Abstract
Purpose
Factors affecting the severity of radiation-induced thrombocytopenia (RIT) are not well-described. We address whether PF4 (a negative paracrine for megakaryopoiesis) affects platelet recovery post-radiation.
Materials and Methods
Using conditioned media from irradiated bone marrow (BM) cells from transgenic mice overexpressing human (h) PF4 (hPF4+), megakaryocyte colony formation was assessed in the presence of this conditioned media and PF4 blocking agents. In a model of radiation-induced thrombocytopenia, irradiated mice with varying PF4 expression levels were treated with anti-hPF4 and/or thrombopoietin (TPO) and platelet count recovery and survival were examined.
Results
Conditioned media from irradiated BM from hPF4+ mice inhibited megakaryocyte colony formation, suggesting that PF4 is a negative paracrine released in RIT. Blocking with an anti-hPF4 antibody restored colony formation of BM grown in the presence of hPF4+ irradiated media as did antibodies that block the megakaryocyte receptor for PF4, Low Density Lipoprotein Receptor Related Protein 1 (LRP1). Irradiated PF4 knockout (KO) mice had higher nadir platelet counts than irradiated hPF4+/KO littermates (651 vs. 328 × 106/mcL, p=0.02) and recovered earlier (15 days vs. 22 days, respectively, p<0.02). When irradiated hPF4+ mice were treated with anti-hPF4 antibody and/or (TPO), they showed less severe thrombocytopenia than untreated, with improved survival and time to platelet recovery, but no additive effect was seen.
Conclusions
Our studies show that in RIT, damaged megakaryocytes release PF4 locally, inhibiting platelet recovery. Blocking PF4 enhances recovery while released PF4 from megakaryocytes limits TPO efficacy, potentially due to increased release of PF4 stimulated by TPO. The clinical value of blocking this negative paracrine pathway post-RIT remains to be determined.
Keywords: Radiation-induced thrombocytopenia, cytokines, chemokines, platelet factor 4, thrombopoietin
Introduction
Megakaryopoiesis is a complex process that is predominantly positively regulated by TPO. However, many other cytokines have been suggested to participate in this process as well(1, 2, 3). In vitro studies of α-granule chemokines have suggested an inhibitory pathway that results in downregulation of megakaryopoiesis(4, 5, 6, 7). We have shown that the abundant platelet α-granule chemokine, PF4, is a physiologic negative paracrine in in vivo murine studies under steady-state conditions and in CIT(4). The mechanism by which PF4 inhibits megakaryocyte development involves binding to surface (LRP1) transiently expressed during megakaryopoiesis(8).
RIT is a significant cause of morbidity and mortality(9). In patients receiving radiation therapy, thrombocytopenia can result in delays of therapy and significant bleeding requiring transfusion of both platelets and packed red blood cells(10). Additionally, in radiation injured persons, bleeding and thrombocytopenia are directly responsible for significant mortality(11, 12). Some studies have shown that platelet count correlates better with survival after radiation exposure than white blood cell count(13). In an era of greater concerns of untoward radiation exposure by the general population, strategies to treat or prevent RIT have gained additional attention and strategies to easily improve survival are needed.
Since we have shown that PF4 levels play an important role in CIT(4), we asked whether a similar effect may be seen in RIT. The recent availability of TPO-receptor (TPO-R) agonists(14, 15) suggests that strategies to treat patients with RIT with such drugs would be efficacious. How a negative feedback loop would affect such therapy and whether a combined therapy would be more efficacious have not been addressed.
Below, we demonstrate that endogenous PF4 levels affect platelet count recovery after radiation-induced injury. Using media conditioned with irradiated BM cells we show that PF4 is the major detectable inhibitor of megakaryopoiesis in our assay. Blocking PF4 increases megakaryopoiesis in vitro and increases platelet counts in vivo, and defines a potential alternative strategy to improve platelet counts post-RIT. Indeed, in an in vivo radiation-induced injury model, treatment with anti-PF4 strategies was as efficacious as treatment with TPO, but surprisingly did not show an additive effect. The clinical implications of these studies are presented.
Material and Methods
Transgenic mice
Animal lines have been described previously, and include homozygous PF4 KO mice generated by replacing the entire coding region for mouse (m) Cxcl4 (also known as Pf4 or Scyb4, LOC56744) (1.2 kb) with a 1.8 kb neomycin resistance gene(16) and a transgenic mouse line that overexpresses human (h) PF4(17). The hPF4+ animals used in the described studies are transgenic with a 10-kb fragment of the human PF4 locus with 5.4 kb of upstream and 3.8 kb of downstream sequence and contain >2-fold the amount of PF4 as human controls(17). All PF4 variant animals had been backcrossed onto a C57BL/6J background for >10 generations. Additionally, hPF4+ animals were then bred with KO animals (hPF4+/KO) and comparative studies for hPF4+/KO and KO were done using littermate controls. PF4 levels were measured by using 100 µL of whole blood collected by retroorbital bleed and centrifuged at 100g for 10 minutes to produce platelet-rich plasma (PRP). The platelets in the PRP were lysed by using 3 rapid free/thaw cycles (dry ice for followed by 37°C water bath each for 10 mins) and then centrifuged at 2000g for 5 minutes to pellet cellular debris. The supernatant was analyzed for PF4 by ELISA (Asserachrome PF4, Diagnostico Stago, Parsippany, NJ) performed according to manufacturer’s instructions.
The mice were housed at the Children’s Hospital of Philadelphia animal facility. All procedures were performed after approval by our institution’s animal use and care committee.
Human intra-individual variability of PF4 studies
Under an IRB approved protocol to study the effect of PF4 on human platelet counts, we collected serial blood samples (2–12 months apart) from 10 healthy pediatric subjects. Whole blood was treated as described for murine samples above and total PF4 levels were analyzed using the Asserachrome PF4 ELISA kit.
In vitro BM mononuclear cell culture (BMMNC) studies
BM from the tibias and femurs of 6–12 week old male mice was isolated and used for in vitro cell culture as previously described(18). Briefly, Iscove’s Modified Dulbecco’s Medium (IMDM; Invitrogen, Carlsbad, CA) without modification was used to flush the marrow cavity of bones harvested from sacrificed mice, and the cells were then passed over a 100-µm nylon filter (BD Biosciences, San Jose, CA). After centrifugation at 1000g, the cell pellet was resuspended in IMDM + 1% Penicillin-Streptomycin (Invitrogen) at a concentration of 2.2 × 106 cells/mL. The cells were then split into two aliquots. One aliquot of cells was irradiated using the MDS Nordion Gammator M38 to a dose of 2261 cGy at a dose rate of 0.2 Gy/min. These cells were allowed to rest for 30 mins after irradiation and then were centrifuged at 1000g for 10 mins to remove cells and cellular debris. The supernatant was then added to cultures of the second aliquot of cells. PF4 levels in the supernatant and conditioned media from routinely cultured BM cells were measured by PF4 ELISA according to manufacturer’s protocol.
Cells were then cultured in a commercially available murine, serum-free BMMNC colony culture system (MegaCult-C, Stem Cell Technologies, Vancouver, Canada). Irradiated cell lysate (10% concentration) was added to some BM cultures. No supportive stromal cells were included in the cultures. In some studies, either rabbit polyclonal anti-PF4 antibodies or rabbit pre-treatment IgG control (Sigma-Genosys, St. Louis, MO) (each at 25 µg/mL) or anti-LRP1 light chain antibody (MA5A6, Molecular Innovations, Novi, MI) (25 µg/mL) was added.
Double-chamber slides were cultured, dried and stained for acetylcholinesterase to mark megakaryocytes per Megacult-C kit protocol. The slides were analyzed by counting the total number of colonies per chamber in addition to the number of colonies showing positive staining for megakaryocytes. A ratio of megakaryocyte-containing colonies/total colonies was calculated for each well and results from duplicate wells were averaged.
Preparation of antibodies for injection
Rabbit anti-human hPF4 serum was obtained from Sigma-Genosys. Control, pre-immunization serum was also provided from the immunized animals. The IgG fraction was purified from the rabbit serum using a Protein G column as previously described(4). A sepharose column with bound hPF4 was prepared to purify anti-hPF4 antibodies from the IgG fraction as previously described(4). These antibodies were then concentrated in phosphate buffered saline (PBS; Invitrogen) using Amicon centrifugation tubes (Millipore, Billerica, MA). The purified antibodies were used in tissue culture experiments. For in vivo studies, F(ab’)2 fragments were prepared from the purified anti-hPF4 antibodies using the ImmunoPure F(ab’)2 kit according to manufacturer instructions (Pierce Bioscience, Rockford, IL). Similar procedures were used to produce anti-murine (m) PF4 for some of the in vivo studies. Endotoxin levels were <1 U per 250 mcg by the LAL method (Cambrex, East Rutherford, NJ).
In vivo studies of platelet counts and response to TPO or antiPF4 strategies
Eight-twelve week old female hPF4+/KO, KO or WT mice were irradiated at 660 cGy in a single dose using an X-RAD 320 biological irradiator (Precision Xray, North Bradford, CT) through a fixed beam at a dose rate of 3 Gy/min at 320kV to animals in a multichamber holder held at a fixed distance from the irradiation source. Twenty minutes after irradiation, animals were injected by tail vein with 250 mg/kg F(ab’)2 fragments of either rabbit control IgG or rabbit anti-human PF4 IgG. Additionally, some animals received treatment with 100 µg/kg murine recombinant TPO (mTPO, R&D systems) by tail vein injection. A second dose of F(ab’)2 fragments ± TPO was given on day 4 (hPF4+/KO animals) or 3 (WT animals) after irradiation. Platelet counts were measured in blood obtained by retro-orbital bleed. Animals were anesthetized and 50 µL of EDTA whole blood was obtained by retro-orbital puncture for complete blood counts measured in an automatic cell counter (HEMAVET, Drew Scientific, Dallas, TX) set for mouse parameters. The MDS Nordion Gammator M38 was used for baseline response to radiation studies where mice were placed in groups of 3 in a canister placed on a turntable and treated with 660 cGy.
Statistics
Differences between groups were compared using the Student’s t-test or one-way ANOVA with Bonferroni’s multiple comparison test as appropriate. The Kusker-Wallis test for inequality was used to compare different treatment arms where appropriate. Statistical analyses were performed using Graph Prism 6 (GraphPad software, La Jolla, CA) or STAT (StatCorp LP, College Station, TX). Differences were considered significant at p values of ≤ 0.05. Sample size calculations showed 83% power to detect a 50% difference in survival at a p<0.05 with 15 animals per arm. Smaller sample sizes were used when statistical significance was reached prior to the 15 animal accrual mark.
Results
In vitro BMMNC studies
Previous studies have shown that exogenous PF4 is able to decrease megakaryocyte colony formation by about 50%(4, 6, 8, 19). Blocking PF4’s effect with either anti-PF4 or anti-LRP1 antibodies can restore megakaryocyte colony formation in the presence of exogenous, recombinant PF4(4, 8). We now ask whether radiation damage leads to release of PF4 into the media of cultured megakaryocytes, and whether this would have an inhibitory effect on further megakaryocyte colony formation. PF4 levels were 10.9±1.8×103 IU/mL for hPF4+/KO-irradiated media compared to 0.22±1.5×103 IU/mL found in regular conditioned media from hPF4+/KO mice. PF4 was not detected in KO conditioned media or KO irradiated cell lysates. When we added hPF4+/KO conditioned media to bone marrow of either KO (Fig 1A) or hPF4+/KO (Fig 1B) mice, we found a significant decrease in megakaryocyte colony formation in both (p<0.001 and p<0.03 respectively), consistent with PF4 being released by irradiation of the cells. When KO conditioned media was added, this inhibitory effect was not seen (Figs 1 A and B), suggesting that the major in vitro inhibitory agent released from the irradiated megakaryocytes is the PF4.
Figure 1. In vitro effect of irradiated cell lysates on megakaryocyte colony formation.
(A) and (B) Effect on megakaryocyte colony numbers of indicated conditioned media from irradiated BM on megakaryocyte colony formation on KO BM (A) and hPF4+/KO BM (B). WT = wildtype BM cells used as a control. N = 4 separate experiments performed in duplicate. Mean ± 1 standard error (SE) is shown. * = p <0.01 compared to no added conditioned media or KO media, ** = p <0.01 compared to WT BM. (C) and (D) Blocking the effects of irradiated hPF4+/KO conditioned media on megakaryocyte colony formation from KO BM (C) and hPF4+/KO BM (D). N = 3 separate experiments performed in duplicate. Mean ± 1 standard error (SE) is shown. (C) p < 0.05 by ANOVA. (D) * = p <0.01 compared to added conditioned media. In (C) and (D), a-LRP is anti-LRP1 antibody and a-PF4 is anti-hPF4 antibody.
If the predominant megakaryocyte colony-suppressing molecule released from irradiated megakaryocytes is PF4, then blocking PF4’s effect should restore colony formation in this in vitro system. Addition of specific anti-PF4 or anti-LRP1 antibodies was able to restore KO colony formation (Fig. 1C) in the presence of PF4 and increased hPF4+/KO colony formation above baseline (Fig. 1D) likely due to blocking endogenous PF4 released by these cells in addition the added PF4. These findings further support that PF4 is the major mediator of megakaryocyte colony formation in the conditioned media from irradiated BM.
In vivo studies of the effect of endogenous PF4 levels in irradiation injury
To test the hypothesis that total platelet PF4 levels are stable over time and not affected by bone marrow injury, we examined platelet PF4 levels over time in two scenarios: First, in animals after recovery from irradiation, we found that PF4/platelet in hPF4+/KO animals did not significantly vary with time even in the setting of irradiation (Fig. e1A); Second, in healthy pediatric subjects, total platelet PF4 levels did not vary within the individual over time, despite significant inter-individual variability (Fig. e1B). KO animals did not have detectable PF4 levels.
To see if our in vitro observations translated to relevant in vivo effects in the setting of radiation injury, we studied the effect of irradiation on platelet count recovery in the two transgenic animal lines. We have previously shown that the level of PF4 content in developing megakaryocytes affects platelet count recovery after chemotherapy.(4) Because irradiation affects not only mature cells, but also progenitor and stem cells, we wanted to see if a similar relationship would be observed if bone marrow injury was the result of irradiation.
Irradiation of animals demonstrated an inverse relationship between platelet PF4 content and platelet count recovery in RIT (Fig 2A). Animals that over-expressed PF4, hPF4+/KO, recovered significantly later than KO littermates with a mean time to recovery of 22.6 ± 1.9 days versus 15.1 ± 0.6 days (p<0.02). Even when corrected for differences in baseline platelet count, the platelet counts were significantly lower in hPF4+/KO animals on days 7 and 12. Consistent with the inverse relationship between PF4 level and platelet count recovery, survival in the hPF4+/KO animals was significantly worse than that of KO animals (Fig 2B) (p<0.05). The etiology of the increased mortality in this and the studies noted below is not clear. Hemoglobin levels, as a reflection of ongoing blood loss and bone marrow suppression, were similar in both groups when measured in surviving animals (Fig. e2). However, hemoglobin level was not measured in mice that succumbed so that exacerbation of blood loss in those animals would not have been appreciated.
Figure 2. Effect of endogenous PF4 levels on platelet count recovery after irradiation.
(A) Platelet counts of irradiated mice after 660cGy irradiation delivered in a single dose. Grey boxes represent mPF4 knockout animals (KO) and black circles represent hPF4 over-expressing animals on the knockout background (hPF4+/KO). Shown is mean ± 1 SE. * = p < 0.05 when corrected for differences in baseline platelet count. (B) Survival of irradiated animals in each group. Survival of KO animals (broken line) was significantly better than hPF4+/KO animals (p<0.05) (solid line). n = 17 KO animals and 10 hPF4+/KO animals.
In vivo studies of the effect of anti-PF4 antibodies
To further demonstrate that this difference in recovery was due to the different PF4 levels between these animal groups, we injected hPF4+/KO animals with F(ab’)2 fragments (either control IgG or anti-hPF4). Anti-hPF4 F(ab’)2 treated animals had significantly improved platelet count recovery with a higher nadir platelet count and significantly shorter duration of thrombocytopenia (Fig. 3A) when compared to animals treated with IgG F(ab’)2 (p<0.009). Platelet count recovery was similar to KO littermates studied simultaneously (Fig. 3A). In addition, none of the animals treated with anti-hPF4 F(ab’)2 died during the course of the experiment while 4 animals in the IgG group and 1 KO animal died (Fig. 3B, p<0.05).
Figure 3. In vivo studies on the effect of anti-hPF4 antibodies in irradiated mice.
(A) Recovery of animals treated with antihPF4 antibodies. hPF4+/KO were treated with either Anti-hPF4 F(ab’)2 fragments (grey circles) or control F(ab’)2 fragments (black circles) on day 0 and 4 after irradiation (250 mg/kg by tail vein injection) shown in comparison with KO animals (white squares). N = 8–12 animals per arm. Shown is mean ± 1 SE. p<0.01 for curves. (B) Survival of the three groups of animals. The grey line represents animals treated with anti-hPF4 antibody. These animals had the best survival although not statistically different from KO animals (solid black line). hPF4+/KO animals treated with control antibody had the worst survival (broken line) p<0.02. N = 8–12 animals per arm.
In vivo studies of the effect of TPO and anti-PF4 antibodies
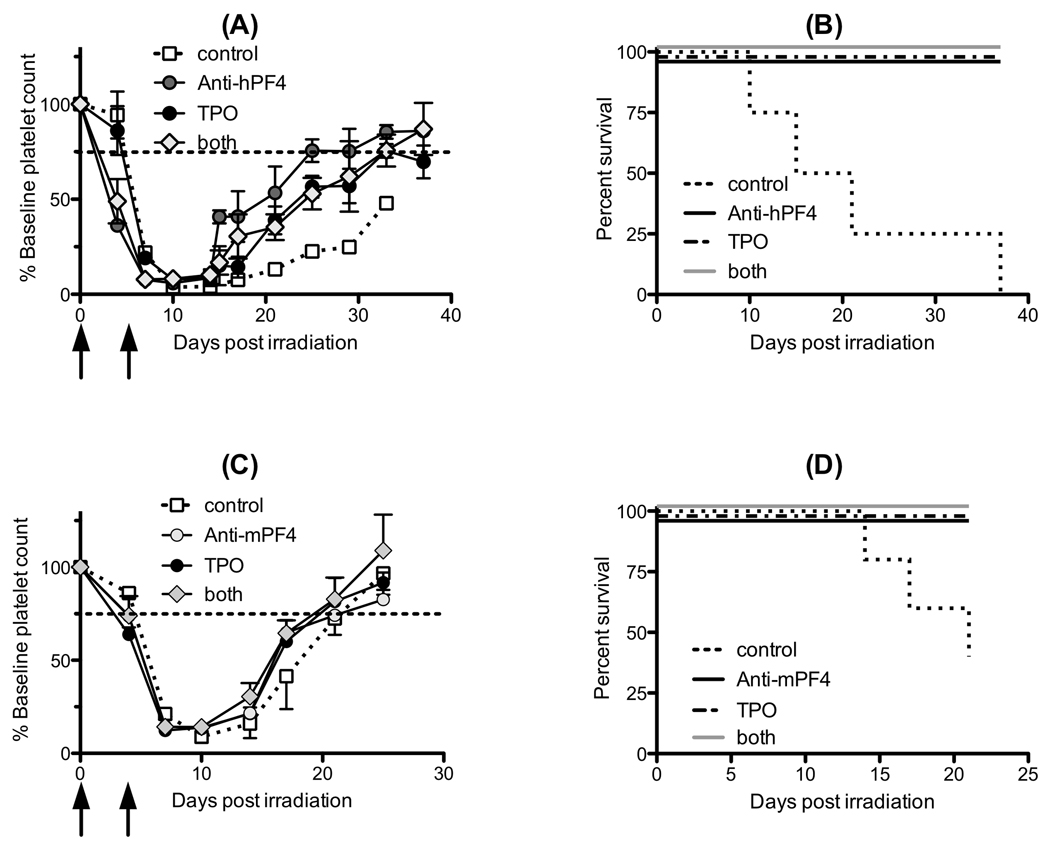
TPO is the major regulator of megakaryopoiesis(20) and clinical trials in the past with recombinant TPO(21) and recently with small molecule TPO-receptor agonists (TPO-mimetics)(14, 15) have shown that platelet count recovery can be enhanced after chemotherapy. Previous murine studies have shown that recombinant mTPO can significantly increase platelet counts(22, 23). We therefore asked whether TPO would be synergistic with blocking PF4 resulting in superior platelet count recovery. We injected anti-hPF4 F(ab’)2 intravenously as above with and without intravenous TPO. Platelet count recovery (Fig. 4A) was improved in all treatment groups when compared to untreated controls. In addition, survival (Fig. 4B) was significantly improved in all treatment groups compared to control (p<0.02). The mean nadir hemoglobin was not different between treatment groups so that the cause of the increased mortality in the control group is not clear (Fig e2A) as discussed above.
Figure 4. In vivo effect of TPO and anti-hPF4 antibodies in irradiated mice.
(A) Platelet count (shown as % baseline count) recovery in hPF4+/KO mice treated with 660 cGy irradiation on day 0. Animals then received anti-hPF4 F(ab’)2 fragments (125 mg/kg iv on days 0 and 4 post irradiation, grey circle) or TPO (100 mcg/kg iv on days 0 and 4 post irradiation, black circle) or both (diamond) and were compared to control animals (open circle and dashed line). N = 4 animals per arm. Shown is mean ± 1 SE. Black arrows represent days of injections. (B) The same data as (A) shown as survival of hPF4+/KO mice after irradiation compared with treated animals. All of the animals that were treated with agents to improve platelet count survived (superimposed solid line) while none of the control animals survived (p<0.002) (broken line). (C) Same as (A) except for WT animals. Animals then received anti-mPF4 F(ab’)2 fragments (250 mg/kg iv on days 0 and 3 post irradiation, grey circle) or TPO (100 mcg/kg iv on days 0 and 3 post irradiation, black circle) or both (diamond) compared to control animals (open square and dashed line). N = 5 animals per arm. Mean ± 1 SE are shown. (D) Same data as (C) but shown as survival of WT animals after irradiation compared with treated animals. All of the animals which were treated with agents to improve platelet count survived (solid line). Two of the control animals survived (p=0.01) (broken line).
To see if these strategies would be effective in mice expressing normal levels of mouse PF4, we examined the ability of anti-mPF4 antibodies and mTPO to alter platelet count recovery in WT animals. Again, platelet count recovery was similar among all treatment groups (Fig. 4C) with shortened duration of thrombocytopenia and higher platelet counts at day 17 (p=0.03). Also, survival was improved over untreated animals (Fig. 4D, p=0.01), suggesting that the PF4 negative paracrine loop may be important in platelet count recovery even in individuals with normal PF4 expression levels and that blocking PF4 may be a novel strategy to alter platelet count recovery after RIT, but was not additive with TPO. Again, the nadir hemoglobin was not significantly different between groups (Fig. e2B).
Discussion
PF4 levels have previously been shown to influence steady-state platelet counts as well as recovery of platelet counts after chemotherapy in mice(4). In both human and mouse in vitro studies, this interaction can be blocked by either blocking PF4 or LRP1(4, 8). In our current studies, we show that both in vitro and in vivo PF4 is an important negative paracrine regulator of the effect of radiation on megakaryocyte formation. In our in vitro studies, we show that irradiation markedly increased the release of PF4 from megakaryocytes and that this chemokine is the major negative regulator of megakaryopoiesis as evidenced by the ability of the anti-PF4 antibody to completely restore colony formation in the presence of hPF4+/KO conditioned media. Studies have shown that there are subpopulations of alpha granules in platelets with different content(24). It is possible that irradiation results in the selective release of PF4 and not other chemokines, but this would need to be formally tested.
In in vivo studies, we show that platelet PF4 levels are inversely related to nadir of platelet count and time of platelet count recovery after irradiation in mice and affect animal survival (Fig. 2). Platelet PF4 levels reflect megakaryocyte levels as platelets have limited protein synthetic capacity (25). Further, platelet PF4 levels do not vary in an animal model after radiation (Fig. e1A), so that the hPF4+/KO mice are likely to have an excess of PF4 released within the bone marrow space compared to WT animals throughout the study.
These effects of PF4 in platelet recovery after irradiation can be blocked by infusion of anti-PF4 F(ab’)2 fragments (Fig. 3). Not shown is that whole anti-PF4 antibodies did not show the same efficacy as F(ab’)2 fragments. We postulate that the smaller F(ab’)2 fragments are better able to reach the bone marrow and block the intramedullary release of PF4 by damaged megakaryocytes. In this same light, heparin that binds PF4 with high avidity(26) was also ineffective (data not shown). We propose that this highly-charged molecule cannot reach high enough concentrations within the intramedullary space to block the negative paracrine effect of the released PF4. We suggest that a small non-charged molecule that can penetrate to the intramedullary space would be needed to bring an anti-PF4-based therapy to clinical efficacy for the treatment of RIT.
We observed significant differences in survival of our animals during the course of these experiments despite the use of similar doses of radiation. The difference in survival between Fig. 2B and Fig. 3B are likely due to the differences in dose rate of irradiation between the two experiments due to the institutional change from one irradiator to another. In the subsequent TPO studies (Fig. 4), apparent differences in survival are due to differences in follow-up with 30-day mortality for hPF4+/KO animals of approximately 50% for all animals treated with the new irradiator. The reason for this decreased survival even after platelet count recovery is not clear; however other studies have shown that platelet count is significantly correlated with survival after radiation even without bleeding(13).
Interestingly, while both TPO and anti-PF4 antibodies improve platelet recovery in irradiated mice, there is no additive effect as seen in both the hPF4+/KO setting (Fig. 4A&B) and in WT mice (Fig. 4C&D). We hypothesize that TPO leads to additional early megakaryocyte recovery and additional PF4 release from these cells so that the intramedullary level of free PF4 is higher after TPO, countering the anti-PF4 antibody effect. Further studies using alternative doses or schedules of anti-PF4 and/or TPO may therefore show additive effects. These studies suggest that both strategies may have a maximal protective effect, and alternative strategies may need to be explored to further reduce mortality and morbidity due to radiation injury.
Clinically, we propose that while TPO is the predominant regulator of platelet production, PF4 inhibition may have an important role in settings associated with increased intra-marrow megakaryocyte turnover such as in CIT and RIT. Further, other clinical scenarios have been shown to have intramedullary apoptosis and lysis of megakaryocytes including immune thrombocytopenia (ITP)(27) and myelodysplastic syndromes (MDS)(28). We propose that in these patients intramedullary released PF4 may influence patient response to TPO-R agonist therapy. It is known that up to 25% of patients with ITP show less than optimal responses to TPO-R agonists (14, 15, 29). Patients having a combination of a high level of megakaryocyte PF4 content and a concurrent rate of intramedullary megakaryocyte apoptosis would be least likely to respond to TPO-based strategy and may benefit from anti-PF4 therapy.
In summary, we present evidence that endogenous PF4 levels affect platelet count recovery after radiation-induced injuries. In the in vitro assays, PF4 is released from radiation-damaged megakaryocytes and appears to be the only biologically relevant inhibitor of megakaryopoiesis in this setting. Blocking PF4’s interaction with its receptor system LRP1 increases megakaryopoiesis in vitro, while blocking PF4 itself increases platelet counts and improves survival in RIT. We propose that total platelet PF4, as a marker intramedullary released PF4, inversely correlates with the degree of thrombocytopenia not only in RIT and CIT, but in ITP and MDS as well. Whether the limitations of TPO-based strategies in these settings is due to a PF4-based effect needs to be tested and whether a combined TPO plus anti-PF4 strategy can be developed that improves outcomes using alternative dosing strategies in these settings remains to be studied.
Supplementary Material
(A) Total platelet PF4 levels in hPF4+/KO animals after irradiation (black squares; mean ± 1SE; left axis) compared to mean change in platelet count (open circles; right axis). n=5 animals. (B) Human PF4 levels over time (specimens collected 2–12 months apart) are stable despite significant inter-individual variability (n=10).
(A) Hemoglobin (gm/dL) for animals after irradiation at the nadir point for hPF4+/KO animals treated with 600 cGy irradiation. There was no difference between the groups. N = 4 animals per arm. (B) The same data as (A) shown for WT animals similarly treated. N = 5 animals per arm.
Acknowledgements
We thank Li Zhai for production of recombinant PF4. This work was supported by funding from the NIH (PO1 HL40387, MP; K23 HL092164, MPL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement:
The authors have no conflicts of interest to declare.
References
- 1.Kaser A, Brandacher G, Steurer W, et al. Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood. 2001;98:2720–2725. doi: 10.1182/blood.v98.9.2720. [DOI] [PubMed] [Google Scholar]
- 2.Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Stromal-derived factor 1 and thrombopoietin regulate distinct aspects of human megakaryopoiesis. Blood. 2000;96:4142–4151. [PubMed] [Google Scholar]
- 3.Weich NS, Fitzgerald M, Wang A, et al. Recombinant human interleukin-11 synergizes with steel factor and interleukin-3 to promote directly the early stages of murine megakaryocyte development in vitro. Blood. 2000;95:503–509. [PubMed] [Google Scholar]
- 4.Lambert MP, Rauova L, Bailey M, et al. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood. 2007;110:1153–1160. doi: 10.1182/blood-2007-01-067116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillai MM, Iwata M, Awaya N, et al. Monocyte-derived CXCL7 peptides in the marrow microenvironment. Blood. 2006;107:3520–3526. doi: 10.1182/blood-2005-10-4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gewirtz AM, Zhang J, Ratajczak J, et al. Chemokine regulation of human megakaryocytopoiesis. Blood. 1995;86:2559–2567. [PubMed] [Google Scholar]
- 7.Majka M, Janowska-Wieczorek A, Ratajczak J, et al. Numerous growth factors, cytokines, and chemokines are secreted by human CD34(+) cells, myeloblasts, erythroblasts, and megakaryoblasts and regulate normal hematopoiesis in an autocrine/paracrine manner. Blood. 2001;97:3075–3085. doi: 10.1182/blood.v97.10.3075. [DOI] [PubMed] [Google Scholar]
- 8.Lambert MP, Wang Y, Bdeir KH, et al. Platelet factor 4 regulates megakaryopoiesis through low density lipoprotein receptor related protein 1 (LRP1) on megakaryocytes. Blood. 2009 doi: 10.1182/blood-2009-04-216473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bick RL, Strauss JF, Frenkel EP. Thrombosis and hemorrhage in oncology patients. Hematol Oncol Clin North Am. 1996;10:875–907. doi: 10.1016/s0889-8588(05)70374-9. [DOI] [PubMed] [Google Scholar]
- 10.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 11.Dainiak N. Hematologic consequences of exposure to ionizing radiation. Exp Hematol. 2002;30:513–528. doi: 10.1016/s0301-472x(02)00802-0. [DOI] [PubMed] [Google Scholar]
- 12.Mettler FA, Jr, Voelz GL. Major radiation exposure--what to expect and how to respond. N Engl J Med. 2002;346:1554–1561. doi: 10.1056/NEJMra000365. [DOI] [PubMed] [Google Scholar]
- 13.Stickney DR, Dowding C, Authier S, et al. 5-androstenediol improves survival in clinically unsupported shesus monkeys with radiation-induced myelosuppression. Int Immunopharmacol. 2007;7:500–505. doi: 10.1016/j.intimp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 14.Kuter DJ. Thrombopoietin and Thrombopoietin Mimetics in the Treatment of Thrombocytopenia. Annu Rev Public Health. 2008 doi: 10.1146/annurev.med.60.042307.181154. [DOI] [PubMed] [Google Scholar]
- 15.Kuter DJ. New drugs for familiar therapeutic targets: thrombopoietin receptor agonists and immune thrombocytopenic purpura. Eur J Haematol Suppl. 2008:9–18. doi: 10.1111/j.1600-0609.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Thornton MA, Kowalska MA, et al. Localization of distal regulatory domains in the megakaryocyte-specific platelet basic protein/platelet factor 4 gene locus. Blood. 2001;98:610–617. doi: 10.1182/blood.v98.3.610. [DOI] [PubMed] [Google Scholar]
- 17.Eslin DE, Zhang C, Samuels KJ, et al. Transgenic mice studies demonstrate a role for platelet factor 4 in thrombosis: dissociation between anticoagulant and antithrombotic effect of heparin. Blood. 2004;104:3173–3180. doi: 10.1182/blood-2003-11-3994. [DOI] [PubMed] [Google Scholar]
- 18.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 19.Gewirtz AM, Calabretta B, Rucinski B, et al. Inhibition of human megakaryocytopoiesis in vitro by platelet factor 4 (PF4) and a synthetic COOH-terminal PF4 peptide. J Clin Invest. 1989;83:1477–1486. doi: 10.1172/JCI114041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaushansky K. Determinants of platelet number and regulation of thrombopoiesis. Hematology Am Soc Hematol Educ Program. 2009:147–152. doi: 10.1182/asheducation-2009.1.147. [DOI] [PubMed] [Google Scholar]
- 21.Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100:3457–3469. doi: 10.1182/blood.V100.10.3457. [DOI] [PubMed] [Google Scholar]
- 22.Ulich TR, del Castillo J, Yin S, et al. Megakaryocyte growth and development factor ameliorates carboplatin-induced thrombocytopenia in mice. Blood. 1995;86:971–976. [PubMed] [Google Scholar]
- 23.Kaushansky K, Lok S, Holly RD, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369:568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- 24.Italiano JE, Jr, Battinelli EM. Selective sorting of alpha-granule proteins. J Thromb Haemost. 2009;7 Suppl 1:173–176. doi: 10.1111/j.1538-7836.2009.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kowalska MA, Rauova L, Poncz M. The role of the platelet chemokine plaetlet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res. 125:292–296. doi: 10.1016/j.thromres.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 26.Denton J, Lane DA, Thunberg L, et al. Binding of platelet factor 4 to heparin oligosaccharides. Biochem J. 1983;209:455–460. doi: 10.1042/bj2090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Houwerzijl EJ, Blom NR, van der Want JJ, et al. Ultrastructural study shows morphologic features of apoptosis and para-apoptosis in megakaryocytes from patients with idiopathic thrombocytopenic purpura. Blood. 2004;103:500–506. doi: 10.1182/blood-2003-01-0275. [DOI] [PubMed] [Google Scholar]
- 28.Delforge M, Raets V, Van Duppen V, et al. CD34+ marrow progenitors from MDS patients with high levels of intramedullary apoptosis have reduced expression of alpha4beta1 and alpha5beta1 integrins. Leukemia. 2005;19:57–63. doi: 10.1038/sj.leu.2403551. [DOI] [PubMed] [Google Scholar]
- 29.Bussel JB, Kuter DJ, Pullarkat V, et al. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113:2161–2171. doi: 10.1182/blood-2008-04-150078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Total platelet PF4 levels in hPF4+/KO animals after irradiation (black squares; mean ± 1SE; left axis) compared to mean change in platelet count (open circles; right axis). n=5 animals. (B) Human PF4 levels over time (specimens collected 2–12 months apart) are stable despite significant inter-individual variability (n=10).
(A) Hemoglobin (gm/dL) for animals after irradiation at the nadir point for hPF4+/KO animals treated with 600 cGy irradiation. There was no difference between the groups. N = 4 animals per arm. (B) The same data as (A) shown for WT animals similarly treated. N = 5 animals per arm.