Summary
Cholinergic modulation of hippocampal synaptic plasticity has been studied extensively by applying receptor agonists or blockers; however the effect of rapid physiological cholinergic stimuli on plasticity is largely unknown. Here we report that septal cholinergic input, activated either by electrical stimulation or via an optogenetic approach, induced different types of hippocampal Schaeffer collateral (SC) to CA1 synaptic plasticity, depending on the timing of cholinergic input relative to the SC input. When the cholinergic input was activated 100 or 10 msec prior to SC stimulation, it resulted in α7 nAChR-dependent long-term potentiation (LTP) or short-term depression, respectively. When the cholinergic stimulation was delayed until 10 msec after the SC stimulation, a muscarinic AChR-dependent LTP was induced. Moreover, these various forms of plasticity were disrupted by Aβ exposure. These results have revealed the remarkable temporal precision of cholinergic functions, providing a novel mechanism for information processing in cholinergic-dependent higher cognitive functions.
Modulatory transmitters, such as acetylcholine, dopamine and serotonin, play a pivotal role in mediating higher cognitive functions, including learning and memory (Reis et al., 2009). Their modulation of synaptic plasticity, a cellular model of learning and memory, has thus been extensively studied. However, the vast majority of knowledge is derived from the use of exogenously applied receptor agonists or blockers. The information about the timing and context of neurotransmitter action is usually lacking, and yet this is critical for information processing and computation (Silberberg et al., 2004; Dan and Poo, 2004; Gradinaru et al., 2010). For example, small shifts in the timing of the same glutamatergic input could result in either long-term potentiation or depression in the case of spike-timing dependent plasticity (Zhang et al., 1998). Although the modulatory transmitters are generally considered to mediate slow synaptic transmission (Greengard, 2001), studies have shown that the timing of exogenously applied acetylcholine is important in modulating high frequency stimulation (HFS)-induced hippocampal synaptic plasticity (Ji et al., 2001; Ge and Dani, 2005), suggesting the potential capability of this neurotransmitter to execute physiological functions with high temporal precision.
Here we have addressed this question by taking advantage of the identifiable cholinergic input pathway from the septum to the hippocampus (Cole and Nicoll, 1983; Cole and Nicoll, 1984; Dutar et al., 1995; Widmer et al., 2006; Wanaverbecq et al., 2007; Zhang and Berg, 2007), and the recently developed optogenetic approach (Tsai et al., 2009; Witten et al., 2010) that allows precise control of specific cholinergic input with high temporal precision. We studied how septal cholinergic inputs, activated either by electrical stimulation or via an optogenetic approach, can regulate the synaptic strength of hippocampal Schaffer collateral (SC) to CA1 synapses. The hippocampal SC to CA1 synapses are among the most studied for synaptic plasticity (Malenka, 2003), a widely recognized cellular model for learning and memory (Bliss and Collingridge, 1993). The hippocampus receives the majority (up to 90%) of its cholinergic inputs from the medial septum via the fimbria/fornix, which enters the hippocampus through the stratum oriens (SO) (Dutar et al, 1995). Alterations of cholinergic function in the hippocampus have been implicated in cognitive dysfunction in Alzheimer's disease, schizophrenia, and nicotine addiction (Kenney and Gould, 2008). Understanding how the septal cholinergic input functions in the hippocampus will provide insights not only for understanding higher brain functions but also for the treatment of these disorders.
As opposed to the previous findings that modulatory neurotransmitters have modulatory effects on pre-existing HFS-induced synaptic plasticity (Jerusalinsky et al., 1997; Power et al., 2003; Dani and Bertrand, 2007; Kenney and Gould, 2008), here we report that single pulses of the septal cholinergic input, activated either by electrical stimulation or more precisely by an optogenetic approach, can directly induce different forms of hippocampal SC to CA1 synaptic plasticity, depending on the timing of cholinergic input relative to the SC input, with a timing precision in the millisecond range. Moreover, these different forms of plasticity are differentially impaired in an Alzheimer's disease model, a disorder of dementia featured with cholinergic dysfunction (Bartus et al., 1982; Terry and Buccafusco, 2003). These results have thus revealed the high temporal precision of cholinergic transmission and its importance in inducing different types of hippocampal synaptic plasticity, providing a novel information processing mechanism underlying higher cognitive functions that involve the hippocampus and cholinergic transmission.
Results
Septal cholinergic inputs induce dynamic hippocampal synaptic plasticity in a timing- and context-dependent manner
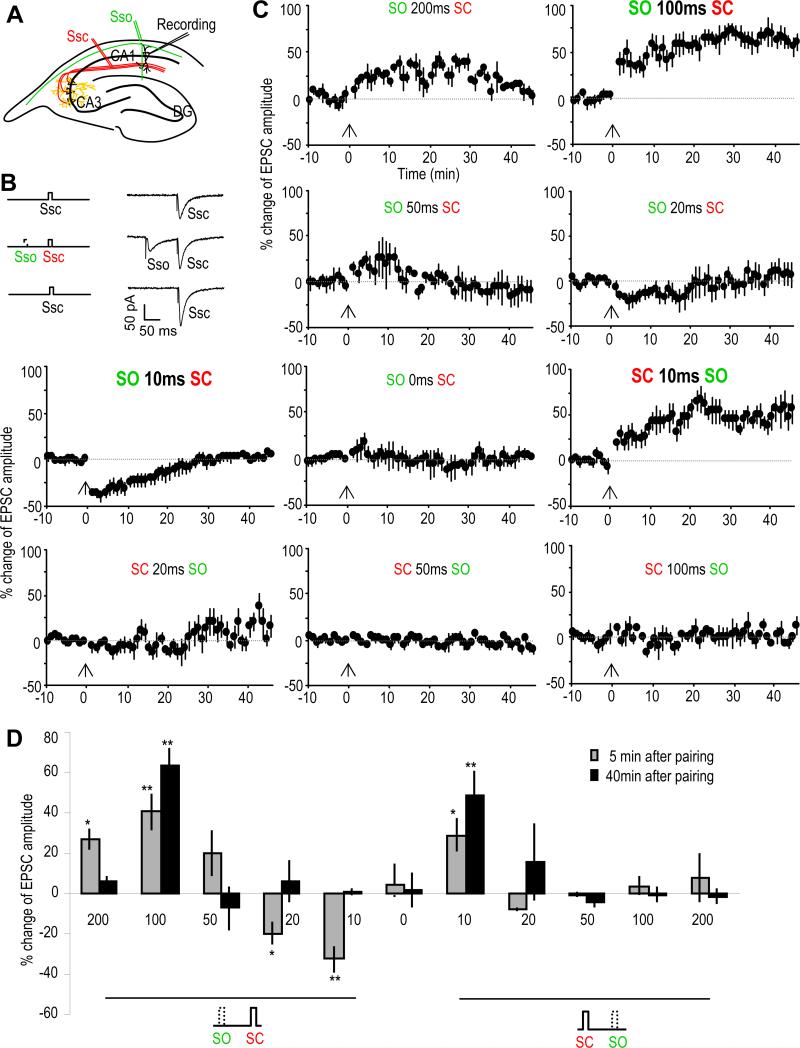
The SC-CA1 synaptic strength was monitored by recording whole cell excitatory postsynaptic currents (EPSCs) from CA1 pyramidal neurons by electrically stimulating the SC pathway with single stimulation pulses in hippocampal slices (Figure 1A). Endogenous acetylcholine (ACh) release was induced by electrically stimulating the stratum oriens (SO) layer, where cholinergic inputs from medial septal nuclei enter the hippocampus (Cole and Nicoll, 1983; Cole and Nicoll, 1984; Dutar et al., 1995; Widmer et al., 2006; Wanaverbecq et al., 2007; Zhang and Berg, 2007). Stimulation of the SO alone, with either single pulses or with high frequency (HFS) or theta burst (TBS) stimulation, produced no significant change of the SC-CA1 EPSC amplitude (Figures S1A-1D). In contrast, a pairing protocol that combined a single stimulation pulse of the SO with that of the SC (Figure 1B), repeated 10 times at 0.033 Hz, resulted in an immediate change of the size of the synaptic response. This effect was temporally-dependent in that by varying the time interval and order of the stimulations, the pairing resulted in various forms of synaptic plasticity of the SC-CA1 synaptic transmission (Figures 1C and 1D).
Fig. 1. SO stimulation induces various types of synaptic plasticity of SC-CA1 synapses.
(A) Schematic diagram showing the placement of recording and stimulating electrodes in the hippocampal slice. EPSCs were recorded from CA1 pyramidal neurons (Recording) by electrically stimulating the SC pathway (Ssc, in red). Cholinergic inputs were activated by electrically stimulating the SO (Sso, in green).
(B) Schematic diagram and sample EPSC traces showing the pairing of SC with SO (middle traces). The SC-EPSCs before (upper traces) and after the pairing protocol (lower traces) were monitored and analyzed.
(C) % change of SC-EPSC amplitude after pairing with the SO (introduced at the 0 min time point as indicated by the arrows) as compare with that before paring. Different types of plasticity were induced depending on the interval and the order of SC-SO pairing.
(D) Bar graph showing the % change of SC-EPSC amplitude 5 or 40 minutes after the pairing as compared with the baseline before the pairing. *p<0.05, **p<0.01, as compared with before pairing protocol, student t-test, n=5-7 in each group.
See also Figure S1.
When pairing SO stimulation 100 ms before the SC stimulation, robust long-term potentiation (LTP) of the EPSC amplitude was induced; intervals of 200 ms and 50 ms were less effective and only produced a short-term potentiation (STP; Figures 1C and 1D). When the interval was shortened to 10 ms, short-term depression (STD) was induced with a less significant effect at a duration of 20 ms. Concurrent stimulation of the SO and SC did not induce any changes in the synaptic response. However, when the SO stimulation was given after the SC stimulation, LTP was induced at the 10 ms time interval, with a slight potentiation at 20 ms and no effect at 50 ms, 100 ms, or 200 ms intervals (Figures 1C and 1D). Interestingly while only 5 pairings were almost as effective as 10 when using ±10 ms intervals (SO before or after SC), 5 pairings induced only STP instead of LTP when pairing SO 100 ms before SC (Figures S1E-1H). The induction of different forms of plasticity stresses the importance of the timing of cholinergic inputs and local synaptic activity in inducing this type of synaptic plasticity.
This plasticity depends on both the timing of the cholinergic input and the activity of local hippocampal synapses receiving the input. Thus one cholinergic input may result in different types of plasticity at different synapses, depending on the local glutamatergic activity in each spine. This timing- and context-dependent mechanism thus provides not only temporal but also spatial precision.
α7 nAChR and mAChR are involved in different types of hippocampal synaptic plasticity, through pre- or post-synaptic mechanisms
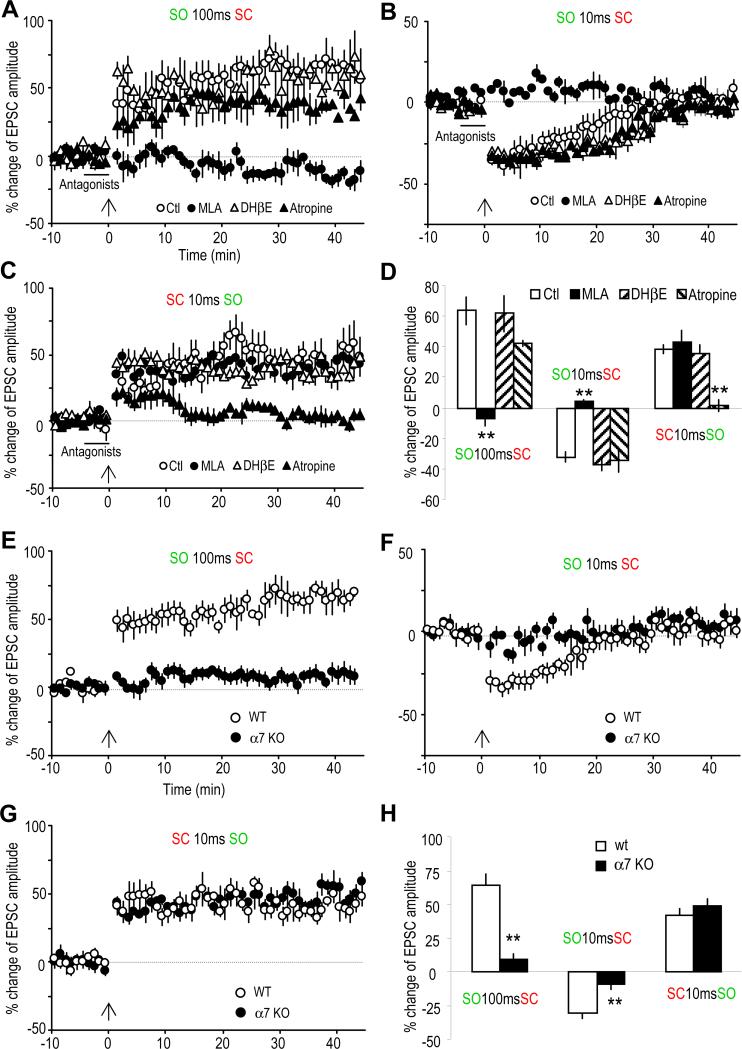
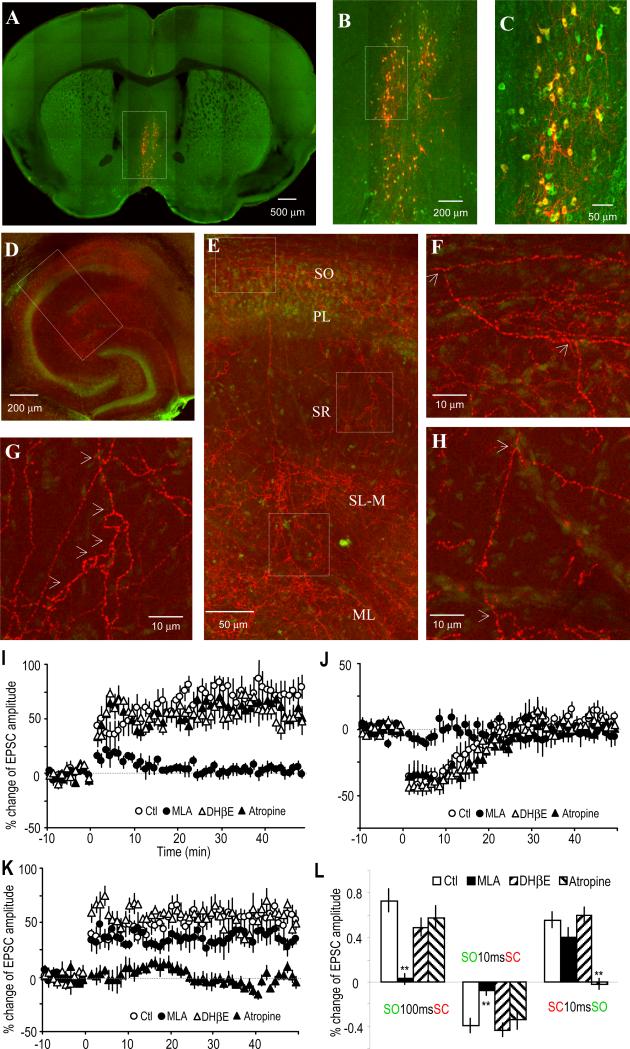
To investigate which ACh receptors might be involved in mediating these forms of plasticity, bath application of cholinergic receptor antagonists was used during the pairing protocol (Figure 2); MLA and DHβE were used to test for the α7 and non-α7 nAChRs (nicotinic AChRs), respectively, and atropine was used to test for the mAChR (muscarinic AChR). The LTP induced by the preceding SO stimulation (100 ms) was completely blocked by MLA (10 nM), whereas DHβE (1 μM) and atropine (5 μM) were ineffective (Figure 2A). Similarly the induction of STD by SO 10 ms before SC was also blocked by MLA, with DHβE and atropine also having no effect (Figure 2B). Therefore, induction of either LTP or STD with prior SO stimulation was due to activation of the α7 nAChR. In contrast, the LTP induced when the SO stimulation occurred after (10 ms) SC stimulation was insensitive to blockade of nAChRs, but was blocked by the mAChR antagonist atropine (Figure 2C), indicating that mAChRs mediated this form of plasticity.
Fig. 2. Involvement of α7 nAChRs and mAChRs in mediating synaptic plasticity.
(A to C) % change of EPSC amplitude showing the effects of three cholinergic antagonists on the three types of plasticity. (A) The LTP induced by pairing the SO 100 ms before the SC was completely blocked by the α7 nAChR antagonist MLA (10 nM), but not by the non-α7 nAChR antagonist DHβE (1 αM) nor the mAChR antagonist atropine (5 μM). (B) The STD induced by pairing the SO 10 ms before the SC was also completely blocked by MLA, but not by DHβE nor atropine. (C) The LTP induced by pairing the SC 10 ms before the SO was blocked by atropine, but not by MLA nor DHβE. (D) Bar graph showing the effects of these three cholinergic antagonists on the three types of synaptic plasticity.
(E to G) % change of EPSC amplitude showing the difference in inducing synaptic plasticity in wild type (WT) and α7 nAChR knockout (KO) mice. (E) LTP was induced by pairing the SO 100 ms before the SC in WT, but not the α7 KO mice. (F) STD was induced by pairing the SO 100 ms before the SC in WT, but not α7 KO mice. (G) The LTP induced by pairing the SC 10 ms before the SO was not affected in the α7 KO mice. (H) Bar graph showing the difference between WT and α7 KO mice. **p<0.01, as compared with WT, student t-test, n=5-6.
The α7 nAChR knockout (KO) mouse was used to further verify the critical role for this receptor in the cholinergic induction of synaptic plasticity. We observed that the LTP and STD induced by SO preceding SC stimulation, which were sensitive to MLA, were entirely absent in slices from the α7 nAChR KO mice, although we observed the plasticity in the wild type littermates (Figures 2E and 2F). Furthermore as expected, the mAChR-dependent LTP was unchanged in the α7 nAChR KO mice (Figure 2G).
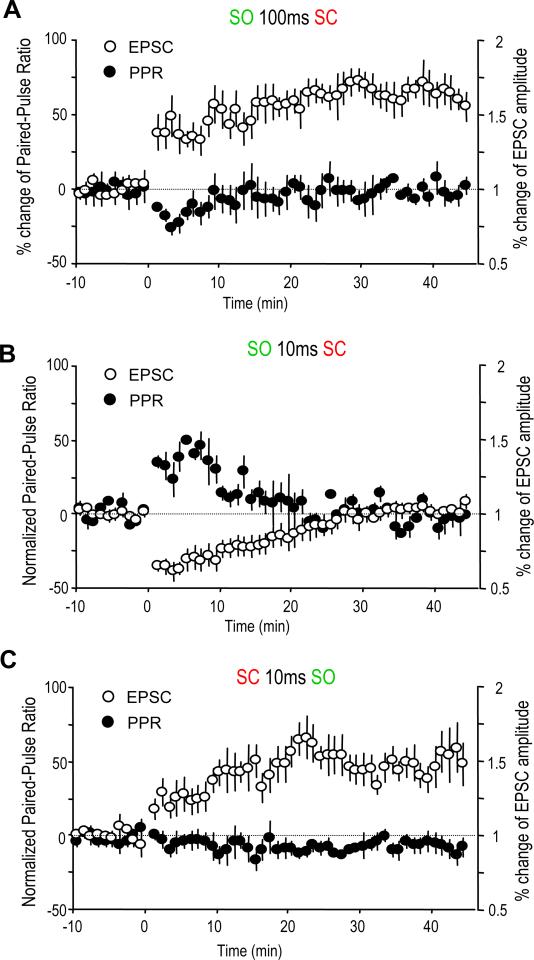
Because AChRs in the hippocampus are located both pre- and postsynaptically, the contribution of both sites to the various forms of plasticity we have observed was examined by comparing the changes of the paired-pulse ratio (PPR); an increase in the PPR, where the second pulse is increased relative to first pulse, suggests decreased presynaptic release, while a decreased ratio suggests increased synaptic release (Dobrunz and Stevens, 1997). For the α7 nAChR-dependent LTP (pairing SO 100 ms before SC), the PPR was decreased initially, and then returned to the baseline (Figure 3A). This suggests that an increased presynaptic release may account for the early potentiation of the EPSCs, but not the late stage. For the α7 nAChR-mediated STD by pairing SO 10 ms before SC, PPR was increased transiently in a time course that fit the time course of the decrease in amplitude of the EPSCs (Figure 3B). This correlation strongly suggests that the STD was mainly mediated through presynaptic inhibition. The PPR was virtually unchanged for the mAChR-mediated LTP (pairing SO 10 ms after SC) (Figure 3C), suggesting a postsynaptic mechanism is more likely to be mediating this particular form of LTP.
Fig. 3. Both pre- and postsynaptic mechanisms are involved in the SO-induced synaptic plasticity.
(A to C) % change of paired-pulse ratio (PPR) and EPSC amplitude after the three pairing protocols which induced synaptic plasticity. The PPR was transiently decreased after pairing SO 100 ms before SC, which induced synaptic LTP (A). The PPR was transiently increased after pairing SO 10 ms before SC, which induced synaptic STD (B). The PPR was not significantly changed after pairing SC 10 ms before SO, which induced synaptic LTP (C).
α7 nAChR-dependent LTP shares similar molecular mechanisms with NMDA receptor-dependent LTP
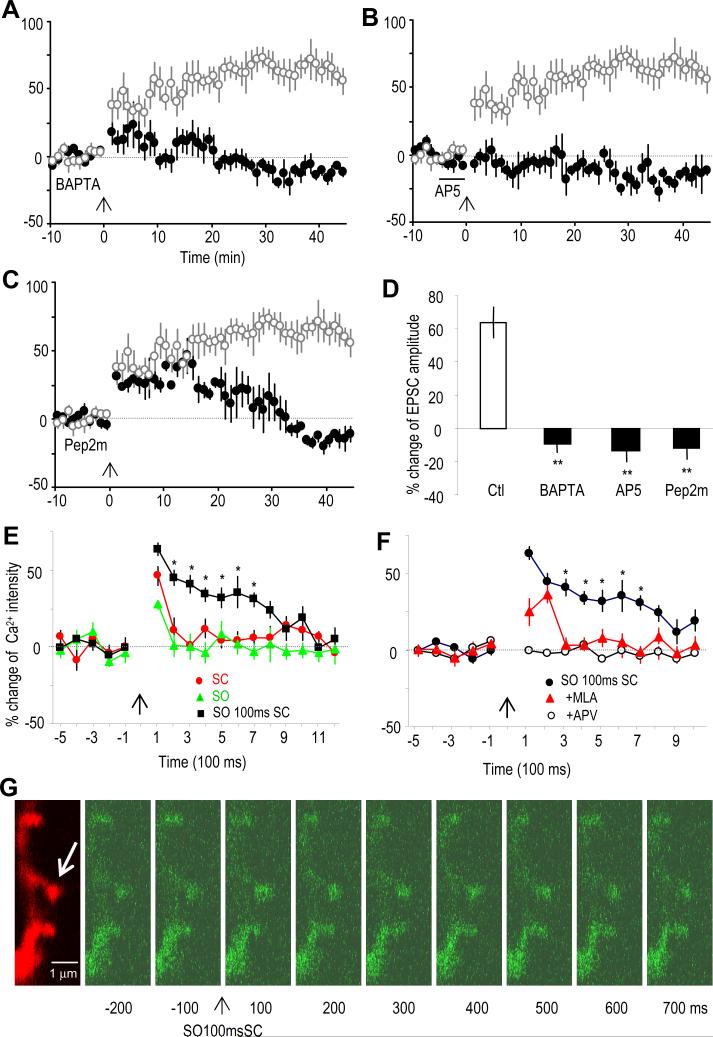
The molecular mechanisms underlying the α7 nAChR-dependent LTP were further studied. Because the activation of the α7 nAChR is known to mediate calcium influx, we first tested whether intracellular calcium chelation could block this form of LTP. Intracellular dialysis of the CA1 pyramidal neuron with the calcium chelator BAPTA (10 mM) completely blocked this form of LTP (Figure 4A), suggesting a mechanism requiring postsynaptic calcium. The α7 nAChR activation may thus act as a source of calcium in inducing this form of LTP. Interestingly this LTP was also blocked by the NMDAR antagonist AP5 (50 μM) (Figure 4B). Thus activation of either the α7 nAChR or NMDAR could serve as a source of calcium. Finally, we tested whether this LTP requires the postsynaptic insertion of GluR2-containing AMPARs, which previously have been shown to mediate LTP in hippocampal CA1 spines (Yao et al., 2008). Indeed dialyzing pyramidal cells with pep2m (100 μM), a peptide containing the NSF (N-ethylmaleimide-sensitive fusion protein) binding site to GluR2 and thus interrupting GluR2-containing AMPAR synaptic insertion, effectively blocked the late stage (about 30 min after the induction of LTP) of the α7 nAChR-dependent LTP (Figure 4C).
Fig. 4. The α7 nAChR-mediated LTP involves NMDAR activation, intracellular calcium increase, and GluR2 AMPAR synaptic insertion.
(A to C) % change of EPSC amplitude showing that the α7 nAChR-mediated LTP (induced by pairing the SO 100 ms before SC) was prevented by (A) including the calcium chelator BAPTA (10 mM) in the recording pipette, (B) the bath application of the NMDA receptor antagonist AP5 (50 μM), and (C) including the peptide pep2m (100 μM) in the recording pipette, which interrupts GluR2 AMPAR synaptic insertion.
(D) Bar graph showing the blockage of the LTP by various treatments. **p<0.01, as compared with control (Ctl), student t-test, n=5-6.
(E) % change of calcium intensity showing that stimulating the SC or the SO alone induced only short term calcium transients, while pairing the SO and SC induced prolonged calcium transients. *p<0.05, as compared with SC or SO alone, student t-test, n=10-15.
(F) The enhanced calcium transient induced by pairing SC with SO was blocked by the α7 nAChR antagonist MLA. The calcium transients were completely blocked by NMDAR antagonist AP5. *p<0.05, as compared with MLA or AP5 treatment, student t-test, n=10-15.
(G) Imaging of a segment of dendrite showing the changes in calcium signals in dendritic spines after pairing the SO 100 ms before the SC. The cell was dialyzed with Alexa 594 (red signal) to visualize the spines. The calcium signal was visualized by fluo-4 (green signal). The arrow points to a spine with significant changes in calcium signal after the pairing.
See also Figure S2.
To investigate whether the calcium levels in spines might be affected by activation of the α7 nAChR, which in conjunction with the NMDAR induces LTP, we examined the calcium transients in postsynaptic CA1 pyramidal spines using the calcium indicator fluo-4 with two-photon laser scanning microscopy (Yasuda et al., 2004). SC or SO stimulation alone induced transient calcium increases, which were blocked by the NMDAR antagonist AP5 (Figures 4E and 4F). Neither was blocked by MLA, the α7 nAChR antagonist (Figure 4F), suggesting that α7 nAChR-mediated calcium transients either do not exist, or more likely were too small to be observed on their own. Interestingly pairing SO stimulation 100 ms before SC stimulation produced a much longer calcium transient than observed with SC stimulation alone (Figures 4E and 4G). This enhancement was blocked by the α7 nAChR antagonist MLA (Figure 4F). Neither pairing at ±10 ms produced the prolongation of calcium transients. Meanwhile stimulating the SC twice with an interval of 100 ms also did not produce the prolongation (Figure S2), indicating that it is specifically the pairing of SO 100 ms before the SC that is required. These data show that properly timed α7 nAChR activation prolongs the NMDAR-mediated calcium transients and thus induces LTP in an NMDAR-dependent manner, which requires calcium increases in the spines and GluR2-containing AMPAR synaptic insertion.
Specific activation of septal cholinergic inputs by an optogenetic approach induces similar dynamic hippocampal synaptic plasticity as induced by electrical stimulation
Electrical stimulation of the SO activates not only septal cholinergic inputs but also other local and external inputs, such as glutamatergic inputs in the hippocampus. To address whether septal cholinergic activation alone is sufficient to account for the SO stimulation-induced hippocampal plasticity, an optogenetic approach was used to replace electrical SO stimulation to specifically activate only cholinergic inputs from septal nuclei (Tsai et al., 2009; Witten et al., 2010). To do this, we selectively expressed the light-activated cation channel channelrhodopsin-2 (ChR2) in medial septal cholinergic neurons. Activation of ChR2 with 488 nm light exposure can induce depolarization and action potentials in the neurons expressing this protein. ChR2 is expressed in the soma, dendrites and axon, and light exposure of the ChR2-expressing axon terminals can thus induce neurotransmitter release. We injected a Cre-inducible adeno-associated virus (AAV) containing a double floxed inverted ChR2 (Tsai et al., 2009; Witten et al., 2010) into the medial septal nuclei of cholineacetyltransferase (ChAT)-Cre transgenic mice. This Cre-inducible AAV is only expressed in Cre-expressing cells (in this case the cholinergic neurons; i.e. the ChAT-positive cells) because the Cre expression is driven by the ChAT promoter. Selective expression of ChR2 (fused with mCherry) in septal cholinergic (ChAT-positive) neurons was verified by immunohistochemistry (Figures 5A to 5C). Functional expression of ChR2 was verified by inducing action potentials with 488 nm laser light exposure of cell bodies or nearby dendrites (Figures S3A and 3B). Septal cholinergic projections in the hippocampus visualized by mCherry showed axons coming from the fimbria/fornix branch in the SO (Figures 5D to 5H). Many of these axon projections send branches to deeper layers of the stratum radiatum, stratum lacunosum-moleculare, and the molecular layer of the dentate gyrus. This pattern confirms our presumption that the upstream primary cholinergic branches innervating the CA1 are located in the SO and supports our use of SO stimulation to activate cholinergic inputs, either electrically or optically, to the CA1.
Fig. 5. Optogenetically activated cholinergic inputs induce dynamic synaptic plasticity of SC-CA1 synapses.
(A to C) Immunostaining of ChAT (green) in a coronal medial septal section showing that ChR2 (fused with mCherry, shown in red) was expressed exclusively in medial septal ChAT-positive neurons.
(D) Horizontal hippocampal slice showing the abundant presence of ChR2-expressing cholinergic terminals (mCherry, red) in hippocampus. The pyramidal layer is visualized with NeuroTrace fluorescent nissl stain (green).
(E) Inset from (D) showing the distribution of cholinergic terminals in different cell layers in (and near) the CA1 region.
(F to H) Insets from (E) showing the bifurcation (indicated by arrows) of the cholinergic terminals, suggesting the primary branches originated in the SO layer. The axon shown in (H) is extended from that in (F). PL, pyramidal cell layer; SL-M, stratum lacunosum-moleculare; ML, molecular layer of dentate gyrus.
(I) LTP was induced by optically activating the cholinergic input 100 ms before stimulating the SC, which was prevented by the α7 nAChR antagonist MLA, but not by the non-α7 nAChR antagonist DHβE nor the mAChR antagonist atropine.
(J) STD was induced by activating the cholinergic input 10 ms before the SC, which was also prevented by MLA, but not by DHβE nor atropine.
(K) LTP was induced by pairing the SC 10 ms before optically activating the cholinergic input, which was prevented by atropine, but not by MLA nor DHβE.
(L) Bar graph showing the three types of synaptic plasticity and the effects of the three AChR antagonists on the plasticity, respectively.
See also Figure S3.
To activate the cholinergic inputs to the CA1, cholinergic terminals in a small region of the SO were exposed to 488 nm light for 20 ms. Activation of the terminals sometimes induced visible nAChR-mediated currents in about 20% of the pyramidal neurons (Figure S3E), usually with a 20 ms delay between the time of light exposure and the cholinergic response. Three time intervals pairing light exposure with SC stimulation were selected to mimic the corresponding pairings of SO and SC electrical stimulation that produced the observed three types of synaptic plasticity described above. Consistent with the results from electrical SO stimulation, when cholinergic input was activated 100 ms (i.e. light exposure 120 ms to take into account the 20 ms delay) before SC stimulation, LTP was induced, which was blocked by the α7 nAChR antagonist MLA, but not by DHβE or atropine (Figure 5I). When cholinergic input was activated 10 ms before SC stimulation, STD was induced, which was also sensitive to MLA but not to DHβE or atropine (Figure 5J). When cholinergic input was activated 10 ms after SC stimulation, LTP was induced, which was blocked by atropine but not by MLA or DHβE (Figure 5K). These results demonstrate that cholinergic input alone, activated by either SO stimulation or by light in cholinergic neurons expressing ChR2, is sufficient to induce the various forms of timing-dependent synaptic plasticity.
α7 nAChR-dependent synaptic plasticity is more vulnerable to Aβ exposure than mAChR-dependent synaptic plasticity
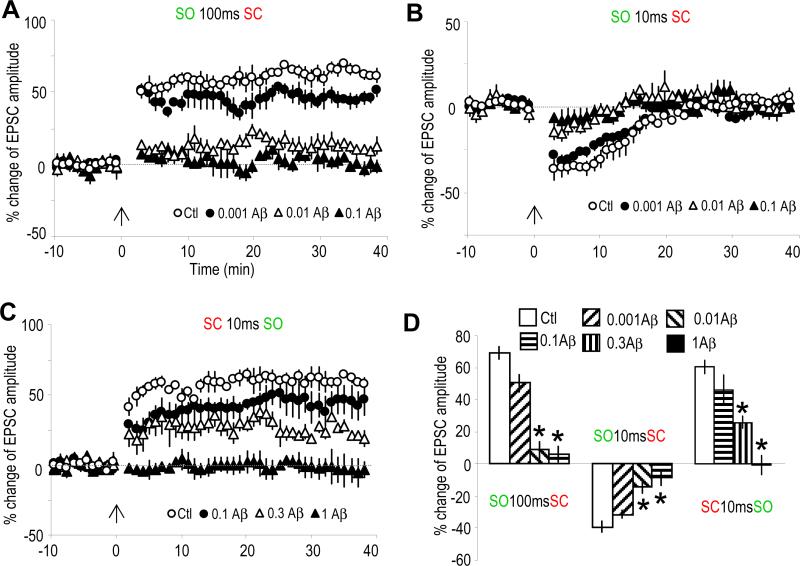
We then investigated the potential implication of this synaptic plasticity in higher cognitive functions. Cholinergic dysfunction has long been hypothesized to be a major cause for the cognitive deficit in Alzheimer's disease (AD) (Bartus et al., 1982; Terry and Buccafusco, 2003). Recent studies strongly suggest that the soluble oligomeric rather than the fibrillar form of β-amyloid (Aβ) causes synaptic and cognitive dysfunction in AD, and the underlying mechanisms have therefore been the focus of current studies (Lue et al., 1999; McLean et al., 1999; Selkoe, 2002; Hsieh et al., 2006; Haass and Selkoe, 2007). Here we show that the α7 nAChR-dependent LTP and STD were largely blocked in slices pre-exposed to 10 nM Aβ for 2 hr (Figures 6A and 6B); our Aβ preparation contains oligomeric, as well as monomeric, Aβ (Lambert et al., 1998). The mAChR-mediated LTP is relatively resistant to 0.1 μM Aβ, but was blocked by higher concentrations of Aβ pre-exposure (partial blockade by 0.3 and complete blockade by 1 μM) (Figure 6C).
Fig. 6. Aβ differentially impaired α7 nAChR- and mAChR-mediated synaptic plasticity.
(A to C) % change of EPSC amplitude showing the effects of 2 hr pre-exposure to various doses of Aβ on the three types of plasticity. Both of the α7 nAChR-mediated LTP (A) and STD (B) were largely blocked by Aβ at a concentration as low as 0.01 μM. The mAChR-mediated LTP (C) was partially blocked by 0.3 μM Aβ and completely blocked by 1 μM Aβ.
(D) Bar graph showing the effects of Aβ at various concentrations on the three types of synaptic plasticity. *p<0.001, as compared with no Aβ treatment, student t-test, n=5-7.
These results thus provide a mechanism for Aβ to impair cholinergic-related synaptic plasticity and cognitive functions. On the other hand, we have recently shown that a dual allosteric modulator, which can simultaneously enhance α7 nAChRs and inhibit α5 subunit-containing γ-aminobutyric acid (A) receptors, not only induces LTP in hippocampal slices but also enhances performance in the radial arm maze and facilitates attentional states in the five choice serial reaction time trial in animals (Johnstone et al., 2011); presumably this is achieved by increasing the possibility of properly timed spontaneous cholinergic and glutamatergic synaptic transmission in the hippocampus. These results strongly suggest that the cholinergic-mediated synaptic plasticity is closely related to cognitive performance, and provides a relevant platform for further testing therapeutic compounds for hippocampus-based cognitive impairment including Alzheimer's disease.
Discussion
Multiple forms of synaptic plasticity have previously been shown to be regulated by both nAChR and mAChR activation. For the nAChRs (and in particular the α7 subtype), the activation of receptors with exogenous ligands in the CA1 and dentate regions enhanced synaptic plasticity (Fujii et al., 1999; Mann and Greenfield, 2003; Welsby et al., 2006; Welsby et al., 2007). Furthermore the effect that the activation of these receptors has on synaptic plasticity can depend on the location of the receptors as well as timing; for example, the activation of α7 nAChRs on hippocampal interneurons can block concurrent STP and LTP in pyramidal cells, whereas presynaptic nAChRs can enhance the release of glutamate and thus increase the probability of inducing LTP (Ji et al., 2001). In addition exogenous ACh may convert HFS-induced STP to LTP or LTD, depending on the timing relative to the SC stimulation (Ge and Dani, 2005). Our current study is in large part consistent with these conclusions, stressing the importance of proper timing of cholinergic activation in shaping hippocampal synaptic plasticity. We have also recently shown that nicotine, acting through the non-α7 nAChRs, was able to enhance synaptic plasticity in deep layers of the entorhinal cortex (Tu et al., 2009). This is consistent with a recent report that α4-containing nAChRs contribute to LTP facilitation in the perforant path (Nashmi et al., 2007). Multiple forms of synaptic plasticity can also be regulated by mAChRs (Maylie and Adelman, 2010). For example, the activation of presynaptic or postsynaptic mAChRs has previously been shown to either enhance or reduce LTP in the hippocampus (Leung et al., 2003; Ovsepian et al., 2004; Seeger et al., 2004; Cobb and Davies, 2005). Recently it was shown that endogenous ACh, acting through the M1 mAChR subtype, facilitates LTP in the hippocampus via inhibition of SK channels (Buchanan et al., 2010).
Here we show that the septal cholinergic input can directly induce hippocampal synaptic plasticity in a timing-dependent manner. When the cholinergic input to the CA1 was activated 100 msec or 10 msec prior to the SC stimulation, it resulted in α7 nAChR-dependent LTP or STD, respectively. This α7 nAChR-dependent LTP was likely due to a postsynaptic effect that required the activation of the NMDAR and prolongation of the NMDAR-mediated calcium transients in the spines, and GluR2-containing AMPAR synaptic insertion. The α7 nAChR-dependent STD appears to be mediated primarily through the presynaptic inhibition of glutamate release (Figure 3). The third and last form of plasticity that we observed was when the cholinergic stimulation was given 10 msec after the SC stimulation; this induced LTP that was dependent on the activation of the mAChR. The underlying mechanism is not clear at this time. PPR study suggests a postsynaptic mechanism (Figure 3), but we have not been able to block this LTP with a calcium chelator dialyzed into the cells under recording (data not shown).
The majority of modulatory transmitter receptors are G-protein coupled receptors which exert functions through intracellular signaling pathways, and are thus considered slow synaptic transmission mediators, as opposed to those receptors that are ligand gated ion-channels (Greengard, 2001). Previous studies have focused on the modulatory effects on existing HFS-induced hippocampal synaptic plasticity by either nAChR or mAChR activation. Our study here clearly shows that cholinergic input, through either its ion channel receptor (α7 nAChR) or the G protein-coupled receptor (mAChR), can directly induce hippocampal synaptic plasticity in a timing- and context-dependent manner. With timing shifts in the millisecond range, different types of synaptic plasticity are induced through different AChR subtypes with different mechanisms (pre- or post-synaptic). These results have thus revealed the striking temporal accuracy of modulatory transmitter systems and the subsequent complex functions achieved based on this capability.
This study also reveals novel physiologically reasonable neural activity patterns that induce synaptic plasticity, a very important question in learning and memory studies (Kandel, 2009). The HFS-induced synaptic plasticity has provided valuable information in underlying molecular mechanisms, but has been questioned as a physiological firing pattern. For this reason, spike timing-dependent plasticity is considered physiologically more reasonable (Markram et al., 1997; Kandel, 2009). Even so, both models focus on manipulating the firing patterns of the same glutamatergic pathway where synaptic plasticity will form. In the present study, synaptic plasticity is induced by an extrinsic input and thus provides a mechanism to integrate information from extrinsic pathways and store it in local synapses. It is thus more relevant to understanding learning and memory, which always involves the precise coordination among multiple brain regions.
Cognitive deficits in AD have long been thought to be caused in part by cholinergic dysfunction (Bartus et al., 1982; Terry and Buccafusco, 2003). Here we have shown that concentrations of oligomeric Aβ as low as 10 nM largely blocks both forms of α7 nAChR-dependent plasticity. For the mAChR-mediated LTP, a concentration of Aβ of nearly 1 μM is required to block this form of plasticity; this concentration is comparable to those who previously have studied Aβ inhibition of hippocampal synaptic plasticity (Lambert et al., 1998; Vitolo et al., 2002; Wang et al., 2004). The synthetic Aβ is much less efficient than naturally secreted Aβ (about 1/200) in inhibiting hippocampal LTP (Wang et al., 2004). The fact that the α7 nAChR-dependent plasticity is more sensitive to Aβ may be due to the previously reported ability of Aβ to bind to the α7 nAChR with high affinity. However the sensitivity of α7 nAChRs to Aβ may vary due to its coassembly with other subunits and/or among different neuronal populations (Liu et al., 2009; Liu et al., 2001; Pettit et al., 2001), and Aβ may be exerting its effect on plasticity through other mechanisms (Nimmrich et al., 2008). Nevertheless, these results thus provide a mechanism for Aβ to impair cholinergic-related synaptic plasticity and cognitive functions, and provides a relevant platform for further testing therapeutic compounds for cholinergic-dependent cognitive impairment including Alzheimer's disease.
Supplementary Material
Acknowledgments
We thank Patricia Lamb for animal genotyping and plasmid preparation, Drs Negin Martin and Charles Romeo for virus packaging, Dr. James M. Wilson at University of Pennsylvania for providing the AAV serotype 9 helper plasmid, Charles J. Tucker and Agnus Janoshazi for assistance with fluorescent microscopy. We also thank Drs Serena Dudek, David Armstrong, Patricia Jensen, and Lutz Birnbaumer for discussions and critical reading of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Methods
Animals and Chemicals
Rats were obtained from Charles River Laboratories. α7 nAChR knockout mice and ChAT-Cre transgenic mice were obtained from Jackson Laboratory. Mice were ear-punched and genotyped at day 19 and used for experiments from day 21. All procedures were approved and performed in compliance with NIEHS/NIH Humane Care and Use of Animals in Research protocols. Unless otherwise indicated, general chemicals or drugs were obtained from Sigma, and fluorescent materials were from Invitrogen. Pep2m was from Tocris.
Slice Preparation and Whole-cell patch-clamp recordings
Animals (2 to 3 weeks old Wistar rats, 3 to 4 weeks old α7 nAChR knockout mice, and 5 to 6 weeks old ChAT-Cre transgenic mice) were anaesthetized with isoflurane and decapitated. Brains were quickly dissected and placed into carboxygenated (95%O2/5%CO2) ice-cold artificial cerebral spinal fluid (ACSF) containing (in mM) 122 NaCl, 2.5 KCl, 1.3 MgCl2, 2 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, 25 glucose. Horizontal brain slices (300μm) corresponding to plate 190~200 of the Paxinos rat brain atlas (Paxinos and Watson, 2007) were cut with a vibratome (Leica, VT1000S). Slices were then stored in ACSF continuously bubbled with carboxygen at room temperature for more than one hour before use.
Slices used for whole-cell patch-clamp studies were continuously perfused with ACSF in a submerged chamber at a rate of 2 ml/min. Whole cell patch clamp was performed under guidance of IR-DIC optics using an Axopatch 200B patch amplifier (Axon Instruments) with a glass pipette filled with an internal solution containing (in mM) 120 potassium gluconate (KGluc), 2 NaCl, 5 MgATP, 0.3 Na2GTP, 20 KCl, 10 HEPES, 1 EGTA and 11.3 D-glucose, with pH ~7.2-7.3 and osmolarity of ~270-280 mOsm. Series resistances ranging from 7-40 MΩ were not compensated for during recordings, but were monitored throughout the experiments. Recordings were discarded when a significant (>20%) change of series resistance was detected. Data were digitized with Digidata 1322A, collected with Clampex and analyzed with Clampfit. Excitatory post-synaptic currents (EPSCs) were recorded under voltage clamp at -60 mV.
Pairing two input pathways by electrical stimulation
Evoked EPSCs were recorded from hippocampal CA1 pyramidal neurons by electrically stimulating the Schaffer collateral (SC) pathway. Cholinergic terminals were activated by electrically stimulating the stratum oriens (SO). The stimulation intensity was adjusted to evoke a post-synaptic current of about 50 -100 pA in amplitude, and the intensity was usually around 20-100 μA for 0.1 ms for SC and 50-200 μA for SO pathway. For LTP, the amplitude of EPSCs at the 40 min time point after the pairing protocol was compared with that before the pairing protocol. For STD, the amplitude at the 5 min time point after pairing was compared with before. Bath applied cholinergic receptor antagonists or other chemicals were applied 5 min before and during the pairing protocol, and were washed away immediately after the pairing procedure.
Calcium imaging in dendritic spines
Calcium imaging was done with the calcium indicator fluo-4 (200 μM included in recording pipette). Alexa 594 (100 μM) was also included in the recording pipette to visualize the dendrites of neurons under recording. Images were acquired with a Zeiss LSM 510 NLO META system coupled to an Axioskop 2FS microscope (Carl Zeiss, Inc., Thornwood, NY) using a Ti:sapphire Chameleon two-photon laser system (Coherent, Inc., Auburn, CA). A wavelength of 810 nm was used to excite both fluo-4 and Alexa 594. An IR Achroplan 63 × objective lens (N.A. 1.1) was used. Emissions were collected using bandpass filters BP 500-550 IR and BP 640-720 IR, respectively (Chroma Technology Corp., Rockingham, VT). Image acquisition and online analysis were performed using Zeiss LSM 510 software.
Stereotaxic injection of viruses into medial septum in mice
Channelrhodopsin-2 was expressed in the medial septum in ChAT (choline acetyl transferase)-Cre mice (expressing Cre under ChAT promoter). AAV-FLEX-rev-ChR2(H134R)-mCherry carrying double floxed ChR2 with reversed sequence (Addgene plasmid 20297 from Karl Deisseroth) were packaged with AAV serotype 9 by the virus core facility at NIEHS. 0.5 μl of virus was injected into 21 days old mice (anaesthetized with 75mg/kg ketamine and 7.5mg/kg xylazine) with the following coordinates: bregma, 0.5 mm; lateral, 0.3 mm tilted at 8 degrees towards the midline; and dorsal-ventral, 4.0 mm. Animals were allowed to recover for at least 12 days, and then hippocampal slices for recordings were prepared as described above.
Activating ChR2-expressing cholinergic neurons by light exposure
The Zeiss LSM 510 NLO META system was also used to generate the light to activate ChR2-expressing cholinergic terminals in the hippocampal SO region, which was co-expressed with mCherry and visualized with 543 nm light that does not activate ChR2. ChR2 was activated with 488 nm laser. The exposure time, intensity, and area were controlled by the LSM 510 system. The intensity of the light used to activate ChR2 in processes was usually 7.5 mW for 20 ms. the cholinergic response (nAChR-mediated currents in CA1 neurons) was usually induced at around 20 ms after initiating the light exposure. To achieve a 100 ms interval for cholinergic inputs before SC inputs, the light exposure was set at 120 ms before SC input.
Immunohistochemistry
100 μm coronal slices of medial septum or horizontal slices of hippocampus were cut from 4% paraformaldehyde fixed brains. After blocking with 5% bovine serum albumin for 1 hr at room temperature (RT), medial septal slices were incubated with goat anti-ChAT antibody (Chemicon, 1:200) at 4°C for 48 hr, and then incubated with secondary Alexa 488 conjugated donkey anti-goat antibody (1:200) for 4 hr at RT. The hippocampal slices were incubated with Neurotrace fluorescent Nissl stain (1:300) for 2 hr at RT to locate the pyramidal layer. Images were then taken with Zeiss LSM 710 Zen system.
Aβ treatment
Human Aβ (1-42) peptide was purchased from Anaspec. It was dissolved in 1% NH4OH at 3 mM. Aliquots were stored at -20°C. Oligomeric Aβ was produced by diluting the stock solution with PBS to 0.1 mM and incubated at 4°C for 48 hr (Lambert et al., 1998). This preparation also contains monomeric Aβ. After brief centrifugation, the supernatant was used to treat hippocampal slices for 2-4 hr before the recording experiments.
Statistics
For whole-cell recordings, the amplitude of SC-EPSC was analyzed with Clampfit. The % changes were calculated by comparing with the average of 10 min baseline recording. For calcium imaging, the averages of 500 ms baseline (5 time points) were used to calculate the % changes. Values were always presented as mean ± SEM. Two-tailed Student t-tests were performed to compare changes with the baselines or controls.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartus RT, Dean RL, 3rd., Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Buchanan KA, Petrovic MM, Chamberlain SE, Marrion NV, Mellor JR. Facilitation of long-term potentiation by muscarinic M(1) receptors is mediated by inhibition of SK channels. Neuron. 2010;68:948–963. doi: 10.1016/j.neuron.2010.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SR, Davies CH. Cholinergic modulation of hippocampal cells and circuits. J Physiol. 2005;562:81–88. doi: 10.1113/jphysiol.2004.076539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983;221:1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Characterization of a slow cholinergic post-synaptic potential recorded in vitro from rat hippocampal pyramidal cells. J Physiol. 1984;352:173–188. doi: 10.1113/jphysiol.1984.sp015285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dutar P, Bassant MH, Senut MC, Lamour Y. The septohippocampal pathway: structure and function of a central cholinergic system. Physiol. Rev. 1995;75:393–427. doi: 10.1152/physrev.1995.75.2.393. [DOI] [PubMed] [Google Scholar]
- Fujii S, Ji Z, Morita N, Sumikawa K. Acute and chronic nicotine exposure differentially facilitate the induction of LTP. Brain Res. 1999;846:137–143. doi: 10.1016/s0006-8993(99)01982-4. [DOI] [PubMed] [Google Scholar]
- Ge S, Dani JA. Nicotinic acetylcholine receptors at glutamate synapses facilitate long-term depression or potentiation. J Neurosci. 2005;25:6084–6091. doi: 10.1523/JNEUROSCI.0542-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer's amyloid beta-peptide. Nature Reviews. Molecular Cell Biology. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, Iwatsubo T, Tomita T, Sisodia S, Malinow R. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerusalinsky D, Kornisiuk E, Izquierdo I. Cholinergic neurotransmission and synaptic plasticity concerning memory processing. Neurochem Res. 1997;22:507–515. doi: 10.1023/a:1027376230898. [DOI] [PubMed] [Google Scholar]
- Ji D, Lape R, Dani JA. Timing and location of nicotinic activity enhances or depresses hippocampal synaptic plasticity. Neuron. 2001;31:131–141. doi: 10.1016/s0896-6273(01)00332-4. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Gu Z, Yoshimura RF, Villegier AS, Hogenkamp DJ, Whittemore ER, Huang JC, Tran MB, Belluzzi JD, Yakel JL, Gee KW. Allosteric modulation of related ligand-gated ion channels synergistically induces long term potentiation in the hippocampus and enhances cognition. J Pharmacol Exp Ther. 2011;336:908–915. doi: 10.1124/jpet.110.176255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER. The biology of memory: a forty-year perspective. J Neurosci. 2009;29:12748–56. doi: 10.1523/JNEUROSCI.3958-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney JW, Gould TJ. Modulation of hippocampus-dependent learning and synaptic plasticity by nicotine. Mol Neurobiol. 2008;38:101–121. doi: 10.1007/s12035-008-8037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. 1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Shen B, Rajakumar N, Ma J. Cholinergic activity enhances hippocampal long-term potentiation in CA1 during walking in rats. J Neurosci. 2003;23:9297–304. doi: 10.1523/JNEUROSCI.23-28-09297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. American Journal of Pathology. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Huang Y, Xue F, Simard A, DeChon J, Li G, Zhang J, Lucero L, Wang M, Sierks M, Hu G, Chang Y, Lukas RJ, Wu J. A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides. J Neurosci. 2009;29:918–29. doi: 10.1523/JNEUROSCI.3952-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kawai H, Berg DK. beta -Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci U S A. 2001;98:4734–4739. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC. Synaptic plasticity and AMPA receptor trafficking. Ann N Y Acad Sci. 2003;1003:1–11. doi: 10.1196/annals.1300.001. [DOI] [PubMed] [Google Scholar]
- Mann EO, Greenfield SA. Novel modulatory mechanisms revealed by the sustained application of nicotine in the guinea-pig hippocampus in vitro. J Physiol. 2003;551:539–550. doi: 10.1113/jphysiol.2003.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Lübke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science. 1997;275:213–215. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Maylie J, Adelman JP. Cholinergic signaling through synaptic SK channels: it's a protein kinase but which one? Neuron. 2010;68:809–11. doi: 10.1016/j.neuron.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of A beta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Annals of Neurology. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional alpha 4 nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–18. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmrich V, Grimm C, Draguhn A, Barghorn S, Lehmann A, Schoemaker H, Hillen H, Gross G, Ebert U, Bruehl C. Amyloid beta oligomers (A beta(1-42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J Neurosci. 2008;28:788–97. doi: 10.1523/JNEUROSCI.4771-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovsepian SV, Anwyl R, Rowan MJ. Endogenous acetylcholine lowers the threshold for long-term potentiation induction in the CA1 area through muscarinic receptor activation: in vivo study. Eur J Neurosci. 2004;20:1267–75. doi: 10.1111/j.1460-9568.2004.03582.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. (6th ed) 2007 doi: 10.1016/0165-0270(80)90021-7. Acadamic Press. [DOI] [PubMed] [Google Scholar]
- Pettit DL, Shao Z, Yakel JL. beta-Amyloid(1-42) peptide directly modulates nicotinic receptors in the rat hippocampal slice. J Neurosci. 2001;21:RC120. doi: 10.1523/JNEUROSCI.21-01-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power AE, Vazdarjanova A, McGaugh JL. Muscarinic cholinergic influences in memory consolidation. Neurobiol Learn Mem. 2003;80:178–193. doi: 10.1016/s1074-7427(03)00086-8. [DOI] [PubMed] [Google Scholar]
- Reis HJ, Guatimosim C, Paquet M, Santos M, Ribeiro FM, Kummer A, Schenatto G, Salgado JV, Vieira LB, Teixeira AL, Palotás A. Neurotransmitters in the central nervous system & their implication in learning and memory processes. Curr. Med. Chem. 2009;16:796–840. doi: 10.2174/092986709787549271. [DOI] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knockout mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci. 2004;24:10117–27. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Silberberg G, Wu C, Markram H. Synaptic dynamics control the timing of neuronal excitation in the activated neocortical microcircuit. J Physiol. 2004;556:19–27. doi: 10.1113/jphysiol.2004.060962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ. The cholinergic hypothesis of age and Alzheimer's disease-related cognitive deficits: recent challenges and their implications for novel drug development. J Pharmacol Exp Ther. 2003;306:821–827. doi: 10.1124/jpet.102.041616. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu B, Gu Z, Shen JX, Lamb PW, Yakel JL. Characterization of a nicotine-sensitive neuronal population in rat entorhinal cortex. J Neurosci. 2009;29:10436–10448. doi: 10.1523/JNEUROSCI.2580-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitolo OV, Sant'Angelo A, Costanzo V, Battaglia F, Arancio O, Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proc Natl Acad Sci USA. 2002;99:13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanaverbecq N, Semyanov A, Pavlov I, Walker MC, Kullmann DM. Cholinergic axons modulate GABAergic signaling among hippocampal interneurons via postsynaptic α7 nicotinic receptors. J. Neurosci. 2007;27:5683–5693. doi: 10.1523/JNEUROSCI.1732-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Rowan MJ, Anwyl R. Beta-amyloid-mediated inhibition of NMDA receptor-dependent long-term potentiation induction involves activation of microglia and stimulation of inducible nitric oxide synthase and superoxide. J Neurosci. 2004;24:6049–6056. doi: 10.1523/JNEUROSCI.0233-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsby P, Rowan M, Anwyl R. Nicotinic receptor-mediated enhancement of long-term potentiation involves activation of metabotropic glutamate receptors and ryanodine-sensitive calcium stores in the dentate gyrus. Eur J Neurosci. 2006;24:3109–3118. doi: 10.1111/j.1460-9568.2006.05187.x. [DOI] [PubMed] [Google Scholar]
- Welsby PJ, Rowan MJ, Anwyl R. Beta-amyloid blocks high frequency stimulation induced LTP but not nicotine enhanced LTP. Neuropharmacology. 2007;53:188–195. doi: 10.1016/j.neuropharm.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Widmer H, Ferrigan L, Davies CH, Cobb SR. Evoked slow muscarinic acetylcholinergic synaptic potentials in rat hippocampal interneurons. Hippocampus. 2006;16:617–628. doi: 10.1002/hipo.20191. [DOI] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Kelly MT, Sajikumar S, Serrano P, Tian D, Bergold PJ, Frey JU, Sacktor TC. PKMζ maintains late long-term potentiation by N-ethylmaleimide-sensitive factor/GluR2-dependent trafficking of postsynaptic AMPA receptors. J. Neurosci. 2008;28:7820–7827. doi: 10.1523/JNEUROSCI.0223-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda R, Nimchinsky EA, Scheuss V, Pologruto TA, Oertner TG, Sabatini BL, Svoboda K. Imaging calcium concentration dynamics in small neuronal compartments. Sci. STKE. 2004;219:pl5. doi: 10.1126/stke.2192004pl5. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berg D. Reversible inhibition of GABAA receptors by α7-containing nicotinic receptors on the vertebrate postsynaptic neurons. J. Physiol. 2007;579:753–763. doi: 10.1113/jphysiol.2006.124578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LI, Tao HW, Holt CE, Harris WA, Poo M. A critical window for cooperation and competition among developing retinotectal synapses. Nature. 1998;395:37–44. doi: 10.1038/25665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.