Abstract
Severe colitis is a well-defined condition that can develop in patients afflicted with ulcerative colitis, but typically responds to a variety of medical therapies. Operative intervention is warranted when massive hemorrhage, perforation, or peritonitis complicates the clinical scenario or medical therapy fails to control the disease. Of the operative options, total/subtotal colectomy and end ileostomy is the usual procedure of choice especially if the operation can be performed through a laparoscopic approach.
Keywords: Ulcerative colitis, toxic colitis, acute colitis, total colectomy, ileostomy
Severe colitis is a well-defined condition that can develop in patients afflicted with ulcerative colitis, but typically responds to a variety of medical therapies. Operative intervention is warranted when massive hemorrhage, perforation, or peritonitis complicates the clinical scenario or medical therapy fails to control the disease. Of the operative options, total/subtotal colectomy and end ileostomy is the usual procedure of choice especially if the operation can be performed through a laparoscopic approach.
DEFINITION
The Working Party of the 2005 Montreal World Congress of Gastroenterology proposed a classification scheme for ulcerative colitis comprised of an assessment of disease extent and severity for an individual relapse.1 The classification categorizes extent in three subgroups (i.e., proctitis, left-sided colitis, extensive colitis) and severity in four subgroups (i.e., clinical remission, mild, moderate, severe). Severe colitis is defined as six or more bloody stools per day plus one sign of systemic toxicity, which includes anemia (<10.5 g/dL), elevated erythrocyte sedimentation rate (ESR) (>30 mm/h), fever (>37.5°C), and tachycardia (90 beats per minute).2
The descriptors “acute severe” and “severe” have largely supplanted other terms such as fulminant or toxic colitis because the former are associated with defined diagnostic criteria, evidence-based practice standards, and reported outcomes. The American College of Gastroenterology,3 the Association of Coloproctology of Great Britain and Ireland,2 and the European Crohn's and Colitis Organization4 have all accepted a nearly identical definition that actually stems from the original description used by Truelove and Witts.5 However, the term fulminant is occasionally used to designate a critical form of severe colitis and is defined as more than 10 stools per day, daily continuous bleeding, blood transfusion requirement, elevated ESR (>30 mm/h), fever (>37.5°C), tachycardia (90 beats per minute), abdominal tenderness and distension, and colonic dilation on abdominal radiographs.6
Toxic megacolon complicating ulcerative colitis represents an extreme in the spectrum of severe colitis7 and complicates the presentation in ∼5% of patients admitted with acute severe colitis.8 It is formally defined as the total or segmental nonobstructive dilatation (>5.5 cm) of the colon associated with systemic toxicity.9,10
INCIDENCE AND PREVALENCE
The incidence of ulcerative colitis is 10.4 to 12 cases per 100,000 people and the prevalence rate is 35 to 100 cases per 100,000 people. The current rates have tended to stabilize in areas of high incidence such as America and northern Europe; areas with a lower incidence such as southern Europe, Asia, and developing countries are experiencing an increasing incidence and prevalence. Of patients presenting for the first time with ulcerative colitis, only a minority (5–8%) manifest symptoms and signs of acute severe colitis.
DIAGNOSIS
A combination of criteria derived from clinical, endoscopic, histologic, and radiologic studies are utilized to make a diagnosis of severe ulcerative colitis and to exclude other differential diagnoses (e.g., Clostridium difficile colitis, Crohn disease, cytomegalovirus infection, drug-induced colitis, ischemic colitis). The earlier described clinical features are used as well as testing that might include a complete blood count, C-reactive protein, electrolytes, liver enzymes and functional parameters, and stool samples.
The clinical variables are also useful in predicting outcome to therapy. In a prospective series where patients incompletely responsive to intravenous (IV) corticosteroids were treated with cyclosporin, an admitting ESR >75 mm/h and temperature >38°C were associated with a 4.6- and 8.8-fold increased risk for colectomy, respectively.11 A similar treatment regimen was used in a series that correlated clinical factors on day 3 with outcome; patients experiencing >8 bloody stools per day or 3 to 8 stools per day plus a CRP >45 mg/L were both associated with a colectomy rate of 85%.12 Three different centers13,14,15 have developed disease activity indices intended to predict outcome and the need for colectomy, but none of these has been universally accepted.
Flexible sigmoidoscopy by an experienced endoscopist using minimal insufflation in an unprepared bowel will often confirm the diagnosis of ulcerative colitis. Endoscopic criteria for severe colitis include extensive mucosal abrasions, deep ulcerations, ulceration edge mucosal detachment, and well-like ulceration.16 Colonoscopy is not usually necessary because the characteristic features can be seen during left-sided endoscopy in most patients, and more proximal inspection risks instigation of toxic megacolon or perforation. Endoscopic biopsy and microscopic identification of multiple intranuclear inclusion bodies on hematoxylin and eosin staining is the most reliable manner of identifying cytomegalovirus infection, especially if confirmed with immunohistochemistry.17
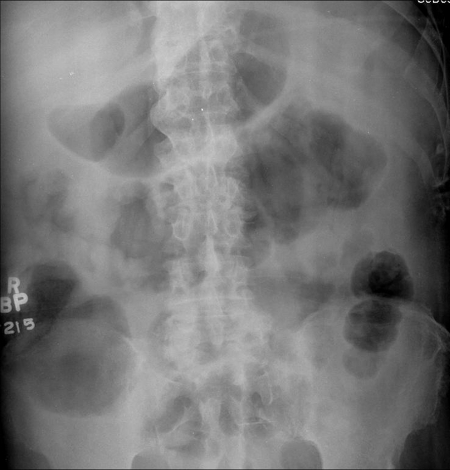
A plain abdominal radiograph should be obtained to estimate disease extent and exclude colonic dilatation (>5.5 cm) suggestive of megacolon (Fig. 1). The typical features of severe disease are mucosal irregularity, loss of the haustrations, and thickening of the colonic wall. Moreover, the presence of mucosal islands12 or small bowel distention18 predicts an increased risk of colectomy during the acute admission. In patients with megacolon, daily abdominal radiographs are warranted until the colonic diameter decreases to an acceptable level or an operation is planned. Repeat radiographs are also appropriate if there is any clinical deterioration during intensive treatment of severe ulcerative colitis.
Figure 1.
A plain abdominal radiograph should be obtained to estimate disease extent. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
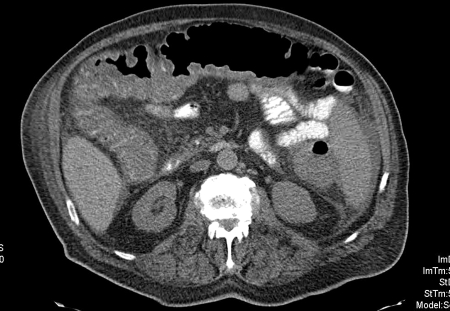
Computed tomography (CT) imaging may elucidate the diagnosis of colitis and give an indication of the underlying etiology in a patient presenting for the first time with acute severe colitis (Fig. 2). This tool can also determine the disease extent and detect otherwise occult complications. Many features such as colonic wall thickness, halo or target sign, and Accordion sign might differentiate inflammatory bowel disease from other causes. The distribution of the colitis can also help determine the correct diagnosis because ulcerative colitis typically affects the colon in a continuous fashion and involves the ileum only as backwash ileitis, which is seen as thickening on CT imaging.
Figure 2.
Computed tomography (CT) imaging showing acute severe colitis. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
MEDICAL THERAPY
Patients with severe colitis should be admitted to hospital and several measures undertaken in addition to the initial diagnostic evaluations. IV fluid and electrolyte replacement are prescribed to correct and prevent dehydration or electrolyte imbalance, blood transfusions are used to maintain a hemoglobin >9 g/dL, subcutaneous minidose heparin is ordered to reduce the risk of venous thromboembolism, enteral nutritional support is started if the patient is malnourished, and IV antibiotics are employed when a high likelihood of infection exists (e.g., signs of toxicity, worsening clinical course). Conversely, anticholinergic medications, antidiarrheal agents, nonsteroidal antiinflammatory drugs, and opioids are discontinued because any of these can precipitate toxic megacolon or worsen the patient's condition.
For reasons previously discussed, the number of bowel movements, including the presence or absence of blood, temperature, and heart rate should be frequently recorded, and routine blood work regularly monitored. Daily physical examinations are appropriate to assess abdominal tenderness and detect signs of peritoneal irritation. More frequent examinations are probably warranted in patients with toxic megacolon because this represents a more critical condition.
Corticosteroids
IV corticosteroids are the mainstay of conventional medical therapy, and their usage should not be delayed while awaiting microbiologic tests. In a systematic review of trials of corticosteroid therapy for acute severe colitis involving 1201 patients from 1974 to 2006, the overall colectomy rate was 34% (95% CI 31–36%); this rate has remained relatively stable for the past 3 decades.19
The prescribed amount of corticosteroids is comparable to 400 mg of daily hydrocortisone and is usually ordered for 5 days. Higher doses are not advantageous and lower doses are less effective,19 bolus administration is equivalent to continuous infusion in terms of efficacy and safety,20 and extending therapy beyond 7 to 10 days adds no benefit.19
Gustavsson and associates21 reported their experience with 61 patients treated with corticosteroids alone for a severe attack of ulcerative colitis. Within 3 months of starting therapy, 28 patients (46%) required colectomy and the 10-year colectomy rate was 64% with no further colectomies occurring after those first 10 years. In the follow-up period extending beyond the first 3 months, the colectomy incidence was not statistically different from that seen in patients with mild or moderate disease requiring IV corticosteroid treatment.
Cyclosporin
Patients not responding to conventional corticosteroid therapy might be candidates for rescue therapy with cyclosporin, which mainly acts by inhibiting T-lymphocyte function that is essential for the propagation of inflammation. Unlike most immunosuppressive agents, cyclosporin does not suppress bone marrow or the activity of other hematopoietic cells, and has a rapid onset of action.
Lichtiger and colleagues22 published the initial placebo-controlled trial supporting the use of cyclosporin in acute severe steroid-refractory ulcerative colitis. They reported that 9 of 11 patients (82%) with severe colitis failing steroid therapy improved with cyclosporin, while none of the 9 patients receiving placebo improved. Furthermore, all 5 patients in the placebo group who later received cyclosporin therapy had a response. In a separate European controlled trial,23 monotherapy with IV cyclosporin was compared with corticosteroids in the treatment of patients with severe ulcerative colitis. After 8 days of therapy, 9 of 14 patients (64%) receiving cyclosporin had a response versus 8 of 15 patients (53%) treated with methylprednisolone. At 12 months, 7 of 9 patients (78%) initially controlled with cyclosporin maintained their remission compared with 3 of 8 (37%) initially treated with methylprednisolone.
These two trials and some of the subsequent studies used 4 mg/kg/day of cyclosporin and that dose was associated with substantial side effects and toxicity. However, 2 mg/kg/day of cyclosporin appears to be equally effective,24 despite an improved safety profile even when used in combination with corticosteroids.25 Random blood sampling should be used to adjust the drug dosage to maintain serum levels of 150 to 250 ng/mL.
In responders, the long-term outcome of IV cyclosporincyclosporin therapy is improved by the transition to several months of oral cyclosporin combined with the introduction of long-term azathioprine or 6-mercaptopurine at the time of discharge from the hospital. In a retrospective cohort study, 40% and 65% of the initial cyclosporin responders had undergone colectomy at 1 and 7 years, respectively. These figures improved to 20% and 40%, respectively, in the subset of patients treated with azathioprine.26 Another study reported that 65% of patients with severe colitis initially responding to cyclosporin had relapsed after 1 year, 90% had relapsed after 3 years, and 58% had undergone colectomy after 7 years of follow-up. At 1 year, nearly 50% of the azathioprine patients remained relapse-free compared with 30% of nonazathioprine patients, but no significant difference was found when comparing time to surgery between the two cohorts.27 Unfortunately, the long-term success rate is substantially lower in patients who have been previously treated with azathioprine or 6-mercaptopurine before initiating cyclosporin therapy.28
Overall, cyclosporin should be avoided in frail or elderly patients with significant co-morbidity as well as patients in whom colectomy is likely to be necessary in the short- to medium-term. Cyclosporin can be considered for patients with acute severe ulcerative colitis who have failed 7 days of conventional corticosteroids or those with fulminant disease that has not responded to 3 days of corticosteroid therapy. Regardless, cyclosporin should not be continued for more than 7 days unless there is a definite response. An initial response rate of nearly 70% is likely, and responders should be discharged on 3 months of oral cyclosporin as a bridge to long-term azathioprine or 6-mercaptopurine therapy. Patients should also be counseled that the likelihood of colectomy is ∼50% over the ensuing 2 to 3 years.25
Infliximab
Patients not responding to corticosteroid therapy might also be candidates for rescue therapy with infliximab that works by binding to tumor necrosis factor α (TNF-α), which is key player in the autoimmune reaction. It appears that infliximab works by blocking the action of TNF-α by preventing it from binding to its receptor in the cell, but it also causes programmed cell death of TNF-α-expressing activated T lymphocytes that mediate inflammation. The data related to the use of infliximab in patients presenting with severe colitis is relatively limited, but one double-blind series included 45 patients receiving IV corticosteroids. Incompletely responsive patients with either moderately severe or severe colitis at days 6- to 8 or fulminant disease at day 3 were randomized to either a single dose of infliximab or placebo.29 At day 90, 29% of the infliximab-treated patients had undergone colectomy compared with 67% of placebo-treated patients. In patients with fulminant disease, 47% of infliximab-treated patients underwent colectomy, compared with 69% of placebo-treated patients.
In a smaller randomized controlled trial of patients failing IV corticosteroids, 4 of 8 patients treated with infliximab clinically responded 2 weeks after a single dose of infliximab, whereas none of three placebo-treated patients responded.30 Comparable short-term results have been achieved in open-label series,31,32 but ∼50% of infliximab-treated patients require colectomy at 5 years.3
In general, infliximab (5 mg/kg) seems to be effective as rescue therapy for severe steroid-refractory colitis in up to 70% of instances, and the clinical response usually occurs within 3 to 7 days of treatment. Infliximab also appears to induce a long-term remission comparable to that seen with cyclosporin. The number of infusions required is not clear, but 2 or 3 infusions seem to be more effective than a single administration for preventing early colectomy.33
Cyclosporin versus Infliximab
No comparative trials have been published, but one series reported on 19 patients with severe colitis who failed one therapy and were then treated with the alternative medication within 4 weeks.34 Three of 9 patients (33%) receiving cyclosporin after failing infliximab and 4 of 10 patients (40%) treated with infliximab after failing cyclosporin achieved remission, which lasted 28.5 and 10.4 months, respectively. One patient who received cyclosporin salvage developed pancreatitis and bacteremia while another suffered herpetic esophagitis; one patient developed sepsis and died after receiving infliximab salvage.
Infliximab may have a better short-term safety profile because it does not provoke seizures or hypertension; however, the long-term profile of both drugs is poorly characterized. Conversely, the advantage of cyclosporin is that it possesses a shorter half-life compared with infliximab. Therefore, if cyclosporin is ineffective, it is quickly metabolized and its effects are ameliorated within a few hours, while infliximab will circulate and remain active for weeks. This difference may become important if the patient does not respond to salvage therapy and requires operative treatment.
OPERATIVE THERAPY
The indications for operative intervention in patients with severe ulcerative colitis include massive hemorrhage, perforation, peritonitis, and unresponsiveness to medical therapy. Although the first three of these are absolute indications, the last is the most common indication and the most difficult to objectively define. Typically, patients are started on IV plus topical corticosteroids and closely observed as previously detailed. If no tangible improvement is witnessed after 3 days of therapy or complete response is not obtained following 7 days of treatment, rescue therapy with cyclosporin or infliximab is initiated or surgery is recommended in those patients unlikely to respond to rescue drugs. If rescue therapy is employed and response is not obtained after 5 or 8 days of treatment with cyclosporin or infliximab, respectively, operative management is typically warranted.
A great deal of uncertainty surrounds those patients who improve, but only partially respond to medical therapy. Continued medical treatment risks creating a progressively unwell, malnourished, and immunosuppressed patient with the associated morbidity and mortality of protracted medical treatment and delayed surgery. Alternatively, urgent surgery is associated with its own risk for morbidity and mortality. In a review of the literature from the current era, the morbidity and mortality rates associated with surgery for severe colitis from all causes are 40.1% and 1.8%, respectively.35 The most frequent surgical complications were wound infection/dehiscence (18.4%), intraabdominal abscess (9.2%), small bowel obstruction (6.2%), ileostomy-related problems (5.5%), and hemorrhage (4.6%), and the most common medical complications were septicemia (18.0%), pneumonia (11.2%), deep venous thrombosis (7.2%), pulmonary embolism (7.0%), and urinary tract infection (4.3%).
In deciding whether to proceed to surgery, the state of the colon and quality of medical therapy prior to the acute attack should be considered. If the patient has chronically active ulcerative colitis despite optimal medical therapy with immunomodulators, colectomy is likely required. On the other hand, if the patient has a history of mild colitis with long periods of steroid-free remission or immunomodulators have not been used, colectomy might be readily avoided. However, this group of patients who only partially respond to medical therapy have an overall 60% likelihood of requiring colectomy during the next year and an 80% risk within 5 years.
Blow-Hole Colostomy and Loop Ileostomy
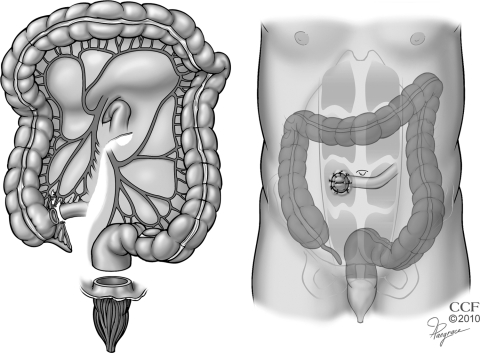
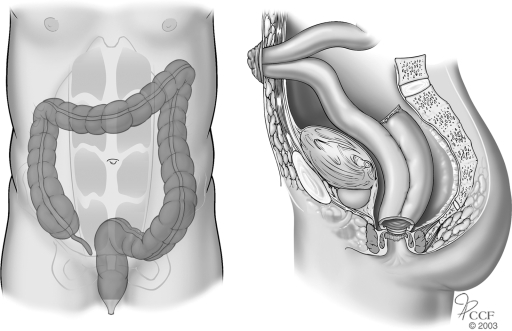
Before the 1950s, ileostomy was the most commonly performed operation for severe colitis. However, this procedure was associated with unsatisfactory results because the dilated colon could perforate despite proximal diversion of the fecal stream. In 1951, Crile and Thomas36 recommended total abdominal colectomy and ileostomy for the treatment of toxic megacolon, and this approach reduced the mortality rate to 14.3% from 63% associated with ileostomy alone. However, selected patients with megacolon are at risk for iatrogenic perforation with fecal spillage caused by manipulation of a friable and edematous colon during colectomy. Accordingly, Turnbull37 advocated colonic decompression and proximal diversion using a skin-level colostomy and loop ileostomy with definitive surgery planned 6 months later. The blow-hole procedure is now rarely performed except in a few high risk situations such as women who are pregnant38 or patients with colonic microperforations, high-lying splenic flexure and dense adhesions, or prohibitive comorbidity.39 The operation is contraindicated in cases of abscess, hemorrhage, or free perforation (Fig. 3).
Figure 3.
Blow-hole colostomy and loop ileostomy. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
The procedure is commenced by entering the abdomen through a limited infraumbilical incision. The presence of a feculent odor or purulent fluid denotes free perforation of the colon, in which case an abdominal colectomy is performed. If no signs of perforation are identified, a loop of terminal ileum is located and delivered through a stoma aperture created at a previously marked site. The incision is closed and attention is directed to the epigastrium where another midline incision is made above the dilated transverse colon. Thickened omentum overlying the transverse colon is commonly encountered and must be dissected away to reveal the serosa of the colon. No attempt is made to deliver the colon to the skin level because the colonic wall is typically friable and can easily perforate. Instead, quarantining sutures are placed between the posterior fascia of the abdominal wall and the seromuscular layer of the colon. A 14-gauge needle is passed through the tenia coli to decompress the colon and allow the bowel to rise into the wound. The colon is then longitudinally opened and matured to the surrounding skin. The loop ileostomy is also matured in a routine everting manner at this time.
Subtotal/Total Colectomy and End Ileostomy
Since the time subtotal/total colectomy and ileostomy were first advocated,36 the operation has evolved into the procedure of choice for most patients requiring surgery for severe ulcerative colitis. The procedure can be performed using a conventional open approach or a minimally invasive laparoscopic approach. Either method avoids the morbidity associated with the pelvic dissection required for proctectomy and allows for future surgical options. In the interim, the patient is able to return to a normal state of health and level of activity while typically discontinuing all medications associated with disease management. Subsequent operations should be delayed several (≥7) months to minimize the associated risk for intraoperative complications and postoperative fistulas,40 but it is undetermined whether an initial laparoscopic colectomy reduces the increased risk or shortens the interval required between procedures.
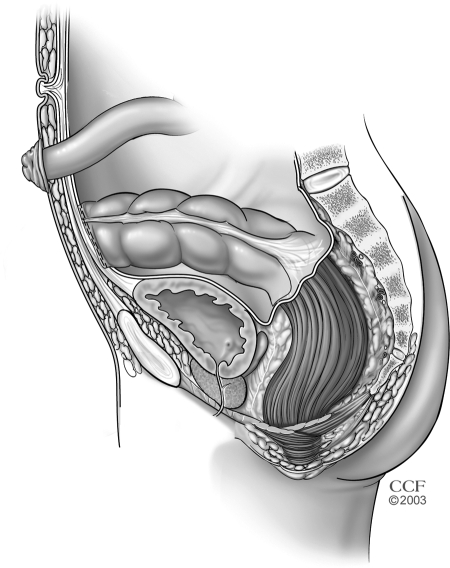
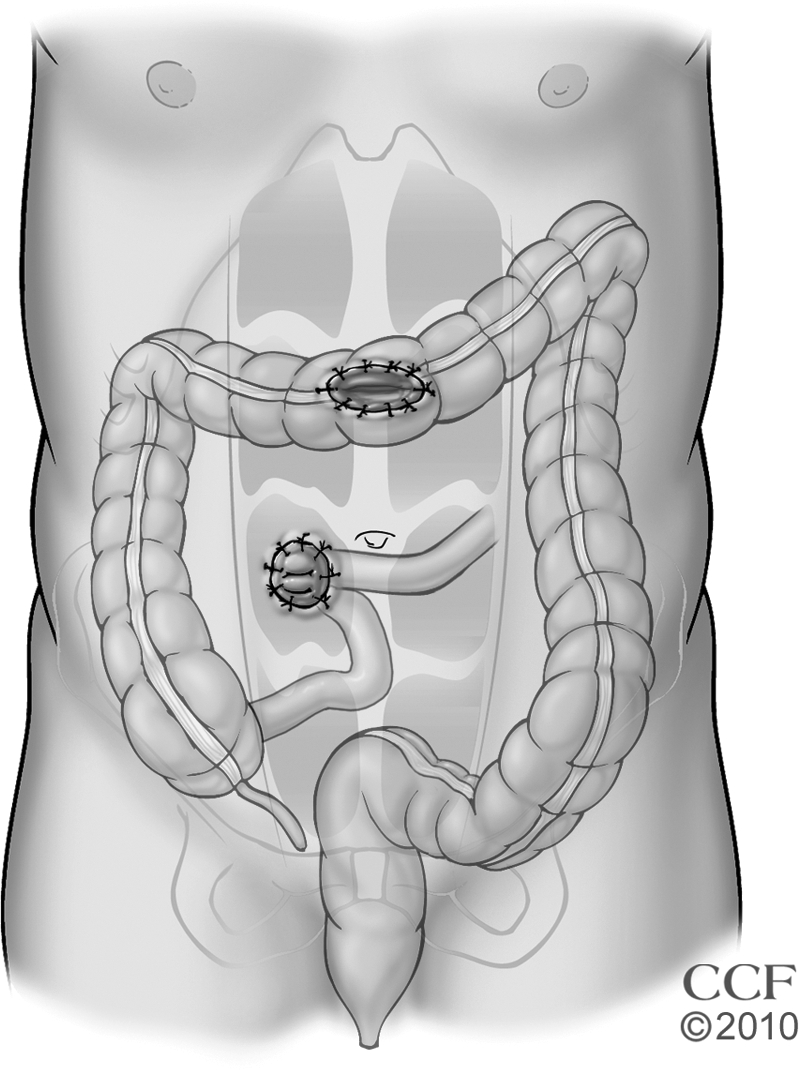
Open subtotal/total colectomy and ileostomy is performed through a midline incision of sufficient length to allow safe mobilization of the entire colon that may be quite friable and easily torn (Fig. 4). The named mesenteric vessels are usually left undisturbed and the small bowel is divided immediately proximal to the ileocecal valve to minimize the risk of injuring retroperitoneal structures and maximize the success rate of future restorative procedures. Preservation of the omentum has been associated with a comparable risk for postoperative small bowel obstruction, but with a lesser rate of postoperative intraabdominal sepsis.41 The omentum is retained by dividing this fatty apron closer to the colon wall than the gastroepiploic vessels; the omentum adherent to the colon as well as the avascular plane between the omentum and colon should be left intact because occult microperforations or abscesses could be otherwise unroofed. Although the colon and distal small bowel are typically mobilized prior to dividing the mesentery, terminal ileum, and distal bowel, it is occasionally better to reverse the order when the colon is particularly fragile and mobilization risks perforation and spillage of feces, especially when mobilizing the splenic flexure. In this reverse manner, contamination is minimized because the injured bowel can be more quickly delivered from the operative field. Following colectomy, the ileostomy is delivered through an aperture in the right rectus muscle and ultimately matured in an everting manner that creates a stoma that is <2.5 cm in diameter and protrudes >2 cm. The small bowel mesentery can be secured to the anterior abdominal wall to avoid internal hernias, but this maneuver is not absolutely required.
Figure 4.
Subtotal/total colectomy and end ileostomy. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
The distal bowel can be managed in one of several manners depending upon the clinical scenario (Fig. 5). The bowel can be divided and closed below the confluence of the tenia coli in the rectum, but some argue this can make future pelvic surgery more difficult and risks pelvic sepsis.42,43,44 The distal sigmoid can be divided, closed, and delivered to the inferior portion of the wound to reside above the fascia level (Fig. 5). If the closure dehisces in this location, a mucous fistula occurs rather than a pelvic abscess.42 With either approach, breakdown of the closure can be lessened by using a rectal tube during the immediate postoperative period.45 In some instances, the colon is too friable to allow closure in which case a primary or delayed mucous fistula is best employed. The bowel is delivered to the inferior aspect of the wound that should be sufficiently remote from the ileostomy site to allow separate pouching without overlap of the adhesive flanges. The delayed mucosa fistula is created by exteriorizing the distal colon >5 cm above the skin level and wrapping the bowel with gauze. This exterior limb is amputated flush with the skin one week later when the bowel is sufficiently adhered to the abdominal wall; the mucous fistula is then created through primary or secondary maturation.
Figure 5.
The distal sigmoid can be divided, closed, and delivered to the inferior portion of the wound to reside above the fascia level. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Laparoscopic resection is an ideal approach for patients with severe colitis because they are often relatively young or concerned about body image. Varied techniques can be employed including the total laparoscopic, laparoscopic-assisted, and hand-assisted laparoscopic approaches. All three techniques are similar in that laparoscopic mobilization of the bowel is required, but vary in how vascular ligation is managed. The total laparoscopic technique features laparoscopic mobilization of the bowel and a small incision is used to deliver the specimen. The laparoscopic-assisted and hand-assisted laparoscopic techniques may use a small infraumbilical midline incision to facilitate control of the mesentery and deliver the resected bowel; the hand-assisted technique differs from the laparoscopic-assisted approach in that the former procedure requires the surgeon to insert a hand into the peritoneal cavity to facilitate much of the operation.
A total laparoscopic approach to severe colitis is feasible and safe especially because no anastomosis is required. In matched cohorts, patients undergoing a total laparoscopic subtotal colectomy compared with an open procedure experienced a shorter length of stay with comparable rates of medical complications and minor/major surgical complications, although the incidence of major surgical complications tended to favor the laparoscopic group.46 Patients managed by laparoscopic-assisted versus open total/subtotal colectomy with end ileostomy for severe ulcerative colitis experience significantly less in-patient narcotic usage, faster return of bowel function, and shorter length of stay with no differences in the perioperative parameters associated with subsequent restorative proctectomy and ileostomy closure.47 Emergent hand-assisted laparoscopic total/subtotal colectomy with creation of end ileostomy in patients with severe ulcerative colitis is also feasible and safe.48 Although the median operative time was significantly longer in the hand-assisted laparoscopic group compared with a matched open group (242 minutes vs 191 minutes), the laparoscopy group experienced significantly reduction in early postoperative complications, risk for reoperation, duration of ileus, and median length of hospital stay. After laparoscopic colectomy, nearly 85% of patients undergoing restoration of intestinal continuity with creation of either ileorectal or ileal pouch-anal anastomosis can be managed through reoperative laparoscopy or elective incision at the site of previous stoma.49,50
In an analysis of pooled data from the literature published in 1975 through 2007,35 the 30-day operative mortality rates after colectomy for colitis of all causes was 9.0%. The most frequent surgical complications were small bowel obstruction/ileus (20.0%), wound infection/dehiscence (18.6%), intraabdominal abscess (17.8%), rectal stump dehiscence (6.7%), and ileostomy-related problems (6.3%), and the most common medical complication was septicemia (9.1%). In the subgroup undergoing a laparoscopic approach, the conversion rate was 3.4% and the main complications were stoma-related problems (10.3%), small bowel obstruction (8.9%), and surgical site infection (6.3%).
Total Proctocolectomy and End Ileostomy
Before the advent of pouch surgery, debate existed related to whether colectomy and ileostomy or proctocolectomy and ileostomy should be performed for the operative management of severe ulcerative colitis.51 Proctocolectomy and end ileostomy has been largely abandoned because removal of the rectum introduces significant difficulties with later attempts at construction of a pouch-anal anastomosis; the anterior pelvic organs tend to collapse onto the posterior pelvis and limit access to the pelvic floor and levator hiatus. However, the operation is warranted in patients with rectal perforation or rectal hemorrhage complicating their severe colitis, but the rectum should be excised only to a point below the problem area to minimize difficulties encountered with future reconstructive surgery. The procedure might also be considered in patients with severe colitis who are not candidates for a restorative operation such as those with significant comorbidity or poor anal sphincter function.
The previously mentioned pooled data analysis35 reported the 30-day operative mortality rates after proctocolectomy for colitis of all causes was 8.3%. The most frequent surgical complications were wound infection/dehiscence (23.7%), hemorrhage (14.3%), pelvic nerve injury (13.3%), intraabdominal abscess (11.3%), and ileostomy-related problems (9.1%), and the most common medical complication was septicemia (28.6%).
The colectomy portion of the procedure is performed similar to the manner previously described. The proctectomy is conducted under direct visualization, and started by sharply mobilizing the fascia propria of the mesorectum from the presacral fascia down to the level of the rectosacral fascia, while avoiding the ureters and sympathetic nerves. The lateral peritoneal reflections and anterior reflection (dorsal to Denonvilliers' fascia) are incised and circumferential mobilization of the rectum and its mesorectum is carried to the pelvic floor while coning in on the distal rectum to minimize risk to the autonomic nerves. The bowel is then divided within the anal canal using a stapling device or an endoanal proctectomy is performed. In the latter instance, a circumferential incision is made at the intersphincteric groove and continued cephalad to meet the prior transabdominal dissection. The pelvic floor, external sphincter, and perianal skin are then closed in a layered manner, taking care to eliminate all dead space that can cause an unhealed perineal wound (Fig. 6).
Figure 6.
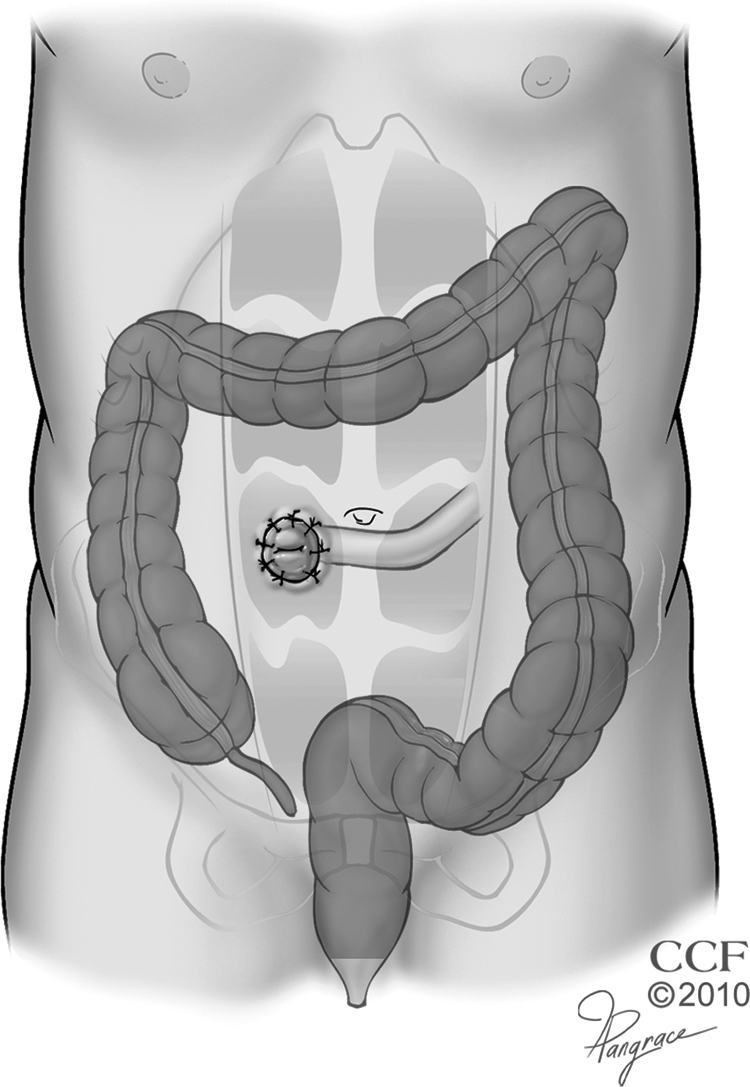
Total proctocolectomy and end ileostomy. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Total Proctocolectomy with Ileal Pouch-Anal Anastomosis and Loop Ileostomy
Although restorative proctocolectomy has become the procedure of choice for patients with ulcerative colitis requiring elective surgery, it likely has little role as the initial procedure in patients with severe colitis. Furthermore, no randomized trials have been published comparing colectomy and ileostomy versus restorative proctocolectomy, and the only existing reports come from an era prior to the introduction of immunosuppressant therapy.
Harms and colleagues52 reported their results with restorative proctocolectomy in 20 patients afflicted by severe ulcerative colitis without signs of sepsis or medical comorbidity. Minor or major complications occurred in more than one-half of patients, and these complications included adrenal insufficiency (15%), small bowel obstruction (15%), pancreatitis (10%), anastomotic leak (5%), and gastrointestinal bleed (5%); no patients experienced pelvic sepsis and no mortalities occurred.
Heyvaert et al53 compared patients in a consecutive series of patients undergoing emergency versus elective proctocolectomy with ileal pouch-anal anastomosis (Fig. 7). The overall complication rate (66% vs 27%) and anastomotic leak rate (41% vs 11%) were higher in the group undergoing an emergent operation leading the authors to conclude that restorative proctocolectomy is contraindicated in emergency circumstances, especially in patients with signs of sepsis on high-dose corticosteroids.
Figure 7.
Total proctocolectomy with ileal pouch-anal anastomosis and loop ileostomy. Reprinted with permission, Cleveland Clinic Center for Medical Art & Photography © 1996–2010. All rights reserved.
Ziv and colleagues54 reported their experience related to 12 patients presenting with severe disease without associated hypotension, megacolon, or tachycardia. These patients were treated by restorative proctocolectomy after failing intensive corticosteroid therapy. This selected group had an outcome comparable to those undergoing elective surgery, and no early septic complications occurred in this small cohort of patients.
CONCLUSIONS
Severe ulcerative colitis is a disease best managed through the combined efforts of physicians and surgeons working with the patient. Medical therapy is the first-line treatment in most cases, but objective measures of improvement and timelines should be agreed upon prior to initiating treatment. If the patient should manifest signs of hemorrhage, perforation, or peritonitis, surgery is immediately indicated. Otherwise, only patients with a worsening course or failure to respond to treatment as previously planned would require operative intervention. Subtotal/total colectomy and ileostomy is the procedure of choice in most instances and a laparoscopic approach is potentially favored when possible and practical.
References
- 1.Silverberg M S, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: Report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5–36. doi: 10.1155/2005/269076. [DOI] [PubMed] [Google Scholar]
- 2.Brown S R, Haboubi N, Hampton J, George B, Travis S PL, ACPGBI The management of acute severe colitis: ACPGBI position statement. Colorectal Dis. 2008;10(Suppl 3):8–29. doi: 10.1111/j.1463-1318.2008.01682.x. [DOI] [PubMed] [Google Scholar]
- 3.Kornbluth A, Sachar D B, Practice Parameters Committee of the American College of Gastroenterology Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501–523, quiz 524. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 4.Travis S PL, Stange E F, Lémann M, et al. Gassull M for the European Crohn's and Colitis Organisation (ECCO). European evidence-based consensus on the management of ulcerative colitis: current management. J Crohn's Colitis. 2008;2:24–62. doi: 10.1016/j.crohns.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Truelove S C, Witts L J. Cortisone in ulcerative colitis; final report on a therapeutic trial. BMJ. 1955;2(4947):1041–1048. doi: 10.1136/bmj.2.4947.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanauer S B. Inflammatory bowel disease. N Engl J Med. 1996;334(13):841–848. doi: 10.1056/NEJM199603283341307. [DOI] [PubMed] [Google Scholar]
- 7.Marshak R H, Lester L J. Megacolon a complication of ulcerative colitis. Gastroenterology. 1950;16(4):768–772. [PubMed] [Google Scholar]
- 8.Gan S I, Beck P L. A new look at toxic megacolon: an update and review of incidence, etiology, pathogenesis, and management. Am J Gastroenterol. 2003;98(11):2363–2371. doi: 10.1111/j.1572-0241.2003.07696.x. [DOI] [PubMed] [Google Scholar]
- 9.Sheth S G, LaMont J T. Toxic megacolon. Lancet. 1998;351(9101):509–513. doi: 10.1016/S0140-6736(97)10475-5. [DOI] [PubMed] [Google Scholar]
- 10.Jones J H, Chapman M. Definition of megacolon in colitis. Gut. 1969;10(7):562–564. doi: 10.1136/gut.10.7.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benazzato L, D'Incà R, Grigoletto F, et al. Prognosis of severe attacks in ulcerative colitis: effect of intensive medical treatment. Dig Liver Dis. 2004;36(7):461–466. doi: 10.1016/j.dld.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Travis S P, Farrant J M, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996;38(6):905–910. doi: 10.1136/gut.38.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo M, Okada M, Yao T, Ueki M, Arima S, Okumura M. An index of disease activity in patients with ulcerative colitis. Am J Gastroenterol. 1992;87(8):971–976. [PubMed] [Google Scholar]
- 14.Lindgren S C, Flood L M, Kilander A F, Löfberg R, Persson T B, Sjödahl R I. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol. 1998;10(10):831–835. doi: 10.1097/00042737-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Ho G T, Mowat C, Goddard C J, et al. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19(10):1079–1087. doi: 10.1111/j.1365-2036.2004.01945.x. [DOI] [PubMed] [Google Scholar]
- 16.Carbonnel F, Gargouri D, Lémann M, et al. Predictive factors of outcome of intensive intravenous treatment for attacks of ulcerative colitis. Aliment Pharmacol Ther. 2000;14(3):273–279. doi: 10.1046/j.1365-2036.2000.00705.x. [DOI] [PubMed] [Google Scholar]
- 17.Criscuoli V, Casà A, Orlando A, et al. Severe acute colitis associated with CMV: a prevalence study. Dig Liver Dis. 2004;36(12):818–820. doi: 10.1016/j.dld.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Chew C N, Nolan D J, Jewell D P. Small bowel gas in severe ulcerative colitis. Gut. 1991;32(12):1535–1537. doi: 10.1136/gut.32.12.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Turner D, Walsh C M, Steinhart A H, Griffiths A M. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007;5(1):103–110. doi: 10.1016/j.cgh.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 20.Bossa F, Fiorella S, Caruso N, et al. Continuous infusion versus bolus administration of steroids in severe attacks of ulcerative colitis: a randomized, double-blind trial. Am J Gastroenterol. 2007;102(3):601–608. doi: 10.1111/j.1572-0241.2006.01007.x. [DOI] [PubMed] [Google Scholar]
- 21.Gustavsson A, Halfvarson J, Magnuson A, Sandberg-Gertzén H, Tysk C, Järnerot G. Long-term colectomy rate after intensive intravenous corticosteroid therapy for ulcerative colitis prior to the immunosuppressive treatment era. Am J Gastroenterol. 2007;102(11):2513–2519. doi: 10.1111/j.1572-0241.2007.01435.x. [DOI] [PubMed] [Google Scholar]
- 22.Lichtiger S, Present D H, Kornbluth A, et al. Cyclosporine in severe ulcerative colitis refractory to steroid therapy. N Engl J Med. 1994;330(26):1841–1845. doi: 10.1056/NEJM199406303302601. [DOI] [PubMed] [Google Scholar]
- 23.D'Haens G, Lemmens L, Geboes K, et al. Intravenous cyclosporine versus intravenous corticosteroids as single therapy for severe attacks of ulcerative colitis. Gastroenterology. 2001;120(6):1323–1329. doi: 10.1053/gast.2001.23983. [DOI] [PubMed] [Google Scholar]
- 24.Assche G Van, D'Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg versus 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology. 2003;125(4):1025–1031. doi: 10.1016/s0016-5085(03)01214-9. [DOI] [PubMed] [Google Scholar]
- 25.Durai D, Hawthorne A B. Review article: how and when to use ciclosporin in ulcerative colitis. Aliment Pharmacol Ther. 2005;22(10):907–916. doi: 10.1111/j.1365-2036.2005.02680.x. [DOI] [PubMed] [Google Scholar]
- 26.Actis G C, Fadda M, David E, Sapino A. Colectomy rate in steroid-refractory colitis initially responsive to cyclosporin: a long-term retrospective cohort study. BMC Gastroenterol. 2007;7:13. doi: 10.1186/1471-230X-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell S, Travis S, Jewell D. Ciclosporin use in acute ulcerative colitis: a long-term experience. Eur J Gastroenterol Hepatol. 2005;17(1):79–84. doi: 10.1097/00042737-200501000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Rowe F A, Walker J H, Karp L C, Vasiliauskas E A, Plevy S E, Targan S R. Factors predictive of response to cyclosporin treatment for severe, steroid-resistant ulcerative colitis. Am J Gastroenterol. 2000;95(8):2000–2008. doi: 10.1111/j.1572-0241.2000.02186.x. [DOI] [PubMed] [Google Scholar]
- 29.Järnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128(7):1805–1811. doi: 10.1053/j.gastro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Sands B E, Tremaine W J, Sandborn W J, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7(2):83–88. doi: 10.1097/00054725-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 31.Bressler B, Law J K, Al Nahdi Sheraisher N, et al. The use of infliximab for treatment of hospitalized patients with acute severe ulcerative colitis. Can J Gastroenterol. 2008;22(11):937–940. doi: 10.1155/2008/749547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lees C W, Heys D, Ho G T, et al. Scottish Society of Gastroenterology Infliximab Group A retrospective analysis of the efficacy and safety of infliximab as rescue therapy in acute severe ulcerative colitis. Aliment Pharmacol Ther. 2007;26(3):411–419. doi: 10.1111/j.1365-2036.2007.03383.x. [DOI] [PubMed] [Google Scholar]
- 33.Kohn A, Daperno M, Armuzzi A, et al. Infliximab in severe ulcerative colitis: short-term results of different infusion regimens and long-term follow-up. Aliment Pharmacol Ther. 2007;26(5):747–756. doi: 10.1111/j.1365-2036.2007.03415.x. [DOI] [PubMed] [Google Scholar]
- 34.Maser E A, Deconda D, Lichtiger S, Ullman T, Present D H, Kornbluth A. Cyclosporine and infliximab as rescue therapy for each other in patients with steroid-refractory ulcerative colitis. Clin Gastroenterol Hepatol. 2008;6(10):1112–1116. doi: 10.1016/j.cgh.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 35.Teeuwen P H, Stommel M W, Bremers A J, der Wilt G J van, de Jong D J, Bleichrodt R P. Colectomy in patients with acute colitis: a systematic review. J Gastrointest Surg. 2009;13(4):676–686. doi: 10.1007/s11605-008-0792-4. [DOI] [PubMed] [Google Scholar]
- 36.Crile G C, Jr, Thomas C Y., Jr The treatment of acute toxic ulcerative colitis by ileostomy and simultaneous colectomy. Gastroenterology. 1951;19(1):58–68. [PubMed] [Google Scholar]
- 37.Turnbull R B, Jr, Hawk W A, Weakley F L. Surgical treatment of toxic megacolon. Ileostomy and colostomy to prepare patients for colectomy. Am J Surg. 1971;122(3):325–331. doi: 10.1016/0002-9610(71)90252-2. [DOI] [PubMed] [Google Scholar]
- 38.Ooi B S, Remzi F H, Fazio V W. Turnbull-Blowhole colostomy for toxic ulcerative colitis in pregnancy: report of two cases. Dis Colon Rectum. 2003;46(1):111–115. doi: 10.1007/s10350-004-6504-6. [DOI] [PubMed] [Google Scholar]
- 39.Remzi F H, Oncel M, Hull T L, Strong S A, Lavery I C, Fazio V W. Current indications for blow-hole colostomy:ileostomy procedure. A single center experience. Int J Colorectal Dis. 2003;18(4):361–364. doi: 10.1007/s00384-002-0453-0. [DOI] [PubMed] [Google Scholar]
- 40.Dinnewitzer A J, Wexner S D, Baig M K, et al. Timing of restorative proctectomy following subtotal colectomy in patients with inflammatory bowel disease. Colorectal Dis. 2006;8(4):278–282. doi: 10.1111/j.1463-1318.2005.00933.x. [DOI] [PubMed] [Google Scholar]
- 41.Ambroze W L, Jr, Wolff B G, Kelly K A, Beart R W, Jr, Dozois R R, Ilstrup D M. Let sleeping dogs lie: role of the omentum in the ileal pouch-anal anastomosis procedure. Dis Colon Rectum. 1991;34(7):563–565. doi: 10.1007/BF02049895. [DOI] [PubMed] [Google Scholar]
- 42.Carter F M, McLeod R S, Cohen Z. Subtotal colectomy for ulcerative colitis: complications related to the rectal remnant. Dis Colon Rectum. 1991;34(11):1005–1009. doi: 10.1007/BF02049965. [DOI] [PubMed] [Google Scholar]
- 43.McKee R F, Keenan R A, Munro A. Colectomy for acute colitis: is it safe to close the rectal stump? Int J Colorectal Dis. 1995;10(4):222–224. doi: 10.1007/BF00346223. [DOI] [PubMed] [Google Scholar]
- 44.Trickett J P, Tilney H S, Gudgeon A M, Mellor S G, Edwards D P. Management of the rectal stump after emergency sub-total colectomy: which surgical option is associated with the lowest morbidity? Colorectal Dis. 2005;7(5):519–522. doi: 10.1111/j.1463-1318.2005.00875.x. [DOI] [PubMed] [Google Scholar]
- 45.Karch L A, Bauer J J, Gorfine S R, Gelernt I M. Subtotal colectomy with Hartmann's pouch for inflammatory bowel disease. Dis Colon Rectum. 1995;38(6):635–639. doi: 10.1007/BF02054125. [DOI] [PubMed] [Google Scholar]
- 46.Ouaïssi M, Lefevre J H, Bretagnol F, Alves A, Valleur P, Panis Y. Laparoscopic 3-step restorative proctocolectomy: comparative study with open approach in 45 patients. Surg Laparosc Endosc Percutan Tech. 2008;18(4):357–362. doi: 10.1097/SLE.0b013e3181772d75. [DOI] [PubMed] [Google Scholar]
- 47.Chung T P, Fleshman J W, Birnbaum E H, et al. Laparoscopic vs. open total abdominal colectomy for severe colitis: impact on recovery and subsequent completion restorative proctectomy. Dis Colon Rectum. 2009;52(1):4–10. doi: 10.1007/DCR.0b013e3181975701. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe K, Funayama Y, Fukushima K, Shibata C, Takahashi K, Sasaki I. Hand-assisted laparoscopic vs. open subtotal colectomy for severe ulcerative colitis. Dis Colon Rectum. 2009;52(4):640–645. doi: 10.1007/DCR.0b013e31819d47b5. [DOI] [PubMed] [Google Scholar]
- 49.Marceau C, Alves A, Ouaissi M, Bouhnik Y, Valleur P, Panis Y. Laparoscopic subtotal colectomy for acute or severe colitis complicating inflammatory bowel disease: a case-matched study in 88 patients. Surgery. 2007;141(5):640–644. doi: 10.1016/j.surg.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Fowkes L, Krishna K, Menon A, Greenslade G L, Dixon A R. Laparoscopic emergency and elective surgery for ulcerative colitis. Colorectal Dis. 2008;10(4):373–378. doi: 10.1111/j.1463-1318.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 51.Binder S C, Miller H H, Deterling R A., Jr Emergency and urgent operations for ulcerative colitis. The procedure of choice. Arch Surg. 1975;110(3):284–289. doi: 10.1001/archsurg.1975.01360090054012. [DOI] [PubMed] [Google Scholar]
- 52.Harms B A, Myers G A, Rosenfeld D J, Starling J R. Management of fulminant ulcerative colitis by primary restorative proctocolectomy. Dis Colon Rectum. 1994;37(10):971–978. doi: 10.1007/BF02049307. [DOI] [PubMed] [Google Scholar]
- 53.Heyvaert G, Penninckx F, Filez L, Aerts R, Kerremans R, Rutgeerts P. Restorative proctocolectomy in elective and emergency cases of ulcerative colitis. Int J Colorectal Dis. 1994;9(2):73–76. doi: 10.1007/BF00699416. [DOI] [PubMed] [Google Scholar]
- 54.Ziv Y, Fazio V W, Church J M, Milsom J W, Schroeder T K. Safety of urgent restorative proctocolectomy with ileal pouch-anal anastomosis for fulminant colitis. Dis Colon Rectum. 1995;38(4):345–349. doi: 10.1007/BF02054219. [DOI] [PubMed] [Google Scholar]