Abstract
Restorative proctocolectomy with ileal pouch-anal anastomosis is the procedure of choice for patients with ulcerative colitis requiring surgery. A J-pouch with a stapled anastomosis has been the preferred technique because it is quicker, safer, and associated with good functional outcomes. A diverting loop ileostomy is usually created at the time of ileal pouch-anal anastomosis. In patients with severe fulminant colitis or toxic megacolon, restorative proctocolectomy with ileal pouch-anal anastomosis is performed in multistages. The technical aspects of ileal pouch-anal anastomosis in patients with ulcerative colitis are reviewed in this article.
Keywords: Proctocolectomy, ileal pouch-anal anastomosis, ulcerative colitis, ileostomy, repeat pouch surgery
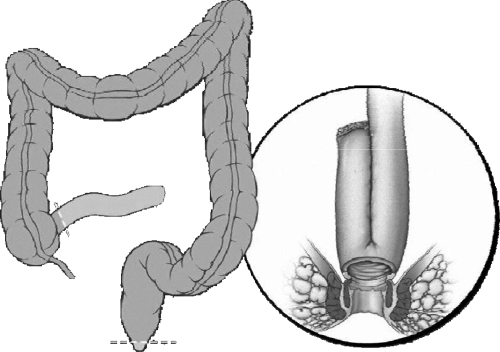
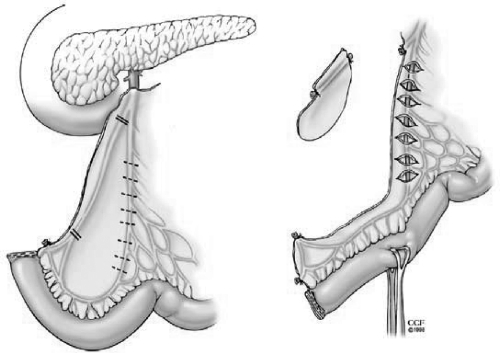
Restorative proctocolectomy with ileal pouch-anal anastomosis (RP/IPAA) is the procedure of choice for patients with ulcerative colitis (UC) requiring surgery (Fig. 1).1,2 RP/IPAA consists of a J, S, or W ileal reservoir anastomosed either by a stapled or hand-sewn technique. It was first described as a combination of an S-pouch and a hand-sewn anastomosis.3 However, a J-pouch and stapled IPAA has been commonly used4 because a J-pouch is easier to create and a stapled anastomosis is associated with better functional outcomes compared with a hand-sewn IPAA.5 Also, preservation of a transitional zone for a stapled IPAA infrequently leads to the development of cancer.6 In patients with severe fulminant colitis or toxic megacolon, RP/IPAA is done in multistages including a subtotal colectomy and an end ileostomy; in approximately 6 months a proctectomy and IPAA with/without a loop ileostomy are performed. In carefully selected patients if there are no adverse features to anastomotic healing, the omission of a diverting ileostomy has proven to be safe.7 In addition to an open IPAA, a laparoscopic IPAA has been reported with favorable short and long-term outcomes.8,9,10,11 Here we report on the technical aspects of IPAA in patients with UC.
Figure 1.
Restorative proctocolectomy and ileal pouch-anal anastomosis. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
OPERATIVE TECHNIQUE
The patient's condition, the extent of colonic disease, and anal sphincter function is preoperatively evaluated by means of manometry and colonoscopic biopsies to rule out Crohn disease (CD), dysplasia, or cancer. The site for a diverting loop ileostomy is marked before IPAA. A complete bowel preparation is usually used. Prophylactic perioperative antibiotics and prophylaxis against deep venous thrombosis should be given. A RP/IPAA consists of removal of the entire colon, the dissection and removal of rectum, the creation of an ileal pouch, and the anastomosis of an ileal pouch to the anal canal using a hand-sewn or stapled technique. The procedure is performed with the patient in a Lloyd Davies position. The rectum is washed out with normal saline in the operating room. A midline vertical incision is performed. The abdomen is explored to see if there are any contraindications to performing a RP/IPAA. The entire colon is mobilized from its retroperitoneal attachments. The terminal ileum is divided just proximal to the ileocecal valve. Mesenteric vessels are ligated and then divided to perform a colectomy.
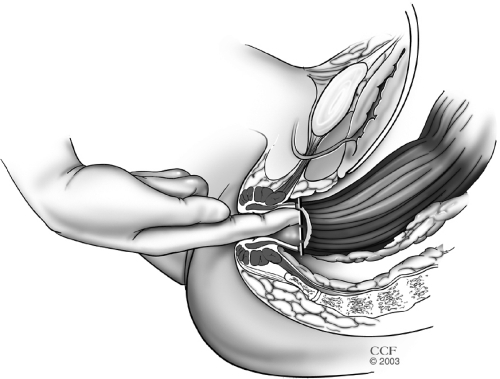
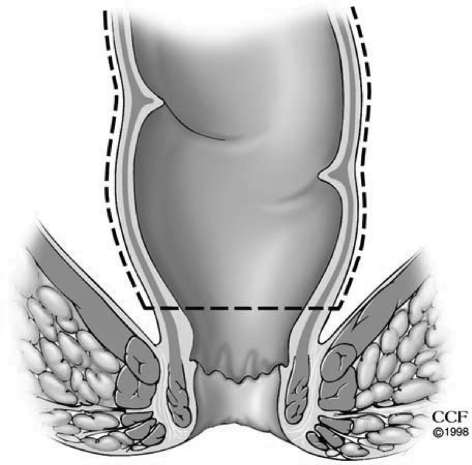
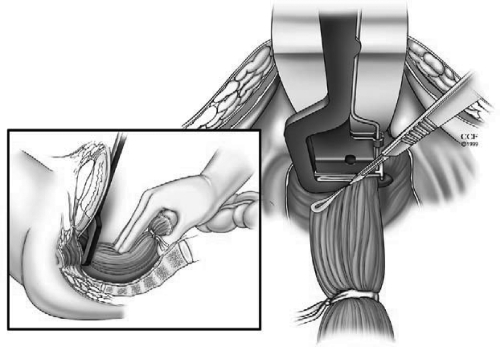
In the presence or concern for dysplasia or cancer, a complete total mesorectal excision (TME) is performed. In benign conditions similar posterior TME dissection is used where presacral fascia is entered between the investing layer of fascia propria of the mesorectum and presacral fascia. The presacral nerves identified at the pelvic rim are preserved. The pelvic dissection is continued in the midline between Waldeyer's fascia and the investing layer of the rectum to the level of the levator muscle. It is crucial not to violate the presacral fascia posteriorly where the lateral and presacral veins can be damaged. However, we prefer to stay close to the rectum anteriorly and laterally to avoid any nerve injury. This starts with a bilateral incision on the pelvic peritoneum and is joined on the anterior rectal wall 1 cm above the peritoneal reflection. An anterior dissection is done to the lower border of the prostate gland or lower one-third of the vagina. The Denonvilliers' fascia is preserved in patients without a carcinoma. The rectum is completely mobilized. A transanal digital evaluation with the tip of a finger is done to mark the level of transection by a linear stapler for double-stapled IPAA or for pursestring sutures for a single-stapled IPAA (Fig. 2). By transecting the rectum at the top of the anal columns for a stapled anastomosis, the anal sensory epithelium is preserved leaving a 1 to 2 cm anal transitional zone (Fig. 3).
Figure 2.
Digital evaluation to mark the level of planned stapled ileal pouch-anal anastomosis. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Figure 3.
Transecting the rectum at the top of the anal columns leaves a 1–2 cm anal transitional zone. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Creation of Ileoanal Pouch and Anastomosis
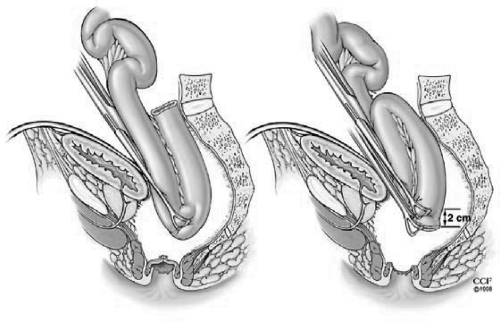
The key to successful pouch surgery is a tension-free anastomosis. For this reason, the small bowel mesentery should be mobilized adequately as far as the third part of the duodenum so that the ileal pouch will reach to the level of the levator floor with no tension. The reach of the ileal pouch to the anal canal in obese patients or in patients who had a prior small bowel resection might be difficult. The technique by grasping the apex of the pouch and simulating the reach down to the anastomosis level is useful to estimate the tension (Fig. 4). Ileocolic vessels ligation at the origin of the superior mesenteric artery can be done to provide an anastomosis with no tension. This is especially a must when one has to do an S-pouch rather than a J-pouch due to reach issues. If there is still tension after these maneuvers, the peritoneal tissue to the right of the superior mesenteric vessels is excised using translumination. Also, small anterior and posterior peritoneal incisions over the superior mesenteric vessels border can be done as an additional maneuver (Fig. 5).
Figure 4.
The clamped pouch apex is delivered into the pelvis. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Figure 5.
Maneuvers to aid reach to the level of the levator floor. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
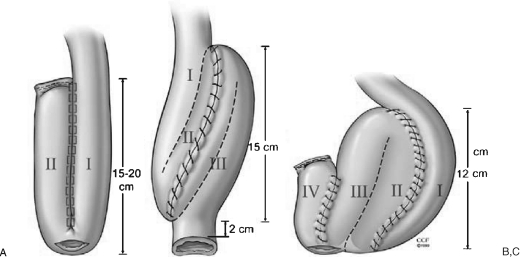
The pouch designs used for RP/IPAA include J, S, or W (Fig. 6). Because a J-pouch is easier to create, it is the most commonly used. The J-pouch is constructed from the terminal 30 to 40 cm of small intestine. This ileum segment is folded into two 15- or 20-cm segments. A 1.5-cm enterotomy is made longitudinally at the pouch apex. A side-to-side anastomosis of the two segments of the ileum is done by using 2 cartridges of ILA 100 linear stapler via enterotomy at the pouch apex. A blind loop of the J-pouch is closed using a linear stapler and is usually reinforced by continuous sutures. The staple lines are checked for hemostasis. After a 0-polypropylene purse string suture is applied to the apical enterotomy, insufflation using normal saline is performed to confirm the integrity of the pouch (Fig. 7).
Figure 6.
(A) J-, (B) S-, and (C) W-pouches. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Figure 7.
Construction of a J-pouch. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
The S-pouch can reach up to 2 to 4 cm further compared with a J-pouch, so it is usually created if there is excessive tension in the IPAA. An S-pouch is constructed using 3 limbs of 12 to 15 cm of terminal small bowel with a 2-cm exit conduit. The ileum segments are approximated by continuous seromuscular sutures. An enterotomy is performed in an S shape. Continuous running full thickness sutures are applied to the two posterior anastomotic lines. The anterior wall is closed with continuous seromuscular sutures. It is then reinforced using interrupted sutures (Fig. 8).
Figure 8.
Construction of an S-pouch. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
An IPAA is constructed using either a stapled or hand-sewn technique. A stapler IPAA is a preferred technique over a hand-sewn IPAA because it is quicker and associated with better outcomes. The stapled IPAA is performed using either single- or double-stapling approaches.
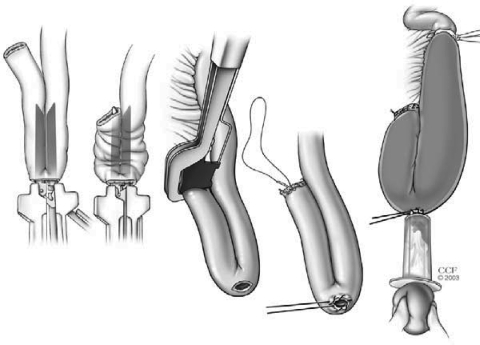
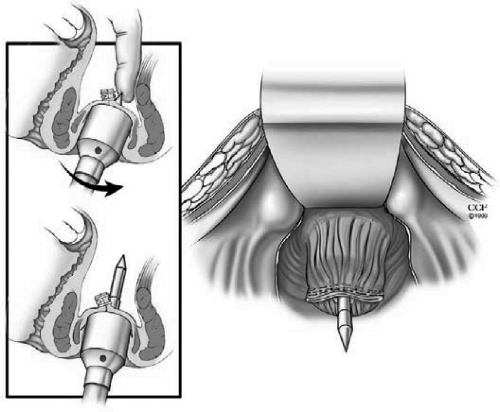
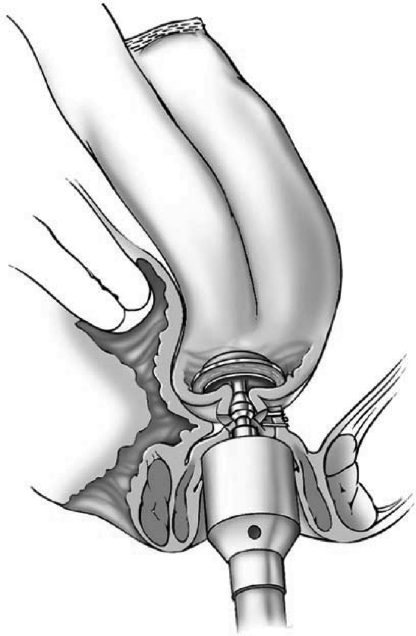
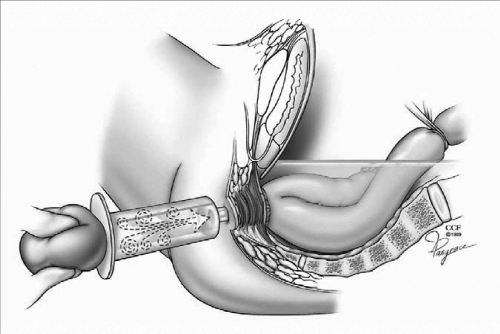
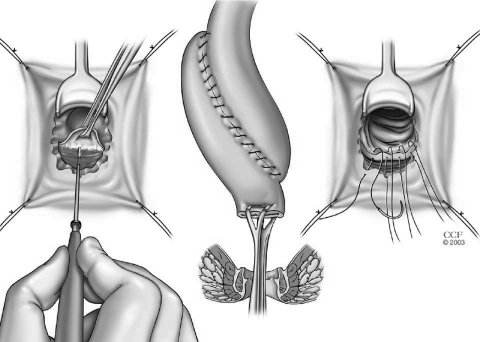
In a double-stapling technique, after firing the linear stapler to close to the distal anorectal stump, the specimen is divided above the staple line (Fig. 9). The anvil of the stapler is placed in the distal and secured with a previously placed purse string suture. A circular stapler is advanced through the anorectal ring just posterior to the linear staple line on the anorectum. Putting the index finger into the anorectal area from the abdominal side and guiding the trocar facilitates the procedure (Fig. 10). The pin of the circular stapler is then mated with the anvil of the circular stapler. The small bowel should be correctly oriented to prevent twisting of the small bowel mesentery. Care must be used to avoid including the posterior vaginal wall within the stapled IPAA in female patients. The ends are approximated and the stapler is fired to complete the anastomosis (Fig. 11). The doughnuts are checked. An air-leak test using normal saline is done to prove the integrity of the anastomosis (Fig. 12).
Figure 9.
After firing the linear stapler to close the distal anorectal stump, the specimen is divided above the staple line. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Figure 10.
Guiding the trocar from the abdominal side just posterior to the linear staple line on the anorectum. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Figure 11.
Construction of double-stapled ileal pouch-anal anastomosis. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Figure 12.
Air-leak test using normal saline is done to prove the integrity of the anastomosis. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
In a single-stapling technique, the anvil of the stapler is placed through the enterotomy at the pouch apex and secured with a purse string suture. A distal purse string with a 0-polypropylene suture is applied to the anorectal stump. The surgical circular stapler is inserted transanally, the pin is advanced completely, and the pursestring is tightened. After the ends are approximated, the stapler is fired. The IPAA is then completed.
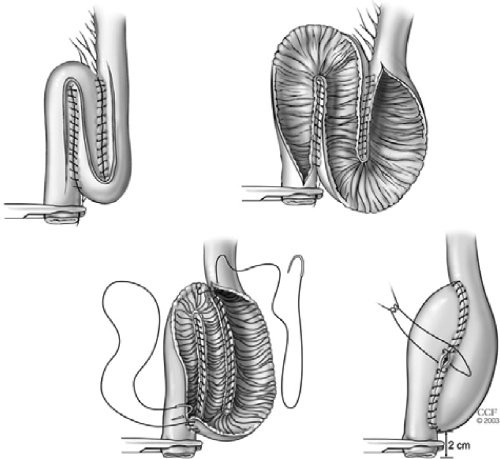
A hand-sewn IPAA is performed after removal of anorectal mucosa from the dentate line up to the level of the anorectal transsection. Sutures in four quadrants are placed to evert the anal verge. An anal retractor is passed. Following injection of 10 to 15 mL of adrenalin solution (1:100,000) to raise the anorectal mucosa off the underlying muscle, removal of mucosa is performed by cautery. Extreme stretching of the anal canal may damage the anal sphincters; therefore, it should be avoided. Then 2–0 polyglycolic acid sutures, each incorporating a small bite of internal anal sphincter, are placed radially at the dentate line. To prevent occurrence of IPAA-vaginal fistula these stitches should not be taken too deeply anteriorly in a female patient. After the pouch is brought down to the anal verge, the previously placed sutures at the dentate line are placed through the full thickness of the apex of the J-pouch or the end of the exit conduit of the S-pouch. After removing the retractor the sutures are tied (Fig. 13).
Figure 13.
Mucosectomy and construction of a hand-sewn ileal pouch-anal anastomosis. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
For most patients, a diverting loop ileostomy is fashioned by bringing the ileum 20 to 25 cm proximal to the pouch to the right lower quadrant of the abdominal wall. Closure of a loop ileostomy is done approximately 3 months after an IPAA. Prior to an ileostomy closure, a water-contrasted pouchogram and pouchoscopy is performed to check the integrity of the ileal pouch and the IPAA. In selected patients, where the risk of the development of anastomotic complications is low, an ileostomy can be omitted. In these patients without a diverting ileostomy, a 32-F mushroom catheter is placed through the anus into the pouch, fixed to the perianal skin, and left in place up to 5 days and irrigated gently with 30 cc of normal saline every 8 hours.
COMPARISON OF OUTCOMES AFTER J- VERSUS S- VERSUS W-POUCH
A J-pouch is the preferred ileal pouch type because it is quicker to construct. An S-pouch and a W-pouch need a hand-sewing; hence, they are more time consuming. In our institution, our preference is a J-pouch. An S-pouch is used when a J-pouch will not reach into the pelvis without excessive tension in the small bowel mesentery.
A recent meta-analysis12 including 18 studies reported 1519 patients with a J-, S-, or W-pouch. Among the 3 types of pouches, there were no significant differences in postoperative complications including leak, stricture, pelvic sepsis, pouchitis, small bowel obstruction, and pouch failure. In terms of functional outcomes, bowel frequency was higher in J-pouch patients than in those with either an S- or W-pouch. In addition, the use of antidiarrheal medication was greater following a J-pouch creation compared with either an S- or a W-pouch. However, those patients with an S- or W-pouch had more difficulty in pouch evacuation, which required intubation, than did patients with a J-pouch. Seepage and incontinence were similar among the 3 types of pouch design. In conclusion, the meta-analysis showed an advantage for the J and W over the S design with respect to spontaneous evacuation, despite the higher bowel frequency with the J-pouch compared with the S- and W-pouch. However, the occurrence of complications among the 3 types of pouches was comparable.
COMPARISON OF OUTCOMES AFTER HAND-SEWN VERSUS STAPLED IPAA
Since the introduction of stapling devices, stapled IPAA has become the favored technique over hand-sewn IPAA. In our institution, stapled IPAA has been commonly used since 1988. We use a hand-sewn IPAA technique in patients with dysplasia or cancer in the lower third of the rectum, those with a failure of stapled technique, or patients requiring a redo IPAA surgery.
A recent meta-analysis13 reported outcomes after hand-sewn versus stapled IPAA in 21 studies with 4183 patients. This study included 2699 patients with a hand-sewn anastomosis and 1484 patients with a stapled IPAA. Most patients had a J-pouch (n = 3184, 80.1%). No significant difference was found in postoperative complications following both techniques. Although bowel frequency was similar, incontinence to liquid stool, seepage at night and the use of pads overnight were higher in those with a hand-sewn IPAA. Also, anorectal physiologic measurements showed significantly lower resting and squeeze pressure in patients undergoing a hand-sewn IPAA. Sexual dysfunction, quality of life, and rate of ATZ dysplasia after IPAA were similar between the two groups.
The most recent study from our institution5 compared outcomes after hand-sewn versus stapled IPAA in 3109 patients from a single institution. Diagnosis was UC in most patients (n = 2222, 72%). Patients with a stapled IPAA had a greater body mass index than those with a hand-sewn IPAA. Groups were otherwise similar in terms of patient characteristics. Complications including pouchitis and wound infection were comparable between the two groups. However, anastomotic stricture, septic complications, small bowel obstruction, and pouch failure were higher in the hand-sewn group compared with the stapled group. When functional outcomes and quality of life data were adjusted for follow-up time, although bowel frequency and rate of urgency were similar between groups, incontinence, seepage, pad usage, dietary, social and work restrictions were greater in patients with a hand-sewn IPAA. Quality of life and happiness with surgical results were significantly higher in the stapled IPAA group. We concluded that stapled IPAA seems to be associated with significantly less complications and better function and quality of life compared with a hand-sewn IPAA.
IPAA WITH OR WITHOUT DIVERTING ILEOSTOMY
A recent meta-analysis14 including 1486 patients from 17 studies compared outcomes in patients with diverting ileostomy versus those without it. The leak rate was significantly higher in patients without stoma whereas anastomotic stricture and failure of IPAA was more common in those with stoma. Pouch-related sepsis, perianal sepsis, and pouchitis were similar. Functional outcomes and sexual function also were comparable between the groups. On sensitivity analysis, the development of pelvic sepsis was found to be higher in the no-stoma group. It was concluded that protective stoma improves outcomes, especially sepsis. However, ileostomy may still be omitted in patients defined as low risk.
A study from our institution including 2002 patients7 compared outcomes after IPAA with ileostomy versus without ileostomy. The pathologic diagnosis was UC/indeterminate colitis in 1755 patients. Patients with an ileostomy were older, more often male, had higher doses of steroids and greater body surface area, needed more blood transfusions, and had shorter length of hospital stay at the time of pouch surgery compared with those without an ileostomy. Anastomotic leak, pelvic sepsis, and the presence of fistula in general were similar between the groups. However, pouch vaginal fistula, small bowel obstruction, hemorrhage, anastomotic stricture, and pouch failure were greater in the ileostomy group, whereas postoperative ileus was higher in no ileostomy group. Two hundred eighty-five patients (17%) developed ileostomy closure-related complications. After adjusting for anastomosis type and age, there were no significant differences in short and long-term functional outcomes and quality of life between the two groups. Authors concluded that in selected patients who fit stringent selection criteria, one stage IPAA is safe and associated with similar or better results compared with two-stage IPAA. Stringent selection criteria used included stapled anastomosis, minimal tension on the IPAA, intact tissue rings, good hemostasis, and absence of air leak, malnutrition (alb <3.5 mg/dL), toxicity, anemia (Hb <13.5 mg/dL), or prolonged consumption of high-dose steroids (prednisone ≥20 mg for longer than 3 months).
Another study from our institution15 reported outcomes of loop ileostomy closure in 1504 patients with IPAA. Complications included small-bowel obstruction (6.4%), wound infection (1.5%), abdominal septic complications (1%), enterocutaneous fistula (0.6%), and others. There was no significant difference in rates of bowel obstruction, wound infection, and anastomotic complications as well as length of hospital stay between patients with a hand-sewn versus stapled ileostomy closure. It was concluded that loop ileostomy closure after IPAA can be done with an acceptable complication rate and a short hospital stay.
REPEAT POUCH SURGERY
Abdominal salvage surgery with/without disconnection of an ileal pouch-anal anastomosis and repeat IPAA (repeat pouch surgery) can be performed in patients with pouch failure due to complications after primary IPAA.
Tekkis et al16 reported 112 patients undergoing 117 pouch salvage procedures with a median follow-up of 46 months. Eighty-six patients had UC. The most common indication for surgery was intraabdominal sepsis (n = 45). Pouch revision/augmentation was the most frequent approach (n = 62). The pouch failure rate after salvage surgery was 21.4%. Failure was most common after surgery for sepsis. Pouch survival rate at 5 years for patients undergoing reconstructive surgery for a nonseptic indication was 85%; the pouch survival rate was 61% in patients with sepsis. All patients with the diagnosis of CD had pouch failure. The type of pouch or anastomosis was not related to pouch survival. The pouch survival rate was 88% and 70% at 1 and 5 years, respectively. Although bowel frequency per 24 hours, incontinence, pad usage, and need for antidiarrheal medication remained unchanged over the time, the number of bowel movements at night and urgency significantly improved after salvage surgery. It was concluded that a successful result after abdominal salvage was more likely for patients with a nonseptic indication compared with those with sepsis. The pouch failure rate after abdominal salvage surgery increased with length of follow-up.
In the meantime, Mathis et al17 identified 51 patients with UC undergoing pouch salvage surgery; however, 22 were found to have CD. Nineteen patients had their initial IPAA elsewhere. Indications for salvage surgery were infectious/inflammatory complications in 65% and mechanical difficulties in 35%. Forty-three percent of patients had complete pouch revision. The median follow-up was 8.2 years. CD and partial revision were found to be associated with an increased risk of complications after pouch reconstruction. The pouch survival rate after salvage surgery was 93% at 1 year and 89% at 5 years. The development of a pelvic abscess was the only variable that predicted pouch failure after pouch revision. Seventy-five percent of patients with pouch failure subsequently had a diagnosis of CD. Patients reported 5 daytime and 1 nighttime bowel movements. Forty-three percent of patients had occasional and 4% had frequent daytime incontinence. Authors concluded that in UC patients with pouch failure, pouch reconstruction was associated with good functional outcomes and quality of life.
A recent study from our institution18 reported the largest number of patients (n = 241) with repeat pouch surgery by the abdominal approach. Of 241, 79 (33%) had primary IPAA at our institution. Pathologic diagnosis was UC in 172 patients. Indications for repeat pouch surgery were chronic fistula (n = 67), leak (n = 65), anastomotic stricture (n = 42), dysfunction/long efferent limb of S-pouch (n = 42), pelvic abscess (n = 25), and others. Seventy-one patients had a new pouch constructed at the time of repeat surgery. In 170 patients the original pouch was salvaged by pouch repair/revision (n = 125), pouch augmentation/advancement/mobilization (n = 28), partial pouch resection (n = 16), and pouchopexy (n = 1). The median follow-up was 5 years (range 0.04–20.8). The pouch failure rate after repeat surgery was 15%. Of patients undergoing pouch salvage surgery because of septic complications as a main indication, 84% had a functioning pouch compared with 87% for a nonseptic indication. Patients had a mean number of bowel movements of 6 per day and 2 per night. Forty-seven percent of patients never/rarely reported urgency. Ninety-nine percent would have repeat pouch surgery again, and 98% would recommend it to other patients. When compared with patients with primary IPAA for functional outcomes and quality of life, although a greater proportion of patients undergoing repeat pouch surgery described pad usage during daytime and seepage episodes during day and nighttime, other functional outcomes and quality of life parameters were comparable between the 2 groups. Authors concluded that pouch failure rate was higher than that after primary IPAA; however, repeat pouch surgery was associated with good functional outcomes and quality of life.
In patients with postoperative IPAA-related complications, the differentiation of CD from these complications might be difficult and patients with these complications may be misdiagnosed as CD. In these patients, redo pouch surgery by the abdominal approach could be associated with good outcomes. We19 reported 33 patients referred from outside institutions with a diagnosis of CD following a primary IPAA. Initial diagnosis before primary IPAA was UC in 31 patients and indeterminate colitis in 2. Of 33, 10 patients received infliximab for CD prior to referral. All 33 patients underwent a redo pouch procedure (laparotomy with creation of new pouch or revision of existing pouch with redo IPAA). Findings on further evaluation and subsequent indications for redo pouch surgery were pouch fistula in 20 patients, anastomotic leak or pelvic sepsis in 17, stricture in 4, refractory pouchitis in 2, long exit conduit in 1, and retained rectal stump in 1. The pouch survival rate was 84.8% after redo pouch surgery with a median follow-up of 1.7 years (range 1–3.5). Five patients developed pouch failure. Seven patients eventually received diagnosis of CD. Functional outcomes and quality of life after redo pouch surgery were compared with patients with a primary IPAA and found to be similar in both groups. We concluded that patients referred with a diagnosis of CD following primary IPAA should be carefully reevaluated because in these patients redo pouch surgery is associated with good long-term outcomes.
CONCLUSIONS
IPAA is the procedure of choice for patients with ulcerative colitis. The creation of J-pouch is easier and has generally a similar rate of complications and functional outcomes when compared with other types of pouch. A stapled IPAA associated with better outcomes compared with a hand-sewn IPAA and risk of development of dysplasia or cancer in ATZ after a stapled IPAA is low. Therefore, a J-pouch with a stapled IPAA is the preferred technique for most surgeons. Omission of ileostomy at the time of IPAA might be considered in carefully selected patients with a low risk of occurrence of complications. In patients with pouch failure due to complications following primary IPAA, repeat pouch surgery can be performed with good results.
References
- 1.Fazio V W, Ziv Y, Church J M, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222(2):120–127. doi: 10.1097/00000658-199508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meagher A P, Farouk R, Dozois R R, Kelly K A, Pemberton J H. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg. 1998;85(6):800–803. doi: 10.1046/j.1365-2168.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 3.Parks A G, Nicholls R J. Proctocolectomy without ileostomy for ulcerative colitis. BMJ. 1978;2(6130):85–88. doi: 10.1136/bmj.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fazio V W, O'Riordain M G, Lavery I C, et al. Long-term functional outcome and quality of life after stapled restorative proctocolectomy. Ann Surg. 1999;230(4):575–584. discussion 584–586. doi: 10.1097/00000658-199910000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirat H T, Remzi F H, Kiran R P, Fazio V W. Comparison of outcomes after hand-sewn versus stapled ileal pouch-anal anastomosis in 3,109 patients. Surgery. 2009;146(4):723–729. discussion 729–730. doi: 10.1016/j.surg.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 6.Remzi F H, Fazio V W, Delaney C P, et al. Dysplasia of the anal transitional zone after ileal pouch-anal anastomosis: results of prospective evaluation after a minimum of ten years. Dis Colon Rectum. 2003;46(1):6–13. doi: 10.1007/s10350-004-6488-2. [DOI] [PubMed] [Google Scholar]
- 7.Remzi F H, Fazio V W, Gorgun E, et al. The outcome after restorative proctocolectomy with or without defunctioning ileostomy. Dis Colon Rectum. 2006;49(4):470–477. doi: 10.1007/s10350-006-0509-2. [DOI] [PubMed] [Google Scholar]
- 8.El-Gazzaz G S, Kiran R P, Remzi F H, Hull T L, Geisler D P. Outcomes for case-matched laparoscopically assisted versus open restorative proctocolectomy. Br J Surg. 2009;96(5):522–526. doi: 10.1002/bjs.6578. [DOI] [PubMed] [Google Scholar]
- 9.McAllister I, Sagar P M, Brayshaw I, Gonsalves S, Williams G L. Laparoscopic restorative proctocolectomy with and without previous subtotal colectomy. Colorectal Dis. 2009;11(3):296–301. doi: 10.1111/j.1463-1318.2008.01590.x. [DOI] [PubMed] [Google Scholar]
- 10.Fichera A, Silvestri M T, Hurst R D, Rubin M A, Michelassi F. Laparoscopic restorative proctocolectomy with ileal pouch anal anastomosis: a comparative observational study on long-term functional results. J Gastrointest Surg. 2009;13(3):526–532. doi: 10.1007/s11605-008-0755-9. [DOI] [PubMed] [Google Scholar]
- 11.Tilney H S, Lovegrove R E, Heriot A G, et al. Comparison of short-term outcomes of laparoscopic vs open approaches to ileal pouch surgery. Int J Colorectal Dis. 2007;22(5):531–542. doi: 10.1007/s00384-006-0177-7. [DOI] [PubMed] [Google Scholar]
- 12.Lovegrove R E, Heriot A G, Constantinides V, et al. Meta-analysis of short-term and long-term outcomes of J, W and S ileal reservoirs for restorative proctocolectomy. Colorectal Dis. 2007;9(4):310–320. doi: 10.1111/j.1463-1318.2006.01093.x. [DOI] [PubMed] [Google Scholar]
- 13.Lovegrove R E, Constantinides V A, Heriot A G, et al. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg. 2006;244(1):18–26. doi: 10.1097/01.sla.0000225031.15405.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weston-Petrides G K, Lovegrove R E, Tilney H S, et al. Comparison of outcomes after restorative proctocolectomy with or without defunctioning ileostomy. Arch Surg. 2008;143(4):406–412. doi: 10.1001/archsurg.143.4.406. [DOI] [PubMed] [Google Scholar]
- 15.Wong K S, Remzi F H, Gorgun E, et al. Loop ileostomy closure after restorative proctocolectomy: outcome in 1,504 patients. Dis Colon Rectum. 2005;48(2):243–250. doi: 10.1007/s10350-004-0771-0. [DOI] [PubMed] [Google Scholar]
- 16.Tekkis P P, Heriot A G, Smith J J, Das P, Canero A, Nicholls R J. Long-term results of abdominal salvage surgery following restorative proctocolectomy. Br J Surg. 2006;93(2):231–237. doi: 10.1002/bjs.5242. [DOI] [PubMed] [Google Scholar]
- 17.Mathis K L, Dozois E J, Larson D W, Cima R R, Wolff B G, Pemberton J H. Outcomes in patients with ulcerative colitis undergoing partial or complete reconstructive surgery for failing ileal pouch-anal anastomosis. Ann Surg. 2009;249(3):409–413. doi: 10.1097/SLA.0b013e31819a697b. [DOI] [PubMed] [Google Scholar]
- 18.Remzi F H, Fazio V W, Kirat H T, Wu J S, Lavery I C, Kiran R P. Repeat pouch surgery by the abdominal approach safely salvages failed ileal pelvic pouch. Dis Colon Rectum. 2009;52(2):198–204. doi: 10.1007/DCR.0b013e31819ad4b6. [DOI] [PubMed] [Google Scholar]
- 19.Garrett K A, Remzi F H, Kirat H T, Fazio V W, Shen B, Kiran R P. Outcome of salvage surgery for ileal pouches referred with a diagnosis of Crohn's disease. Dis Colon Rectum. 2009;52(12):1967–1974. doi: 10.1007/DCR.0b013e3181b77d1e. [DOI] [PubMed] [Google Scholar]