Abstract
The objectives are to evaluate the applicability of the Pittsburgh staging system (PSS) (designed for primary temporal bone malignancies) to advanced periauricular cutaneous malignancies with temporal bone involvement and to study treatment outcomes and prognostic factors predicting recurrence-free survival. Ten patients with advanced periauricular cutaneous malignancy with temporal bone involvement were identified. Patients with primary temporal bone or parotid gland malignancies were excluded. All patients were clinically T4 at presentation by the American Joint Committee on Cancer (AJCC) staging system. Using Pittsburgh staging, six were T1 (stage I) and four were T4 (stage III). The mean follow-up was 13.6 months (3 to 24 months). Patients with basal cell carcinoma were managed with wide local excision and lateral temporal bone resection (WLE/LTBR) without adjuvant therapy. Two of three (66%) are alive and free of disease; one patient died of other causes. Treatment for squamous cell carcinoma patients involved multimodality therapy. Kaplan–Meier survival curves show a worse prognosis in terms of disease-specific survival for patients with higher-staged PSS tumors. This did not reach statistical significance. The PSS may provide additional prognostic information on advanced cutaneous malignancies of the temporal bone over the more widely used AJCC staging system. However, further prospective multicenter studies with larger sample size are required to validate our findings. Basal cell carcinoma was well controlled with WLE/LTBR alone without adjuvant therapy, whereas squamous cell carcinoma required multimodality therapy: WLE/LTBR and postoperative radiation with or without chemotherapy.
Keywords: Pittsburgh staging system, periauricular cutaneous malignancy, temporal bone
Skin cancer is the most common malignancy in the United States. More than one million cases of nonmelanoma skin cancers were expected to be diagnosed in 2009.1 The head and neck region is a largely sun-exposed area and accounts for the location of at least 75% of skin cancers. The auricle and periauricular area are the site of origin in ∼5% to 10% of all nonmelanoma skin cancers.2 Basal cell carcinoma (BCC) is the most common histological type, accounting for ∼85% of all skin cancers, whereas squamous cell carcinoma (SCC) accounts for ∼10% of skin cancers. Some studies have shown a significantly higher ratio of SCC/BCC in the periauricular area.3
Staging of nonmelanoma skin malignancies utilizes the American Joint Committee on Cancer (AJCC) system4 (Table 1). This system utilizes measurements in centimeters to categorize lesions as T1 to T3 tumors (T1 = < 2 cm, T2 = 2 to 5 cm, T3 >5 cm) and involvement of any deep extradermal structures upstages a lesion to T4 status.
Table 1.
AJCC Staging Criteria for Cutaneous Carcinoma
| Tx | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| Tis | Carcinoma in situ |
| T1 | Tumor less than 2 cm in greatest dimension |
| T2 | Tumor between 2 cm and 5 cm in greatest dimension |
| T3 | Tumor greater than 5 cm in greatest dimension |
| T4 | Tumor invading deep extradermal structures (i.e., cartilage, bone, muscle) |
Staging of periauricular tumors is problematic because even small, early tumors have easy access to cartilage, thereby giving them T4 status according to current AJCC staging criteria. The AJCC staging system groups tumors with deep temporal bone involvement into the same category as tumors that superficially invade the auricular cartilage despite what is likely a vast difference in prognosis. This issue of lumping heterogeneous tumors in the AJCC T4 stage has been previously described.5
A majority of skin carcinomas of the head and neck can be managed with a range of treatment options such as traditional surgical excision, Mohs micrographic surgery, electrodessication and curettage, or radiation therapy. All of these modalities have been shown to provide excellent local control rates. The rate of nodal metastasis from these cancers ranges between 0.5 and 2%.
Behavior of periauricular tumors is widely documented to be much more aggressive than other areas of cutaneous SCC.6,7 Periauricular tumors have a much higher rate of recurrence (4 to 7%)6,7 and approximately a sevenfold higher risk of nodal metastasis. The prevalence of occult metastases has prompted some authors to advocate prophylactic neck dissections in the setting of advanced periauricular SCC. The morbidity associated with regional metastasis in periauricular cutaneous malignancies is also significantly higher than with other locations.8 Reasons for this increased aggressiveness of periauricular tumors are multiple. The presence of multiple embryonic fusion planes in the periauricular region facilitates tumor spread.6,9 Also, the increased exposure of the periauricular area promotes increased actinic damage of the surrounding skin. Third, the skin in this area is thin and this proximity to underlying cartilage and bone can make complete resection more challenging.6 It is our opinion, however, that even though this early cartilage invasion may negatively impact prognosis, the medial extent of the disease is likely a more important prognostic indicator. This was our reason for pursuing the use of the Pittsburgh staging system (PSS) in these tumors.
Advanced periauricular tumors with temporal bone involvement have traditionally been surgically managed with wide local excision (WLE) of the cutaneous component and resection of the temporal bone (sleeve resection, lateral [LTBR], subtotal, or total temporal bone resection [TBR]), the extent of which is dependent on the extent of temporal bone involvement. This has been variously combined with neck dissections, parotidectomy, adjuvant radiation, and/or chemotherapy, depending on the extent of disease, presence of nodal metastasis, histological subtype and features, and physician preference.6,9,10
In contrast to cutaneous cancers invading the temporal bone, malignancy of temporal bone origin is exceedingly rare with an estimated incidence of six cases per million.11 SCC is the most common histological type of malignancy in the temporal bone, but the risk factors are different from auricular carcinoma with chronic irritation or inflammation likely playing the major causal role.12 Because of its rarity, a strong, statistically supported treatment algorithm has not been reached. For most authorities, surgical therapy in the form of TBR is first-line treatment with potential roles for radiation and chemotherapy as adjuncts. There are several staging systems for temporal bone carcinoma, but the most widely used is the modified PSS13; this system accounts for “depth” of tumor invasion to more anatomically “medial” regions (Table 2). Early (T1 and T2) lesions have high survival rates (80 to 100%, 2 years), whereas late lesions (T3 and T4) have much poorer prognoses (<50%, 2 years) despite more aggressive surgical resection.14 Of note is the staggering drop off of survival after the external auditory canal (EAC) is breached full thickness or invasion of the inner ear and prominent skull base neurovascular structures occurs.
Table 2.
Pittsburgh Staging System for Temporal Bone Carcinoma
| T1 | Tumor limited to the external auditory canal without bony erosion or evidence of soft-tissue extension |
| T2 | Tumor with limited external auditory canal bony erosion (not full thickness) or radiographic finding consistent with limited (<0.5 cm) soft-tissue involvement |
| T3 | Tumor eroding the osseous external auditory canal (full thickness) with limited (<0.5 cm) soft-tissue involvement, or tumor involving middle ear and/or mastoid, or patients presenting with facial paralysis |
| T4 | Tumor eroding the cochlea, petrous apex, medial wall of the middle ear, carotid canal, jugular foramen or dura, or with extensive (>0.5 cm) soft-tissue involvement |
We report on 10 patients who presented with advanced periauricular cutaneous carcinomas with temporal bone involvement. We examine potential prognostic factors in these aggressive skin malignancies, with special focus on the application of the PSS in further substaging AJCC T4 disease.
MATERIALS AND METHODS
A retrospective chart review institutional review board (IRB) approved study was conducted to identify patients with known extensive cutaneous carcinoma of the periauricular area with temporal bone invasion that presented to one of the Louisiana State University Health Sciences Center (LSU HSC) tertiary care facilities from January 1, 2001, to December 31, 2009. Inclusion criteria included documentation of preoperative staging (i.e., imaging, physical exam), documentation of operative findings and histology for those patients who underwent surgical intervention, and documented follow-up. Patient age, gender, tobacco use, site of lesion, histological type (BCC or SCC), and tumor features including nodal spread, perineural invasion, and lymphovascular invasion were recorded. The primary tumor was staged by both the AJCC system and the PSS. We did provide one exception to the PSS: the amount of soft-tissue invasion was disregarded, as all tumors in the study would have been PSS T4. We also evaluated lesions based on the individual components of the PSS (extent of EAC involvement, involvement of the temporomandibular joint, dura, parotid, middle and inner ear, internal jugular vein, carotid artery, and cranial nerves). Any surgical, radiologic, or chemotherapeutic interventions were documented, and their impact on survival was statistically evaluated. Length of follow-up and disease status at last follow-up were documented. Patients were classified as one of the following: alive and with no evidence of disease (NED), died of other causes without evidence of disease (DOC), active disease, or died of disease (DOD). Any incidences of recurrence or distant metastasis were documented.
The statistical analysis included the use of binomial probabilities, the Cox proportional hazards model, odds ratios, and Kaplan–Meier analysis to evaluate the effects of explanatory variables on survival and oncological outcomes.
RESULTS
Tumor Location
The most common locations for the tumors were preauricular (4/10) and on the conchal bowl (3/10). All 3 BCCs originated within, the auricle itself, whereas the SCCs all arose in the surrounding periauricular skin. Of note, 8/10 lesions were left-sided. Further investigation of cases that were excluded because of lack of documentation revealed that 10/12 lesions encountered were left-sided, which would be unusual (p = 0.019) under the null hypothesis that a lesion has an equal probability of occurring on either side.
Basal Cell Carcinoma (N = 3)
Three patients had extensive periauricular cutaneous BCC with involvement of the temporal bone. All patients were T4N0M0 by AJCC and T1N0M0 by PSS. They were down-staged by the Pittsburgh system because they had only superficial involvement of the external auditory canal and did not involve any deeper structures. As previously mentioned, volume of soft-tissue disease was not used as a staging criteria. All patients were treated surgically with WLE and LTBR. None of these patients received adjuvant radiation therapy or chemotherapy. Two of three patients underwent superficial parotidectomy, but none of these patients had evidence of disease within the parotid. None of these patients underwent an elective neck dissection. Of note, none of our BCC patients had worrisome histological features such as perineural or lymphovascular invasion.
All three of these patients were locoregionally controlled. Two out of three are alive with NED; the third patient died of COPD, but had NED at the time of his death. Mean follow-up for this group was 19 months (9 to 24 months).
Squamous Cell Carcinoma (N = 7)
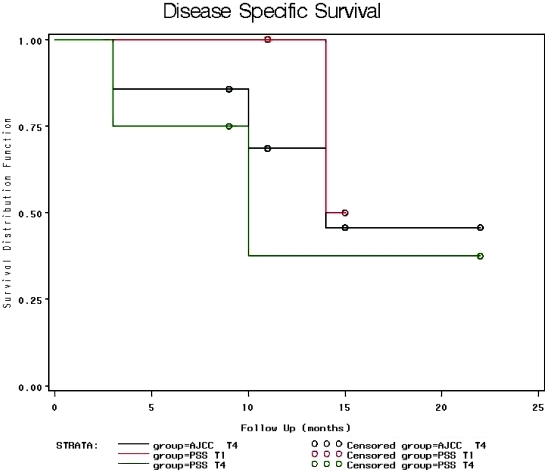
All seven were staged T4 by AJCC, staging was changed to T1 by the Pittsburgh system in three patients for the same reasons mentioned above (Fig. 1). At a mean follow-up of 12 months for seven patients with SCC, 4/7 were either alive with NED or died of other causes with NED. Three patients with SCC died (3, 10, and 14 months), whereas a fourth patient recurred at 8 months, but currently has NED after undergoing a second procedure.
Figure 1.
Kaplan–Meier disease specific survival plots for AJCC T4, PSS T1, and PSS T4 patients. Black line indicates disease-specific survival curve for all SCC patients in the study (AJCC T4). Red line indicates survival curve for patients with PSS T1 disease (n = 3). Green line indicates survival curve for patients with PSS T4 disease. Circles indicate censorship points.
Of the seven SCC's, three presented with T1 disease by Pittsburgh criteria. Two of these received surgical intervention and they are 15 months and 11 months postoperative and both currently have NED. One of these recurred 8 months postoperatively, then underwent a second TBR and WLE, and currently has NED. The other patient with T1 disease had to have surgical intervention delayed secondary to systems-based issues, so he received preoperative radiation therapy and his tumor rapidly grew despite seemingly adequate preoperative treatment. He progressed to T3 status and chose palliative care over the more extensive TBR that would have been required to control his disease. He expired 14 months after diagnosis.
The other four patients with SCC presented with T4 disease by Pittsburgh criteria. Three received surgical intervention, and one was placed on hospice care because of advanced tumor with dural, carotid, and jugular involvement. Mean follow-up for this group was 9.8 months (3 to 22 months). Two of these patients have died at 3 and 10 months, respectively. The other two patients have NED at 9 and 22 months. Several specific findings are worth reporting but none of these findings with regards to staging reached statistical significance:
The two patients with PSS T1 disease who received primary surgical therapy have been controlled and currently NED.
The only PSS T1 patient who died did not receive surgical therapy.
Preoperative radiation therapy was not effective in the one case in which it was used.
PSS T4 disease at presentation resulted in two mortalities out of four patients.
Kaplan–Meier curves show worse disease-specific survival for higher-staged PSS tumors, but a thorough statistical analysis showed Pittsburgh staging did not have a statistically significant impact on recurrence or survival.
The only case of widely metastatic disease occurred in a patient with a PSS T4 tumor.
Prognostic Features
TOBACCO USE
Tobacco use did not clinically correlate with tumor staging or prognosis.
NODAL METASTASIS
Only three patients in our study presented with nodal disease. One was PSS T1 and has done very well postoperatively. The other two were PSS T4. One has died of metastatic disease. The third patient with nodal disease was PSS T4 and is currently NED. Nodal disease, therefore, did not have any significant impact on survival (p = NS).
PERINEURAL INVASION
Three of five patients (60%) undergoing surgical intervention for SCC had perineural invasion on histological evaluation. Of these three patients, one recurred with active metastatic disease at 3 months and is DOD, one locoregionally recurred at 8 months, but has NED at 11 months following salvage TBR, and one has NED at 9 months. Of note, the patient that died from metastatic disease had microscopically positive margins on the facial nerve in the specimen. The two operated patients without perineural invasion on their specimens are NED at 15 and 22 months postoperatively. Statistically, there was insufficient evidence to conclude that perineural invasion is associated with a higher probability of disease recurrence (p = 0.400).
ANGIOLYMPHATIC INVASION
Of the five patients with SCC who received surgical intervention, two had angiolymphatic invasion on permanent histology. One has done well postoperatively and is 15 months out and has NED, the other has died of metastatic disease. Three of five patients with surgically treated SCC did not have angiolymphatic invasion on histology. They have NED at 9, 11, and 22 months postoperative. All clinically have NED, one has recurred, but has NED following a repeat TBR. There was no statistically significant relationship with angiolymphatic invasion and survival.
Parotid involvement was present in 2/7 (29%) patients with SCC. One died at 3 months, and the other has died of metastatic disease. Dural, jugular, and carotid involvement were present in one patient. This patient died at 3 months.
DISCUSSION
Tumor Location
Only one study by Shotton et al,10 available for comparison within the limited literature on the topic, listed the locations and histology of their cases. Their findings do not corroborate with ours as they had two BCCs, one of which was preauricular and one of which was in the EAC. They also documented multiple SCCs within the auricle itself as well as some which originated from the periauricular skin. Eight of 10 of our lesions were left-sided. Further review of cases excluded for various reasons revealed that 10/12 lesions were left-sided (p = 0.019). No study encountered in the author's literature review has yet commented on the laterality of SCC of the head and neck. It does, however, seem logical that Americans would accumulate more overall actinic damage to the left side of the face because of exposure while driving an automobile. This would be an interesting point for further evaluation in future studies. A comparative study with a cohort of patients with increased right ear exposure could be incorporated to evaluate a difference in laterality of head and neck cutaneous malignancies.
Basal Cell Carcinoma
Our study revealed that extensive BCC with temporal bone involvement can be effectively managed with surgical therapy alone. Good survival and recurrence-free prognosis can be expected if appropriate surgical excision is performed. None of our patients showed aggressive histological features and therefore were managed with primary surgical excision alone.
Cutaneous BCC has long been known to be relatively easily managed by complete surgical excision with adequate margins. Management of extensive periauricular BCC was once a significant challenge because of the difficulty of securing a medial margin. Numerous articles have documented that surgical excision with some form of TBR is appropriate and effective for treating these lesions.8,9,10 Previous literature has also documented improved survival when comparing BCC versus SCC. Our study corroborates both of these previous findings, as our BCC patients tended to have improved postoperative outcomes. This did not reach statistical significance, likely due to the small number of patients in the study.
With regards to staging of BCC, all of our tumors were T4 AJCC and T1 PSS. It could be argued that the improved survival seen in this group was due to their clinical stage and not the histology, although in our opinion, histology probably played the more important role.
Squamous Cell Carcinoma
STAGING
Using Kaplan–Meier survival curve analysis, disease-specific survival seemed to be improved with lower-stage PSS tumors, but a thorough statistical analysis of the PSS versus AJCC staging system did not reveal any statistically valid differences. This is likely at least partly attributable to the small number of patients in our series. The obvious heterogeneity of AJCC T4 tumors of periauricular origin leads one to believe that the PSS would offer significant prognostic advantages, but the rarity of these advanced tumors makes any large statistical analysis difficult. Multicenter data pooling or a meta-analysis would be helpful in obtaining statistically significant results.
PROGNOSTIC FEATURES
Tobacco use and nodal disease at presentation did not seem to have any statistical impact on oncological or survival outcomes. This is surprising since nodal disease has long been recognized as a very important prognostic forms in many forms of cancer, including the head and neck. This could represent limited follow-up as well as limited overall numbers in the study.
CONCLUSION
Advanced periauricular cutaneous carcinoma is a rare disease with little available literature regarding epidemiology, prognostic markers, and management protocols. The PSS may provide improved prognostic information with regards to the staging of these tumors as compared with the more widely used AJCC system. Further studies with higher patient numbers obtained through multicenter data pooling or meta-analysis will be critical in elucidating the specific role of the PSS in these tumors. BCC is well managed with surgical extirpation alone, unless aggressive histological features dictate more aggressive management. SCC is best managed with surgery followed by postoperative radiation therapy.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Estrem S A, Renner G J. Special problems associated with cutaneous carcinoma of the ear. Otolaryngol Clin North Am. 1993;26(2):231–245. [PubMed] [Google Scholar]
- Ahmad I, Das Gupta A R. Epidemiology of basal cell carcinoma and squamous cell carcinoma of the pinna. J Laryngol Otol. 2001;115(2):85–86. doi: 10.1258/0022215011907497. [DOI] [PubMed] [Google Scholar]
- Skin Cancer Treatment National Cancer Institute Website. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/skin/HealthProfessional/page4. Accessed February 8, 2010. Available at: http://www.cancer.gov/cancertopics/pdq/treatment/skin/HealthProfessional/page4
- Dinehart S M, Peterson S. Evaluation of the American Joint Committee on Cancer staging system for cutaneous squamous cell carcinoma and proposal of a new staging system. Dermatol Surg. 2005;31(11 Pt 1):1379–1384. doi: 10.2310/6350.2005.31201. [DOI] [PubMed] [Google Scholar]
- Niparko J K, Swanson N A, Baker S R, Telian S A, Sullivan M J, Kemink J L. Local control of auricular, periauricular, and external canal cutaneous malignancies with Mohs surgery. Laryngoscope. 1990;100(10 Pt 1):1047–1051. doi: 10.1288/00005537-199010000-00004. [DOI] [PubMed] [Google Scholar]
- Gal T J, Futran N D, Bartels L J, Klotch D W. Auricular carcinoma with temporal bone invasion: outcome analysis. Otolaryngol Head Neck Surg. 1999;121(1):62–65. doi: 10.1016/s0194-5998(99)70126-9. [DOI] [PubMed] [Google Scholar]
- Clark R R, Soutar D S. Lymph node metastases from auricular squamous cell carcinoma. A systematic review and meta-analysis. J Plast Reconstr Aesthet Surg. 2008;61(10):1140–1147. doi: 10.1016/j.bjps.2008.04.036. [DOI] [PubMed] [Google Scholar]
- Levine H. Cutaneous carcinoma of the head and neck: management of massive and previously uncontrolled lesions. Laryngoscope. 1983;93(1):87–105. doi: 10.1288/00005537-198301000-00017. [DOI] [PubMed] [Google Scholar]
- Shotton J C, Sergeant R J, Tanner N S, Allen J P. Lateral temporal bone resection for extensive pinnal malignancy. Has anything changed in forty years? J Laryngol Otol. 1993;107(8):697–702. doi: 10.1017/s002221510012417x. [DOI] [PubMed] [Google Scholar]
- Prasad S, Janecka I P. Efficacy of surgical treatments for squamous cell carcinoma of the temporal bone: a literature review. Otolaryngol Head Neck Surg. 1994;110(3):270–280. doi: 10.1177/019459989411000303. [DOI] [PubMed] [Google Scholar]
- Kinney S E, Wood B G. Malignancies of the external ear canal and temporal bone: surgical techniques and results. Laryngoscope. 1987;97(2):158–164. doi: 10.1288/00005537-198702000-00006. [DOI] [PubMed] [Google Scholar]
- Arriaga M A, Curtin H, Takahashi H, Hirsch B E, Kamerer D B. Staging proposal for external auditory meatus carcinoma based on preoperative clinical examination and computed tomography findings. Ann Otol Rhinol Laryngol. 1990;99(9 Pt 1):714–721. doi: 10.1177/000348949009900909. [DOI] [PubMed] [Google Scholar]
- Moody S A, Hirsch B E, Myers E N. Squamous cell carcinoma of the external auditory canal: an evaluation of a staging system. Am J Otol. 2000;21(4):582–588. [PubMed] [Google Scholar]