Abstract
Although restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) has become the surgical treatment of choice for patients with refractory ulcerative colitis (UC) or UC with dysplasia, surgical, inflammatory, and noninflammatory adverse sequelae are common. Pouchitis, representing a spectrum of disease phenotypes, is the most common long-term complication of IPAA. De novo Crohn disease (CD) of the pouch can occur in patients with a preoperative diagnosis of UC. Differential diagnosis between fibrostenotic or fistulizing CD and surgery-associated strictures, sinuses, and fistulas often requires a combined assessment of symptom, endoscopy, histology, radiography, and examination under anesthesia. There is a role for endoscopic therapy for stricturing complications of IPAA. Chronic antibiotic-refractory pouchitis, refractory cuffitis, as well as fibrostenotic or fistulizing CD of the pouch are the leading late-onset causes for pouch failure.
Keywords: Complication, ileal pouch, inflammatory bowel disease, restorative proctocolectomy
Even if the era for biological therapy for inflammation bowel disease (IBD) has arrived, restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) is still the surgical treatment of choice for the majority of patients with ulcerative colitis (UC) who fail medical therapy or develop dysplasia or neoplasia as well as for patients with familial adenomatous polyposis (FAP). Although this bowel-anatomy-altering procedure improves patients' health-related quality of life and substantially reduces the risk for UC-associated neoplasia, mechanical/surgical, inflammatory, and functional complications are common. Some of these complications can lead to pouch failure with pouch excision or permanent diversion. In a meta-analysis of 43 studies of 9,317 patients from mostly tertiary-care settings, the frequency of pouch failure was estimated to be 7% with a median follow-up of 37 months; the frequency increased to 9% after more than 60 months.1 The most common causes for early-onset pouch failure are pouch leaks, abscess, and pelvic sepsis.2,3 Whereas the leading causes for late-onset pouch failure are chronic pouchitis, Crohn disease (CD) of the pouch,4 refractory cuffitis,5 and chronic deep, complex pouch sinus.6 Recognition and appropriate diagnosis of these pouch-related conditions are imperative for proper management and prognosis.
SURGERY-ASSOCIATED COMPLICATIONS
Pouch leak can occur after IPAA and the most common locations are the pouch-anal anastomosis and the tip of the “J.”7 Patients with immediate postoperative leak usually present with symptoms related to pelvic sepsis.2 Soluble contrast pouchogram, pelvic magnetic resonance imaging (MRI), and examination under anesthesia can help detect the location, feature, and depth of the leak.8 Immediate postoperative pelvic sepsis and pelvic abscess occurs in 5 to 20% of patients undergoing restorative proctocolectomy with IPAA, and ∼30% of these patients will eventually have pouch failure.1,2,4,9 Pelvic sepsis was associated with anastomotic leak in 34%, fistulas in 25%, and even mortality in 3% of patients.2 Pelvic sepsis often requires surgical intervention. There is a limited role for practicing gastroenterologists in the diagnosis and management of acute anastomotic leaks.
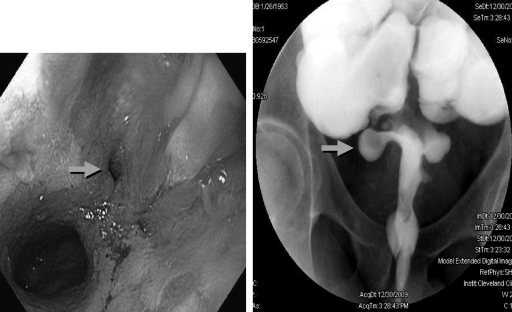
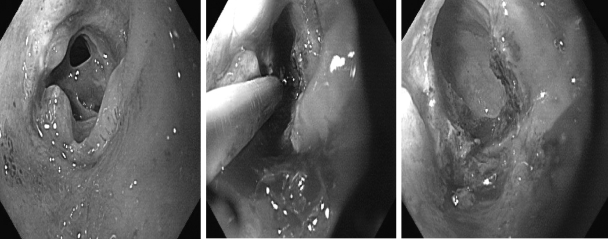
Pouch sinus is typically a later presentation of an initial anastomotic leak. The most common location of a pouch sinus is the pouch-anal anastomotic site, which is frequently located at the presacral space (Fig. 1). Some patients can present with symptoms suggestive of pelvic sepsis, pouchitis, CD of the pouch, or refractory cuffitis; others may be asymptomatic. Common symptoms in patients with pouch sinus are perianal pain, pelvic pressure and discomfort, and tailbone pain. Severe and complex sinus may result in intermittent fever, weight loss, and anemia. In extreme cases, chronic deep pouch sinus can lead to osteomyelitis of tailbone posteriorly and pouch-vaginal or pouch-vesicular fistulas anteriorly. For patients with clinical presentations suspected of pouch sinus, a careful pouchoscopy should be performed with attention to the anastomosis and the tip of the “J.” However, abdominal imaging with water-soluble contrasted pouchogram and/or MRI of the pelvis is often needed to delineate the complexity, depth, and possible complicating abscess. Sinus opening and sinus tract can be detected by a combined assessment of pouchoscopy, contrasted pouchogram, examination under anesthesia and pelvic MRI.10 Treatment usually includes periodic incision and drainage of the chronically infected superficial sinuses to promote secondary healing and closure. It may take up to 9 to 12 months before these sinuses heal. Fibrin glue injection of the sinus may be attempted.11 Patients with a long sinus track who do not have complete healing following ileostomy closure, are usually candidates for a redo pouch procedure.11,12 Our group started using endoscopic needle knife therapy for the treatment of simple, shallow (<5 cm in depth) presacral sinus (Fig. 2). The procedure has been performed in patients with or without protecting diverting ileostomy.13 Our anecdotal experience suggests that ∼50% of patients with a simple, short sinus can be treated with endoscopy.
Figure 1.
Pouch sinus on pouch endoscopy and contrasted pouchogram (arrows).
Figure 2.
Endoscopic needle knife therapy for a distal pouch sinus.
Surgery-associated fistulas can originate at any levels of the pouch with the most common location being at the anastomosis, and they can extend into any adjacent organs or to the skin. The distinction between surgery-induced fistulas and CD-associated fistulas can be challenging. Pouch-vaginal fistula (PVF) is a unique and yet common condition with IPAA and a major source of morbidity and a common cause for pouch failure.14 Although diagnosis of PVF is mainly based on symptoms, endoscopic and radiographic documentation is often needed for the differential diagnosis and direction of treatment. Our recent study found that examination under anesthesia is more accurate than pouch endoscopy, MRI, computed tomography (CT), and water-contrasted pouchogram.15 A diagnosis of CD should be suspected in patients with PVF, if the patients have a preoperative diagnosis of CD before IPAA, the presence of a fistula 6 to 12 months after IPAA in the absence of immediate postoperative leaks, pelvic abscess or pelvis sepsis, a complex fistula outside the anastomosis or dentate line, concurrent ulcers in the proximal small bowel or afferent limb, or repeated failure for fistula repair or fibrin glue therapy. However, surgical intervention is often required for PVF, despite the etiology of PVF.16
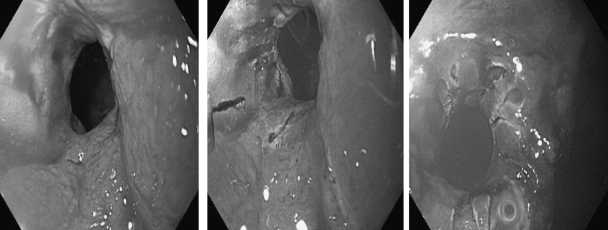
Strictures after IPAA are common. In a series of 1,884 cases with IPAA, pouch strictures occurred in 213 patients (11%).17 However, most of those strictures are self-limiting web-type strictures that are recognized and dilated at the time of stoma closure. Common locations are at the anastomosis, pouch inlet, and site of prior ileostomy. Contributing etiological factors include surgery-associated ischemia, use of nonsteroidal antiinflammatory drugs (NSAIDs), and CD. Strictures can be treated with medical therapy (including cessation of NSAIDs and pharmaceutical treatment for CD) as well as endoscopic18 or bougie dilations, endoscopic needle knife therapy (Fig. 3), surgical stricturoplasty, bowel resection and anastomosis, or pouch diversion or excision.19
Figure 3.
Endoscopic needle knife therapy for a pouch inlet stricture.
POUCHITIS
Pouchitis, a nonspecific inflammation of the ileal reservoir, is the most common long-term complication in patients with IPAA. It significantly affects patients' health-related quality of life.20 Reported cumulative frequency rates of pouchitis 10 to 11 years after IPAA surgery range from 23 to 46%,21,22 with an incidence of 40% within the first 12 months after ileostomy closure.23 Pouchitis almost exclusively occurs in patients with underlying UC and is rarely seen in patients with FAP.24,25 Although its etiology and pathogenesis are not entirely clear, multiple lines of evidence suggest that an abnormal mucosal immune response to altered commensal bacterial flora (dysbiosis) leads to acute or chronic inflammation of the pouch reservoir in a genetically susceptible host.26,27,28 Reported risk factors for pouchitis include genetic polymorphisms of IL-1 receptor antagonist29,30,31 and NOD2/CARD15;32 noncarrier status of tumor-necrosis factor allele 2;31 extensive UC22,33,34 backwash ileitis;33 precolectomy thrombocytosis;35,36 preoperative corticosteroid use;36 extraintestinal manifestations, especially PSC and arthropathy;21,34,36,37,38,39 the presence of perinuclear antineutrophil cytoplasmic antibodies;40,41,42 being a nonsmoker;36,43,44 and regular use of NSAIDs.34,44 Some of these risk factors are therapeutically modifiable.
Pouchitis is not a homogenous disease entity. Rather, it represents a disease spectrum with a wide range of clinical presentations, endoscopic and histologic features, disease courses, and prognoses. Increased stool frequency, urgency, incontinence, nocturnal seepage, abdominal cramping, pelvic discomfort, and arthralgia are the most common presenting symptoms.17 The severity of symptoms does not necessarily correlate with the degree of endoscopic or histologic inflammation of the pouch.45,46 In addition, these symptoms are not specific for pouchitis, as they can be presented in patients with other inflammatory or functional disorders of the pouch, such as CD, cuffitis, pouch sinus, and irritable pouch syndrome (IPS). Endoscopy is the most reliable tool for diagnosis and differential diagnosis of pouchitis.45 Ideally, a combined assessment of symptoms and endoscopic and histologic features should be performed to diagnose pouchitis.
It is imperative to classify the phenotype of pouchitis before initiating therapy. Although there is no validated and universally accepted classification system, pouchitis, from different clinical perspectives, may be categorized into (1) idiopathic versus secondary, (2) remission versus active, (3) acute versus chronic with a cutoff duration of 4 weeks, (4) infrequent episodes (e.g., <4 episodes a year) versus relapsing (≥4 episodes a year) versus continuous course, and (5) antibiotic-responsive, antibiotic-dependent versus antibiotic-refractory refractory.47,48,49 Chronic antibiotic-refractory pouchitis is one of the leading causes for pouch failure, resulting in permanent diversion or pouch excision. In a study of 100 consecutive UC with IPAA, 5 developed chronic pouchitis, of whom 2 had pouch failure.47 One would expect that there will be an increasing number of patients with chronic antibiotic-refractory pouchitis in clinical practice, as a cumulative number of patients with IPAA are expanding. For patients with a disease course refractory to conventional antibiotic therapy, secondary etiologies should be evaluated. These secondary etiologic factors include infection from intestinal pathogens (e.g., Clostridium difficile,50 Candida,51 cytomegalovirus,52,53 and Campylobacter species,54 NSAID use,55 concurrent autoimmune disorders (e.g., celiac disease),56 and pouch ischemia.57
Treatment strategies vary in different types of pouchitis.58 A common scenario is that a patient with active endoscopic inflammation of the pouch may be asymptomatic. However, it is advisable to still treat the patient, as chronic smoldering inflammation may lead to a “stiff” pouch. For antibiotic-responsive pouchitis, the first-line therapy generally includes a 14-day course of metronidazole (15–20 mg/kg/day) or ciprofloxacin (1000 mg/day).59,60 Other agents were reported in open-label trials including tetracycline, doxycycline, clarithromycin, amoxicillin/clavulanic acid, oral or topical budesonide, alicaforsen enemas (an antisense inhibitor of intercellular adhesion molecule-1), leukocytapheresis,61 probiotics,62 and a carbon microsphere adsorbent agent.63
Patients with antibiotic-dependent pouchitis often require long-term maintenance therapy to keep disease in remission. Maintenance agents include probiotics (such as VSL#3®, VSL Pharmaceuticals, Gaithersburg, MD64,65) and a low dose of antibiotics (such as rifaximin).66 Although the probiotic agents have been shown to be highly effective in primary and secondary prophylaxis of pouchitis in randomized controlled trials, the results of postmarket routine clinical use have been disappointing.67
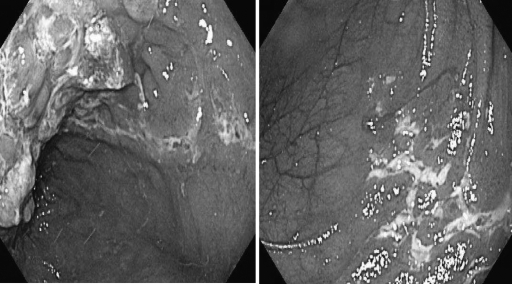
Treatment of antibiotic-refractory pouchitis is often challenging. It is important to investigate contributing factors related to failure of antibiotic therapy, such as Clostridium difficile and cytomegalovirus infection, NSAID use, and current autoimmune disorders. Recently, we also found that some patients with pouchitis had ischemic features on endoscopy and abdominal imaging. A classic endoscopic feature of ischemic pouchitis is the presence of inflammation in the half of the pouch body sparing the rest half of the pouch with a sharp demarcation between inflamed and noninflamed parts along the suture line (Fig. 4).57 After exclusion of infection of the specific bacterial or viral pathogens, patients with antibiotic-refractory pouchitis can be treated with a prolonged course of combined antibiotic agents. Options could be combined ciprofloxacin (1000 mg/d) with rifaximin (2000 mg/d),68,69 metronidazole (1000 mg/d),70 or tinidazole (1000–1500 mg/d) for 4 weeks.71 However, maintenance of remission in this group of patients after the induction therapy with dual antibiotics remains challenging.72 These agents include bismuth carbomer enemas,73 short-chain fatty acid enemas,74 glutamine enemas, oral budesonide,75 mesalamine enemas, 6-mercaptopurine or azothioprine, and infliximab.76
Figure 4.
Ischemic pouchitis on endoscopy with sharp demarcation of inflamed and noninflamed parts of pouch along the suture line (left) and triangular-shaped disease distribution (right).
Cuffitis
Cuffitis, considered to be a variant form of UC in the rectal cuff, is common in patients with IPAA, particularly in those with stapled anastomosis without mucosectomy. Clinical symptoms of cuffitis are similar to those in pouchitis. In addition, patients with cuffitis often present with small-quantity bloody bowel movements. Cuffitis can be treated with mesalamine suppositories,77 or topical lidocaine/corticosteroid agents. In our clinical practice, we occasionally treat refractory cuffitis with endoscopic injection of long-acting corticosteroids. Systemic agents are rarely needed. Of note, cuffitis may not be a simple residual ulcerative colitis, as surgery-associated ischemia may contribute inflammation at the anal transitional zone. Patients with cuffitis refractory to topical mesalamine and/or corticosteroid therapy should be evaluated for other disease processes at or around the cuff, such as fistula and chronic anastomotic leaks. Refractory cuffitis can also be a sign of CD of the pouch. Chronic cuffitis can be a contributing factor for anastomotic stricture. Refractory cuffitis associated with CD or surgery-complications can lead to pouch failure.78
Crohn Disease of the Pouch
Reported cumulative frequencies of CD of the pouch ranged from 2.7 to 13%.48 CD of the pouch can occur after IPAA, which is intentionally performed in a selected group of patients with Crohn colitis with no previous small intestinal or perianal disease;79 CD is also inadvertently found in colectomy specimens of patients with a preoperative diagnosis of UC. CD of the pouch diagnosed in these two settings may be classified into early-onset CD of the pouch.80 De novo CD of the pouch may develop weeks to years after IPAA for UC and a reassessment of the proctocolectomy specimens may show no evidence of CD. De novo CD developed months to years after IPAA may be considered as late-onset CD.80Patients with late-onset CD of the pouch typically appear to have a poorer prognosis with a higher risk for pouch failure than those with early onset CD.80
The etiology and pathogenesis of CD of the pouch are not clear. It has been postulated that IPAA with components of procedure-associated ischemia, anastomosis, and fecal stasis may create a CD-friendly environment.81 Therefore, extensive preoperative endoscopic, serological, histologic, and radiographic evaluation, which would have excluded CD, does not guarantee nondevelopment of CD after IPAA. On the other hand, patients with Crohn colitis, a relative contraindication for IPAA, who had the procedure intentionally performed, may not necessarily have developed CD of the pouch.82 Therefore, the natural history of CD of the pouch is poorly defined.
Assessment of risk factors for the development of CD of the pouch may help the decision of whether, when, and which surgical procedure is performed. Reported risk factors for CD of the pouch include family history of CD and smoking.83,84 Intraoperative or immediate postsurgical histopathological evaluation of colectomy specimen for neural hypertrophy may be predictive of the development of CD of the pouch.85 The role of IBD serology in predicting postoperative development of CD of the pouch warrants further evaluation with confirmation studies.83
CD of the pouch can be classified into inflammatory, fibrostenotic, or fistulizing phenotypes.48 The clinical phenotypes of CD of the pouch are associated with different risk factors and clinical presentations.86 Presentation of CD of the pouch, however, often overlap with that from other inflammatory disorders of the pouch (e.g., pouchitis and cuffitis) and surgery-associated strictures, sinuses, and fistulas. Therefore, the diagnosis of CD of the pouch should be based on a combined assessment of symptom, endoscopy, histology, abdominal imaging, and sometimes examination under anesthesia. Endoscopic and/or histologic documentation of upper GI disease with esophagogastroduodenoscopy may be helpful for the diagnosis of CD. The role of serology in the diagnosis and differential diagnosis of CD of the pouch needs further investigation.
Although symptoms from various disease conditions of the pouch often overlap, there are symptoms and signs that may suggest a diagnosis of CD, particularly fibrostenotic and fistulizing CD. Clinical presentations of CD of the pouch can be persistent abdominal pain, nausea, anemia, weight loss, failure to thrive, fever, and fistular drainage. It is critical to differentiate NSAID-induced ileitis/pouchitis from CD ileitis and backwash ileitis with diffuse pouchitis. It is also important to distinguish surgical ischemia-associated stricture and fistulizing complications from fibrostenotic or fistulizing CD. However, these distinctions can be difficult to make. In clinical practice, CD of the pouch should be suspected if a patient develops de novo fistula more than 6 to 12 months after ileostomy take-down in the absence of postoperative leak, abscess, and sepsis.
There are scant data on the treatment of CD of the pouch. Patients should be strongly encouraged to avoid cigarette smoking and NSAID use. The majority of CD patients require life-long medical therapy to avoid permanent ileostomy. Stratification of patients based on clinical phenotypes is important for treatment. CD can be treated with a combined medical,87 endoscopic (e.g., balloon dilation of stricture),88 and surgical (e.g., stricturoplasty)89 therapies. Inflammatory CD of the pouch may be treated with topical and oral 5-aminosalicylate agents, oral or topical corticosteroids, antibiotics, immunomodulators, and biologics. Commonly used agents include oral mesalamines and budesonide. Break-capsule mesalamines or budesonide mixed with applesauce may provide better coverage for patients with proximal small bowel disease. In patients whose disease is refractory to these agents, particularly when they have concurrent extraintestinal symptoms, biological agents such as infliximab and adalimumab may be used.
For fibrostenotic CD of the pouch, endoscopic therapy together along with medical therapy is often needed. Although medical therapy alone has a minimum therapeutic impact on mechanical strictures, it may help reduce mucosal inflammation in between the strictures. Endoscopic balloon dilation can be attempted for CD-associated stricture. Endoscopic balloon dilation with or without guidewire can be safely performed without fluoroscopic guidance.18 For patients with long and high-grade strictures, endoscopic needle knife “stricturoplasty” treatment, particularly with Doppler ultrasound guidance may be attempted in experienced hands. Refractory, multiple, and angulated strictures may be treated with surgical stricturoplasty, resection and anastomosis, or proximal diversion.
The management of fistulizing CD of the pouch has been difficult. Antibiotics, immunomodulators, and biologics may be tried. Biologics such as infliximab and adalimumab appeared to be effective in short-term induction, with decreased fistular drainage.87,90 However, whether the biological therapy could maintain the disease in remission in the long term or have a positive impact on the natural history of CD of the pouch remains to be seen. Medical therapy for PVF and pouch-bladder fistula has been disappointing. Surgical intervention is usually required.
IRRITABLE POUCH SYNDROME
Irritable pouch syndrome is a functional disorder in patients with IPAA.91 The disease entity has a significant negative impact on health-related quality of life.20 Its etiology and pathophysiology are not clear. It may be attributed to psychosocial factors,20 visceral hypersensitivity as measured by electronic barostat,92 and enterochromaffin cell hyperplasia of the pouch mucosa.93 Currently, IPS is a diagnosis of exclusion. Treatment is empiric, and therapeutic agents include antidiarrheals, antispasmodics, tricyclic antidepressants, and oral or topical narcotic agents.
POUCH NEOPLASIA
Although proctocolectomy with IPAA substantially reduces the risk for dysplasia and cancer in UC patients, dysplasia or adenocarcinoma can still occur in the rectal cuff (anal transitional zone) or pouch body, presenting as flat or polypoid lesions.94,95,96,97 Reported risk factors for dysplasia or cancer in the ileal pouch or cuff include a longer duration of UC (irrespective of duration of IPAA),98 a preoperative or intraoperative diagnosis of dysplasia or cancer from underlying UC,99 the presence of pancolitis with backwash ileitis,100,101 villous atrophy of the pouch mucosa or histologic type C mucosa (pouch mucosa with persistent severe atrophy),94,98,100,102 chronic pouchitis,98 and primary sclerosing cholangitis.98 Our recent study of 3203 pouch patients with underlying IBD showed the cumulative incidences for pouch neoplasia at 5, 10, 15, 20, and 25 years were 0.9%, 1.3%, 1.9%, 4.2%, and 5.1%, respectively. In multivariable analysis, the risk factors associated with pouch neoplasia was a preoperative diagnosis of UC-associated cancer or dysplasia, with adjusted hazard ratios of 13.43 (95% confidence interval [CI] = 3.96–45.53, p < 0.001) and 3.62 (95% CI = 1.59–8.23, p = 0.002), respectively. Pouch endoscopy with biopsy, the current gold standard for surveillance, may still miss the detection of neoplasia at the dysplasia stage.103
In summary, restorative proctocolectomy IPAA is a technically challenging procedure that requires appropriate skills and expertise to minimize surgery-associated complications. Adverse sequelae of IPAA are common. Accurate diagnosis, classification, and differential diagnosis of pouch disorders and associated complications are important for proper management and prognosis and for improving long-term surgical outcome. De novo CD of the pouch can occur. The risk for neoplasia in patients with UC and IPAA is small, but it not eliminated by colectomy or mucosectomy. A preoperative diagnosis of dysplasia or cancer of colon or rectum is a risk factor for pouch dysplasia or adenocarcinoma.
References
- 1.Hueting W E, Buskens E, der Tweel I van, Gooszen H G, Laarhoven C J van. Results and complications after ileal pouch anal anastomosis: a meta-analysis of 43 observational studies comprising 9,317 patients. Dig Surg. 2005;22(1-2):69–79. doi: 10.1159/000085356. [DOI] [PubMed] [Google Scholar]
- 2.Sagap I, Remzi F H, Hammel J P, Fazio V W. Factors associated with failure in managing pelvic sepsis after ileal pouch-anal anastomosis (IPAA)—a multivariate analysis. Surgery. 2006;140(4):691–703. discussion 703–704. doi: 10.1016/j.surg.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 3.Prudhomme M, Dehni N, Dozois R R, Tiret E, Parc R. Causes and outcomes of pouch excision after restorative proctocolectomy. Br J Surg. 2006;93(1):82–86. doi: 10.1002/bjs.5147. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls R J. Review article: ulcerative colitis—surgical indications and treatment. Aliment Pharmacol Ther. 2002;16(Suppl 4):25–28. doi: 10.1046/j.1365-2036.16.s4.5.x. [DOI] [PubMed] [Google Scholar]
- 5.Shen B, Lian L, Remzi F H, et al. Natural history of cuffitis in ulcerative colitis patients with restorative proctocolectomy and ileal pouch-anal anastomosis. New Orleans, LA: Poster presented at: Digestive Disease Week; May 1–10, 2010.
- 6.Garrett K A, Remzi F H, Kirat H T, Fazio V W, Shen B, Kiran R P. Outcome of salvage surgery for ileal pouches referred with a diagnosis of Crohn's disease. Dis Colon Rectum. 2009;52(12):1967–1974. doi: 10.1007/DCR.0b013e3181b77d1e. [DOI] [PubMed] [Google Scholar]
- 7.Paye F, Penna C, Chiche L, Tiret E, Frileux P, Parc R. Pouch-related fistula following restorative proctocolectomy. Br J Surg. 1996;83(11):1574–1577. doi: 10.1002/bjs.1800831127. [DOI] [PubMed] [Google Scholar]
- 8.Hedrick T L, Sawyer R G, Foley E F, Friel C M. Anastomotic leak and the loop ileostomy: friend or foe? Dis Colon Rectum. 2006;49(8):1167–1176. doi: 10.1007/s10350-006-0602-6. [DOI] [PubMed] [Google Scholar]
- 9.Meagher A P, Farouk R, Dozois R R, Kelly K A, Pemberton J H. J ileal pouch-anal anastomosis for chronic ulcerative colitis: complications and long-term outcome in 1310 patients. Br J Surg. 1998;85(6):800–803. doi: 10.1046/j.1365-2168.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 10.Tang L, Cai H, Moore L, Shen B. Evaluation of endoscopic and imaging modalities in the diagnosis of structural disorders of the ileal pouch. Inflamm Bowel Dis. 2010;16(9):1526–1531. doi: 10.1002/ibd.21199. [DOI] [PubMed] [Google Scholar]
- 11.Swain B T, Ellis C N. Fibrin glue treatment of low rectal and pouch-anal anastomotic sinuses. Dis Colon Rectum. 2004;47(2):253–255. doi: 10.1007/s10350-003-0040-7. [DOI] [PubMed] [Google Scholar]
- 12.Whitlow C B, Opelka F G, Gathright J B, Jr, Beck D E. Treatment of colorectal and ileoanal anastomotic sinuses. Dis Colon Rectum. 1997;40(7):760–763. doi: 10.1007/BF02055427. [DOI] [PubMed] [Google Scholar]
- 13.Lian L, Geisler D, Shen B. Endoscopic needle knife treatment of chronic presacral sinus at the anastomosis at an ileal pouch-anal anastomosis. Endoscopy. 2010;42(Suppl 2):E14. doi: 10.1055/s-0029-1215257. [DOI] [PubMed] [Google Scholar]
- 14.Shah N S, Remzi F, Massmann A, Baixauli J, Fazio V W. Management and treatment outcome of pouch-vaginal fistulas following restorative proctocolectomy. Dis Colon Rectum. 2003;46(7):911–917. doi: 10.1007/s10350-004-6684-0. [DOI] [PubMed] [Google Scholar]
- 15.Rochey H, Navaneethan U, Kiran R, Shen B. Evaluation of diagnostic modalities for pouch vaginal fistula in patients with inflammatory bowel disease and restorative proctocolectomy. San Antonio,TX: Paper presented at: American College of Gastroenterology Annual Meeting; October 18–20, 2010.
- 16.Burke D, Laarhoven C J van, Herbst F, Nicholls R J. Transvaginal repair of pouch-vaginal fistula. Br J Surg. 2001;88(2):241–245. doi: 10.1046/j.1365-2168.2001.01663.x. [DOI] [PubMed] [Google Scholar]
- 17.Prudhomme M, Dozois R R, Godlewski G, Mathison S, Fabbro-Peray P. Anal canal strictures after ileal pouch-anal anastomosis. Dis Colon Rectum. 2003;46(1):20–23. doi: 10.1007/s10350-004-6491-7. [DOI] [PubMed] [Google Scholar]
- 18.Shen B, Fazio V W, Remzi F H, et al. Endoscopic balloon dilation of ileal pouch strictures. Am J Gastroenterol. 2004;99(12):2340–2347. doi: 10.1111/j.1572-0241.2004.40604.x. [DOI] [PubMed] [Google Scholar]
- 19.Lucha P A, Jr, Fticsar J E, Francis M J. The strictured anastomosis: successful treatment by corticosteroid injections—report of three cases and review of the literature. Dis Colon Rectum. 2005;48(4):862–865. doi: 10.1007/s10350-004-0838-y. [DOI] [PubMed] [Google Scholar]
- 20.Shen B, Fazio V W, Remzi F H, et al. Comprehensive evaluation of inflammatory and noninflammatory sequelae of ileal pouch-anal anastomoses. Am J Gastroenterol. 2005;100(1):93–101. doi: 10.1111/j.1572-0241.2005.40778.x. [DOI] [PubMed] [Google Scholar]
- 21.Penna C, Dozois R, Tremaine W, et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996;38(2):234–239. doi: 10.1136/gut.38.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fazio V W, Ziv Y, Church J M, et al. Ileal pouch-anal anastomoses complications and function in 1005 patients. Ann Surg. 1995;222(2):120–127. doi: 10.1097/00000658-199508000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003;124(5):1202–1209. doi: 10.1016/s0016-5085(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 24.Penna C, Tiret E, Kartheuser A, Hannoun L, Nordlinger B, Parc R. Function of ileal J pouch-anal anastomosis in patients with familial adenomatous polyposis. Br J Surg. 1993;80(6):765–767. doi: 10.1002/bjs.1800800638. [DOI] [PubMed] [Google Scholar]
- 25.Tjandra J J, Fazio V W, Church J M, Oakley J R, Milsom J W, Lavery I C. Similar functional results after restorative proctocolectomy in patients with familial adenomatous polyposis and mucosal ulcerative colitis. Am J Surg. 1993;165(3):322–325. doi: 10.1016/s0002-9610(05)80834-7. [DOI] [PubMed] [Google Scholar]
- 26.Gosselink M P, Schouten W R, Lieshout L MC van, Hop W C, Laman J D, Ruseler-van Embden J G. Delay of the first onset of pouchitis by oral intake of the probiotic strain Lactobacillus rhamnosus GG. Dis Colon Rectum. 2004;47(6):876–884. doi: 10.1007/s10350-004-0525-z. [DOI] [PubMed] [Google Scholar]
- 27.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(2):305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 28.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53(1):108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter M J, Di Giovine F S, Cox A, et al. The interleukin 1 receptor antagonist gene allele 2 as a predictor of pouchitis following colectomy and IPAA in ulcerative colitis. Gastroenterology. 2001;121(4):805–811. doi: 10.1053/gast.2001.28017. [DOI] [PubMed] [Google Scholar]
- 30.Brett P M, Yasuda N, Yiannakou J Y, et al. Genetic and immunological markers in pouchitis. Eur J Gastroenterol Hepatol. 1996;8(10):951–955. doi: 10.1097/00042737-199610000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Aisenberg J, Legnani P E, Nilubol N, et al. Are pANCA, ASCA, or cytokine gene polymorphisms associated with pouchitis? Long-term follow-up in 102 ulcerative colitis patients. Am J Gastroenterol. 2004;99(3):432–441. doi: 10.1111/j.1572-0241.2004.04107.x. [DOI] [PubMed] [Google Scholar]
- 32.Meier C B, Hegazi R A, Aisenberg J, et al. Innate immune receptor genetic polymorphisms in pouchitis: is CARD15 a susceptibility factor? Inflamm Bowel Dis. 2005;11(11):965–971. doi: 10.1097/01.mib.0000186407.25694.cf. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt C M, Lazenby A J, Hendrickson R J, Sitzmann J V. Preoperative terminal ileal and colonic resection histopathology predicts risk of pouchitis in patients after ileoanal pull-through procedure. Ann Surg. 1998;227(5):654–662. discussion 663–665. doi: 10.1097/00000658-199805000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Achkar J P, Al-Haddad M, Lashner B, et al. Differentiating risk factors for acute and chronic pouchitis. Clin Gastroenterol Hepatol. 2005;3(1):60–66. doi: 10.1016/s1542-3565(04)00604-4. [DOI] [PubMed] [Google Scholar]
- 35.Okon A, Dubinsky M, Vasiliauskas E A, et al. Elevated platelet count before ileal pouch-anal anastomosis for ulcerative colitis is associated with the development of chronic pouchitis. Am Surg. 2005;71(10):821–826. [PubMed] [Google Scholar]
- 36.Fleshner P, Ippoliti A, Dubinsky M, et al. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2007;5(8):952–958, quiz 887. doi: 10.1016/j.cgh.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Shepherd N A, Hultén L, Tytgat G NJ, et al. Pouchitis. Int J Colorectal Dis. 1989;4(4):205–229. doi: 10.1007/BF01644986. [DOI] [PubMed] [Google Scholar]
- 38.Lohmuller J L, Pemberton J H, Dozois R R, Ilstrup D, Heerden J van. Pouchitis and extraintestinal manifestations of inflammatory bowel disease after ileal pouch-anal anastomosis. Ann Surg. 1990;211(5):622–627. discussion 627–629. [PMC free article] [PubMed] [Google Scholar]
- 39.Hata K, Watanabe T, Shinozaki M, Nagawa H. Patients with extraintestinal manifestations have a higher risk of developing pouchitis in ulcerative colitis: multivariate analysis. Scand J Gastroenterol. 2003;38(10):1055–1058. doi: 10.1080/00365520310005938. [DOI] [PubMed] [Google Scholar]
- 40.Fleshner P R, Vasiliauskas E A, Kam L Y, et al. High level perinuclear antineutrophil cytoplasmic antibody (pANCA) in ulcerative colitis patients before colectomy predicts the development of chronic pouchitis after ileal pouch-anal anastomosis. Gut. 2001;49(5):671–677. doi: 10.1136/gut.49.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuisma J, Järvinen H, Kahri A, Färkkilä M. Factors associated with disease activity of pouchitis after surgery for ulcerative colitis. Scand J Gastroenterol. 2004;39(6):544–548. doi: 10.1080/00365520410004668. [DOI] [PubMed] [Google Scholar]
- 42.Hui T, Landers C, Vasiliauskas E, et al. Serologic responses in indeterminate colitis patients before ileal pouch-anal anastomosis may determine those at risk for continuous pouch inflammation. Dis Colon Rectum. 2005;48(6):1254–1262. doi: 10.1007/s10350-005-0013-0. [DOI] [PubMed] [Google Scholar]
- 43.Merrett M N, Mortensen N, Kettlewell M, Jewell D O. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut. 1996;38(3):362–364. doi: 10.1136/gut.38.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen B, Fazio V W, Remzi F H, et al. Risk factors for diseases of ileal pouch-anal anastomosis in patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2006;4:81–89. doi: 10.1016/j.cgh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Shen B, Achkar J-P, Lashner B A, et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. 2001;121(2):261–267. doi: 10.1053/gast.2001.26290. [DOI] [PubMed] [Google Scholar]
- 46.Moskowitz R L, Shepherd N A, Nicholls R J. An assessment of inflammation in the reservoir after restorative proctocolectomy with ileoanal ileal reservoir. Int J Colorectal Dis. 1986;1(3):167–174. doi: 10.1007/BF01648445. [DOI] [PubMed] [Google Scholar]
- 47.Sandborn W J. Pouchitis: risk factors, frequency, natural history, classification and public health prospective. In: In: Me Leod RS, Martin F, Sutherland LR, Wallace JL, Williams CN, editor. Trends in Inflammatory Bowel Disease 1996. Lancaster, UK: Kluwer Academic Publishers; 1997. pp. 51–63. [Google Scholar]
- 48.Shen B, Remzi F H, Lavery I C, Lashner B A, Fazio V W. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin Gastroenterol Hepatol. 2008;6(2):145–158. quiz 124. doi: 10.1016/j.cgh.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 49.Shen B. Diagnosis and treatment of patients with pouchitis. Drugs. 2003;63(5):453–461. doi: 10.2165/00003495-200363050-00002. [DOI] [PubMed] [Google Scholar]
- 50.Shen B O, Jiang Z-D, Fazio V W, et al. Clostridium difficile infection in patients with ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2008;6(7):782–788. doi: 10.1016/j.cgh.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 51.Kühbacher T, Ott S J, Helwig U, et al. Bacterial and fungal microbiota in relation to probiotic therapy (VSL#3) in pouchitis. Gut. 2006;55(6):833–841. doi: 10.1136/gut.2005.078303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muñoz-Juarez M, Pemberton J H, Sandborn W J, Tremaine W J, Dozois R R. Misdiagnosis of specific cytomegalovirus infection of the ileoanal pouch as refractory idiopathic chronic pouchitis: report of two cases. Dis Colon Rectum. 1999;42(1):117–120. doi: 10.1007/BF02235196. [DOI] [PubMed] [Google Scholar]
- 53.Moonka D, Furth E E, MacDermott R P, Lichtenstein G R. Pouchitis associated with primary cytomegalovirus infection. Am J Gastroenterol. 1998;93(2):264–266. doi: 10.1111/j.1572-0241.1998.00264.x. [DOI] [PubMed] [Google Scholar]
- 54.Shen B. Campylobacter infection in patients with ileal pouches. Am J Gastroenterol. 2010;105(2):472–473. doi: 10.1038/ajg.2009.550. [DOI] [PubMed] [Google Scholar]
- 55.Shen B, Fazio V W, Bennett A E, et al. Effect of withdrawal of non-steroidal anti-inflammatory drug use in patients with the ileal pouch. Dig Dis Sci. 2007;52:3321–3328. doi: 10.1007/s10620-006-9710-3. [DOI] [PubMed] [Google Scholar]
- 56.Shen B, Remzi F H, Nutter B, et al. Association between immune-associated disorders and adverse outcomes of ileal pouch-anal anastomosis. Am J Gastroenterol. 2009;104(3):655–664. doi: 10.1038/ajg.2008.76. [DOI] [PubMed] [Google Scholar]
- 57.Shen B, Plesec T P, Remer E, et al. Asymmetric endoscopic inflammation of the ileal pouch: a sign of ischemic pouchitis? Inflamm Bowel Dis. 2010;16(5):836–846. doi: 10.1002/ibd.21129. [DOI] [PubMed] [Google Scholar]
- 58.Nicholls R J. Review article: ulcerative colitis—surgical indications and treatment. Aliment Pharmacol Ther. 2002;16(Suppl 4):25–28. doi: 10.1046/j.1365-2036.16.s4.5.x. [DOI] [PubMed] [Google Scholar]
- 59.Madden M V, McIntyre A S, Nicholls R J. Double-blind crossover trial of metronidazole versus placebo in chronic unremitting pouchitis. Dig Dis Sci. 1994;39(6):1193–1196. doi: 10.1007/BF02093783. [DOI] [PubMed] [Google Scholar]
- 60.Shen B, Achkar J P, Lashner B A, et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis. 2001;7(4):301–305. doi: 10.1097/00054725-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Araki Y, Mitsuyama K, Nagae T, et al. Leukocytapheresis for the treatment of active pouchitis: a pilot study. J Gastroenterol. 2008;43(7):571–575. doi: 10.1007/s00535-008-2199-0. [DOI] [PubMed] [Google Scholar]
- 62.Gionchetti P, Rizzello F, Morselli C, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum. 2007;50(12):2075–2082, discussion 2082–2084. doi: 10.1007/s10350-007-9068-4. [DOI] [PubMed] [Google Scholar]
- 63.Shen B, Pardi D S, Bennett A E, et al. The efficacy and tolerability of AST-120 (spherical carbon adsorbent) in active pouchitis. Am J Gastroenterol. 2009;104(6):1468–1474. doi: 10.1038/ajg.2009.138. [DOI] [PubMed] [Google Scholar]
- 64.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119(2):305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 65.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004;53(1):108–114. doi: 10.1136/gut.53.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen B, Remzi F H, Lopez A R, Queener E. Rifaximin for maintenance therapy in antibiotic-dependent pouchitis. BMC Gastroenterol. 2008;8:26. doi: 10.1186/1471-230X-8-26. [electronic journal] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen B, Brzezinski A, Fazio V W, et al. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther. 2005;22(8):721–728. doi: 10.1111/j.1365-2036.2005.02642.x. [DOI] [PubMed] [Google Scholar]
- 68.Gionchetti P, Rizzello F, Venturi A, et al. Antibiotic combination therapy in patients with chronic, treatment-resistant pouchitis. Aliment Pharmacol Ther. 1999;13(6):713–718. doi: 10.1046/j.1365-2036.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- 69.Abdelrazeq A S, Kelly S M, Lund J N, Leveson S H. Rifaximin-ciprofloxacin combination therapy is effective in chronic active refractory pouchitis. Colorectal Dis. 2005;7(2):182–186. doi: 10.1111/j.1463-1318.2004.00746.x. [DOI] [PubMed] [Google Scholar]
- 70.Mimura T, Rizzello F, Helwig U, et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther. 2002;16(5):909–917. doi: 10.1046/j.1365-2036.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 71.Shen B, Fazio V W, Remzi F H, et al. Combined ciprofloxacin and tinidazole therapy in the treatment of chronic refractory pouchitis. Dis Colon Rectum. 2007;50(4):498–508. doi: 10.1007/s10350-006-0828-3. [DOI] [PubMed] [Google Scholar]
- 72.Viscido A, Kohn A, Papi C, Caprilli R. Management of refractory fistulizing pouchitis with infliximab. Eur Rev Med Pharmacol Sci. 2004;8(5):239–246. [PubMed] [Google Scholar]
- 73.Tremaine W J, Sandborn W J, Wolff B G, Carpenter H A, Zinsmeister A R, Metzger P P. Bismuth carbomer foam enemas for active chronic pouchitis: a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther. 1997;11(6):1041–1046. doi: 10.1046/j.1365-2036.1997.00253.x. [DOI] [PubMed] [Google Scholar]
- 74.Wischmeyer P, Pemberton J H, Phillips S F. Chronic pouchitis after ileal pouch-anal anastomosis: responses to butyrate and glutamine suppositories in a pilot study. Mayo Clin Proc. 1993;68(10):978–981. doi: 10.1016/s0025-6196(12)62270-8. [DOI] [PubMed] [Google Scholar]
- 75.Gionchetti P, Rizzello F, Poggioli G, et al. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment Pharmacol Ther. 2007;25(10):1231–1236. doi: 10.1111/j.1365-2036.2007.03306.x. [DOI] [PubMed] [Google Scholar]
- 76.Calabrese C, Gionchetti P, Rizzello F, et al. Short-term treatment with infliximab in chronic refractory pouchitis and ileitis. Aliment Pharmacol Ther. 2008;27(9):759–764. doi: 10.1111/j.1365-2036.2008.03656.x. [DOI] [PubMed] [Google Scholar]
- 77.Shen B, Lashner B A, Bennett A E, et al. Treatment of rectal cuff inflammation (cuffitis) in patients with ulcerative colitis following restorative proctocolectomy and ileal pouch-anal anastomosis. Am J Gastroenterol. 2004;99(8):1527–1531. doi: 10.1111/j.1572-0241.2004.30518.x. [DOI] [PubMed] [Google Scholar]
- 78.Shen B, Lian L, Remzi F H, et al. Natural history of cuffitis in ulcerative colitis patients with restorative proctocolectomy and ileal pouch-anal anastomosis. New Orleans, LA: Poster presented at: Digestive Diseases Week; May 1–5, 2010.
- 79.Panis Y, Poupard B, Nemeth J, Lavergne A, Hautefeuille P, Valleur P. Ileal pouch/anal anastomosis for Crohn's disease. Lancet. 1996;347(9005):854–857. doi: 10.1016/s0140-6736(96)91344-6. [DOI] [PubMed] [Google Scholar]
- 80.Melton G B, Fazio V W, Kiran R P, et al. Long-term outcomes with ileal pouch-anal anastomosis and Crohn's disease: pouch retention and implications of delayed diagnosis. Ann Surg. 2008;248(4):608–616. doi: 10.1097/SLA.0b013e318187ed64. [DOI] [PubMed] [Google Scholar]
- 81.Shen B. Crohn's disease of the ileal pouch: reality, diagnosis, and management. Inflamm Bowel Dis. 2009;15(2):284–294. doi: 10.1002/ibd.20661. [DOI] [PubMed] [Google Scholar]
- 82.Shen B, Patel S, Lian L. Natural history of Crohn's disease in patients who underwent intentional restorative proctocolectomy with ileal pouch-anal anastomosis. Aliment Pharmacol Ther. 2010;31(7):745–753. doi: 10.1111/j.1365-2036.2009.04227.x. [DOI] [PubMed] [Google Scholar]
- 83.Melmed G Y, Fleshner P R, Bardakcioglu O, et al. Family history and serology predict Crohn's disease after ileal pouch-anal anastomosis for ulcerative colitis. Dis Colon Rectum. 2008;51(1):100–108. doi: 10.1007/s10350-007-9158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen B, Remzi F H, Hammel J P, et al. Family history of Crohn's disease is associated with an increased risk for Crohn's disease of ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2009;15:163–170. doi: 10.1002/ibd.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nasseri Y, Melmed G, Wang H L, Targan S, Fleshner P. Rigorous histopathological assessment of the colectomy specimen in patients with inflammatory bowel disease unclassified does not predict outcome after ileal pouch-anal anastomosis. Am J Gastroenterol. 2010;105(1):155–161. doi: 10.1038/ajg.2009.510. [DOI] [PubMed] [Google Scholar]
- 86.Shen B, Fazio V W, Remzi F H, et al. Risk factors for clinical phenotypes of Crohn's disease of the ileal pouch. Am J Gastroenterol. 2006;101(12):2760–2768. doi: 10.1111/j.1572-0241.2006.00838.x. [DOI] [PubMed] [Google Scholar]
- 87.Colombel J-F, Ricart E, Loftus E V, Jr, et al. Management of Crohn's disease of the ileoanal pouch with infliximab. Am J Gastroenterol. 2003;98(10):2239–2244. doi: 10.1111/j.1572-0241.2003.07675.x. [DOI] [PubMed] [Google Scholar]
- 88.Shen B, Fazio V W, Remzi F H, et al. Endoscopic balloon dilation of ileal pouch strictures. Am J Gastroenterol. 2004;99(12):2340–2347. doi: 10.1111/j.1572-0241.2004.40604.x. [DOI] [PubMed] [Google Scholar]
- 89.Matzke G M, Kang A S, Dozois E J, Sandborn W J. Mid pouch strictureplasty for Crohn's disease after ileal pouch-anal anastomosis: an alternative to pouch excision. Dis Colon Rectum. 2004;47(5):782–786. doi: 10.1007/s10350-003-0105-7. [DOI] [PubMed] [Google Scholar]
- 90.Shen B, Remzi F H, Lavery I C, et al. Administration of adalimumab in the treatment of Crohn's disease of the ileal pouch. Aliment Pharmacol Ther. 2009;29(5):519–526. doi: 10.1111/j.1365-2036.2008.03920.x. [DOI] [PubMed] [Google Scholar]
- 91.Shen B, Achkar J-P, Lashner B A, et al. Irritable pouch syndrome: a new category of diagnosis for symptomatic patients with ileal pouch-anal anastomosis. Am J Gastroenterol. 2002;97(4):972–977. doi: 10.1111/j.1572-0241.2002.05617.x. [DOI] [PubMed] [Google Scholar]
- 92.Shen B, Sanmiguel C, Bennett A E, et al. Irritable pouch syndrome is characterized by visceral hypersensitivity. Inflamm Bowel Dis. 2010 Aug 3 doi: 10.1002/ibd.21412. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 93.Shen B, Liu W, Remzi F H, et al. Enterochromaffin cell hyperplasia in irritable pouch syndrome. Am J Gastroenterol. 2008;103(9):2293–2300. doi: 10.1111/j.1572-0241.2008.01990.x. [DOI] [PubMed] [Google Scholar]
- 94.Freeman H. Dysplasia-associated polypoid mucosal lesion in a pelvic pouch after restorative proctocolectomy for ulcerative colitis. Can J Gastroenterol. 2001;15(7):485–488. doi: 10.1155/2001/431437. [DOI] [PubMed] [Google Scholar]
- 95.Negi S S, Chaudhary A, Gondal R. Carcinoma of pelvic pouch following restorative proctocolectomy: report of a case and review of the literature. Dig Surg. 2003;20(1):63–65. doi: 10.1159/000068855. [DOI] [PubMed] [Google Scholar]
- 96.Laureti S, Ugolini F, D'Errico A, Rago S, Poggioli G. Adenocarcinoma below ileoanal anastomosis for ulcerative colitis: report of a case and review of the literature. Dis Colon Rectum. 2002;45(3):418–421. doi: 10.1007/s10350-004-6194-0. [DOI] [PubMed] [Google Scholar]
- 97.Hyman N. Rectal cancer as a complication of stapled IPAA. Inflamm Bowel Dis. 2002;8(1):43–45. doi: 10.1097/00054725-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 98.Das P, Johnson M W, Tekkis P P, Nicholls R J. Risk of dysplasia and adenocarcinoma following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2007;9(1):15–27. doi: 10.1111/j.1463-1318.2006.01148.x. [DOI] [PubMed] [Google Scholar]
- 99.Sarigol S, Wyllie R, Gramlich T, et al. Incidence of dysplasia in pelvic pouches in pediatric patients after ileal pouch-anal anastomosis for ulcerative colitis. J Pediatr Gastroenterol Nutr. 1999;28(4):429–434. doi: 10.1097/00005176-199904000-00015. [DOI] [PubMed] [Google Scholar]
- 100.Veress B, Reinholt F P, Lindquist K, Löfberg R, Liljeqvist L. Long-term histomorphological surveillance of the pelvic ileal pouch: dysplasia develops in a subgroup of patients. Gastroenterology. 1995;109(4):1090–1097. doi: 10.1016/0016-5085(95)90566-9. [DOI] [PubMed] [Google Scholar]
- 101.Heuschen U A, Hinz U, Allemeyer E H, et al. Backwash ileitis is strongly associated with colorectal carcinoma in ulcerative colitis. Gastroenterology. 2001;120(4):841–847. doi: 10.1053/gast.2001.22434. [DOI] [PubMed] [Google Scholar]
- 102.Gullberg K, Ståhlberg D, Liljeqvist L, et al. Neoplastic transformation of the pelvic pouch mucosa in patients with ulcerative colitis. Gastroenterology. 1997;112(5):1487–1492. doi: 10.1016/s0016-5085(97)70029-5. [DOI] [PubMed] [Google Scholar]
- 103.Kariv R, Remzi F H, Lian L, et al. Preoperative colorectal neoplasia increases risk for pouch neoplasia in patients with restorative proctocolectomy. Gastroenterology. 2010;139(3):806–812. 812, e1–e2. doi: 10.1053/j.gastro.2010.05.085. [DOI] [PubMed] [Google Scholar]