Abstract
Background
Peroxynitrite is increasingly proposed as a contributor to defence system in marine bivalve. It can be formed by combinaison of superoxide and nitric oxide, and can react with tyrosine residues of proteins giving rise to 3-nitrotyrosine.
Results
The present article describes a competitive ELISA for the measurement of 3-nitrotyrosine contents of plasma proteins from marine bivalves by means of a monoclonal anti 3-nitrotyrosine antibody mouse IgG.
Conclusions
This assay is sensitive enough to determine the amounts of 3-nitrotyrosine in plasma proteins from one animal only.
Using the C-ELISA, we have shown that the phagocytosis of zymosan particles increased the 3-nitrotyrosine levels of plasma proteins from mussel M. galloprovincialis and oyster C. gigas 5.8 and 7.5 times respectively.
Background
Bivalves, unlike vertebrates, do not have humoral antigen specific active compounds such as antibodies and their self-defence systems are based on non-specific defensive compounds and phagocytosis by haemocytes [1, 2].
During phagocytic burst or after in vitro stimulation with PMA or LPS, haemocytes produce superoxide anions, i.e. the initial species of reactive oxygen intermediates (ROI) and nitric oxide (NO).
ROI generation has been reported in Patinopecten vessoensis [3] Crassostrea virginica [4], Crassostrea gigas [5], Ostrea edulis, Pecten maximus [6], Mytilus edulis [7] and Mytilus galloprovincialis [8]. NO-synthase activity was detected in haemocytes of M. edulis [9] and C. gigas [10] and peroxynitrite production by M. galloprovincialis haemocytes has been recently reported [8, 11, 12].
In the presence of superoxide anions, nitric oxide generates peroxynitrite, a strong oxidant which kills bacteria [13] and parasitic protozoa [10, 14, 15]. Moreover, peroxynitrite is a nitrating agent, that converts tyrosine in 3-nitrotyrosine [16]. Such nitration has been observed in proteins from human polymorphonuclear cells [17] and 3-nitrotyrosine has been used as a marker to assess peroxynitrite involvement in pathological processes such as adult respiratory distress syndrome [18], rheumatoid arthritis [19] and celiac disease [15].
To determine levels of protein-associated 3-nitrotyrosine in human plasma or serum, Khan et al. [20] developed a competitive enzyme-linked immuno-assay (C-ELISA) for 3-nitrotyrosine using a polyclonal anti-3-nitrotyrosine rabbit IgG raised against nitrated KLH. In the present study, we slightly modified this C-ELISA assay to investigate 3-nitrotyrosine levels in plasma proteins from mussel M. galloprovincialis and oyster C. gigas before and after zymosan phagocytosis.
Results
ELISA standard curve construction
We developed a competitive ELISA to quantify 3-nitrotyrosine residues in plasma proteins from marine bivalves . A standard curve was constructed by determining the binding inhibition of the anti-3-nitrotyrosine antibody to a synthetic antigen (BSANT) immobilised on coated microtitration plates in the presence of serial dilutions of the free antigen BSANT in solution.
Figure 1 shows that the curve obtained was linear from 0.2 to 0.003 μM BSANT / assay. In contrast, unmodified BSA showed no significant binding inhibition of the 3-nitrotyrosine antibody and no significant cross-reaction of KLH, an invertebrate protein model was observed.
Figure 1.
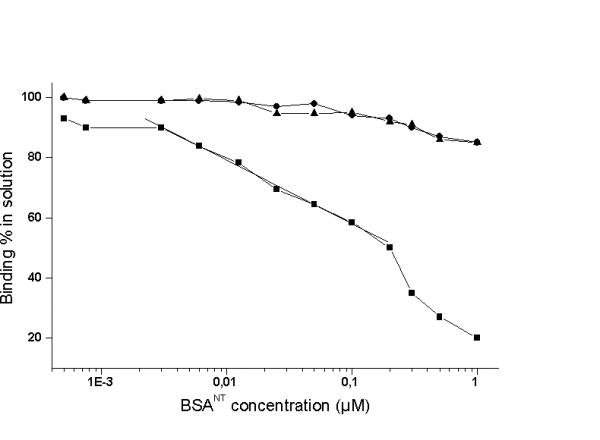
Comparison of standard curves for the inhibition of anti-3-nitrotyrosine antibody binding by various proteins in the C-ELISA. The curves show competition for the anti-3-nitrotyrosine antibody between the immobilized BSANT and competing free proteins in solution: ■ BSANT (n = 3); ● BSA (n = 2); ▲ KLH (n = 2). The percentage inhibition of maximum antibody binding (absorbance at 490 nm in the absence of competition) is plotted against the competing free protein concentration.
Effect of in vivo stimulation of mussel hemocytes with PMA on 3-nitrotyrosine levels in plasma proteins
The 3-nitrotyrosine concentration in proteins from M. galloprovincialis plasma samples was quantified by C-ELISA and expressed as BSANT equivalents using the standard curve of Fig. 1. As shown in table 1, marked individual variations were observed and a mean concentration (n = 20) of 0.037 ± 0.025 μM BSANT equivalents was estimated. After 1 h incubation of mussel hemocytes with PMA, the in vitro level of 3-nitrotyrosine in plasma increased to a mean value (n = 20) of 0.118 ± 0.024 μM BSANT equivalents (3.2-fold enhancement). To confirm that the increase of BSANT equivalents was dependent on NO production, we incubated mussel hemocytes with L-NIO, a NO-synthase inhibitor. When these hemocytes were stimulated with PMA, mean 3-nitrotyrosine concentrations of 0.082 ± 0.024 μM BSANT equivalents were obtained (Table 1). They corresponded to 69% inhibition when compared to hemocytes untreated with NO-synthase inhibitor.
Table 1.
Mean 3-nitrotyrosine levels in plasma from mussels (n = 20) before and after PMA-stimulation. 3-nitrotyrosine contents of plasma proteins were determined in triplicate by C-ELISA as described in the Material and Methods and expressed as BSANT equivalents. "unstimulated": plasma from unstimulated haemolymph, "+ PMA": plasma 30 min after addition of 10 μL PMA (1 mg mL-1) to haemolymph, "PMA + L-NIO": plasma after 5 min incubation of haemolymph with L-NIO and 30 min with PMA.
| Unstimulated | + PMA | + PMA + L-NIO |
| 0.037 ± 0.025 (n = 20) | 0.118 ± 0.024 (n = 20) | 0.082 ± 0.024 (n = 20) |
Measurement of 3-nitrotyrosine content in plasma proteins from marine bivalve before and after in vitro phagocytosis of zymosan particles
As shown in table 2, the phagocytosis of zymosan particles by M. galloprovincialis haemocytes promoted a marked increase in the 3-nitrotyrosine concentration in mussel plasma, reaching mean concentrations (n = 20) of 0.214 ± 0.023 μM BSANT equivalents (5.8-fold enhancement). The addition of DPI, a strong inhibitor of both NO-synthase and NADPH-oxidase, before phagocytosis lowered the 3-nitrotyrosine contents of plasma proteins to a mean concentration (n = 20) of 0.117 ± 0.036 μM BSANT equivalents (54.6 % inhibition).
Table 2.
Mean 3-nitrotyrosine levels in plasma from mussels (n = 20) and oysters (n = 18) before and after phagocytosis of zymosan particles. 3-nitrotyrosine contents of plasma proteins were determined in triplicate by C-ELISA as described in the Material and Methods and expressed as BSANT equivalents. "unstimulated": plasma from unstimulated haemolymph, "zymosan": plasma 30 min after addition of 50 μL zymosan (40 mg mL-1) to haemolymph, "zymosan + DPI": plasma after 5 min incubation of haemolymph with DPI and 30 min with zymosan particles.
| Unstimulated | Zymosan | Zymosan + DPI | |
| Mussels | 0.037 ± 0.025 (n = 20) | 0.214 ± 0.023 (n = 20) | 0.117 ± 0.036 (n = 20) |
| Oysters | 0.023 ± 0.017 (n = 18) | 0.176 ± 0.022 (n = 18) | 0.127 ± 0.037 (n = 18) |
As with mussel (M. galloprovincialis), the concentration of 3-nitrotyrosylated proteins in plasma samples from oysters (C. gigas) was quantified by ELISA and expressed as BSANT equivalents using the standard curve of Fig. 1. Substantial individual variations were observed and a mean concentration (n = 18) of 0.023 ± 0.017 μM BSANT equivalents was estimated (Table 2). In vitro Phagocytosis of zymosan particles by hemocytes, increased the mean 3-nitrotyrosine content of plasma to 0.176 ± 0.022 μM BSANT equivalents (7.5-fold enhancement).
As in the mussel experiments, the increase in the 3-nitrotyrosine level was strongly inhibited by incubation of haemolymph with DPI (72 % inhibition), before the addition of zymosan particles (Table 2).
Discussion
This study describes a C-ELISA for the detection of 3-nitrotyrosine residues in proteins from marine bivalve plasma.
Concentrations of 3-nitrotyrosine residues in the plasma proteins from untreated mussels and oysters varied between animals but they always increased markedly after in vitro PMA-stimulation of haemocytes or after phagocytosis of zymosan particles. These increases were inhibited by haemolymph preincubation with DPI or L-NIO.
The low levels of endogenous 3-nitrotyrosine residues in the plasma of untreated animals could have been due to peroxynitrite, a reactive nitrogen species that can modify tyrosine residues of proteins into 3-nitrotyrosine, as shown by Beckman and Koppenol [14] when studying human blood cells. Other reactive nitrogen species such as nitric oxide and nitrite anion could also be involved [21]. The 3-nitrotyrosine levels detected in proteins from untreated mussel and oyster haemolymph could thus reflect the exposure of proteins to all the nitrating agents produced by cells and thus confirm the generation of nitric oxide by these species.
The increased 3-nitrotyrosine levels observed after PMA-stimulation of haemocytes and zymosan particle phagocytosis, and inhibition with DPI and L-NIO, confirmed our previous results [8, 11, 12] and showed that peroxynitrite is generated by haemocytes in response to activation of both NADPH-oxidase and NO-synthase metabolic pathways.
Conclusion
The C-ELISA method we developed is sensitive enough to determine the amounts of 3-nitrotyrosine in plasma proteins of a single animal and to measure variations in 3-nitrotyrosine contents promoted by haemocyte stimulation or zymosan particle phagocytosis.
However, this method remains semi-quantitative since the 3-nitrotyrosine antibody may not bind all 3-nitrotyrosine residues in a sample containing a mixture of proteins due to inaccessibility to some 3-nitrotyrosine residues because of the influence of adjacent aminoacids on antibody binding. We used the C-ELISA method to detect and quantify the stress of mussels and oysters exposed to environmental variations.
Materials and methods
Chemicals and buffers
Phorbol myristate acetate (PMA), bovine serum albumin (BSA), keyhole limpet haemocyanin (KLH) and zymosan were purchased from Sigma Chemical Co. (St. Louis, USA). L-N5-(1-iminoethyl)-ornithine (L-NIO) and diphenylene iodonium chloride (DPI) were products of Alexis Corporation (Switzerland). Phosphate buffered saline was prepared using Dulbecco PBS salt using HPLC-grade distilled water. The pH and osmolarity were adjusted to 8.3 and 1100 mOsm, respectively. All other chemicals and reagents used were analytical grade.
Anti-nitrotyrosine antibody (clone 1A6: mouse monoclonal IgG) was from Upstate Biotechnology Inc. (Lake Placid, USA) and anti-mouse IgG (H + L), peroxidase-conjugate antibody was from Jackson Immunochemical Laboratory, Inc. (Baltimore, USA).
Experimental animals
Two year old mussels (M. galloprovincialis) and oysters (C. gigas) raised at Palavas (France), were maintained in laboratory in a running sea water system (salinity: 33??, 17°C) with continuous aeration.
Haemolymph collection
One ml haemolymph from each animal was withdrawn (from the posterior adductor muscle of mussels and from the pericardial cavity of oysters) in disposable plastic syringes (2 ml, 21G needle) and transferred to polypropylene test tubes kept on ice.
Haemocyte stimulation
Haemocytes were stimulated by the addition of 50 μl of the zymosan suspension (40 mg mL-1, final pH 7.1) or by 10 μl of PMA (1 mg mL-1). In inhibition experiments, haemolymph was preincubated for 5 min with DPI, L-NIO or filtrated sea water (control). The haemolymph was centrifuged (180 g, 5 min., 4°C) and the supernatant (plasma) collected by carefull pipetting using Pasteur pipettes. The viability of cells in the presence of various added agents was tested before the experiments by trypan blue exclusion assay.
Synthesis of nitrosylated BSA (BSANT)
3-nitrotyrosine was coupled to carboxylic groups of BSA using N-hydroxysuccinimide/N-ethyl-N'-(dimethyl-aminopropyl)-carbodiimide reagent at pH 8.3. After incubation overnight under stirring at 4°C, the solution was extensively dialysed against PBS. The 3-nitrotyrosine content of BSANT was determined by absorbance at 438 nm (pH 9.0) using a molar extinction coefficient of 4300 M-1 cm-1. It was in the 2-3 mol nitrotyrosine / mol BSA range.
C-ELISA immunoassays
Each well from the 96-well plates was coated with 50 μL BSANT (10 μg mL-1 in PBS pH = 7.2), incubated overnight at 4°C, washed with 250 μL PBS / 0.1% Tween buffer and then blocked for 1 hr at 37°C with 5% skimmed milk to prevent nonspecific binding.
Serial dilutions of BSANT (0.2 to 0.003 μM) in PBS (for standard curve determination) or plasma sample from individual marine bivalves were incubated v/v overnight at 4°C under stirring with monoclonal anti 3-nitrotyrosine mouse IgG (1:2000). These solutions were then poured into coated wells (50 μL / well). After 2 h incubation at 37°C, 3-nitrotyrosine antibody bound to BSANT was labelled by the addition of anti-mouse IgG peroxidase-conjugate (1: 4000, 50 μL / well) and incubated for 90 min at 37°C. Then colour development was initiated by the addition of peroxidase substrate (orthophenylene diamine), allowed to develop for up to 15 min at room temperature and terminated by the addition of 4 N sulphuric acid. Antibody bound to BSANT was determined from the absorbance at 490 nm and expressed as BSANT equivalents.
Contributor Information
Jean Torreilles, Email: jtorreil@univ-montp2.fr.
Bernard Romestand, Email: romestand@univ-montp2.fr.
References
- Gourdon I, Guérin M-C, Torreilles J. Cellular and molecular mechanisms of the stress response in marine bivalves. C R Soc Biol. 1998;192:749–774. [PubMed] [Google Scholar]
- Torreilles J, Guérin M-C. Espèces oxygénées réactives et système de défense des bivalves marins. C R Acad Sc, Paris. 1996;318:209–218. [PubMed] [Google Scholar]
- Nakamura M, Mori K, Inooka S, Nomura T. In vitro production of hydrogen peroxide by the amoebocytes of the scallop Patinopecten yessoensis (Jay). Dev Comp Immunol. 1985; 9:407–417. doi: 10.1016/0145-305X(85)90004-7. [DOI] [PubMed] [Google Scholar]
- Larson KG, Robertson BS, Hetrick FM. Effect of environmental polluants on the chemiluminescence of hemocytes from the American oyster Crassostrea virginica. Dis Aquatn Org. 1989;6:131–136. [Google Scholar]
- Bachère E, Hervio D, Mialhe E. Luminol-dependent chemiluminescence by hemocytes of two marine bivalves, Ostrea edulis and Crassostrea gigas. Dis Aquat Org. 1991;11:173–180. [Google Scholar]
- Le Gall G, Bachère E, Miahle E. Chemiluminescence analysis of the activity of Pecten maximus hemocytes stimulated with zymosan and host-specific Rickettsiales-like organisms. Dis Aquat Org. 1991;11:181–186. [Google Scholar]
- Pipe RK. Generation of reactive oxygen metabolites by the haemocytes of the mussel Mytilus edulis. Dev CompImmunol. 1992;16:111–122. doi: 10.1016/0145-305X(92)90012-2. [DOI] [PubMed] [Google Scholar]
- Torreilles J, Guérin M-C. Production of peroxynitrite by zymosan stimulation of Mytilus galloprovincialis haemocytes in vitro. Fish and Shellfish Immunol. 1999; 9:509–518. doi: 10.1006/fsim.1998.0200. [DOI] [Google Scholar]
- Ottaviani E, Paemen LR, Stefano GB. Evidence for nitric oxide production and utilization as a bactericidal agent by invertebrate immunocytes. Eur J Pharmacol. 1993;248:319–324. doi: 10.1016/0926-6917(93)90006-C. [DOI] [PubMed] [Google Scholar]
- Nakayama K, Maruyama T. Differential production of active oxygen species in photo-symbiotic and non-symbiotic bivalves. Dev Comp Immunol. 1998;22:151–159. doi: 10.1016/S0145-305X(97)00060-8. [DOI] [PubMed] [Google Scholar]
- Arumugam M, Romestand B, Torreilles J, Roch P. In vitro production of superoxide and nitric oxide (as nitrite and nitrate) by Mytilus galloprovincialis haemocytes upon incubation with PMA or laminarin or during yeast phagocytosis. Eur J cell Biol. 2000;79:513–519. doi: 10.1078/0171-9335-00068. [DOI] [PubMed] [Google Scholar]
- Arumugam M, Romestand B, Torreilles J. Nitrite release in haemocytes from Mytilus galloprovincialis, Crassostrea gigas and Ruditapes decussatus upon stimulation with phorbol myristate acetate. Aquat Living Resour. 2000;13:173–177. doi: 10.1016/S0990-7440(00)00150-9. [DOI] [Google Scholar]
- Zhu L, Gunn C, Beckman JS. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;27:1424–1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Ter Steege JCA, Koster-Kamphuis L, Van Straaten EA, Forget PP, Buurman WA. Nitrotyrosine in plasma of celiac disease patients as detected by a new sandwich ELISA. Free Rad Biol Med. 1998;25:953–963. doi: 10.1016/S0891-5849(98)00184-1. [DOI] [PubMed] [Google Scholar]
- Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- Salman-Tabcheh S, Guérin M-C, Torreilles J. Nitration of tyrosyl-residues from extra and intracellular proteins in human whole blood. Free Rad Biol Med. 1995;19:695–698. doi: 10.1016/0891-5849(95)00075-9. [DOI] [PubMed] [Google Scholar]
- Haddad IY, Pataki G, Hu P, Galliani C, Beckman J, Matalon S. Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest. 1994;94:2407–2413. doi: 10.1172/JCI117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur H, Halliwell B. Nitrotyrosine in serum and synovial fluid from rheumatoid patients. FEBS Lett. 1994;350:9–12. doi: 10.1016/0014-5793(94)00722-5. [DOI] [PubMed] [Google Scholar]
- Khan J, Brennan DM, Bradley N, Gao B, Bruckdorfer R, Jacobs M. 3-nitrotyrosine in the proteins of human plasma determined by an ELISA method. Biochem J. 1998;330:795–801. doi: 10.1042/bj3300795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–160. doi: 10.1016/S0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]


