Abstract
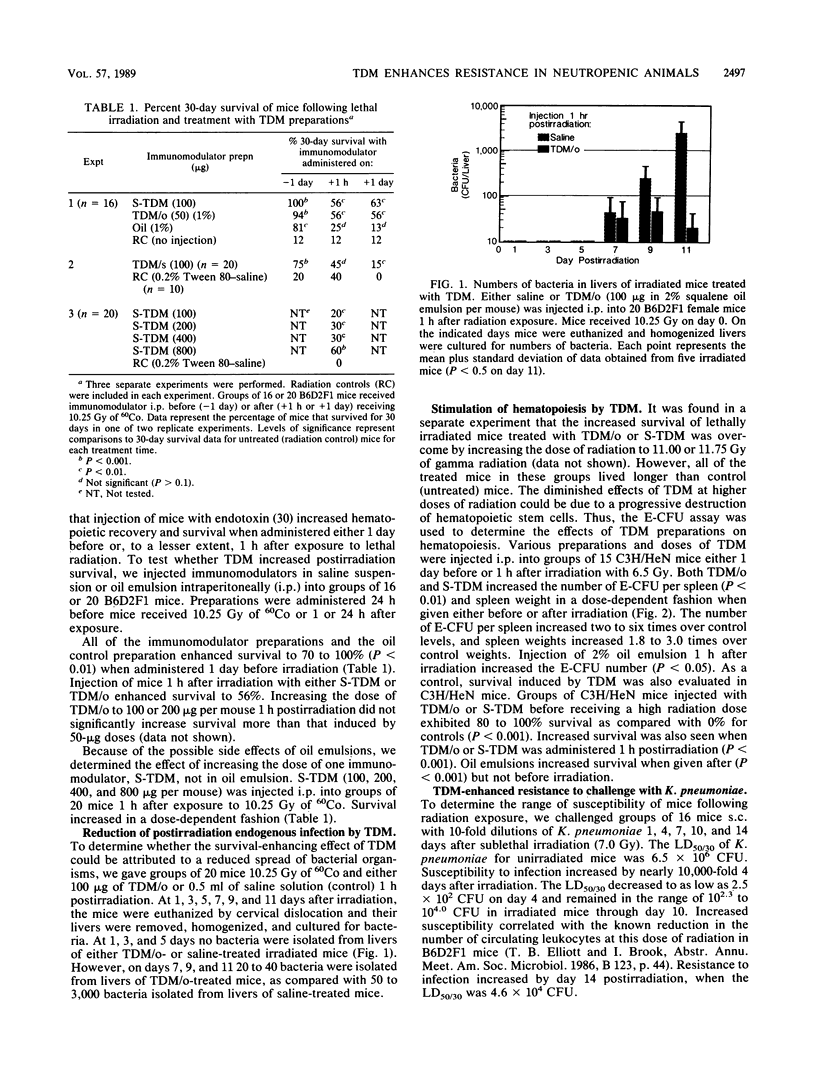
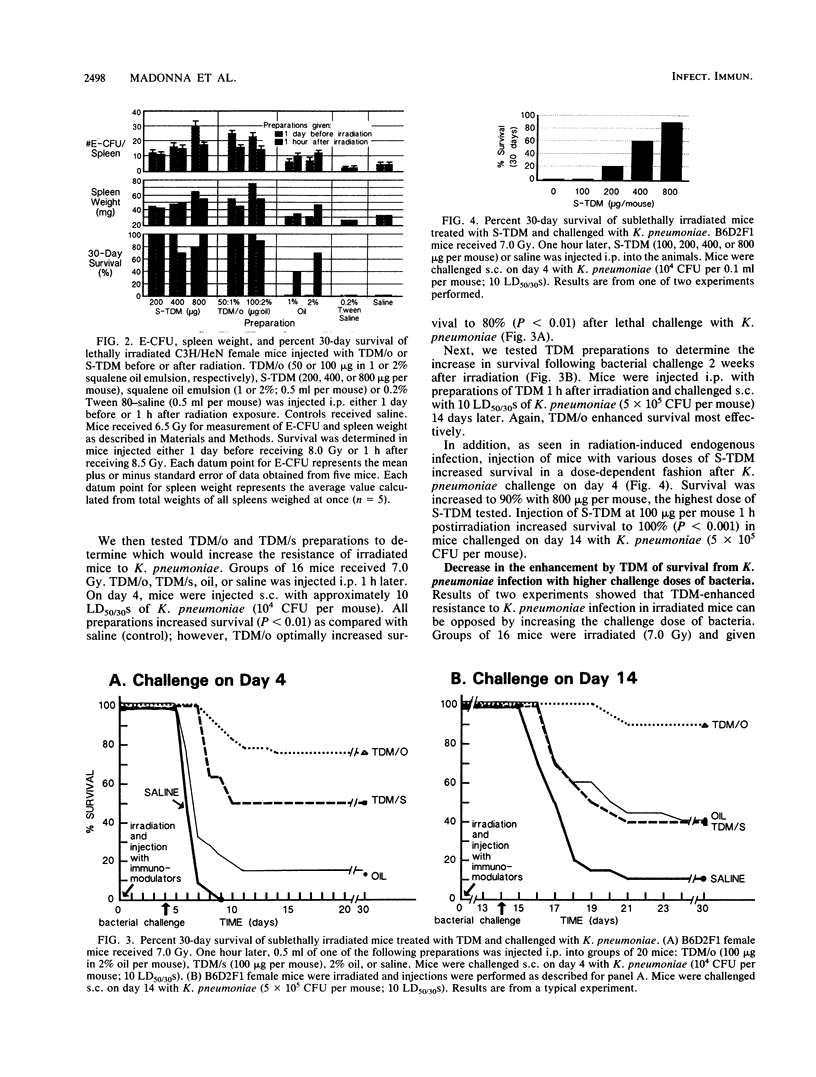
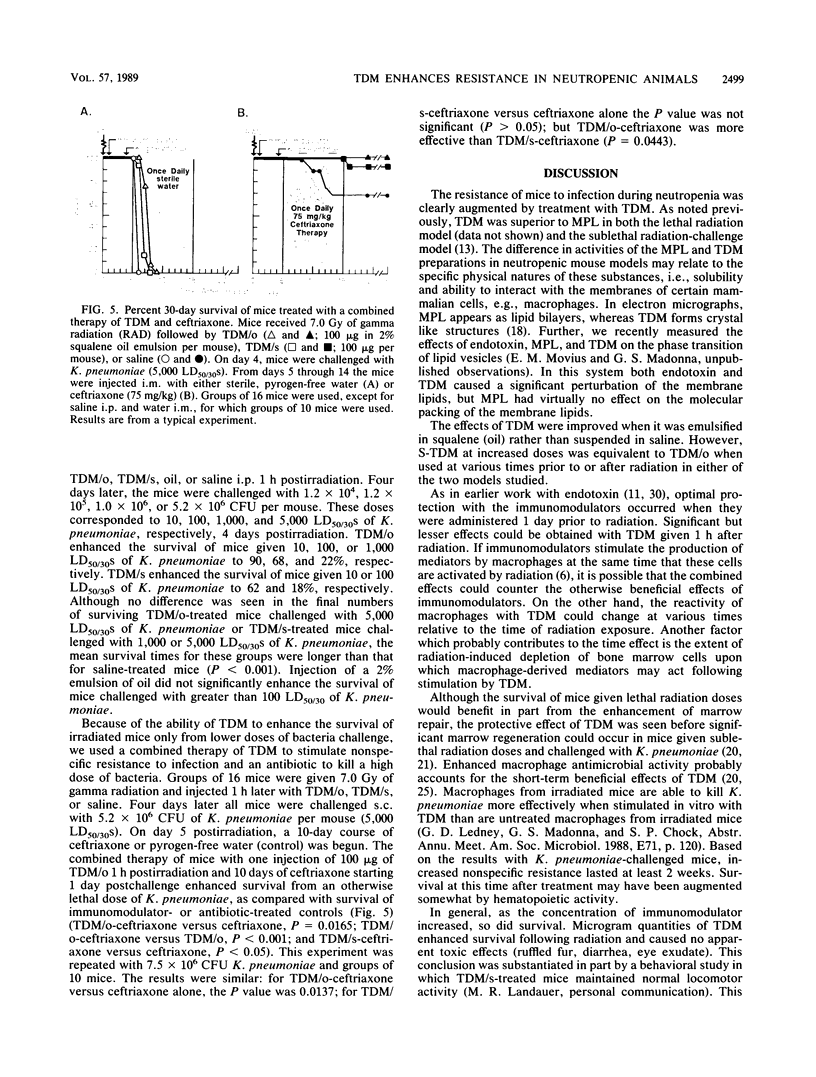
Bacterial infections are lethal complications of neutropenia, and antibiotics alone are inadequate therapy for these infections. Irradiated mice become severely neutropenic and remain susceptible to infection for 2 to 3 weeks, depending on the dose and quality of radiation. Some bacterial cell wall derivatives stimulate nonspecific host defense mechanisms against a variety of microbes which might cause postirradiation infection. In this study we determined if the cell wall glycolipid trehalose dimycolate (TDM), derived from Mycobacterium phlei, or a synthetic preparation of TDM was able to (i) enhance survival in mice when given before or after lethal doses of 60Co radiation and (ii) increase nonspecific resistance to postirradiation infection. Treatment with TDM oil-in-water emulsions and with synthetic TDM significantly enhanced survival before and after lethal doses of 60Co irradiation. This result correlated with the ability of TDM to reduce the translocation of intestinal bacteria and to stimulate hematopoiesis. With respect to nonspecific resistance to infection, TDM injected 1 h after sublethal irradiation increased resistance to a lethal Klebsiella pneumoniae challenge (10 50% lethal doses of K. pneumoniae in 30 days [LD50/30]) 4 or 14 days later. Increasing the dose of K. pneumoniae to 5,000 LD50/30 on day 4 overwhelmed the ability of TDM-treated mice to overcome infection. However, TDM treatment 1 h postirradiation combined with ceftriaxone antibiotic therapy (days 5 through 14) enhanced survival, even when the higher dose of bacteria (5,000 LD50/30) was used. These results indicate that in irradiated mice, TDM can be used to enhance survival and, as a potent stimulant of nonspecific resistance to infection in neutropenic mice, can act synergistically with antibiotic therapy to reduce sepsis and mortality.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper T. Low oxygen enhancement ratios for radiosensitive bacterial strains, and the probable interaction of two types of primary lesion. Nature. 1968 Mar 2;217(5131):862–863. doi: 10.1038/217862a0. [DOI] [PubMed] [Google Scholar]
- Azuma I., Ribi E. E., Meyer T. J., Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. 1974 Jan;52(1):95–101. doi: 10.1093/jnci/52.1.95. [DOI] [PubMed] [Google Scholar]
- Batist G., Reynaud A., Katki A. G., Travis E. L., Shoemaker M. C., Greene R. F., Myers C. E. Enzymatic defense against radiation damage in mice. Effect of selenium and vitamin E depletion. Biochem Pharmacol. 1986 Feb 15;35(4):601–606. doi: 10.1016/0006-2952(86)90354-0. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Knuppel R. A., Shepherd J. H., Anderson R., Rao P. S. Septic shock and the obstetrician/gynecologist. South Med J. 1982 Jul;75(7):809–813. doi: 10.1097/00007611-198207000-00010. [DOI] [PubMed] [Google Scholar]
- Geiger B., Gallily R. Effect of X-irradiation on various functions of murine macrophages. Clin Exp Immunol. 1974 Apr;16(4):643–655. [PMC free article] [PubMed] [Google Scholar]
- KAPLAN H. S., SPECK R. S., JAWETZ E. Impairment of antimicrobial defense following total body irradiation of mice. J Lab Clin Med. 1952 Nov;40(5):682–691. [PubMed] [Google Scholar]
- Kierszenbaum F., Zenian A., Wirth J. J. Macrophage activation by cord factor (trehalose 6,6'-dimycolate): enhanced association with and intracellular killing of Trypanosoma cruzi. Infect Immun. 1984 Feb;43(2):531–535. doi: 10.1128/iai.43.2.531-535.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEDNEY G. D., WILSON R. PROTECTION INDUCED BY BACTERIAL ENDOTOXIN AGAINST WHOLE-BODY X-IRRADIATION IN GERMFREE AND CONVENTIONAL MICE. Proc Soc Exp Biol Med. 1965 Apr;118:1062–1065. doi: 10.3181/00379727-118-30046. [DOI] [PubMed] [Google Scholar]
- Landauer M. R., Davis H. D., Dominitz J. A., Weiss J. F. Dose and time relationships of the radioprotector WR-2721 on locomotor activity in mice. Pharmacol Biochem Behav. 1987 Jul;27(3):573–576. doi: 10.1016/0091-3057(87)90370-4. [DOI] [PubMed] [Google Scholar]
- Lemaire G., Tenu J. P., Petit J. F., Lederer E. Natural and synthetic trehalose diesters as immunomodulators. Med Res Rev. 1986 Jul-Sep;6(3):243–274. doi: 10.1002/med.2610060302. [DOI] [PubMed] [Google Scholar]
- Madonna G. S., Peterson J. E., Ribi E. E., Vogel S. N. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986 Apr;52(1):6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masihi K. N., Lange W., Brehmer W., Ribi E. Immunobiological activities of nontoxic lipid A: enhancement of nonspecific resistance in combination with trehalose dimycolate against viral infection and adjuvant effects. Int J Immunopharmacol. 1986;8(3):339–345. doi: 10.1016/0192-0561(86)90116-5. [DOI] [PubMed] [Google Scholar]
- Numata F., Nishimura K., Ishida H., Ukei S., Tone Y., Ishihara C., Saiki I., Sekikawa I., Azuma I. Lethal and adjuvant activities of cord factor (trehalose-6,6'-dimycolate) and synthetic analogs in mice. Chem Pharm Bull (Tokyo) 1985 Oct;33(10):4544–4555. doi: 10.1248/cpb.33.4544. [DOI] [PubMed] [Google Scholar]
- Olds G. R., Chedid L., Lederer E., Mahmoud A. A. Induction of resistance to Schistosoma mansoni by natural cord factor and synthetic lower homologues. J Infect Dis. 1980 Apr;141(4):473–478. doi: 10.1093/infdis/141.4.473. [DOI] [PubMed] [Google Scholar]
- Parant M., Parant F., Chedid L., Drapier J. C., Petit J. F., Wietzerbin J., Lederer Enhancement of nonspecific immunity to bacterial infection by cord factor (6,6'-trehalose dimycolate). J Infect Dis. 1977 May;135(5):771–777. doi: 10.1093/infdis/135.5.771. [DOI] [PubMed] [Google Scholar]
- Patchen M. L., D'Alesandro M. M., Brook I., Blakely W. F., MacVittie T. J. Glucan: mechanisms involved in its "radioprotective" effect. J Leukoc Biol. 1987 Aug;42(2):95–105. doi: 10.1002/jlb.42.2.95. [DOI] [PubMed] [Google Scholar]
- Patchen M. L., MacVittie T. J., Wathen L. M. Effects of pre- and post-irradiation glucan treatment on pluripotent stem cells, granulocyte, macrophage and erythroid progenitor cells, and hemopoietic stromal cells. Experientia. 1984 Nov 15;40(11):1240–1244. doi: 10.1007/BF01946654. [DOI] [PubMed] [Google Scholar]
- Qureshi N., Takayama K., Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982 Oct 10;257(19):11808–11815. [PubMed] [Google Scholar]
- Ribi E., Cantrell J. L., Takayama K., Qureshi N., Peterson J., Ribi H. O. Lipid A and immunotherapy. Rev Infect Dis. 1984 Jul-Aug;6(4):567–572. doi: 10.1093/clinids/6.4.567. [DOI] [PubMed] [Google Scholar]
- SMITH W. W., ALDERMAN I. M., GILLESPIE R. E. Hematopoietic recovery induced by bacterial endotoxin in irradiated mice. Am J Physiol. 1958 Mar;192(3):549–556. doi: 10.1152/ajplegacy.1958.192.3.549. [DOI] [PubMed] [Google Scholar]
- Stewart D. A., Ledney G. D., Baker W. H., Daxon E. G., Sheehy P. A. Bone marrow transplantation of mice exposed to a modified fission neutron (N/G-30:1) field. Radiat Res. 1982 Nov;92(2):268–279. [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. Early repair processes in marrow cells irradiated and proliferating in vivo. Radiat Res. 1963 Jan;18:96–105. [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Ribi E., Cantrell J. L. Separation and characterization of toxic and nontoxic forms of lipid A. Rev Infect Dis. 1984 Jul-Aug;6(4):439–443. doi: 10.1093/clinids/6.4.439. [DOI] [PubMed] [Google Scholar]
- Tenu J. P., Lederer E., Petit J. F. Stimulation of thymocyte mitogenic protein secretion and of cytostatic activity of mouse peritoneal macrophages by trehalose dimycolate and muramyldipeptide. Eur J Immunol. 1980 Aug;10(8):647–653. doi: 10.1002/eji.1830100813. [DOI] [PubMed] [Google Scholar]
- Vosika G. J., Gray G. R. Phase I study of iv mycobacterial cell wall skeleton and cell wall skeleton combined with trehalose dimycolate. Cancer Treat Rep. 1983 Sep;67(9):785–790. [PubMed] [Google Scholar]
- Walker R. I., Ledney G. D., Galley C. B. Aseptic endotoxemia in radiation injury and graft-vs-host diesease. Radiat Res. 1975 May;62(2):242–249. [PubMed] [Google Scholar]
- Yarkoni E., Bekierkunst A. Nonspecific resistance against infection with Salmonella typhi and Salmonella typhimurium induced in mice by cord factor (trehalose-6,6'-dimycolate) and its analogues. Infect Immun. 1976 Nov;14(5):1125–1129. doi: 10.1128/iai.14.5.1125-1129.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni E., Wang L., Bekierkunst A. Stimulation of macrophages by cord factor and by heat-killed and living BCG. Infect Immun. 1977 Apr;16(1):1–8. doi: 10.1128/iai.16.1.1-8.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman J. J., Dietrich K. A. Current perspectives on septic shock. Pediatr Clin North Am. 1987 Feb;34(1):131–163. doi: 10.1016/s0031-3955(16)36186-7. [DOI] [PubMed] [Google Scholar]




