonen A, Glatz JF, Luiken JJ. Differential regulation of cardiac glucose and fatty acid uptake by endosomal ph and actin filaments. Am J Physiol Cell Physiol. 2010;298(6):C1549–C1559. doi: 10.1152/ajpcell.00334.2009. [DOI] [PubMed] [Google Scholar]
- 5.Schwenk RW, Dirkx E, Coumans WA, Bonen A, Klip A, Glatz JF, Luiken JJ. Requirement for distinct vesicle-associated membrane proteins in insulin- and amp-activated protein kinase (ampk)-induced translocation of glut4 and cd36 in cultured cardiomyocytes. Diabetologia. 2010;53(10):2209–2219. doi: 10.1007/s00125-010-1832-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell LK, Finck BN, Kelly DP. Mouse models of mitochondrial dysfunction and heart failure. J Mol Cell Cardiol. 2005;38(1):81–91. doi: 10.1016/j.yjmcc.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Hue L, Taegtmeyer H. The randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297(3):E578–E591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shepherd PR, Kahn BB. Glucose transporters and insulin action–implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341(4):248–257. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 9.Watson RT, Pessin JE. Intracellular organization of insulin signaling and glut4 translocation. Recent Prog Horm Res. 2001;56:175–193. doi: 10.1210/rp.56.1.175. [DOI] [PubMed] [Google Scholar]
- 10.Schwenk RW, Luiken JJ, Bonen A, Glatz JF. Regulation of sarcolemmal glucose and fatty acid transporters in cardiac disease. Cardiovasc Res. 2008;79(2):249–258. doi: 10.1093/cvr/cvn116. [DOI] [PubMed] [Google Scholar]
- 11.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human cd36. J Biol Chem. 1993;268(24):17665–17668. [PubMed] [Google Scholar]
- 12.Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, Yamada KA, Brunet S, Xu H, Nerbonne JM, Welch MJ, Fettig NM, Sharp TL, Sambandam N, Olson KM, Ory DS, Schaffer JE. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res. 2005;96(2):225–233. doi: 10.1161/01.RES.0000154079.20681.B9. [DOI] [PubMed] [Google Scholar]
- 13.Gimeno RE, Ortegon AM, Patel S, Punreddy S, Ge P, Sun Y, Lodish HF, Stahl A. Characterization of a heart-specific fatty acid transport protein. J Biol Chem. 2003;278(18):16039–16044. doi: 10.1074/jbc.M211412200. [DOI] [PubMed] [Google Scholar]
- 14.Olson AL, Pessin JE. Structure, function, and regulation of the mammalian facilitative glucose transporter gene family. Annu Rev Nutr. 1996;16:235–256. doi: 10.1146/annurev.nu.16.070196.001315. [DOI] [PubMed] [Google Scholar]
- 15.Russell C, Stagg SM. New insights into the structural mechanisms of the copii coat. Traffic. 2009;11(3):303–310. doi: 10.1111/j.1600-0854.2009.01026.x. [DOI] [PubMed] [Google Scholar]
- 16.Muller H, Deckers K, Eckel J. The fatty acid translocase (fat)/cd36 and the glucose transporter glut4 are localized in different cellular compartments in rat cardiac muscle. Biochem Biophys Res Commun. 2002;293(2):665–669. doi: 10.1016/S0006-291X(02)00276-0. [DOI] [PubMed] [Google Scholar]
- 17.Habets DD, Coumans WA, Voshol PJ, den Boer MA, Febbraio M, Bonen A, Glatz JF, Luiken JJ. Ampk-mediated increase in myocardial long-chain fatty acid uptake critically depends on sarcolemmal cd36. Biochem Biophys Res Commun. 2007;355(1):204–210. doi: 10.1016/j.bbrc.2007.01.141. [DOI] [PubMed] [Google Scholar]
- 18.Habets DDJ (2008) Regulation of cardiac long-chain fatty acid and glucose utilization, chapter 5: Aicar stimulates long-chain fatty acid uptake and oxidation in mouse heart independent of cd36.89–101. Thesis, Maastricht University, the Netherlands
- 19.Chabowski A, Coort SL, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Bonen A. The subcellular compartmentation of fatty acid transporters is regulated differently by insulin and by aicar. FEBS Lett. 2005;579(11):2428–2432. doi: 10.1016/j.febslet.2004.11.118. [DOI] [PubMed] [Google Scholar]
- 20.Coort SL, Willems J, Coumans WA, van der Vusse GJ, Bonen A, Glatz JF, Luiken JJ. Sulfo-n-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (fat/cd36)-mediated cellular fatty acid uptake. Mol Cell Biochem. 2002;239(1–2):213–219. doi: 10.1023/A:1020539932353. [DOI] [PubMed] [Google Scholar]
- 21.McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51(1):7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- 22.Glatz JF, Bonen A, Ouwens DM, Luiken JJ. Regulation of sarcolemmal transport of substrates in the healthy and diseased heart. Cardiovasc Drugs Ther. 2006;20(6):471–476. doi: 10.1007/s10557-006-0582-8. [DOI] [PubMed] [Google Scholar]
- 23.Shulman GI. Cellular mechanisms of insulin resistance in humans. Am J Cardiol. 1999;84(1A):3J–10J. doi: 10.1016/S0002-9149(99)00350-1. [DOI] [PubMed] [Google Scholar]
- 24.Luiken JJ, Coort SL, Koonen DP, van der Horst DJ, Bonen A, Zorzano A, Glatz JF. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch. 2004;448(1):1–15. doi: 10.1007/s00424-003-1199-4. [DOI] [PubMed] [Google Scholar]
- 25.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, Noon GP, Frazier OH, Taegtmeyer H. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. FASEB J. 2004;18(14):1692–1700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 26.Coort SL, Hasselbaink DM, Koonen DP, Willems J, Coumans WA, Chabowski A, van der Vusse GJ, Bonen A, Glatz JF, Luiken JJ. Enhanced sarcolemmal fat/cd36 content and triacylglycerol storage in cardiac myocytes from obese zucker rats. Diabetes. 2004;53(7):1655–1663. doi: 10.2337/diabetes.53.7.1655. [DOI] [PubMed] [Google Scholar]
- 27.Carley AN, Atkinson LL, Bonen A, Harper ME, Kunnathu S, Lopaschuk GD, Severson DL. Mechanisms responsible for enhanced fatty acid utilization by perfused hearts from type 2 diabetic db/db mice. Arch Physiol Biochem. 2007;113(2):65–75. doi: 10.1080/13813450701422617. [DOI] [PubMed] [Google Scholar]
- 28.Aguer C, Mercier J, Man CY, Metz L, Bordenave S, Lambert K, Jean E, Lantier L, Bounoua L, Brun JF, Raynaud de Mauverger E, Andreelli F, Foretz M, Kitzmann M. Intramyocellular lipid accumulation is associated with permanent relocation ex vivo and in vitro of fatty acid translocase (fat)/cd36 in obese patients. Diabetologia. 2010;53(6):1151–1163. doi: 10.1007/s00125-010-1708-x. [DOI] [PubMed] [Google Scholar]
- 29.Chabowski A, Chatham JC, Tandon NN, Calles-Escandon J, Glatz JF, Luiken JJ, Bonen A. Fatty acid transport and fat/cd36 are increased in red but not in white skeletal muscle of zdf rats. Am J Physiol Endocrinol Metab. 2006;291(3):E675–E682. doi: 10.1152/ajpendo.00096.2006. [DOI] [PubMed] [Google Scholar]
- 30.Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal fat/cd36. FASEB J. 2004;18(10):1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- 31.Bonen A, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ. The fatty acid transporter fat/cd36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes. 2006;30(6):877–883. doi: 10.1038/sj.ijo.0803212. [DOI] [PubMed] [Google Scholar]
- 32.Severson DL. Diabetic cardiomyopathy: recent evidence from mouse models of type 1 and type 2 diabetes. Can J Physiol Pharmacol. 2004;82(10):813–823. doi: 10.1139/y04-065. [DOI] [PubMed] [Google Scholar]
- 33.van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, Bax JJ, de Roos A, Kamp O, Paulus WJ, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119(15):2069–2077. doi: 10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- 34.Holland WL, Knotts TA, Chavez JA, Wang LP, Hoehn KL, Summers SA. Lipid mediators of insulin resistance. Nutr Rev. 2007;65(6 Pt 2):S39–46. doi: 10.1111/j.1753-4887.2007.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 35.DeFronzo RA. Insulin resistance, lipotoxicity, type 2 diabetes, atherosclerosis: the missing links. The claude bernard lecture 2009. Diabetologia. 2010;53(7):1270–1287. doi: 10.1007/s00125-010-1684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the framingham heart study. Circulation. 1983;67(5):968–977. doi: 10.1161/01.CIR.67.5.968. [DOI] [PubMed] [Google Scholar]
- 37.Taha M, Lopaschuk GD. Alterations in energy metabolism in cardiomyopathies. Ann Med. 2007;39(8):594–607. doi: 10.1080/07853890701618305. [DOI] [PubMed] [Google Scholar]
- 38.Kusminski CM, Shetty S, Orci L, Unger RH, Scherer PE. Diabetes and apoptosis: lipotoxicity. Apoptosis. 2009;14(12):1484–1495. doi: 10.1007/s10495-009-0352-8. [DOI] [PubMed] [Google Scholar]
- 39.van Dis I, Kromhout D, Geleijnse JM, Boer JM, Verschuren WM. Body mass index and waist circumference predict both 10-year nonfatal and fatal cardiovascular disease risk: study conducted in 20,000 dutch men and women aged 20–65 years. Eur J Cardiovasc Prev Rehabil. 2009;16(6):729–734. doi: 10.1097/HJR.0b013e328331dfc0. [DOI] [PubMed] [Google Scholar]
- 40.Rudich A, Klip A. Push/pull mechanisms of glut4 traffic in muscle cells. Acta Physiol Scand. 2003;178(4):297–308. doi: 10.1046/j.1365-201X.2003.01163.x. [DOI] [PubMed] [Google Scholar]
- 41.Zeigerer A, McBrayer MK, McGraw TE. Insulin stimulation of glut4 exocytosis, but not its inhibition of endocytosis, is dependent on rabgap as160. Mol Biol Cell. 2004;15(10):4406–4415. doi: 10.1091/mbc.E04-04-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang J, Holman GD. Insulin and contraction stimulate exocytosis, but increased amp-activated protein kinase activity resulting from oxidative metabolism stress slows endocytosis of glut4 in cardiomyocytes. J Biol Chem. 2005;280(6):4070–4078. doi: 10.1074/jbc.M410213200. [DOI] [PubMed] [Google Scholar]
- 43.Klip A. The many ways to regulate glucose transporter 4. Appl Physiol Nutr Metab. 2009;34(3):481–487. doi: 10.1139/H09-047. [DOI] [PubMed] [Google Scholar]
- 44.Fazakerley DJ, Lawrence SP, Lizunov VA, Cushman SW, Holman GD. A common trafficking route for glut4 in cardiomyocytes in response to insulin, contraction and energy-status signalling. J Cell Sci. 2009;122(Pt 5):727–734. doi: 10.1242/jcs.041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tremblay F, Dubois MJ, Marette A. Regulation of glut4 traffic and function by insulin and contraction in skeletal muscle. Front Biosci. 2003;8:d1072–d1084. doi: 10.2741/1137. [DOI] [PubMed] [Google Scholar]
- 46.Lee W, Ryu J, Spangler RA, Jung CY. Modulation of glut4 and glut1 recycling by insulin in rat adipocytes: kinetic analysis based on the involvement of multiple intracellular compartments. Biochemistry. 2000;39(31):9358–9366. doi: 10.1021/bi0007021. [DOI] [PubMed] [Google Scholar]
- 47.Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter glut4. Nat Rev Mol Cell Biol. 2002;3(4):267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- 48.Kaburagi Y, Satoh S, Tamemoto H, Yamamoto-Honda R, Tobe K, Veki K, Yamauchi T, Kono-Sugita E, Sekihara H, Aizawa S, Cushman SW, Akanuma Y, Yazaki Y, Kadowaki T. Role of insulin receptor substrate-1 and pp60 in the regulation of insulin-induced glucose transport and glut4 translocation in primary adipocytes. J Biol Chem. 1997;272(41):25839–25844. doi: 10.1074/jbc.272.41.25839. [DOI] [PubMed] [Google Scholar]
- 49.Fasshauer M, Klein J, Ueki K, Kriauciunas KM, Benito M, White MF, Kahn CR. Essential role of insulin receptor substrate-2 in insulin stimulation of glut4 translocation and glucose uptake in brown adipocytes. J Biol Chem. 2000;275(33):25494–25501. doi: 10.1074/jbc.M004046200. [DOI] [PubMed] [Google Scholar]
- 50.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296(5573):1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 51.Le Marchand-Brustel Y, Gautier N, Cormont M, Van Obberghen E. Wortmannin inhibits the action of insulin but not that of okadaic acid in skeletal muscle: comparison with fat cells. Endocrinology. 1995;136(8):3564–3570. doi: 10.1210/en.136.8.3564. [DOI] [PubMed] [Google Scholar]
- 52.Luiken JJ, Dyck DJ, Han XX, Tandon NN, Arumugam Y, Glatz JF, Bonen A. Insulin induces the translocation of the fatty acid transporter fat/cd36 to the plasma membrane. Am J Physiol Endocrinol Metab. 2002;282(2):E491–E495. doi: 10.1152/ajpendo.00419.2001. [DOI] [PubMed] [Google Scholar]
- 53.Luiken JJ, Koonen DP, Willems J, Zorzano A, Becker C, Fischer Y, Tandon NN, Van Der Vusse GJ, Bonen A, Glatz JF. Insulin stimulates long-chain fatty acid utilization by rat cardiac myocytes through cellular redistribution of fat/cd36. Diabetes. 2002;51(10):3113–3119. doi: 10.2337/diabetes.51.10.3113. [DOI] [PubMed] [Google Scholar]
- 54.Shisheva A. Phosphoinositides in insulin action on glut4 dynamics: not just ptdins(3, 4, 5)p3. Am J Physiol Endocrinol Metab. 2008;295(3):E536–E544. doi: 10.1152/ajpendo.90353.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shepherd PR. Mechanisms regulating phosphoinositide 3-kinase signalling in insulin-sensitive tissues. Acta Physiol Scand. 2005;183(1):3–12. doi: 10.1111/j.1365-201X.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 56.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, 3rd, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase akt2 (pkb beta) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 57.Mora A, Sakamoto K, McManus EJ, Alessi DR. Role of the pdk1-pkb-gsk3 pathway in regulating glycogen synthase and glucose uptake in the heart. FEBS Lett. 2005;579(17):3632–3638. doi: 10.1016/j.febslet.2005.05.040. [DOI] [PubMed] [Google Scholar]
- 58.Bandyopadhyay G, Standaert ML, Galloway L, Moscat J, Farese RV. Evidence for involvement of protein kinase c (pkc)-zeta and noninvolvement of diacylglycerol-sensitive pkcs in insulin-stimulated glucose transport in l6 myotubes. Endocrinology. 1997;138(11):4721–4731. doi: 10.1210/en.138.11.4721. [DOI] [PubMed] [Google Scholar]
- 59.Luiken JJ, Ouwens DM, Habets DD, van der Zon GC, Coumans WA, Schwenk RW, Bonen A, Glatz JF. Permissive action of protein kinase c-zeta in insulin-induced cd36- and glut4 translocation in cardiac myocytes. J Endocrinol. 2009;201(2):199–209. doi: 10.1677/JOE-09-0046. [DOI] [PubMed] [Google Scholar]
- 60.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase balpha. Curr Biol. 1997;7(4):261–269. doi: 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 61.Dong LQ, Liu F. Pdk2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289(2):E187–E196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 62.Miinea CP, Sano H, Kane S, Sano E, Fukuda M, Peranen J, Lane WS, Lienhard GE. As160, the akt substrate regulating glut4 translocation, has a functional rab gtpase-activating protein domain. Biochem J. 2005;391(Pt 1):87–93. doi: 10.1042/BJ20050887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roach WG, Chavez JA, Miinea CP, Lienhard GE. Substrate specificity and effect on glut4 translocation of the rab gtpase-activating protein tbc1d1. Biochem J. 2007;403(2):353–358. doi: 10.1042/BJ20061798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu XJ, Yang C, Gupta N, Zuo J, Chang YS, Fang FD. Protein kinase c-zeta regulation of glut4 translocation through actin remodeling in cho cells. J Mol Med. 2007;85(8):851–861. doi: 10.1007/s00109-007-0232-z. [DOI] [PubMed] [Google Scholar]
- 65.Kovacic S, Soltys CL, Barr AJ, Shiojima I, Walsh K, Dyck JR. Akt activity negatively regulates phosphorylation of amp-activated protein kinase in the heart. J Biol Chem. 2003;278(41):39422–39427. doi: 10.1074/jbc.M305371200. [DOI] [PubMed] [Google Scholar]
- 66.Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF, Goodyear LJ. Distinct signals regulate as160 phosphorylation in response to insulin, aicar, and contraction in mouse skeletal muscle. Diabetes. 2006;55(7):2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- 67.Luiken JJ, Momken I, Habets DD, El Hasnaoui M, Coumans WA, Koonen DP, Glatz JF, Bonen A. Arsenite modulates cardiac substrate preference by translocation of glut4, but not cd36, independent of mitogen-activated protein kinase signaling. Endocrinology. 2006;147(11):5205–5216. doi: 10.1210/en.2006-0849. [DOI] [PubMed] [Google Scholar]
- 68.Luiken JJ, Coort SL, Koonen DP, Bonen A, Glatz JF. Signalling components involved in contraction-inducible substrate uptake into cardiac myocytes. Proc Nutr Soc. 2004;63(2):251–258. doi: 10.1079/PNS2004333. [DOI] [PubMed] [Google Scholar]
- 69.Habets DD, Coumans WA, El Hasnaoui M, Zarrinpashneh E, Bertrand L, Viollet B, Kiens B, Jensen TE, Richter EA, Bonen A, Glatz JF, Luiken JJ. Crucial role for lkb1 to ampkalpha2 axis in the regulation of cd36-mediated long-chain fatty acid uptake into cardiomyocytes. Biochim Biophys Acta. 2009;1791(3):212–219. doi: 10.1016/j.bbalip.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 70.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the lkb1 tumor suppressor, strad alpha/beta and mo25 alpha/beta are upstream kinases in the amp-activated protein kinase cascade. J Biol. 2003;2(4):28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sakamoto K, Zarrinpashneh E, Budas GR, Pouleur AC, Dutta A, Prescott AR, Vanoverschelde JL, Ashworth A, Jovanovic A, Alessi DR, Bertrand L. Deficiency of lkb1 in heart prevents ischemia-mediated activation of ampkalpha2 but not ampkalpha1. Am J Physiol Endocrinol Metab. 2006;290(5):E780–E788. doi: 10.1152/ajpendo.00443.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for amp-activated protein kinase. Cell Metab. 2005;2(1):9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of amp-activated protein kinase in mammalian cells. Cell Metab. 2005;2(1):21–33. doi: 10.1016/j.cmet.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 74.Abbott MJ, Edelman AM, Turcotte LP. Camkk is an upstream signal of amp-activated protein kinase in regulation of substrate metabolism in contracting skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2009;297(6):R1724–R1732. doi: 10.1152/ajpregu.00179.2009. [DOI] [PubMed] [Google Scholar]
- 75.Chen HC, Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert M, Farese RV, Jr, Farese RV. Activation of the erk pathway and atypical protein kinase c isoforms in exercise- and aminoimidazole-4-carboxamide-1-beta-d-riboside (aicar)-stimulated glucose transport. J Biol Chem. 2002;277(26):23554–23562. doi: 10.1074/jbc.M201152200. [DOI] [PubMed] [Google Scholar]
- 76.Farese RV, Sajan MP, Yang H, Li P, Mastorides S, Gower WR, Jr, Nimal S, Choi CS, Kim S, Shulman GI, Kahn CR, Braun U, Leitges M. Muscle-specific knockout of pkc-lambda impairs glucose transport and induces metabolic and diabetic syndromes. J Clin Invest. 2007;117(8):2289–2301. doi: 10.1172/JCI31408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raney MA, Turcotte LP. Evidence for the regulation of contraction-induced fatty acid oxidation via extracellular signal-regulated kinase 1/2 activation independent of changes in fatty acid uptake. Metabolism. 2007;56(9):1192–1200. doi: 10.1016/j.metabol.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 78.Luiken JJ, Vertommen D, Coort SL, Habets DD, El Hasnaoui M, Pelsers MM, Viollet B, Bonen A, Hue L, Rider MH, Glatz JF. Identification of protein kinase d as a novel contraction-activated kinase linked to glut4-mediated glucose uptake, independent of ampk. Cell Signal. 2008;20(3):543–556. doi: 10.1016/j.cellsig.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 79.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of tbc1d1 and as160 by growth factors, insulin and ampk activators. Biochem J. 2008;409(2):449–459. doi: 10.1042/BJ20071114. [DOI] [PubMed] [Google Scholar]
- 80.Kanzaki M, Pessin JE. Insulin-stimulated glut4 translocation in adipocytes is dependent upon cortical actin remodeling. J Biol Chem. 2001;276(45):42436–42444. doi: 10.1074/jbc.M108297200. [DOI] [PubMed] [Google Scholar]
- 81.Olson AL, Trumbly AR, Gibson GV. Insulin-mediated glut4 translocation is dependent on the microtubule network. J Biol Chem. 2001;276(14):10706–10714. doi: 10.1074/jbc.M007610200. [DOI] [PubMed] [Google Scholar]
- 82.Torok D, Patel N, Jebailey L, Thong FS, Randhawa VK, Klip A, Rudich A. Insulin but not pdgf relies on actin remodeling and on vamp2 for glut4 translocation in myoblasts. J Cell Sci. 2004;117(Pt 22):5447–5455. doi: 10.1242/jcs.01421. [DOI] [PubMed] [Google Scholar]
- 83.Chiu TT, Patel N, Shaw AE, Bamburg JR, Klip A. Arp2/3- and cofilin-coordinated actin dynamics is required for insulin-mediated glut4 translocation to the surface of muscle cells. Mol Biol Cell. 2010;21(20):3529–3539. doi: 10.1091/mbc.E10-04-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baus D, Heermeier K, De Hoop M, Metz-Weidmann C, Gassenhuber J, Dittrich W, Welte S, Tennagels N. Identification of a novel as160 splice variant that regulates glut4 translocation and glucose-uptake in rat muscle cells. Cell Signal. 2008;20(12):2237–2246. doi: 10.1016/j.cellsig.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 85.St-Denis JF, Cushman SW. Role of snare’s in the glut4 translocation response to insulin in adipose cells and muscle. J Basic Clin Physiol Pharmacol. 1998;9(2–4):153–165. doi: 10.1515/JBCPP.1998.9.2-4.153. [DOI] [PubMed] [Google Scholar]
- 86.Larance M, Ramm G, Stockli J, van Dam EM, Winata S, Wasinger V, Simpson F, Graham M, Junutula JR, Guilhaus M, James DE. Characterization of the role of the rab gtpase-activating protein as160 in insulin-regulated glut4 trafficking. J Biol Chem. 2005;280(45):37803–37813. doi: 10.1074/jbc.M503897200. [DOI] [PubMed] [Google Scholar]
- 87.Martin LB, Shewan A, Millar CA, Gould GW, James DE. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter glut4 in 3t3–l1 adipocytes. J Biol Chem. 1998;273(3):1444–1452. doi: 10.1074/jbc.273.3.1444. [DOI] [PubMed] [Google Scholar]
- 88.Lamb CA, McCann RK, Stockli J, James DE, Bryant NJ. Insulin-regulated trafficking of glut4 requires ubiquitination. Traffic. 2010;11(11):1445–1454. doi: 10.1111/j.1600-0854.2010.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Su X, Abumrad NA. Cellular fatty acid uptake: a pathway under construction. Trends Endocrinol Metab. 2009;20(2):72–77. doi: 10.1016/j.tem.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chidlow JH, Jr, Sessa WC. Caveolae, caveolins, and cavins: complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86(2):219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dancourt J, Barlowe C. Protein sorting receptors in the early secretory pathway. Annu Rev Biochem. 2010;79:777–802. doi: 10.1146/annurev-biochem-061608-091319. [DOI] [PubMed] [Google Scholar]
- 92.Beck R, Rawet M, Wieland FT, Cassel D. The copi system: molecular mechanisms and function. FEBS Lett. 2009;583(17):2701–2709. doi: 10.1016/j.febslet.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 93.Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83(5):703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 94.Chakrabarti R, Buxton J, Joly M, Corvera S. Insulin-sensitive association of glut-4 with endocytic clathrin-coated vesicles revealed with the use of brefeldin A. J Biol Chem. 1994;269(11):7926–7933. [PubMed] [Google Scholar]
- 95.Lachaal M, Moronski C, Liu H, Jung CY. Brefeldin a inhibits insulin-induced glucose transport stimulation and glut4 recruitment in rat adipocytes. J Biol Chem. 1994;269(38):23689–23693. [PubMed] [Google Scholar]
- 96.Martin S, Ramm G, Lyttle CT, Meerloo T, Stoorvogel W, James DE. Biogenesis of insulin-responsive glut4 vesicles is independent of brefeldin a-sensitive trafficking. Traffic. 2000;1(8):652–660. doi: 10.1034/j.1600-0854.2000.010809.x. [DOI] [PubMed] [Google Scholar]
- 97.Kono-Sugita E, Satoh S, Suzuki Y, Egawa M, Udaka N, Ito T, Sekihara H. Insulin-induced glut4 recycling in rat adipose cells by a pathway insensitive to brefeldin A. Eur J Biochem. 1996;236(3):1033–1037. doi: 10.1111/j.1432-1033.1996.01033.x. [DOI] [PubMed] [Google Scholar]
- 98.Bao S, Smith RM, Jarett L, Garvey WT. The effects of brefeldin a on the glucose transport system in rat adipocytes. Implications regarding the intracellular locus of insulin-sensitive glut4. J Biol Chem. 1995;270(50):30199–30204. doi: 10.1074/jbc.270.50.30199. [DOI] [PubMed] [Google Scholar]
- 99.Watson RT, Pessin JE. Subcellular compartmentalization and trafficking of the insulin-responsive glucose transporter, glut4. Exp Cell Res. 2001;271(1):75–83. doi: 10.1006/excr.2001.5375. [DOI] [PubMed] [Google Scholar]
- 100.Pohl J, Ring A, Korkmaz U, Ehehalt R, Stremmel W. Fat/cd36-mediated long-chain fatty acid uptake in adipocytes requires plasma membrane rafts. Mol Biol Cell. 2005;16(1):24–31. doi: 10.1091/mbc.E04-07-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ros-Baro A, Lopez-Iglesias C, Peiro S, Bellido D, Palacin M, Zorzano A, Camps M. Lipid rafts are required for glut4 internalization in adipose cells. Proc Natl Acad Sci USA. 2001;98(21):12050–12055. doi: 10.1073/pnas.211341698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shigematsu S, Watson RT, Khan AH, Pessin JE. The adipocyte plasma membrane caveolin functional/structural organization is necessary for the efficient endocytosis of glut4. J Biol Chem. 2003;278(12):10683–10690. doi: 10.1074/jbc.M208563200. [DOI] [PubMed] [Google Scholar]
- 103.Ralston E, Ploug T. Caveolin-3 is associated with the t-tubules of mature skeletal muscle fibers. Exp Cell Res. 1999;246(2):510–515. doi: 10.1006/excr.1998.4305. [DOI] [PubMed] [Google Scholar]
- 104.Mattern HM, Raikar LS, Hardin CD. The effect of caveolin-1 (cav-1) on fatty acid uptake and cd36 localization and lipotoxicity in vascular smooth muscle (vsm) cells. Int J Physiol Pathophysiol Pharmacol. 2009;1(1):1–14. [PMC free article] [PubMed] [Google Scholar]
- 105.Ring A, Le Lay S, Pohl J, Verkade P, Stremmel W. Caveolin-1 is required for fatty acid translocase (fat/cd36) localization and function at the plasma membrane of mouse embryonic fibroblasts. Biochim Biophys Acta. 2006;1761(4):416–423. doi: 10.1016/j.bbalip.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 106.Brozinick JT, Jr, Hawkins ED, Strawbridge AB, Elmendorf JS. Disruption of cortical actin in skeletal muscle demonstrates an essential role of the cytoskeleton in glucose transporter 4 translocation in insulin-sensitive tissues. J Biol Chem. 2004;279(39):40699–40706. doi: 10.1074/jbc.M402697200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Condrescu M, Reeves JP. Actin-dependent regulation of the cardiac na(+)/ca(2+) exchanger. Am J Physiol Cell Physiol. 2006;290(3):C691–C701. doi: 10.1152/ajpcell.00232.2005. [DOI] [PubMed] [Google Scholar]
- 108.Tong P, Khayat ZA, Huang C, Patel N, Ueyama A, Klip A. Insulin-induced cortical actin remodeling promotes glut4 insertion at muscle cell membrane ruffles. J Clin Invest. 2001;108(3):371–381. doi: 10.1172/JCI12348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Olson AL, Eyster CA, Duggins QS, Knight JB. Insulin promotes formation of polymerized microtubules by a phosphatidylinositol 3-kinase-independent, actin-dependent pathway in 3t3–l1 adipocytes. Endocrinology. 2003;144(11):5030–5039. doi: 10.1210/en.2003-0609. [DOI] [PubMed] [Google Scholar]
- 110.Khayat ZA, Tong P, Yaworsky K, Bloch RJ, Klip A. Insulin-induced actin filament remodeling colocalizes actin with phosphatidylinositol 3-kinase and glut4 in l6 myotubes. J Cell Sci. 2000;113(Pt 2):279–290. doi: 10.1242/jcs.113.2.279. [DOI] [PubMed] [Google Scholar]
- 111.JeBailey L, Rudich A, Huang X, Di Ciano-Oliveira C, Kapus A, Klip A. Skeletal muscle cells and adipocytes differ in their reliance on tc10 and rac for insulin-induced actin remodeling. Mol Endocrinol. 2004;18(2):359–372. doi: 10.1210/me.2003-0294. [DOI] [PubMed] [Google Scholar]
- 112.Huang J, Imamura T, Babendure JL, Lu JC, Olefsky JM. Disruption of microtubules ablates the specificity of insulin signaling to glut4 translocation in 3t3–l1 adipocytes. J Biol Chem. 2005;280(51):42300–42306. doi: 10.1074/jbc.M510920200. [DOI] [PubMed] [Google Scholar]
- 113.Shigematsu S, Khan AH, Kanzaki M, Pessin JE. Intracellular insulin-responsive glucose transporter (glut4) distribution but not insulin-stimulated glut4 exocytosis and recycling are microtubule dependent. Mol Endocrinol. 2002;16(5):1060–1068. doi: 10.1210/me.16.5.1060. [DOI] [PubMed] [Google Scholar]
- 114.Ai H, Ralston E, Lauritzen HP, Galbo H, Ploug T. Disruption of microtubules in rat skeletal muscle does not inhibit insulin- or contraction-stimulated glucose transport. Am J Physiol Endocrinol Metab. 2003;285(4):E836–E844. doi: 10.1152/ajpendo.00238.2002. [DOI] [PubMed] [Google Scholar]
- 115.Shimoni Y, Rattner JB. Type 1 diabetes leads to cytoskeleton changes that are reflected in insulin action on rat cardiac k(+) currents. Am J Physiol Endocrinol Metab. 2001;281(3):E575–E585. doi: 10.1152/ajpendo.2001.281.3.E575. [DOI] [PubMed] [Google Scholar]
- 116.Sun-Wada GH, Wada Y, Futai M. Diverse and essential roles of mammalian vacuolar-type proton pump atpase: toward the physiological understanding of inside acidic compartments. Biochim Biophys Acta. 2004;1658(1–2):106–114. doi: 10.1016/j.bbabio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 117.Forgac M. Vacuolar atpases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8(11):917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 118.Merkulova M, Bakulina A, Thaker YR, Gruber G, Marshansky V. Specific motifs of the v-atpase a2-subunit isoform interact with catalytic and regulatory domains of arno. Biochim Biophys Acta. 2010;1797(8):1398–1409. doi: 10.1016/j.bbabio.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 119.Chinni SR, Shisheva A. Arrest of endosome acidification by bafilomycin a1 mimics insulin action on glut4 translocation in 3t3–l1 adipocytes. Biochem J. 1999;339(Pt 3):599–606. doi: 10.1042/0264-6021:3390599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yang J, Gillingham AK, Hodel A, Koumanov F, Woodward B, Holman GD. Insulin-stimulated cytosol alkalinization facilitates optimal activation of glucose transport in cardiomyocytes. Am J Physiol Endocrinol Metab. 2002;283(6):E1299–E1307. doi: 10.1152/ajpendo.00341.2002. [DOI] [PubMed] [Google Scholar]
- 121.Choi YO, Park JH, Song YS, Lee W, Moriyama Y, Choe H, Leem CH, Jang YJ. Involvement of vesicular h+-atpase in insulin-stimulated glucose transport in 3t3–f442a adipocytes. Endocr J. 2007;54(5):733–743. doi: 10.1507/endocrj.K07-090. [DOI] [PubMed] [Google Scholar]
- 122.Riskin A, Nannegari VH, Mond Y. Acute effectors of glut1 glucose transporter subcellular targeting in cit3 mouse mammary epithelial cells. Pediatr Res. 2008;63(1):56–61. doi: 10.1203/PDR.0b013e31815b440b. [DOI] [PubMed] [Google Scholar]
- 123.Hurtado-Lorenzo A, Skinner M, El Annan J, Futai M, Sun-Wada GH, Bourgoin S, Casanova J, Wildeman A, Bechoua S, Ausiello DA, Brown D, Marshansky V. V-atpase interacts with arno and arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol. 2006;8(2):124–136. doi: 10.1038/ncb1348. [DOI] [PubMed] [Google Scholar]
- 124.Schimmoller F, Simon I, Pfeffer SR. Rab gtpases, directors of vesicle docking. J Biol Chem. 1998;273(35):22161–22164. doi: 10.1074/jbc.273.35.22161. [DOI] [PubMed] [Google Scholar]
- 125.Takai Y, Sasaki T, Matozaki T. Small gtp-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. [DOI] [PubMed] [Google Scholar]
- 126.Cormont M, Le Marchand-Brustel Y. The role of small g-proteins in the regulation of glucose transport (review) Mol Membr Biol. 2001;18(3):213–220. doi: 10.1080/09687680110077541. [DOI] [PubMed] [Google Scholar]
- 127.Cormont M, Bortoluzzi MN, Gautier N, Mari M, van Obberghen E, Le Marchand-Brustel Y. Potential role of rab4 in the regulation of subcellular localization of glut4 in adipocytes. Mol Cell Biol. 1996;16(12):6879–6886. doi: 10.1128/mcb.16.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schwenk RW, Luiken JJ, Eckel J. Fip2 and rip11 specify rab11a-mediated cellular distribution of glut4 and fat/cd36 in h9c2-hir cells. Biochem Biophys Res Commun. 2007;363(1):119–125. doi: 10.1016/j.bbrc.2007.08.111. [DOI] [PubMed] [Google Scholar]
- 129.Uhlig M, Passlack W, Eckel J. Functional role of rab11 in glut4 trafficking in cardiomyocytes. Mol Cell Endocrinol. 2005;235(1–2):1–9. doi: 10.1016/j.mce.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 130.Kessler A, Tomas E, Immler D, Meyer HE, Zorzano A, Eckel J. Rab11 is associated with glut4-containing vesicles and redistributes in response to insulin. Diabetologia. 2000;43(12):1518–1527. doi: 10.1007/s001250051563. [DOI] [PubMed] [Google Scholar]
- 131.Schwenk RW, Eckel J. A novel method to monitor insulin-stimulated gtp-loading of rab11a in cardiomyocytes. Cell Signal. 2007;19(4):825–830. doi: 10.1016/j.cellsig.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 132.Shibata H, Omata W, Kojima I. Insulin stimulates guanine nucleotide exchange on rab4 via a wortmannin-sensitive signaling pathway in rat adipocytes. J Biol Chem. 1997;272(23):14542–14546. doi: 10.1074/jbc.272.23.14542. [DOI] [PubMed] [Google Scholar]
- 133.Li L, Omata W, Kojima I, Shibata H. Direct interaction of rab4 with syntaxin 4. J Biol Chem. 2001;276(7):5265–5273. doi: 10.1074/jbc.M003883200. [DOI] [PubMed] [Google Scholar]
- 134.Hales CM, Vaerman JP, Goldenring JR. Rab11 family interacting protein 2 associates with myosin vb and regulates plasma membrane recycling. J Biol Chem. 2002;277(52):50415–50421. doi: 10.1074/jbc.M209270200. [DOI] [PubMed] [Google Scholar]
- 135.Prekeris R, Klumperman J, Scheller RH. A rab11/rip11 protein complex regulates apical membrane trafficking via recycling endosomes. Mol Cell. 2000;6(6):1437–1448. doi: 10.1016/S1097-2765(00)00140-4. [DOI] [PubMed] [Google Scholar]
- 136.Welsh GI, Leney SE, Lloyd-Lewis B, Wherlock M, Lindsay AJ, McCaffrey MW, Tavare JM. Rip11 is a rab11- and as160-rabgap-binding protein required for insulin-stimulated glucose uptake in adipocytes. J Cell Sci. 2007;120(Pt 23):4197–4208. doi: 10.1242/jcs.007310. [DOI] [PubMed] [Google Scholar]
- 137.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103(32):11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sakamoto K, Holman GD. Emerging role for as160/tbc1d4 and tbc1d1 in the regulation of glut4 traffic. Am J Physiol Endocrinol Metab. 2008;295(1):E29–E37. doi: 10.1152/ajpendo.90331.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sano H, Kane S, Sano E, Miinea CP, Asara JM, Lane WS, Garner CW, Lienhard GE. Insulin-stimulated phosphorylation of a rab gtpase-activating protein regulates glut4 translocation. J Biol Chem. 2003;278(17):14599–14602. doi: 10.1074/jbc.C300063200. [DOI] [PubMed] [Google Scholar]
- 140.Thong FS, Bilan PJ, Klip A. The rab gtpase-activating protein as160 integrates akt, protein kinase c, and amp-activated protein kinase signals regulating glut4 traffic. Diabetes. 2007;56(2):414–423. doi: 10.2337/db06-0900. [DOI] [PubMed] [Google Scholar]
- 141.Randhawa VK, Ishikura S, Talior-Volodarsky I, Cheng AW, Patel N, Hartwig JH, Klip A. Glut4 vesicle recruitment and fusion are differentially regulated by rac, as160, and rab8a in muscle cells. J Biol Chem. 2008;283(40):27208–27219. doi: 10.1074/jbc.M804282200. [DOI] [PubMed] [Google Scholar]
- 142.Sun Y, Bilan PJ, Liu Z, Klip A. Rab8a and rab13 are activated by insulin and regulate glut4 translocation in muscle cells. Proc Natl Acad Sci USA. 2010;107(46):19909–19914. doi: 10.1073/pnas.1009523107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sano H, Roach WG, Peck GR, Fukuda M, Lienhard GE. Rab10 in insulin-stimulated glut4 translocation. Biochem J. 2008;411(1):89–95. doi: 10.1042/BJ20071318. [DOI] [PubMed] [Google Scholar]
- 144.Hong W. Snares and traffic. Biochim Biophys Acta. 2005;1744(3):493–517. [PubMed] [Google Scholar]
- 145.Jahn R, Scheller RH. Snares–engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 146.Chen Y, Gan BQ, Tang BL. Syntaxin 16: unraveling cellular physiology through a ubiquitous snare molecule. J Cell Physiol. 2010;225(2):326–332. doi: 10.1002/jcp.22286. [DOI] [PubMed] [Google Scholar]
- 147.Randhawa VK, Bilan PJ, Khayat ZA, Daneman N, Liu Z, Ramlal T, Volchuk A, Peng XR, Coppola T, Regazzi R, Trimble WS, Klip A. Vamp2, but not vamp3/cellubrevin, mediates insulin-dependent incorporation of glut4 into the plasma membrane of l6 myoblasts. Mol Biol Cell. 2000;11(7):2403–2417. doi: 10.1091/mbc.11.7.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M. Role of snap23 in insulin-induced translocation of glut4 in 3t3–l1 adipocytes. Mediation of complex formation between syntaxin4 and vamp2. J Biol Chem. 2000;275(11):8240–8247. doi: 10.1074/jbc.275.11.8240. [DOI] [PubMed] [Google Scholar]
- 149.Rose AJ, Jeppesen J, Kiens B, Richter EA. Effects of contraction on localization of glut4 and v-snare isoforms in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2009;297(5):R1228–R1237. doi: 10.1152/ajpregu.00258.2009. [DOI] [PubMed] [Google Scholar]
- 150.McMahon HT, Mills IG. Cop and clathrin-coated vesicle budding: different pathways, common approaches. Curr Opin Cell Biol. 2004;16(4):379–391. doi: 10.1016/j.ceb.2004.06.009. [DOI] [PubMed] [Google Scholar]