Abstract
Transcranial motor evoked potential (MEP) for the facial nerve (facial MEP) has been recognized as a good method for quantitative monitoring of facial nerve function in skull base surgery. To improve the feasibility and safety of facial MEP monitoring, a peg-screw electrode and a “threshold-level” method were investigated. From 2007 to 2009, intraoperative facial MEP monitoring with the peg-screw electrode and threshold-level method was successfully achieved in 26 of 29 patients who underwent surgery for the posterior fossa extra-axial tumor. The relationship between the change in the facial MEP threshold level and the postoperative function of the facial nerve was analyzed in 23 patients who had no facial palsy preoperatively. There were no complications associated with facial MEP monitoring. Nine patients who had stable facial MEP threshold had no facial palsy. Fourteen patients who had worsened but measurable facial MEP threshold had mild palsy at discharge. Two of three patients who had severely worsened and unmeasurable facial MEP threshold had severe facial palsy. The change in the facial MEP was well correlated with the postoperative facial function. The peg-screw electrode and threshold-level method are good options for facial MEP monitoring.
Keywords: Intraoperative electrophysiological monitoring, facial nerve, motor evoked potential, skull base
Surgery of skull base tumors still poses some challenges owing to the difficult anatomy and intimate association with the cranial nerves. Postoperative cranial nerve palsy in skull base surgery remains a significant clinical problem, although various monitoring techniques have been used routinely. Facial nerve function is currently monitored during surgery using various electrophysiological methods. Among many electrophysiological monitoring methods for the facial nerve, transcranial motor evoked potential (MEP) for the facial nerve (facial MEP) has been recently recognized as a safe and valuable quantitative method for monitoring the inherent variability of facial nerve function.1,2,3,4
It is difficult to measure MEP of the facial nerve compared with that of the extremities. Because the stimulation point of the skull is near the recording point of the face, high-frequency, multipulse transcranial stimulation will affect the facial electromyogram (EMG), which will cause difficulty in reading the MEP wave.1,2,3 To decrease the current spread effect, a cranial peg-screw electrode and a “threshold-level” stimulation method, which have already been utilized to monitor intraoperative transcranial MEP, were adopted for convenient measuring and monitoring of the facial MEP.5,6,7 Our methods and results of facial MEP monitoring and its correlation with postoperative facial function are reported.
MATERIALS AND METHODS
From February 2007 to April 2009, 29 consecutive patients with extra-axial tumors in the posterior fossa requiring EMG monitoring for the facial nerve were treated at Shinshu University Hospital and its affiliated hospitals. Facial MEP was measured and monitored in these subjects. The majority of patients (n = 18) underwent resection of vestibular schwannomas. Seven patients with petrous and clival meningiomas, three patients with jugular foramen neurinomas, and one patient with trigeminal neurinoma were included.
MEP Monitoring Technique
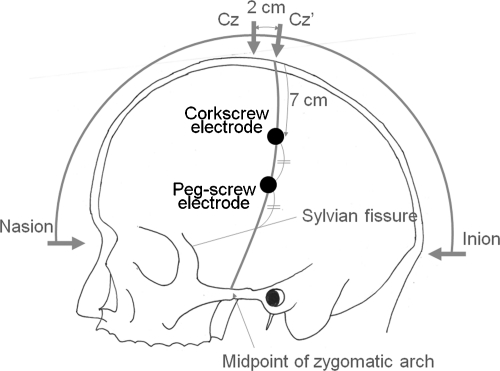
MEB 2216 (Nihonkohden Co. Ltd., Tokyo, Japan) was used as an electrophysiological device. After induction of general anesthesia, the scalp corkscrew electrodes were placed at C3′ and C4′, which were 7 cm lateral from the midline on the line between Cz′ (2 cm caudal from Cz) and midpoint of the zygomatic arch. The cranial peg-screw electrode was placed at the midpoint on the line mentioned above and between C3′ or C4′ in the contralateral side and the imaginary sylvian fissure, which is lined from the external angle of eyelid to 6 cm above the external auditory canal (Figs. 1 and 2).7 The ipsilateral side of the scalp corkscrew electrode was for cathode, and each contralateral anodal electrode was chosen when the scalp corkscrew was selected for the MEP of the upper extremity; the cranial peg-screw electrode was used for the facial MEP. An anodal constant current stimulation (mA) was used for transcranial electrical stimulation. A 4- or 5-pulse stimulus train was used with each stimulus duration of 0.2 or 0.3 milliseconds and an initial interstimulus interval of 1.7 to 2.0 milliseconds. Compound muscle potentials were recorded from a pair of the needle electrodes placed on the ipsilateral side of the face (musculus frontalis, orbicularis oculi, orbicularis oris) and bilateral abductor pollicis brevis (APB). Signals were amplified and filtered (20 to 2000 Hz) before display.
Figure 1.
Illustration depicting the placement of stimulating scalp corkscrew electrode and cranial peg-screw electrode.
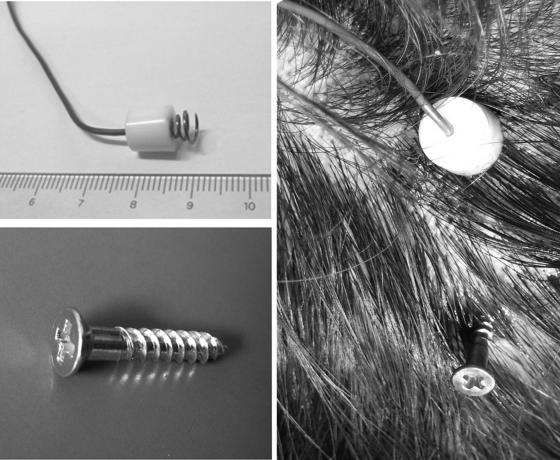
Figure 2.
(Upper left) Photo showing the scalp corkscrew electrode. (Lower left) Photo showing the cranial peg-screw electrode. (Right) Photo showing the scalp electrode and peg-screw electrode placed in their approximate position on the patient's head. After fixing the head position, each electrode is connected to the electrical stimulator.
General anesthesia was introduced by injection of a short-acting barbiturate or propofol and neuromuscular blocking agent to intubate the subject. Inhalational agents and neuromuscular blockade were avoided after intubation. The anesthesia was maintained by constant infusion of propofol (100 to 300 mg/kg/min). Narcosis (e.g., fentanyl, remifentanil) was also induced by either constant infusion or intermittent bolus. In most cases, nitrous oxide (<50%) was also given.
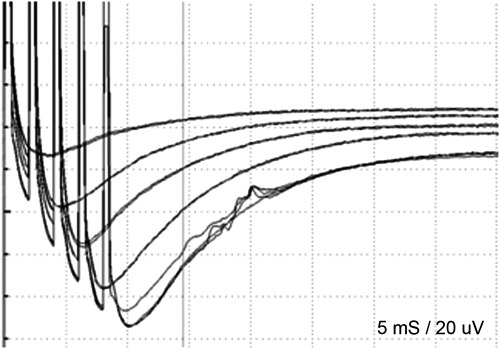
Threshold-level stimulation method was attempted to monitor the facial MEPs. This method has been proposed by Calancie et al.5,6 A threshold response was any evoked muscle response that exceeded ∼20 μV in peak-to-peak amplitude and that had appropriate response latency (e.g., latency to orbicularis oris was ∼15 to 20 milliseconds and latency to APB was ∼20 to 25 milliseconds). To evaluate whether the response was evoked by the motor pathway, disappearance of the response was confirmed by the times of pulse stimulus train decrease in the same electric intensity (Fig. 3). Baseline thresholds were established for all muscle groups being monitored and were determined at multiple times throughout the surgical procedure. The stimulus intensity was limited below 100 mA. The surgeon was notified whenever the threshold level of facial MEP changed, even if threshold level of the MEP of APB was equal.
Figure 3.
The waveform of facial MEP. Stimulation time increased one by one with the same stimulation intensity; five-train stimulation shows the response.
Other electrophysiological monitoring devices were also applied. Triggered compound muscle action potentials (tCMAP), 4 Hz of bipolar stimulation with 0.1 mA of electrical intensity and 0.1 milliseconds of duration, were utilized for detecting the location of the facial nerve. Sounds of free-running EMG of the face were made for continuous monitoring of facial function whenever the facial MEP was not recorded. Auditory brain stem response and cochlear nerve action potentials were measured for monitoring hearing function.
All the patients tolerated the procedure well and had good postoperative courses. There were no complications, such as cerebrospinal fluid leakage, brain injury, intracranial hemorrhage, and wound infection, associated with surgical procedures.
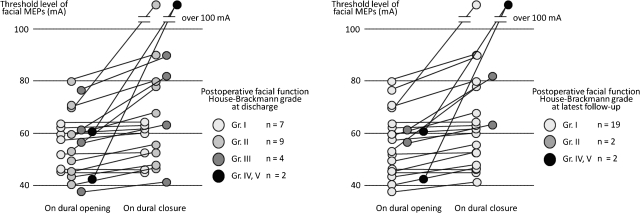
Intraoperative facial MEP was monitored by a threshold-level method in 26 patients. In the initial three patients, facial MEP was unable to be recorded due to technical failure. The changing pattern of facial MEP was divided in three groups: the unchanged group, the worsened but measurable (less than 100 mA) group, and the severely worsened and unmeasurable (over 100 mA) group. Correlation between intraoperative facial MEP findings and postoperative facial function was analyzed in 23 cases; the remaining three patients, as they had facial palsy preoperatively, were excluded. Postoperative facial function was graded clinically using the House-Brackmann (H&B) grading system.8 The facial function was examined at discharge and at least 6 months after operation. At discharge, seven patients had H&B grade I (normal) nine had grade II, four had grade III, and two had grade IV and V palsy, respectively. Mean follow-up period was 17 months. At follow-up, 19 patients had H&B grade I, 2 had grade II, and 2 had grade IV and V palsy, respectively. Grade II facial palsy at discharge disappeared in all nine patients. Two patients with grade III facial palsy at discharge remained grade II facial palsy after at least 6 months of follow-up.
RESULTS
There were no complications, such as skin injury, infection, skull fracture, and epi- or subdural hematoma, associated with the placement of the electrodes and electrical stimulation. Facial MEP was recorded from the orbicularis oris, because facial MEP was sometimes not recognized from the frontalis and orbicularis oculi. The facial MEP threshold level fluctuated during microsurgery, even when the MEP of the APB threshold level was stable. Attenuation of facial MEP following compression and movement of the tumor was almost recovered soon after a short interrupting manipulation. On the other hand, facial MEP sometimes suddenly attenuated during dissection of the facial nerve from the tumor. This type of attenuation was difficult to recover.
The individual change of facial MEP threshold level is shown in Fig. 4. All patients in the unchanged group had no facial palsy during the postoperative period. All patients in the worsened but measurable group had postoperative H&B grade II or III at discharge, and two patients in this group remained grade II at follow-up. Two of three patients in the severely worsened and unmeasurable group had H&B grade IV or higher.
Figure 4.
The individual data of the change in the facial MEP threshold level. (Left) Relationship with postoperative facial palsy at discharge. (Right) Relationship with postoperative facial palsy at latest follow-up.
DISCUSSION
Problems Measuring the Facial MEP
Measuring MEP of the facial nerve is more difficult than measuring MEP of the extremities. Because the stimulation point of the skull is near the recording point of the face, high-frequency multipulse transcranial stimulation will affect the facial EMG, which will cause difficulty in reading the MEP wave.1,2,3 The peripheral seventh nerve is stimulated directly without conduction through the central motor pathway from the cortex. Furthermore, baseline facial EMG is distorted by the condenser effect of the scalp and the skull. Facial EMG is frequently contaminated by temporalis muscle contraction by direct electrical stimulation. Although placing the stimulation electrode just above the related somatotopy of the face is effective for decreasing the stimulation current intensity, the temporalis muscle just above the primary motor area of the face emphasizes the current spread effect. To avoid these problems of the current spread, scalp electrodes for the cathode on Cz, not ipsilateral C3′ or C4′, have been proposed.1,2,3 However, electric intensity to stimulate the central cortex may need to be stronger than that of conventional transcranial stimulation point.
Merits of Using Peg-Screw Electrodes and Threshold-level Stimulation Methods for Facial MEP
The concept of the current pathways for stimulation of peg-screw electrodes was represented by Watanabe et al.7 Although much of the stimulus current spreads laterally through the scalp from the scalp electrodes because of the high resistance of the skull, the stimulus current passes through the skull more effectively from the peg-screw electrodes. The use of peg-screw electrodes can reduce the condenser effect of the skin, therefore reducing the current intensity. The peg-screw electrode can be placed just above the primary motor area of the face, due to less contraction of the temporalis muscle. This means that the effect of current spread decreases.
MEP is monitored by the final-baseline ratio of amplitude in many institutes. On the other hand, the threshold-level stimulation method is another approach to monitor the MEP. Reduced electric intensity is adequate to stimulate the central cortex because the baseline amplitude of MEP is smaller compared with that of conventional MEP monitoring. Reduced electric intensity can decrease the problems of the current spread effect. Furthermore, the threshold-level method may provide earlier warning of, and be more sensitive to, impending deterioration in the central motor conduction.5
Compared with the conventional approach, facial MEP can be recorded conveniently and is judged easily. Convenient monitoring leads to easy routine use and judgment of waveform change. It also prevents misjudging of the waveform.
Facial MEP with Other Intraoperative Facial Monitoring Methods
There are no doubts that evaluation of the intraoperative facial nerve function by electrophysiological monitoring improves postoperative facial nerve function preservation rate. Facial MEP monitoring is not independent from other facial nerve monitoring methods. tCMAP, free-running electromyography, and facial MEP are widely used for prediction of postoperative facial nerve function, because each of them has its merits and demerits. tCMAP is easily available and widely used in facial nerve preservation surgery; however, it is questionable whether tCMAP monitoring predicts the facial function or not. Recording tCMAP interrupts the surgical procedures. It is impossible to record the baseline of tCMAP before securing the route exit zone of the facial nerve. An unstable stimulation electrode is unreliable for quantitative tCMAP evaluation. Supramaximal stimulation for quantitative evaluation might cause electrical injury to the facial nerve. tCMAP might be used for facial nerve mapping. Although free-running EMG signals may sensitively monitor damage of the facial nerve, they are still uncertain for quantitative analysis. Free-running EMG should be used as an early worsening sign of the facial nerve function.
Facial MEP can be monitored throughout the operation without interrupting the surgical procedure. In facial MEP monitoring, recording and stimulation electrodes can be placed stably and do not disturb the operative field. These characteristics of the facial MEP lead to a high possibility of quantitative monitoring. Our results showed that the initial threshold level was stable in all patients in the postoperative no facial palsy group. This indicates that facial function was intact. However, our report has some limitations because of the small sample size and lack of a control group, which limits the statistical analysis. A surgeon can perform the procedure near the facial nerve with confidence in the facial nerve function, even if the facial nerve at the route exit zone is not secured. Monitoring facial MEP can improve a surgeon's confidence level and thus lead to better surgical results.
CONCLUSION
Intraoperative facial MEP was monitored using a peg-screw electrode and threshold-level method. No attenuation of the facial MEP indicated no postoperative facial palsy, and severe attenuation of facial MEP meant a possibility of permanent severe facial palsy. Together with other electrophysiological monitoring methods, the facial MEP has an important role for preserving the facial nerve function in skull base surgery.
NOTES
The abstract of this paper was presented at the 21st Annual Meeting of Japanese Society for the Skull Base Surgery and submission to Skull Base: An Interdisciplinary Approach was recommended by the committee.
ACKNOWLEDGMENTS
The authors thank Dr. Nunung Nur Rahmah for editing the manuscript.
References
- Akagami R, Dong C C, Westerberg B D. Localized transcranial electrical motor evoked potentials for monitoring cranial nerves in cranial base surgery. Neurosurgery. 2005;57(1 Suppl):78–85. discussion 78–85. doi: 10.1227/01.neu.0000163486.93702.95. [DOI] [PubMed] [Google Scholar]
- Dong C CJ, Macdonald D B, Akagami R, et al. Intraoperative facial motor evoked potential monitoring with transcranial electrical stimulation during skull base surgery. Clin Neurophysiol. 2005;116:588–596. doi: 10.1016/j.clinph.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Oishi M, Takao T, Saito A, Fujii Y. Facial nerve motor-evoked potential monitoring during skull base surgery predicts facial nerve outcome. J Neurol Neurosurg Psychiatry. 2008;79:1066–1070. doi: 10.1136/jnnp.2007.130500. [DOI] [PubMed] [Google Scholar]
- Liu B Y, Tian Y J, Liu W, et al. Intraoperative facial motor evoked potentials monitoring with transcranial electrical stimulation for preservation of facial nerve function in patients with large acoustic neuroma. Chin Med J (Engl) 2007;120:323–325. [PubMed] [Google Scholar]
- Calancie B, Molano M R. Alarm criteria for motor-evoked potentials: what's wrong with the “presence-or-absence” approach? Spine (Phila Pa 1976) 2008;33:406–414. doi: 10.1097/BRS.0b013e3181642a2f. [DOI] [PubMed] [Google Scholar]
- Calancie B, Harris W, Brindle G F, Green B A, Landy H J. Threshold-level repetitive transcranial electrical stimulation for intraoperative monitoring of central motor conduction. J Neurosurg. 2001;95(2 Suppl):161–168. doi: 10.3171/spi.2001.95.2.0161. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Watanabe T, Takahashi A, Saito N, Hirato M, Sasaki T. Transcranial electrical stimulation through screw electrodes for intraoperative monitoring of motor evoked potentials. Technical note. J Neurosurg. 2004;100:155–160. doi: 10.3171/jns.2004.100.1.0155. [DOI] [PubMed] [Google Scholar]
- House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]