Summary
Elongating E. coli RNAP is modulated by NusA protein. The C-terminal domain (CTD) of the RNAP α-subunit (αCTD) interacts with the acidic CTD 2 (AR2) of NusA, releasing the autoinhibitory blockade of the NusA S1-KH1-KH2 motif and allowing NusA to bind nascent nut spacer RNA. We determined the solution conformation of the AR2:αCTD complex. The αCTD residues that interface with AR2 are identical to those that recognize UP promoter elements A nusA-ΔAR2 mutation does not affect UP-dependent rrnH transcription initiation in vivo. Instead, the mutation inhibits Rho-dependent transcription termination at phage λ tR1, which lies adjacent to the λ nutR sequence. The Rho-dependent λ timm terminator, which is not preceded by a λ nut sequence, is fully functional. We propose that constitutive binding of NusA-ΔAR2 to λ nutR occludes Rho. In addition, the mutation confers a dominant defect in exiting stationary phase.
Introduction
The three distinct steps of transcription, that is initiation, elongation, and termination, are highly regulated by transcription factors and genetic signals encoded in DNA or RNA (Severinov, 2000). In E. coli, transcription initiation is mediated by the six subunit (σα2ββ’ω) RNA polymerase holoenzyme (RNAP) (Mooney et al., 2005). The N-terminal domains (NTD) of the two α-subunits are part of the structurally well-defined RNAP nucleus, whereas transcription initiation is controlled, in part, by the α-subunit C-terminal domain (αCTD). Regulatory factors such as catabolite activator protein (CAP), MarA, or SoxR bind to αCTD and increase initiation rates at specific promoters (Benoff et al., 2002; Shah and Wolf, 2004; Zou et al., 1992). The αCTD also interacts with cis-acting DNA sequences upstream of rrn promoters (UP-elements), thereby increasing the basal transcription level by about two orders of magnitude (Ross et al., 1993). Structurally, a key motif of the αCTD is a compact globular domain of two helix-hairpin-helix motifs linked via a connecting helix, the (Helix-hairpin-Helix)2, or (HhH)2, fold (Jeon et al., 1995). This fold was originally identified as a nucleic acid binding motif, but it is also known as a module for protein-protein interaction (Shao and Grishin, 2000).
The highly conserved 55 kDa monomeric NusA protein attaches to RNAP shortly after transcription initiation (Mooney et al., 2009). NusA slows the rate of transcription elongation and stimulates intrinsic and, possibly, Rho-dependent transcription termination(Cardinale et al., 2008). It is essential for viability in wild-type E. coli (Cardinale et al., 2008; Schmidt and Chamberlin, 1984). Efficient termination is required to silence cryptic prophage; thus, a nusA mutation deleted beyond aminoacid 127 (referred to below as ΔnusA) can be introduced into strain MDS42, which is deleted for these elements (Cardinale et al., 2008). NusA also plays a central role in the architecture of the phage λ N processive antitermination complex (Mah et al., 1999). NusA is composed of an NTD, three RNA-binding motifs S1, KH1, KH2, and two C-terminal acidic repeats (AR1, AR2) (Liu et al., 1996; Nudler and Gottesman, 2002) that, like αCTD, form an (HhH)2 fold (Eisenmann et al., 2005). The NTD interacts with RNAP, and two different binding sites in RNAP have been proposed. Whereas structural similarity and competitive binding with σ70 suggest that the NTD interacts directly with the RNAP β’ clamp helices (Borukhov et al., 2005), single particle electron microscopy and protein-RNA crosslinking suggest a binding site for NusA NTD close to the RNAP RNA exit channel, possibly by direct interaction with the flap-tip helix (Ha et al., 2010; Vassylyev, 2009; Yang et al., 2009). As part of the antitermination complex, NusA binds to the spacer region of the λ nut RNA sequence (Prasch et al., 2009). In contrast, isolated full-length NusA does not bind RNA, but a deletion mutant lacking AR2 does bind, indicating an autoinhibitory function of AR2. NusA association with αCTD also permits RNA binding, suggesting that the interaction between αCTD and AR2 relieves NusA autoinhibition (Mah et al., 2000). Although their global structures are nearly identical (Eisenmann et al., 2005), AR1 and AR2 exhibit entirely different protein binding specificities. AR1 binds λN (Bonin et al., 2004; Eisenmann et al., 2005; Mah et al., 1999), whereas no interaction of AR1 with αCTD or of AR2 with λN has been reported.
Here we describe the solution structure of the complex between AR2 and the αCTD of RNAP as well as the inhibitory interaction between AR2 and the NusA RNA-binding motifs that is released as a consequence of AR2:αCTD complex formation.
Results
Structure determination
Initial solution nuclear magnetic resonance (NMR) based interaction studies using a construct containing 15N-enriched NusA AR1 and AR2 (Eisenmann et al., 2005) resulted in complete disappearance of AR2 resonances in the 15N, 1H heteronuclear single quantum coherence (HSQC) spectra upon addition of RNAP αCTD(249-329), demonstrating the binding of αCTD to AR2 but at the same time rendering a determination of the conformation of the AR2: αCTD complex with this complete AR1-AR2 construct by solution NMR impossible. Using a construct containing only AR2 greatly improves the quality of the spectra, and complete resonance assignment of AR2 and αCTD was possible by application of heteronuclear double- and triple-resonance NMR experiments. NMR titration experiments using 15N enriched αCTD allow an upper limit estimate of the apparent dissociation constant KD in the range of 1-10 μM (Figure 1), consistent with earlier results determined by gel shift assays (Mah et al., 2000). Experimentally derived structural restraints (Table 1) allowed the calculation of an ensemble of structures with reasonable local geometry without restraint violation. The backbone (N, Cα, C’) coordinates superimpose with a root mean square deviation (rmsd) of 0.89 Å for the overall complex (0.36 Å within AR2, residues 430-491, and 0.61 Å within αCTD, residues 249-321).
Figure 1. Affinity of AR2:αCTD.
A: Chemical shift changes in an expanded region of the 1H,15N HSQC of 15N labeled AR2 upon addition of unlabeled αCTD. The arrows mark the direction of signal shifts during the titration series.
B: Titration curves for selected residues. Data fitting using a bimolecular two-state binding model results in a KD in a range of 1 μM - 5 μM (Leu434: 2.8 μM; Ala468: 3.4 μM; Ala480: 1.2 μM). Due to the high concentration required for the NMR experiments these results represent an estimate of the upper limit of the binding affinity.
Table 1. Structural statistics for AR2:αCTD complex.
| Total NOE Distance Restraints | 2470 |
|---|---|
| Intermolecular (AR2 – αCTD) | 33 |
| Intramolecular (AR2) | 1241 |
| Intraresidual | 366 |
| Interresidual Sequential, medium, long |
323, 330, 222 |
| Hydrogen Bond Restraints | 22 |
| Dihedral Angle Restraints | 7 |
| Intramolecular (αCTD) | 1196 |
| Intraresidual | 470 |
| Interresidual Sequential, medium, long |
281, 244, 201 |
| Hydrogen Bond Restraints | 16 |
| Dihedral Angle Restraints | 33 |
| <RMSD> from mean structure | |
| Backbone/heavy atom for AR2 ::αCTD (Å) | 0.89 |
| Backbone/heavy atom for AR2 (Å) | 0.36 |
| Backbone/heavy atom for αCTD (Å) | 0.61 |
| Ramachandran statistics (% residues) | |
| Most Favourable Region | 89.3 |
| Additionally Allowed Region | 8.5 |
| Generously Allowed Region | 2.0 |
| Disallowed Region | 0.3 |
Structure of the AR2:αCTD complex
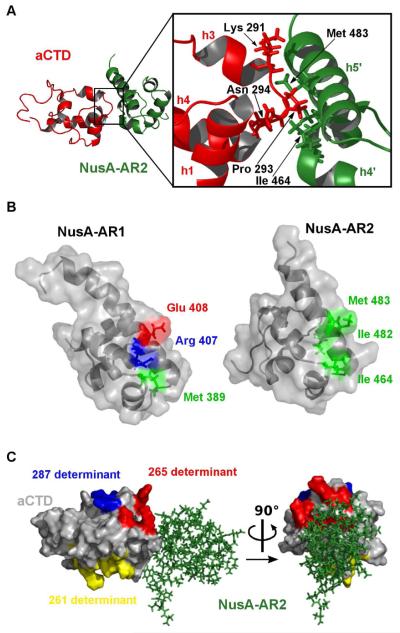
We determined the solution structure of the 19 kDa complex between AR2(426-495) and αCTD(249-329) by heteronuclear double- and triple-resonance NMR spectroscopy (Figure 2A, 2B). The structures of AR2 and αCTD in the complex superimpose well with their structures in the unbound state (AR2, pdb entry 1WCN: rmsd 0.94 Å for residues 429-491; αCTD pdb entry 1COO: rmsd 1.9 Å for residues 256-310). Thus, complex formation does not induce significant rearrangement within the domains and can be described as rigid body interaction. The recognition surface of AR2:αCTD is composed of a hydrophobic groove at AR2 which contains residues Ile464 of helix 4 and Ile482, Met483 of helix 5. The αCTD counterpart consists of a hairpin, Lys291-Gly294, between helix 3 and helix 4 (Figure 3A), with Pro293 forming a tip that is deeply buried in the AR2 pocket.
Figure 2. Solution structure of the AR2:αCTD complex.
A: Superposition of the structural ensemble of the AR2 (green):αCTD (red) complex.
B: Ribbon representation of the lowest energy structure. Helices are labeled h1 through h4 for αCTD and h1′ through h5′ for NusA-AR2, colors as in (A).
Figure 3. Binding interface of the αCTD:AR2 complex.
A: The hairpin of αCTD, red, interacts with h4 and h5 of AR2, green. Pro293 and Lys291 of αCTD, red, interact mainly with Met483 and Ile464 of AR2, green.
B: The differences between the highly homologous domains NusA AR1 (surface representation: left) and AR2 are evident. Residues 356 to 414 of NusA-AR1 have been fitted onto residue 431 to 490 of AR2 in complex with αCTD (right; negatively charged residues, red; positively charged residues, blue; hydrophobic residues, green).
C: αCTD, grey, in complex with AR2, green. The 261 determinant, yellow, interacts mainly with the σ70 factor, the 265 determinant, red, interacts mainly with UP-elements, and the 287 determinant, blue, interacts with regulatory proteins such as CAP (Benoff, Yang, et al, 2002). In the αCTD:AR2 complex, the 265 determinant is blocked by AR2, green, whereas the 287 and 261 determinants are accessible.
The small AR2:αCTD interaction interface of 694 Å2 supports our finding that no large rearrangement takes place upon complex formation (Lo Conte et al., 1999). The structure of the AR2:αCTD is the second example of a protein-protein complex involving two domains with the (HhH)2 fold, in addition to the ERCC1:XPF complex (Tripsianes et al., 2005; Tsodikov et al., 2005). In the AR2:αCTD complex only the loop of the second HhH motif of αCTD interacts with both helices of the second HhH motif of AR2, whereas in ERCC1:XPF (Tripsianes et al., 2005; Tsodikov et al., 2005) the (HhH)2 domains are arranged as an intermolecular four helix bundle with a pseudo-2-fold symmetry axis and a comparatively large interaction surface. These different architectures of complexes made up of two identical interaction motifs seem, however, optimized for their respective physiological functions: ERCC1:XPF forms a stable heterodimeric complex that is required for a functional endonuclease (Tripsianes et al., 2005; Tsodikov et al., 2005), whereas NusA and RNAP form a weak and transient complex appropriate for a regulatory function.
Binding specificity of NusA acidic repeats
Although AR1 and AR2 share high sequence homology and virtually identical three-dimensional structures, αCTD recognizes AR2 with high specificity. Interaction of αCTD with AR1 has not been observed. The role of AR1 is not entirely clear; it binds λN but is not required for N activity in vitro (Bonin et al., 2004; Eisenmann et al., 2005; Mah et al., 1999).
The differences in target recognition by AR1 and AR2 become explicable by closer scrutiny of their respective interaction surfaces: In the AR1:λN recognition peptide complex, the crucial feature of AR1 is a hydrophobic patch involving Phe369, Val372, and Leu398 (Bonin et al., 2004) that forms essential contacts to Leu40 from λN. An analogous hydrophobic patch on AR2 would be interrupted by Lys447. Similarly, the crucial feature allowing AR2:αCTD complex formation is a hydrophobic interaction surface formed by AR2 Ile464, Ile482, and Met483 (Figure 3B), roughly opposite the AR1:λN recognition site. In AR1, Glu408 and Arg407 replace Met483 and Ile482 in AR2 (Figure 3B), again abrogating formation of a hydrophobic patch.
The two acidic repeats of NusA and RNAP αCTD share the (HhH)2 fold. Their interactions with other proteins demonstrate that despite similar folds, different regions of the proteins are used for target protein recognition, although in all three cases the interaction site is represented by small convex or concave hydrophobic regions. These differences suggest a versatile role of the (HhH)2 fold as a general protein recognition motif, but will make prediction of these binding sites challenging.
Transcription regulation at αCTD
The αCTD contains three discrete sites that bind elements which regulate RNAP transcriptional activities (Ebright and Busby, 1995; Gaal et al., 1996; Ishihama, 1993; Jeon et al., 1995; Murakami, Fujita, and Ishihama, 1996; Murakami et al., 1997). The 287 determinant is important for interaction with activator proteins such as CAP, the 261 determinant interacts with σ70-factor, and the 265 determinant recognizes UP-elements and the activator proteins MarA and SoxS (Dangi et al., 2004; Gaal et al., 1996; Shah and Wolf, 2004). SoxS binding reduces αCTD attachment to UP-elements, diverting RNAP to specific SoxS promoter sequences (Shah and Wolf, 2004). In obvious analogy, the AR2 interaction site is also located close to the 265 determinant and occludes the DNA binding site of αCTD, rendering binding of αCTD to AR2 and binding of αCTD to UP-elements mutually exclusive (Figure 3C).
Preventing or reversing αCTD binding to UP-elements would be expected to affect transcription of rrn operons. By inhibiting binding, NusA might direct transcription away from rrn towards other promoters, as is suggested for SoxS (Shah and Wolf 2004). Conversely, reversing αCTD binding might stimulate escape from rrn promoters. Gel shift experiments demonstrate that stepwise addition of AR2 released αCTD from a complex with DNA carrying an UP-element, as did full-length NusA. In contrast, AR1 alone did not release αCTD (Figure 4). This is consistent with the higher affinity of αCTD for AR2 (KD<10 μM) compared to the affinity of αCTD to a typical UP-element such as rrnB P1 (KD=120 μM) (Yasuno et al., 2001). But it should be noted that this in vitro experiment uses isolated protein domains and DNA. This may not reflect the real situation, where the components are tethered together through the RNA polymerase.
Figure 4. NusA AR2 displaces αCTD from the UP-element.
A: Gel retardation experiments detecting displacement of αCTD from an UP-element by purified AR2 domain. All reactions contain 25 μM 32P-labeled wild-type UP-element DNA and an increasing amount of αCTD (up to 100 μM) and AR2 (up to 250 μM).
B: Gel retardation experiments detecting displacement of αCTD from an UP-element by full-length NusA and control with NusA AR1. All reactions contain 25 μM 32P-labeled wild-type UP-element DNA, increasing amount of αCTD (up to 100 μM), NusA AR1 (up to 250 μM), and NusA wt (up to 200 μM).
We tested AR2 interaction with UP-elements in vivo, using an rrnH – 16S - luc fusion that carries the rrnH UP-element. A nusA-ΔAR2 mutant was constructed by recombineering in the MDS42 background (Sharan et al., 2009). The mutant grows almost as well as wild-type MDS42, and better than a ΔnusA strain. MDS42 wildtype, ΔnusA, and nusA-ΔAR2 were assayed for luciferase in log-phase or after overnight growth. No difference in luciferase activity was observed among the three strains, indicating that NusA does not affect UP-element activity in vivo (Table 2). This implies that NusA does not interact with αCTD until the rrn promoter has been cleared, and is consistent with the report that NusA competes with σ for RNAP core binding (Greenblatt and Li, 1981).
Table 2. NusA does not control rrnH transcription.
Shown are luciferase values (× 10−6). MDS42 and MDS42 mutants carrying an rrnH 16S - luc fusion were grown overnight in LB at 37°C (stationary phase), diluted 1:50, and incubated at 37°C to OD600 0.20 – 0.45 (log phase). Initial OD600 and viability were equivalent for the three strains. Shown is a representative experiment; assays were performed at least 4 times with equivalent results.
| strain | nus | Stationary | Log |
|---|---|---|---|
| 10916 | + | 1.2 | 30.5 |
| 10918 | nusA-ΔAR2 | 1.2 | 31.2 |
| 10935 | ΔnusA | 1.9 | 33.0 |
Although NusA deletions do not affect UP-element activity, they dramatically slow the rate of exit from stationary phase (Fig 5). MDS42 wild-type, ΔnusA, and nusA-ΔAR2 overnight cultures were diluted into fresh medium and their growth followed optically. Both ΔnusA and nusA-ΔAR2 strains entered log phase only after a prolonged lag. In the case of the ΔnusA strain, expression of NusA from a plasmid suppressed the outgrowth phenotype. In contrast, the defect of the nusA-ΔAR2 mutation was dominant. The dominance of the AR2 deletion suggests that the mutation confers a gain-of-function, i.e. constitutive RNA binding, phenotype on NusA.
Figure 5. NusA deletions slow the rate of exit from stationary phase.
Strain MDS42 and its nusA mutant derivatives were grown overnight at 37 °C in LB or, for plasmid-bearing strains, LB + ampicillin (50 μg/ml) and then diluted 100-fold into LB or for plasmid-bearing strains, LB + ampicillin + 0.5 mM IPTG and grown at 37 °C for the times indicated. Initial OD600’s and viable counts for the 6 strains were equivalent. Strains: 10323 = wild-type (wt); 10324 = ΔnusA; 10881 = nusA-ΔAR2; 10875 = wt/ptac-NusA+; 10876 = ΔnusA/ptac-NusA+; 10889 = nusA-ΔAR2/ptac-NusA+.
Transcription termination defect of nusA-ΔAR2
Since NusA-ΔAR2 is expected to bind λnut RNA constitutively, we asked if it affected termination at λtr1, a Rho-dependent terminator that is immediately promoter-distal to λnutR. Termination was significantly impaired in the nusA-ΔAR2 mutant (Table 3) compared to wild-type or ΔnusA strains. The defect was not suppressed by expression of NusA from a multicopy plasmid, as expected if deletion of AR2 generates a gain-of-function phenotype. In contrast to termination at λtR1, Rho-dependent termination at λtimm was unaffected by deletion of AR2 (Table 4). This terminator does not lie adjacent to a nut site. We propose that constitutive binding of NusA-ΔAR2 to nutR prevents access of Rho to λtR1. Note also that the activity of the λpL promoter is not reduced in the nusA-ΔAR2 strain. λpL, like the rrnH promoters, includes an UP-element (Giladi, Koby, et al, 1998).
Table 3. NusA-ΔAR2 dominant transcription termination defects at λnutRtR1.
Strains are derivatives of MDS42 and where indicated, carry a plac-NusA+ plasmid. lacZ is expressed from the fusion λcI857 – pR – cro (ΔRBS) – nutR – tR1 – cII::lacZ. Cells are assayed for β-galactosidase activity (Miller units) after overnight growth at 42°C. Strains carrying plasmids were induced with IPTG (0.5 mM). Shown is a representative experiment. Assays were performed at least twice with equivalent results.
| strain | nusA | plasmid | β-gal units |
|---|---|---|---|
| 10323 | + | − | 176 |
| 10324 | Δ | − | 343 |
| 10881 | ΔAR2 | − | 902 |
| 10875 | + | plac-NusA | 227 |
| 10876 | Δ | plac-NusA | 158 |
| 10889 | ΔAR2 | plac-NusA | 1114 |
Table 4. NusA-ΔAR2 does not affect termination at timm, a Rho-dependent terminator lacking a nut site.
Strains are MDS42 derivatives that carry the fusion: λpRM - λcI857 – timm – pL – nutL – N::lacZ. At 32°C, pRM is active and pL is repressed; at 42°C, pRM is repressed and pL is active. pRM transcripts terminate at the Rho-dependent timm terminator. Cells were assayed for β-galactosidase activity (Miller units) after overnight growth at 32°C +/− BCM (bicyclomycin, 20μg/ml) or at 42°C. At this concentration, BCM entirely inhibits Rho-dependent termination. Shown is a representative experiment. Assays were performed at least twice with equivalent results.
| strain | nusA | Temp (°C) | BCM | β-gal units |
|---|---|---|---|---|
| 10900 | + | 32 | − | 51 |
| 10900 | + | 32 | + | 378 |
| 10900 | + | 42 | − | 913 |
| 10901 | ΔAR2 | 32 | − | 31 |
| 10901 | ΔAR2 | 32 | + | 625 |
| 10901 | ΔAR2 | 42 | − | 430 |
Autoinhibition of NusA is mediated by AR2-KH1 interaction and released by αCTD
AR2 blocks RNA access to the NusA-S1-KH1-KH2 (SKK) domain (Mah et al., 2000; Nudler and Gusarov, 2003). We performed NMR titration experiments with isotope-labeled NusA SKK and AR2. The backbone resonances of NusA-SKK could be assigned by application of multidimensional heteronuclear transverse relaxation optimized spectroscopy (TROSY) based triple resonance NMR. Secondary structure elements derived from chemical shifts are consistent with known NusA structures from other bacteria (Gopal et al., 2001; Worbs et al., 2001), allowing the use of these structures as models for E. coli NusA-SKK. HSQC NMR titration of 2H, 15N labeled SKK with AR2 resulted in significant chemical shift changes of resonances from the KH1 region of SKK (Figure 6A). In contrast to KH1, resonances from the S1 and KH2 regions were not affected, suggesting that AR2 interacts exclusively with the KH1 domain (Figure 6A,7A and 7C). Addition of αCTD to the SKK:AR2 complex reversed the chemical shift changes induced in the KH1 signals on AR2 addition (Figure 7B), confirming that αCTD promotes NusA binding to RNA by displacing AR2 from SKK.
Figure 6. Chemical shift perturbation of NusA-SKK by AR2 and nutL RNA.
A: Chemical shift changes of NusA-SKK upon binding to AR2 as a function of primary sequence.
B: Chemical shift changes of NusA-SKK upon binding to λ nutL RNA as a function of primary sequence; X = residues not assigned. Dotted line represents the significance level of 0.04 ppm; bars represent the three RNA binding domains.
Figure 7. NusA-SKK interacts with AR2 and nutL RNA.
A: Binding of AR2 to SKK. Chemical shift changes of residues Arg270 and Gly249 during an NMR titration experiment of 2H, 15N labeled NusA-SKK with unlabeled AR2 demonstrate binding of AR2 to SKK.
B: Release of autoinhibition. The direction of titration could be reversed by adding αCTD showing the release of NusA autoinhibition by AR2:αCTD interaction.
C: KH1 is interaction domain with AR2 and nutL RNA. Because of the 52% homology of E. coli and Thermotoga maritima NusA-SKK (Worbs et al., 2001), we used the crystal structure of the latter as a template and mapped the observed chemical shift changes onto the surface, highlighting the binding interface of AR2 (C) and λ nutL RNA (D) (S1, light blue; KH1, gray; KH2 blue; Residues with resonances showing chemical shift changes of 0.04 ppm < Δδ < 0.1 ppm, green; those with Δδ > 0.1 ppm, red; unassigned resonances, yellow). Bottom: structure of nutL RNA as used in the titration experiment.
RNA binding of NusA-SKK
The λnutL and λnutR RNA sequences are located downstream of the early λ promotors pL and pR, respectively. The nut sequences form stable antitermination complexes with NusA, NusB, NusE, NusG, and λN (Nudler and Gottesman, 2002). Within the complex, NusA binds to spacer RNA (Prasch et al., 2009). We mapped the chemical shift perturbations of 2H, 15N labeled SKK by λnutL boxA-spacer-boxB RNA. Nearly all 1HN and 15N resonances in both KH domains were affected upon binding to λnutL RNA (Figure 6B and 7D). The most prominent changes, however, were observed in residues Arg210 to Trp276 within the KH1 domain. The binding surface of KH1 for λnutL boxA-spacer-boxB RNA exactly matches the binding surface of KH1 for AR2, indicating the precision of the autoinhibitory interaction. Our results also lend support to earlier mutational data (Zhou et al., 2001; Zhou et al., 2002b) which showed that RNA binding was abolished by mutating the first glycine in the conserved GXXG motifs of KH1 (G253D) and KH2 (G319D). Both glycines are part of the binding interface, since their resonances show intermediate to slow exchange on the NMR time scale in the SKK:nutL RNA complex. A third mutation, R199A, located in the S1 domain, also abolishes nut RNA binding (Zhou et al., 2001). Although our data show that R199 is not directly involved in binding nut RNA, it is located in the hinge connecting S1 and KH1. The R199A mutation may impede KH1 RNA binding by changing the relative positions of S1 and KH1. These results with E. coli contrast studies of a complex between the M. tuberculosis NusA and rrn boxC RNA(Beuth et al., 2005). In this complex, rrn BoxC RNA interacts predominantly with KH2.
Discussion
E. coli NusA forms a complex with RNAP near the start site of transcription. The complex is stabilized by interactions between the NusA NTD and the β or β’ RNAP subunits, as well as by an interaction between NusA AR2 and RNAP αCTD. Interaction with the αCTD reverses autoinhibition by AR2 of the RNA binding motif of NusA, allowing NusA to bind RNA.
We have solved the structure of E. coli NusA AR2 in complex with the RNAP αCTD. In this complex, the NusA AR2 domain interacts with the αCTD 265 determinant, which also binds UP-elements at ribosomal RNA promoters. Indeed, NusA or isolated AR2 displaces αCTD from UP-element DNA in vitro. Nevertheless, the rrnH operon, which contains an UP-element, was expressed normally in strains lacking NusA or carrying NusA with an AR2 deletion, as monitored with an rrnH – 16S RNA – luciferase fusion. As in wild-type E. coli, the operon was highly active in log phase cells, and down-regulated in stationary phase. We suggest that NusA does not affect transcription initiation at rrn promoters because it enters the transcription elongation complex after the promoters have been cleared and sigma released, consistent with the finding that NusA attaches to the transcription elongation complex subsequent to Rho (Mooney et al., 2009).
Although it appears not to affect ribosomal RNA promoter activity, NusA is known to reduce globally the rate of transcription elongation and to stimulate termination, particularly at intrinsic termination signals. Whether interaction between the AR2 domain and the αCTD plays a role in these reactions is not known.
We have further examined the phenotypes of ΔnusA (a truncation carrying only the N-terminal domain) and nusA-ΔAR2 mutants. Both lag in exiting stationary phase. NusA expressed from a plasmid suppresses the ΔnusA but not the nusA-ΔAR2 mutation. This indicates that loss of AR2 confers a dominant gain-of-function phenotype, presumably the ability of NusA-ΔAR2 to bind RNA constitutively. This phenotype suggests that NusA N-terminal interactions are sufficient to allow NusA to bind RNAP in the absence of AR2 interactions with αCTD, although this has not been directly demonstrated.
We also found that termination at the Rho-dependent λtimm terminator was unaffected by the nusA-ΔAR2 mutation. Unlike λtimm, termination at λtR1, a Rho-dependent terminator that lies just promoter-distal to λnutR, was compromised in a dominant fashion by the AR2 deletion. In contrast to nusA-ΔAR2, ΔnusA did not reduce termination at λtR1. This is most simply explained by competition between NusA-ΔAR2 and Rho for binding to the λnutR/tR1 region. We suggest that constitutive RNA binding by the NusA-ΔAR2 underlies this competition. That nusA-ΔAR2 has a phenotype affecting transcription elongation implies that the interaction of wild-type NusA with αCTD may be reversible during elongation, at least at the λnutR sequence.
The structure of AR2 bound to the NusA RNA-binding motif SKK indicates that AR2 uses the same interface to interact with the αCTD and with KH1, indicating that AR2 binding to αCTD completely precludes binding to the KH1 motif. Interestingly, the interactions between AR2 and the RNA-binding motif are exclusively with KH1. This suggests that KH1 is the dominant RNA-binding motif, consistent with analysis of NusA point mutant proteins, although the S1 motif is also thought to participate in RNA binding (Zhou et al., 2002a).
It will be interesting to determine the mechanistic defects of the nusA point mutations, nusA1 and nusA100, which block λ N and/or HK022 Nun function and which lie in the S1 motif.
Experimental Procedures
Protein preparation
E. coli AR1 (residues 339-426) and AR2 (residues 424-495) were expressed as a deca-histidine-tagged protein in BL21(DE3) from pET-19b (Novagen) and purified as described previously (Eisenmann et al., 2005; Prasch et al., 2006). E. coli αCTD (residues 233-329) was expressed as a deca-histidine-tagged protein in BL21(DE3) from pET-19b (Novagen) and purified as described previously (Eisenmann et al., 2005). E. coli NusA-SKK (residues 132-348) was expressed as a penta-histidine-tagged protein in BL21(DE3) from pET-11a (Novagen) as described previously (Mah et al., 1999).
Briefly, strains carrying these constructs were grown in M9 minimal medium supplemented with 15NH4Cl and 0.2% 13C D-glucose as the sole nitrogen and carbon sources, respectively (Marley et al., 2001). To improve the resolution and sensitivity of the NMR spectra, deuterated M9 minimal medium supplemented with 15NH4Cl and 0.2% 13C D-glucose has been used for NusA-SKK.
In the case of AR1, AR2, and αCTD the recombinant proteins were purified under native conditions by nickel-affinity chromatography (5 mL His-trap chelating column, GE Health Care) and eluted by applying an imidazole step gradient. Peak fractions containing the constructs were dialyzed against 50 mM Tris/HCl at pH 8.0, 150 mM NaCl, and 1 mM DTT. After dialysis, the N-terminal deca-His tag was cleaved off using PreScission protease. The cleaved proteins were dialyzed against 50 mM Tris/HCl at pH 7.4 and 1 mM DTT and further purified via a QXL column (GE Health Care) by a NaCl step gradient in the same buffer with up to 1 M NaCl. The eluted fractions containing the proteins were concentrated with Vivaspin concentrators (Vivascience, MWCO 5000 Da) and dialyzed against NMR buffer. NMR samples contained 0.5-1.5 mM AR1, AR2, or αCTD in 10 mM potassium phosphate (pH 6.8), 50 mM NaCl, 0.02% sodium azide, and 10% D2O. For the complex sample we used a stoichiometric ratio of AR2:αCTD of 1:3, or vice versa.
In the case of NusA-SKK, the recombinant protein was purified under native conditions by nickel-affinity chromatography (5 ml His-trap chelating column, GE Health Care) and eluted by applying an imidazole step gradient. Peak fractions containing the constructs were dialyzed against 50 mM sodium phosphate buffer, pH 7.6, 100 mM NaCl, 10 mM β-mercaptoethanol, 0.5 mM EDTA, and 1 % glycerol (v/v) and concentrated with Vivaspin concentrators (Vivascience, MWCO 10000 Da).
NMR spectroscopy
All NMR spectra were recorded at 298 K on Bruker Avance 700 MHz and Avance 800 MHz spectrometers with cryogenically-cooled triple-resonance probes equipped with pulsed field-gradient capabilities.
For resonance assignment of NusA-AR2 and αCTD in their complex standard double and triple resonance through-bond experiments were recorded (Sattler et al., 1999) using differential isotopically labeled samples (uniformly 13C,15N labeled AR2 and unlabeled αCTD and vice versa). For NusA-SKK, assignment was done using TROSY-type NMR experiments (Pervushin et al., 1997; Salzmann et al., 1998) and 15N edited NOESY spectra. For obtaining distance restraints 15N-separated and 13C-separated nuclear Overhauser enhancement (NOE) experiments were recorded (Ikura et al., 1991; Talluri and Wagner, 1996). Intermolecular NOEs were recorded with one partner 15N/13C-labeled and the other unlabeled in D2O using three-dimensional 13C-separated/12C-filtered NOE experiments (Zwahlen et al., 1997). NMR data were processed using in-house routines and visualized with NMRView (Johnson, 2004). Dissociations constants were determined from chemical shift changes of residues showing fast exchange behaviour during a titration experiment using a two-state model.
Structure determination
Distance restraints for structure calculation were derived from 15N-NOESY-HSQC and 13C-NOESY-HSQC spectra with mixing times of 100-120 ms. NOESY cross peaks were classified according to their relative intensities and converted to distance restraints with upper limits of 3.0 Å (strong), 4.0 Å (medium), 5.0 Å (weak), and 6.0 Å (very weak). For ambiguous distance restraints the r−6 summation over all assigned possibilities defined the upper distance limit.
Hydrogen bonds were included for backbone amide protons in regular secondary structure if the amide proton did not show a water exchange cross peak in the 15N-edited NOESY spectrum or showing slow exchange in H/D exchange experiments. The structure calculations were performed with the program XPLOR-NIH 1.2.1(Schwieters et al., 2003) using a three-step simulated annealing protocol with floating assignment of prochiral groups including a conformational database potential. The structures were analyzed with the programs XPLOR-NIH 1.2.1 and PROCHECK (Laskowski et al., 1996). NMR resonance assignments were deposited in the BMRB under the accession codes 15614. Coordinates and restraints for structure calculation were deposited in the PDB under accession code 2JZB.
Competition gel retardation assay
Synthetic oligonucleotides were purchased from biomers.net (Ulm, Germany). Annealing was promoted by incubation of the appropriate oligonucleotides at 95°C for 5 min, with cooling down at room temperature in NMR buffer (10 mM potassium phosphate (pH 6.8), 50 mM NaCl). The oligonucleotides were 5′-end labelled with [32P] ATP using T4 polynucleotide kinase (NEB). Unincorporated label was removed using a 1 ml G25 spin column (GE Healthcare).
Competition gel retardation assays (10 μl) were performed in NMR buffer (10 mM potassium phosphate (pH 6.8), 50 mM NaCl). Binding reactions typically contained 100 μM unlabelled DNA with 0.16 μM [32P] labelled DNA, with increasing concentrations of binding protein or competitor protein in a final reaction volume of 10 μl. The reactions were analysed in 6 % non-denaturing polyacrylamide gels (Novex Pre-Cast DNA Retardation Gels, Invitrogen) in 1/2x TBE buffer, and electrophoresed at 80 V for ~90 min at 4°C and visualized with a phosphoimager.
Construction of 16S rRNA-luc and aspU-luc gene fusions
The 16S rRNA-luc gene fusion was constructed in the rrnH rRNA gene operon of the E. coli chromosome to assay the transcription activity within rRNA gene operons in vivo. The construction of the fusion was done in two steps using recombineering (Thomason et al., 2007). First, the luc/amp gene cassette was assembled in the chromosome of JS470 so that the luc gene could be transferred between various genetic backgrounds using amp as a genetic marker. Then, the luc/amp cassette was used to make the rrnH-luc fusion in NB363 replacing the rrsH-aspU region, rrsH-aspU<>luc/amp.
The luc/amp gene cassette was assembled by placing the ampicillin resistance amp gene just downstream to the firefly luciferase luc gene in the galK<>luc chromosomal locus of JS470 (kindly provided by Dr. J. Sawitzke), a derivative of the recombinogenic DY330 strain containing a defective λ prophage expressing the red recombination genes. The amp gene with its own promoter was amplified by PCR (Platinum Taq DNA Polymerase High Fidelity, Invitrogen, CA) from pBR322 using the forward primer CGGAAAGATCGCCGTGTAAACGAAACTCCCGCACTGGCACCCGATCATTCAAATATGTATCCGCTC and the reverse primer CATCCCTGCGTTGTTACGCAAAGTTAACAGTCGGTACGGCTGACCTTACCAATGCTTAATC AGTGAGGC. These bipartite primers were designed so that the last 23 bases at the 3′ end of the primers (shown in bold) provided sequences for amplification of the amp gene, whereas the first 45 bases provided sequences homologous to the galK<>luc downstream target region which are required for recombination of the PCR-amplified amp gene into that region of the E. coli chromosome. The resulting amp gene PCR product was purified by the PCR Purification Kit (Qiagen) and 100 ng of the product was used to transform JS470 heat-induced for recombineering at A600 ~ 0.4 for 15 min. as previously described in detail for DY330 (Bubunenko et al., 2007). The transformed cells were grown for 3 hours at 32°C and plated on L plates containing 30 μg/ml Amp (Ap30). The plates were incubated for 2 days at 32°C and the recombinants analyzed by PCR for amp using a pair of checking primers flanking the chromosomal amp insertion: AACTCGACGCAAGAAAAATCAGAG and GTCGCACCCCAGTCCATCAGCGTG. The resulting NB371 cells were used as a source of the luc/amp cassette.
To make the 16S rRNA-luc fusion, the luc/amp cassette was amplified by PCR using the forward primer AATTCATTACAAAGTTTAATTCTTTGAGCATCAAACTTTTGAAGGAGATATTCATATGGAAGACGCCAAAAACATAAAG and the reverse primer CTTATTAAGAAGCCTCGAGTTAACGCTCGAGGTTTTTTTTCGTCTTTACCAATGCTTAATCA GTGAGGC. In these bipartite primers, the last 24 bases (shown in bold) provided the regions for amplification of luc/amp. The first 55 bases of the forward primers and 45 bases of the reverse primer provided the regions of homology to the respective target regions of the chromosomal rrnH rRNA gene operon. The PCR-amplified fragments were purified and used to transform NB363, a W3110 derivative containing a miniλ-tet expressing the red recombination genes (Court et al., 2003). Recombineering was done as described above for JS470. The cells were plated on Ap30 plates and the recombinants screened for the luc-amp insertions by PCR using a pair of checking primers flanking the rrsH-aspU region, TACCAAGTCTCAAGAGTGAACACG and GCAGGGATAGCCATAATATGCCTC or the aspU gene, CGCCGAAGCTGTTTTGGCGGATTG and GCAGGGATAGCCATAATATGCCTC respectively. The miniλ-tet was removed from the final NB375 (W3110 rrsH-aspU<>luc/amp) strain by growing cells at 37 °C and selecting for TcS colonies.
Strains and enzyme assays
Strains were derivatives of MDS42, which lacks prophages an2d insertion elements (Posfai, Plunkett, et al, 2006). A pBR322-based plasmid (pRW1; (Watnick, and Gottesman, 1998)) that expressed LacZ under ptaq control was used for complementation studies. Standard β-galactosidase and luciferase assays were used. Details are described in the Table Legends.
Acknowledgements
Financial support by the Deutsche Forschungsgemeinschaft to PR (Ro617/17-1) and by NIH to MEG (GM037219) is gratefully acknowledged. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and in part by a Trans National Institutes of Health/Food and Drug Administration Intramural Biodefense Program Grant of National Institutes of Allergy and Infectious Disease (to Donald L. Court). This project has been also partly funded with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benoff B, Yang H, Lawson CL, Parkinson G, Liu J, Blatter E, Ebright YW, Berman HM, Ebright RH. Structural basis of transcription activation: the CAP-alpha CTD-DNA complex. Science. 2002;5586:1562–1566. doi: 10.1126/science.1076376. [DOI] [PubMed] [Google Scholar]
- Beuth B, Pennell S, Arnvig KB, Martin SR, Taylor IA. Structure of a Mycobacterium tuberculosis NusA-RNA complex. EMBO J. 2005;20:3576–3587. doi: 10.1038/sj.emboj.7600829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin I, Muhlberger R, Bourenkov GP, Huber R, Bacher A, Richter G, Wahl MC. Structural basis for the interaction of Escherichia coli NusA with protein N of phage lambda. Proc. Natl. Acad. Sci. U. S. A. 2004;38:13762–13767. doi: 10.1073/pnas.0405883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borukhov S, Lee J, Laptenko O. Bacterial transcription elongation factors: new insights into molecular mechanism of action. Mol. Microbiol. 2005;5:1315–1324. doi: 10.1111/j.1365-2958.2004.04481.x. [DOI] [PubMed] [Google Scholar]
- Bubunenko M, Baker T, Court DL. Essentiality of ribosomal and transcription antitermination proteins analyzed by systematic gene replacement in Escherichia coli. J Bacteriol. 2007;7:2844–53. doi: 10.1128/JB.01713-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinale CJ, Washburn RS, Tadigotla VR, Brown LM, Gottesman ME, Nudler E. Termination factor Rho and its cofactors NusA and NusG silence foreign DNA in E. coli. Science. 2008;5878:935–938. doi: 10.1126/science.1152763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court DL, Swaminathan S, Yu D, Wilson H, Baker T, Bubunenko M, Sawitzke J, Sharan SK. Mini-lambda: a tractable system for chromosome and BAC engineering. Gene. 2003:63–69. doi: 10.1016/s0378-1119(03)00728-5. [DOI] [PubMed] [Google Scholar]
- Dangi B, Gronenborn AM, Rosner JL, Martin RG. Versatility of the carboxy-terminal domain of the alpha subunit of RNA polymerase in transcriptional activation: use of the DNA contact site as a protein contact site for MarA. Mol. Microbiol. 2004;1:45–59. doi: 10.1111/j.1365-2958.2004.04250.x. [DOI] [PubMed] [Google Scholar]
- Ebright RH, Busby S. The Escherichia coli RNA polymerase alpha subunit: structure and function. Curr. Opin. Genet. Dev. 1995;2:197–203. doi: 10.1016/0959-437x(95)80008-5. [DOI] [PubMed] [Google Scholar]
- Eisenmann A, Schwarz S, Prasch S, Schweimer K, Rösch P. The E. coli NusA carboxy-terminal domains are structurally similar and show specific RNAP- and λ N interaction. Protein Sci. 2005;8:2018–2029. doi: 10.1110/ps.051372205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaal T, Ross W, Blatter EE, Tang H, Jia X, Krishnan VV, Assa-Munt N, Ebright RH, Gourse RL. DNA-binding determinants of the alpha subunit of RNA polymerase: novel DNA-binding domain architecture. Genes Dev. 1996;1:16–26. doi: 10.1101/gad.10.1.16. [DOI] [PubMed] [Google Scholar]
- Giladi H, Koby S, Prag G, Engelhorn M, Geiselmann J, Oppenheim AB. Participation of IHF and a distant UP element in the stimulation of the phage lambda PL promoter. Mol. Microbiol. 1998;2:443–451. doi: 10.1046/j.1365-2958.1998.01079.x. [DOI] [PubMed] [Google Scholar]
- Gopal B, Haire LF, Gamblin SJ, Dodson EJ, Lane AN, Papavinasasundaram KG, Colston MJ, Dodson G. Crystal structure of the transcription elongation/anti-termination factor NusA from Mycobacterium tuberculosis at 1.7 A resolution. J. Mol. Biol. 2001;5:1087–1095. doi: 10.1006/jmbi.2000.5144. [DOI] [PubMed] [Google Scholar]
- Greenblatt J, Li J. Interaction of the sigma factor and the nusA gene protein of E. coli with RNA polymerase in the initiation-termination cycle of transcription. Cell. 1981;2:421–428. doi: 10.1016/0092-8674(81)90332-9. [DOI] [PubMed] [Google Scholar]
- Ha KS, Toulokhonov I, Vassylyev DG, Landick R. The NusA N-terminal domain is necessary and sufficient for enhancement of transcriptional pausing via interaction with the RNA exit channel of RNA polymerase. J. Mol. Biol. 2010 doi: 10.1016/j.jmb.2010.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura M, Spera S, Barbato G, Kay LE, Krinks M, Bax A. Secondary structure and side-chain proton and carbon-13 resonance assignments of calmodulin in solution by heteronuclear multidimensional NMR spectroscopy. Biochemistry. 1991;38:9216–9228. doi: 10.1021/bi00102a013. [DOI] [PubMed] [Google Scholar]
- Ishihama A. Protein-protein communication within the transcription apparatus. J. Bacteriol. 1993;9:2483–2489. doi: 10.1128/jb.175.9.2483-2489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YH, Negishi T, Shirakawa M, Yamazaki T, Fujita N, Ishihama A, Kyogoku Y. Solution structure of the activator contact domain of the RNA polymerase alpha subunit. Science. 1995;5241:1495–1497. doi: 10.1126/science.270.5241.1495. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Using NMRView to visualize and analyze the NMR spectra of macromolecules. Methods Mol. Biol. 2004:313–352. doi: 10.1385/1-59259-809-9:313. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR. 1996;4:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- Liu K, Zhang Y, Severinov K, Das A, Hanna MM. Role of Escherichia coli RNA polymerase alpha subunit in modulation of pausing, termination and anti-termination by the transcription elongation factor NusA. EMBO J. 1996;1:150–161. [PMC free article] [PubMed] [Google Scholar]
- Lo Conte L, Chothia C, Janin J. The atomic structure of protein-protein recognition sites. J. Mol. Biol. 1999;5:2177, 2198. doi: 10.1006/jmbi.1998.2439. [DOI] [PubMed] [Google Scholar]
- Mah TF, Kuznedelov K, Mushegian A, Severinov K, Greenblatt J. The alpha subunit of E. coli RNA polymerase activates RNA binding by NusA. Genes Dev. 2000;20:2664–2675. doi: 10.1101/gad.822900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah TF, Li J, Davidson AR, Greenblatt J. Functional importance of regions in Escherichia coli elongation factor NusA that interact with RNA polymerase, the bacteriophage lambda N protein and RNA. Mol. Microbiol. 1999;3:523–537. doi: 10.1046/j.1365-2958.1999.01618.x. [DOI] [PubMed] [Google Scholar]
- Marley J, Lu M, Bracken C. A method for efficient isotopic labeling of recombinant proteins. J. Biomol. NMR. 2001;1:71–75. doi: 10.1023/a:1011254402785. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Darst SA, Landick R. Sigma and RNA polymerase: an on-again, off-again relationship? Mol. Cell. 2005;3:335–345. doi: 10.1016/j.molcel.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Mooney RA, Davis SE, Peters JM, Rowland JL, Ansari AZ, Landick R. Regulator Trafficking on Bacterial Transcription Units In Vivo. Mol Cell. 2009:97–108. doi: 10.1016/j.molcel.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Fujita N, Ishihama A. Transcription factor recognition surface on the RNA polymerase alpha subunit is involved in contact with the DNA enhancer element. EMBO J. 1996;16:4358–4367. [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Owens JT, Belyaeva TA, Meares CF, Busby SJ, Ishihama A. Positioning of two alpha subunit carboxy-terminal domains of RNA polymerase at promoters by two transcription factors. Proc. Natl. Acad. Sci. U. S. A. 1997;21:11274–11278. doi: 10.1073/pnas.94.21.11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudler E, Gottesman ME. Transcription termination and anti-termination in E. coli. Genes Cells. 2002;8:755–768. doi: 10.1046/j.1365-2443.2002.00563.x. [DOI] [PubMed] [Google Scholar]
- Nudler E, Gusarov I. Analysis of the intrinsic transcription termination mechanism and its control. Methods Enzymol. 2003:369–382. doi: 10.1016/S0076-6879(03)71028-3. [DOI] [PubMed] [Google Scholar]
- Pervushin K, Riek R, Wider G, Wuthrich K. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proc. Natl. Acad. Sci. U. S. A. 1997;23:12366–12371. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posfai G, Plunkett G, 3rd, Feher T, Frisch D, Keil GM, Umenhoffer K, Kolisnychenko V, Stahl B, Sharma SS, de Arruda M, et al. Emergent properties of reduced-genome Escherichia coli. Science. 2006;5776:1044–1046. doi: 10.1126/science.1126439. [DOI] [PubMed] [Google Scholar]
- Prasch S, Jurk M, Washburn RS, Gottesman ME, Wöhrl BM, Rösch P. RNA-binding specificity of E. coli NusA. Nucl. Acids Res. 2009;14:4736–4742. doi: 10.1093/nar/gkp452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasch S, Schwarz S, Eisenmann A, Wöhrl BM, Schweimer K, Rösch P. Interaction of the intrinsically unstructured phage lambda N Protein with E. coli NusA. Biochemistry. 2006;14:4542–4549. doi: 10.1021/bi0523411. [DOI] [PubMed] [Google Scholar]
- Ross W, Gosink KK, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse RL. A third recognition element in bacterial promoters: DNA binding by the alpha subunit of RNA polymerase. Science. 1993;5138:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- Salzmann M, Pervushin K, Wider G, Senn H, Wuthrich K. TROSY in triple-resonance experiments: new perspectives for sequential NMR assignment of large proteins. Proc. Natl. Acad. Sci. U. S. A. 1998;23:13585–13590. doi: 10.1073/pnas.95.23.13585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Schleucher J, Griesinger C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. Nucl. Magn. Reson. Spectrosc. 1999:39–158. [Google Scholar]
- Schmidt MC, Chamberlin MJ. Binding of rho factor to Escherichia coli RNA polymerase mediated by nusA protein. J Biol Chem. 1984;24:15000–2. [PubMed] [Google Scholar]
- Schwieters CD, Kuszewski JJ, Tjandra N, Clore GM. The Xplor-NIH NMR molecular structure determination package. J. Magn. Reson. 2003:66–74. doi: 10.1016/s1090-7807(02)00014-9. [DOI] [PubMed] [Google Scholar]
- Severinov K. RNA polymerase structure-function: insights into points of transcriptional regulation. Curr. Opin. Microbiol. 2000;2:118–125. doi: 10.1016/s1369-5274(00)00062-x. [DOI] [PubMed] [Google Scholar]
- Shah IM, Wolf RE., Jr. Novel protein--protein interaction between Escherichia coli SoxS and the DNA binding determinant of the RNA polymerase alpha subunit: SoxS functions as a co-sigma factor and redeploys RNA polymerase from UP-element-containing promoters to SoxS-dependent promoters during oxidative stress. J. Mol. Biol. 2004;3:513–532. doi: 10.1016/j.jmb.2004.08.057. [DOI] [PubMed] [Google Scholar]
- Shao X, Grishin NV. Common fold in helix-hairpin-helix proteins. Nucleic Acids Res. 2000;14:2643–2650. doi: 10.1093/nar/28.14.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharan SK, Thomason LC, Kuznetsov SG, Court DL. Recombineering: a homologous recombination-based method of genetic engineering. Nat. Protoc. 2009;2:206–223. doi: 10.1038/nprot.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talluri S, Wagner G. An optimized 3D NOESY-HSQC. J. Magn. Reson. B. 1996;1064-1866(2):200–205. doi: 10.1006/jmrb.1996.0132. [DOI] [PubMed] [Google Scholar]
- Thomason L, Court DL, Bubunenko M, Costantino N, Wilson H, Datta S, Oppenheim A. Recombineering: genetic engineering in bacteria using homologous recombination. Curr. Protoc. Mol. Biol. 2007 doi: 10.1002/0471142727.mb0116s78. Unit 1.16. [DOI] [PubMed] [Google Scholar]
- Tripsianes K, Folkers G, Ab E, Das D, Odijk H, Jaspers NG, Hoeijmakers JH, Kaptein R, Boelens R. The structure of the human ERCC1/XPF interaction domains reveals a complementary role for the two proteins in nucleotide excision repair. Structure. 2005;12:1849–1858. doi: 10.1016/j.str.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Tsodikov OV, Enzlin JH, Scharer OD, Ellenberger T. Crystal structure and DNA binding functions of ERCC1, a subunit of the DNA structure-specific endonuclease XPF-ERCC1. Proc. Natl. Acad. Sci. U. S. A. 2005;32:11236–11241. doi: 10.1073/pnas.0504341102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassylyev DG. Elongation by RNA polymerase: a race through roadblocks. Curr. Opin. Struct. Biol. 2009;6:691–700. doi: 10.1016/j.sbi.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick RS, Gottesman ME. Escherichia coli NusA is required for efficient RNA binding by phage HK022 nun protein. Proc Natl Acad Sci U S A. 1998;4:1546–51. doi: 10.1073/pnas.95.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worbs M, Bourenkov GP, Bartunik HD, Huber R, Wahl MC. An extended RNA binding surface through arrayed S1 and KH domains in transcription factor NusA. Mol. Cell. 2001;6:1177–1189. doi: 10.1016/s1097-2765(01)00262-3. [DOI] [PubMed] [Google Scholar]
- Yang X, Molimau S, Doherty GP, Johnston EB, Marles-Wright J, Rothnagel R, Hankamer B, Lewis RJ, Lewis PJ. The structure of bacterial RNA polymerase in complex with the essential transcription elongation factor NusA. EMBO Rep. 2009;9:997–1002. doi: 10.1038/embor.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuno K, Yamazaki T, Tanaka Y, Kodama TS, Matsugami A, Katahira M, Ishihama A, Kyogoku Y. Interaction of the C-terminal domain of the E. coli RNA polymerase alpha subunit with the UP element: recognizing the backbone structure in the minor groove surface. J. Mol. Biol. 2001;2:213–225. doi: 10.1006/jmbi.2000.4369. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Mah TF, Greenblatt J, Friedman DI. Evidence that the KH RNA-binding domains influence the action of the E. coli NusA protein. J. Mol. Biol. 2002a;5:1175–1188. doi: 10.1016/s0022-2836(02)00238-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Mah TF, Greenblatt J, Friedman DI. Evidence that the KH RNA-binding domains influence the action of the E. coli NusA protein. J Mol Biol. 2002b;5:1175–88. doi: 10.1016/s0022-2836(02)00238-3. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Mah TF, Yu YT, Mogridge J, Olson ER, Greenblatt J, Friedman DI. Interactions of an Arg-rich region of transcription elongation protein NusA with NUT RNA: implications for the order of assembly of the lambda N antitermination complex in vivo. J. Mol. Biol. 2001;1:33–49. doi: 10.1006/jmbi.2001.4722. [DOI] [PubMed] [Google Scholar]
- Zou C, Fujita N, Igarashi K, Ishihama A. Mapping the cAMP receptor protein contact site on the alpha subunit of Escherichia coli RNA polymerase. Mol. Microbiol. 1992;18:2599–2605. doi: 10.1111/j.1365-2958.1992.tb01437.x. [DOI] [PubMed] [Google Scholar]
- Zwahlen C, Legault P, Vincent SJF, Greenblatt J, Konrat R, Kay LE. Methods for Measurement of Intermolecular NOEs by Multinuclear NMR Spectroscopy: Application to a Bacteriophage λ N-Peptide/boxB RNA Complex. J. Am. Chem. Soc. 1997;29:6711–6721. [Google Scholar]