Abstract
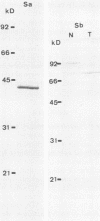
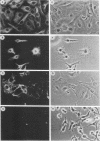
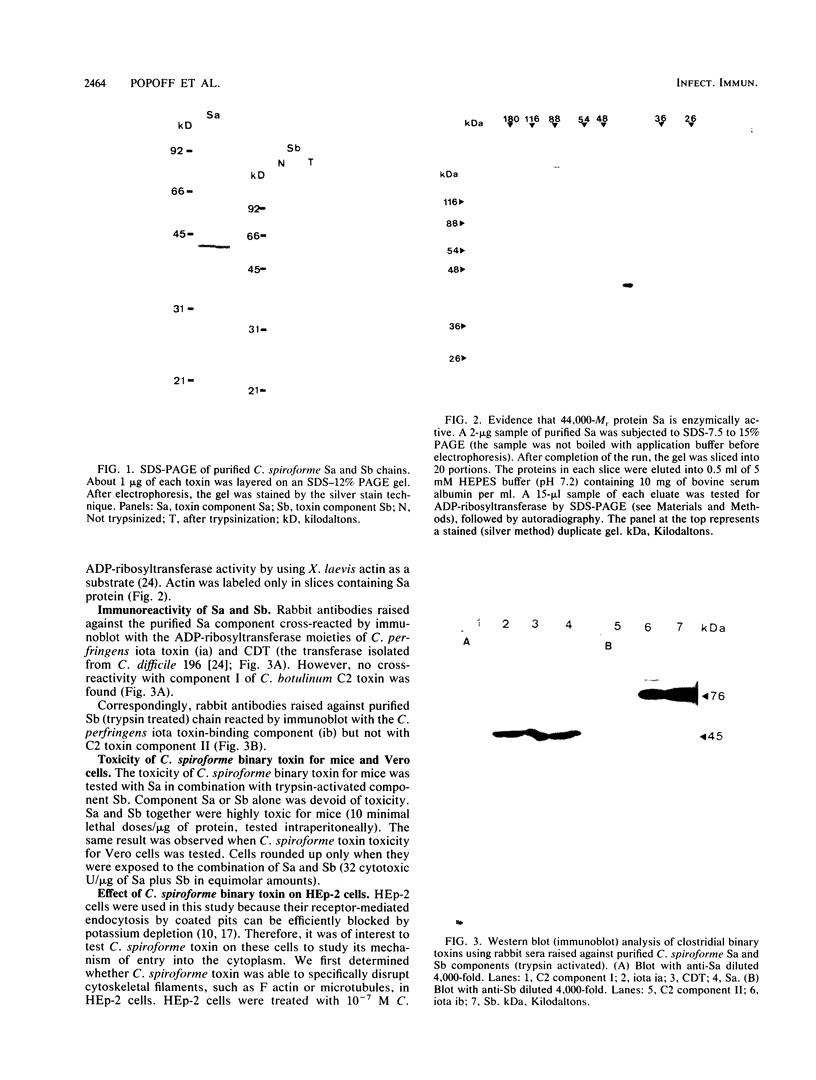
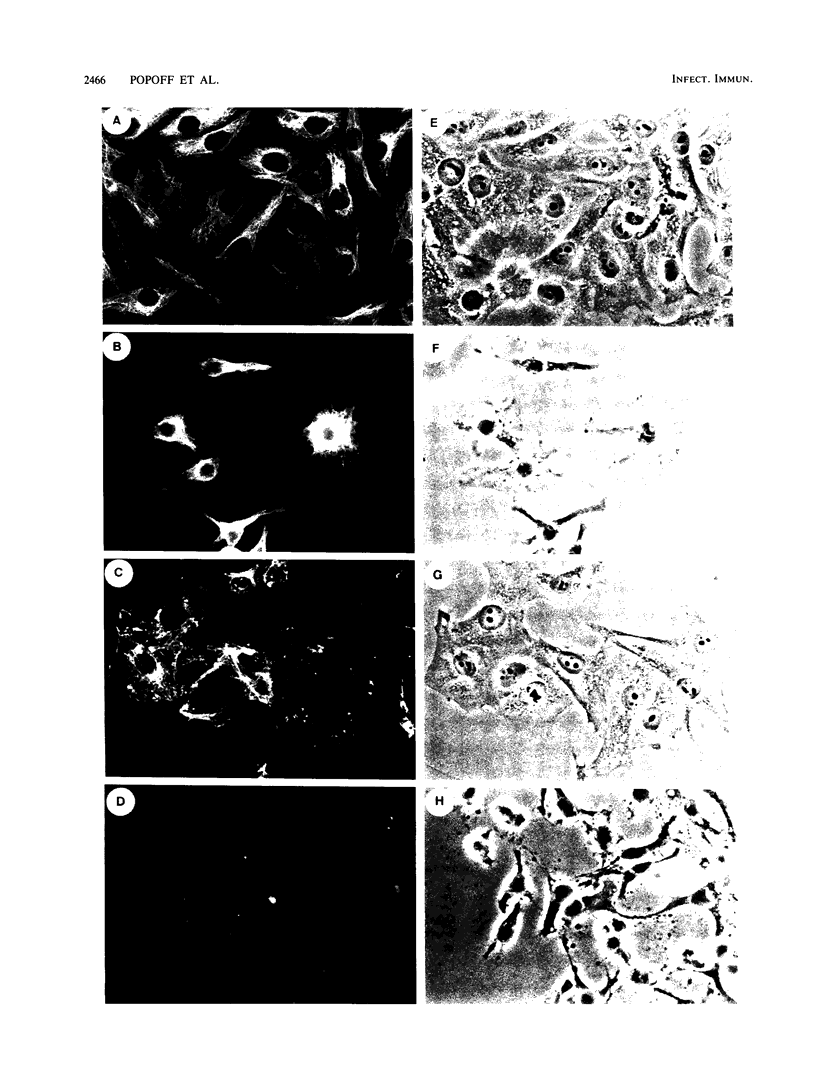
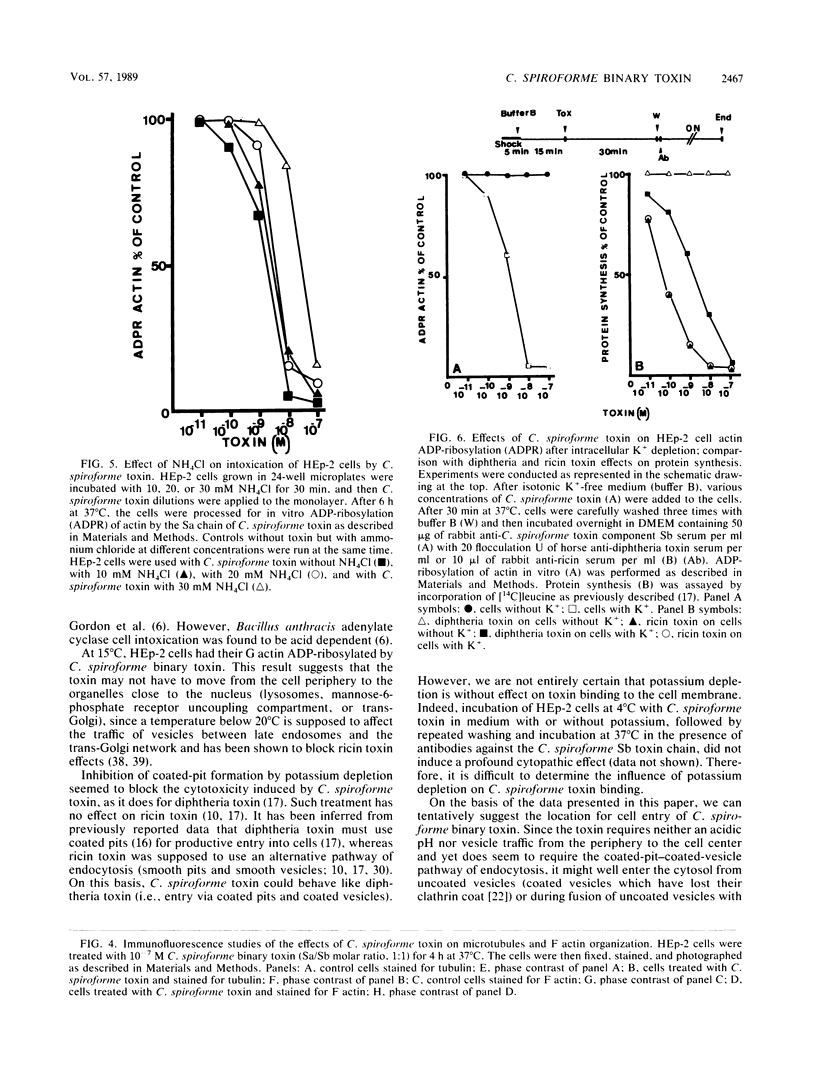
The two components Sa (Mr, 44,000) and Sb (Mr, 92,000) of Clostridium spiroforme toxin were identified and characterized. Serological data permitted the identification of two groups of actin ADP-ribosylating clostridial toxins. The first consists of only C. botulinum C2. The second group includes spiroforme toxin, iota toxin of C. perfringens E, and an enzyme called CDT found in one strain of C. difficile, antibodies against which cross-react with all of the members of both groups. C. spiroforme toxin acted on cells by disrupting microfilaments by ADP-ribosylation of G actin. Toxicity was not blocked by 10 or 20 mM ammonium chloride and was only moderately inhibited by 30 mM NH4Cl. Inhibition of coated-pit formation in HEp-2 cells by potassium depletion strongly protected against the effect of C. spiroforme toxin. Toxicity was not blocked by incubation of HEp-2 cells and spiroforme toxin at 15 degrees C. These results suggest that this new binary toxin enters cells via the coated-pit-coated-vesicle pathway and might reach the cytoplasm at the same time as or before transfer to early endosomes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986 Jul 24;322(6077):390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- Anderson R. G., Pathak R. K. Vesicles and cisternae in the trans Golgi apparatus of human fibroblasts are acidic compartments. Cell. 1985 Mar;40(3):635–643. doi: 10.1016/0092-8674(85)90212-0. [DOI] [PubMed] [Google Scholar]
- Buck C. A., Horwitz A. F. Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol. 1987;3:179–205. doi: 10.1146/annurev.cb.03.110187.001143. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Draper R. K., Simon M. I. The entry of diphtheria toxin into the mammalian cell cytoplasm: evidence for lysosomal involvement. J Cell Biol. 1980 Dec;87(3 Pt 1):849–854. doi: 10.1083/jcb.87.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon V. M., Leppla S. H., Hewlett E. L. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect Immun. 1988 May;56(5):1066–1069. doi: 10.1128/iai.56.5.1066-1069.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Hoflack B., Simons K., Mellman I., Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988 Feb 12;52(3):329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986 Oct 24;234(4775):438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- Kim K., Groman N. B. In vitro inhibition of diphtheria toxin action by ammonium salts and amines. J Bacteriol. 1965 Dec;90(6):1552–1556. doi: 10.1128/jb.90.6.1552-1556.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madshus I. H., Sandvig K., Olsnes S., van Deurs B. Effect of reduced endocytosis induced by hypotonic shock and potassium depletion on the infection of Hep 2 cells by picornaviruses. J Cell Physiol. 1987 Apr;131(1):14–22. doi: 10.1002/jcp.1041310104. [DOI] [PubMed] [Google Scholar]
- Marnell M. H., Shia S. P., Stookey M., Draper R. K. Evidence for penetration of diphtheria toxin to the cytosol through a prelysosomal membrane. Infect Immun. 1984 Apr;44(1):145–150. doi: 10.1128/iai.44.1.145-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Bolzau E., Helenius A. Penetration of Semliki Forest virus from acidic prelysosomal vacuoles. Cell. 1983 Mar;32(3):931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Maxfield F. R. Weak bases and ionophores rapidly and reversibly raise the pH of endocytic vesicles in cultured mouse fibroblasts. J Cell Biol. 1982 Nov;95(2 Pt 1):676–681. doi: 10.1083/jcb.95.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekada E., Uchida T., Okada Y. Methylamine stimulates the action of ricin toxin but inhibits that of diphtheria toxin. J Biol Chem. 1981 Feb 10;256(3):1225–1228. [PubMed] [Google Scholar]
- Mellman I., Fuchs R., Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Morris R. E., Gerstein A. S., Bonventre P. F., Saelinger C. B. Receptor-mediated entry of diphtheria toxin into monkey kidney (Vero) cells: electron microscopic evaluation. Infect Immun. 1985 Dec;50(3):721–727. doi: 10.1128/iai.50.3.721-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya M., Dautry-Varsat A., Goud B., Louvard D., Boquet P. Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. J Cell Biol. 1985 Aug;101(2):548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I. Activation of botulinum C2 toxin by trypsin. Infect Immun. 1987 Jun;55(6):1461–1465. doi: 10.1128/iai.55.6.1461-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Tsuyama S. ADP-ribosylation of nonmuscle actin with component I of C2 toxin. Biochem Biophys Res Commun. 1986 Apr 29;136(2):802–806. doi: 10.1016/0006-291x(86)90511-5. [DOI] [PubMed] [Google Scholar]
- Olsnes S., Sandvig K. How protein toxins enter and kill cells. Cancer Treat Res. 1988;37:39–73. doi: 10.1007/978-1-4613-1083-9_4. [DOI] [PubMed] [Google Scholar]
- Popoff M. R., Boquet P. Clostridium spiroforme toxin is a binary toxin which ADP-ribosylates cellular actin. Biochem Biophys Res Commun. 1988 May 16;152(3):1361–1368. doi: 10.1016/s0006-291x(88)80435-2. [DOI] [PubMed] [Google Scholar]
- Popoff M. R., Rubin E. J., Gill D. M., Boquet P. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect Immun. 1988 Sep;56(9):2299–2306. doi: 10.1128/iai.56.9.2299-2306.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuner K. H., Presek P., Boschek C. B., Aktories K. Botulinum C2 toxin ADP-ribosylates actin and disorganizes the microfilament network in intact cells. Eur J Cell Biol. 1987 Feb;43(1):134–140. [PubMed] [Google Scholar]
- Rubin E. J., Gill D. M., Boquet P., Popoff M. R. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988 Jan;8(1):418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Brown J. E. Ionic requirements for entry of Shiga toxin from Shigella dysenteriae 1 into cells. Infect Immun. 1987 Feb;55(2):298–303. doi: 10.1128/iai.55.2.298-303.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Brown J. E., Petersen O. W., van Deurs B. Endocytosis from coated pits of Shiga toxin: a glycolipid-binding protein from Shigella dysenteriae 1. J Cell Biol. 1989 Apr;108(4):1331–1343. doi: 10.1083/jcb.108.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Fuchs R., Male P., Mellman I. Two distinct subpopulations of endosomes involved in membrane recycling and transport to lysosomes. Cell. 1988 Jan 15;52(1):73–83. doi: 10.1016/0092-8674(88)90532-6. [DOI] [PubMed] [Google Scholar]
- Simpson L. L., Stiles B. G., Zepeda H. H., Wilkins T. D. Molecular basis for the pathological actions of Clostridium perfringens iota toxin. Infect Immun. 1987 Jan;55(1):118–122. doi: 10.1128/iai.55.1.118-122.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L., Stiles B. G., Zepeda H., Wilkins T. D. Production by Clostridium spiroforme of an iotalike toxin that possesses mono(ADP-ribosyl)transferase activity: identification of a novel class of ADP-ribosyltransferases. Infect Immun. 1989 Jan;57(1):255–261. doi: 10.1128/iai.57.1.255-261.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles B. G., Wilkins T. D. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon. 1986;24(8):767–773. doi: 10.1016/0041-0101(86)90101-7. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Chaponnier C., Ezzell R. M., Hartwig J. H., Janmey P. A., Kwiatkowski D. J., Lind S. E., Smith D. B., Southwick F. S., Yin H. L. Nonmuscle actin-binding proteins. Annu Rev Cell Biol. 1985;1:353–402. doi: 10.1146/annurev.cb.01.110185.002033. [DOI] [PubMed] [Google Scholar]
- Tycko B., Maxfield F. R. Rapid acidification of endocytic vesicles containing alpha 2-macroglobulin. Cell. 1982 Mar;28(3):643–651. doi: 10.1016/0092-8674(82)90219-7. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Petersen O. W., Olsnes S., Sandvig K. Delivery of internalized ricin from endosomes to cisternal Golgi elements is a discontinuous, temperature-sensitive process. Exp Cell Res. 1987 Jul;171(1):137–152. doi: 10.1016/0014-4827(87)90257-6. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Sandvig K., Petersen O. W., Olsnes S., Simons K., Griffiths G. Estimation of the amount of internalized ricin that reaches the trans-Golgi network. J Cell Biol. 1988 Feb;106(2):253–267. doi: 10.1083/jcb.106.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]