Abstract
As endoscopic skull base resections have advanced, appropriate reconstruction has become paramount. The reconstructive options for the skull base include both avascular and vascular grafts. We review these and provide an algorithm for endoscopic skull base reconstruction. One hundred and sixty-six skull base dural defects, reconstructed with an endonasal vascular flap, were examined. As an adjunct, avascular reconstruction techniques are discussed to illustrate all options for endonasal skull base reconstruction. Cerebrospinal fluid (CSF) leak rates are also discussed. Small CSF leaks may be successfully repaired with various avascular grafting techniques. Endoscopic endonasal approaches (EEAs) to the skull base often have larger dural defects with high-flow CSF leaks. Success rates for some EEA procedures utilizing avascular grafts approach 90%, yet in high-flow leak situations, success rates are much lower (50 to 70%). Defect location and complexity guides vascularized flap choice. When nasoseptal flaps are unavailable, anterior/sellar defects are best managed with an endoscopically harvested pericranial flap, whereas clival/posterior defects may be reconstructed with an inferior turbinate or temporoparietal flap. An endonasal skull base reconstruction algorithm was constructed and points to increased use of various vascularized reconstructions for more complex skull base defects.
Keywords: Skull base reconstruction, nasoseptal flap, AlloDerm, CSF leak rate, algorithm, skull base, pericranial flap, vascular reconstruction
Over the course of the past 10 years, endoscopic resection of skull base tumors has advanced.1 As the complexity of skull base defect size and location has increased, the need for more robust and reliable reconstructive options has also increased. The challenge is to recreate the barrier between the cranial vault and the nasal cavity to prevent and eliminate cerebrospinal fluid (CSF) leaks and protect the brain from exposure to infectious sources. Reconstruction of small and idiopathic defects of the skull base can be reliably performed with a variety of known techniques with a high degree of success1,2 (90 to 97%).
Endoscopic endonasal approaches (EEAs) can be divided into six sagittal plane corridors: transfrontal, transcribiform, transplanum, transsellar, transclival, and transodontoid.3 EEAs and the resultant skull base defect poses a more complex challenge in the face of reconstruction. This is, in part, due to the nature of the more extreme bone removal and the resultant size of the dural defect.
Due to the complexity and size of skull base defects after EEA, the same techniques that are used for repairing small defects through which CSF leaks no longer apply. However, there are reconstructive options available. The current options available for reconstruction have expanded and the decision to reconstruct must take into account the anticipated location, size, and shape of the skull base defect. The ladder for skull base reconstructions includes avascular grafts, nasoseptal pedicled flaps, turbinate flaps, and novel endoscopic regional flaps.4,5,6 Given these recent advances, we present a portion of our prospective data of EEA skull base defects repaired with vascularized flaps, discuss all options for reconstruction, and provide an algorithm for endoscopic skull base reconstruction.
METHODS
A literature review using PubMed identified series that involved intradural skull base defects reconstructed after tumor resection using EEA. Reconstruction for noniatrogenic spontaneous CSF leaks was excluded. Each series was reviewed to identify the tumor location and size, skull base defect size, and the reconstruction method. After obtaining Institutional Review Board approval, we also include our series of nasoseptal flap (NSF) reconstructions that involved intradural skull base defects reconstructed after tumor resection using EEA. We performed 166 cases employing reconstruction of intradural skull base defects after an EEA from June 2007 to December 2008. The skull base defect was repaired with a vascular pedicled flap of the nasal septum mucoperiosteum and mucoperichondrium based on the nasoseptal artery as described previously.7 When the NSF flap was unavailable secondary to tumor invasion, the location of the anticipated defect was the impetus for choosing another vascularized flap. Anterior skull base defects were repaired with a pericranial flap (PCF). The technique for this flap has been previously described.4 Posterior and clival skull base defects were repaired with a transposed temporoparietal fascia flap (TPFF) or inferior turbinate flap (ITF).5,6 Size of the skull base defects after EEA, complications after reconstruction, and presence of lumbar drain were analyzed. A literature search identified a single study of 75 EEA patients that underwent NSF reconstruction of the skull base after EEA between January 30, 2006, to January 30, 2007.8 The CSF leak percentage was calculated for each type of repair. An algorithm was constructed based on the available data and our (University of North Carolina and University of Pittsburgh) current vascular flap usage.
RESULTS
The NSF was used to reconstruct skull base defects in 150 patients in a prospective series. Leak rates stratified by defect site versus quality of CSF leak are listed in Table 1. Six (4.0%) of the 150 patients had a postoperative CSF leak. Of the 150 patients, 59 had high-flow leak rates as a direct result of opening the ventricle or from entering the arachnoid cistern. Of the group with high-flow leak rates, 6.7% (four of 59) had a postoperative CSF leak. The four failures in this group were found to have the following tumor pathology: (1) olfactory meningioma resected via transcribriform approach, (2) pituitary macroadenoma resected via transphenoid approach, (3) craniopharyngioma resected via transphenoid approach, and (4) clival chordoma resected via transclival approach. Each of the four failures required a return trip to the operating room to reposition and bolster the NSF with a fat graft. Intraoperative lumbar drain was also placed. One NSF was found to have necrosis and required a temporoparietal fascia flap to repair the CSF fistula. The flap loss was in the setting of a patient treated with preoperative proton beam radiation for a clival chordoma who had significant osteoradionecrosis of the periclival and pterygoid bones.
Table 1.
Quality and Location of Cerebrospinal Fluid (CSF) Leak after Skull Base Reconstruction in 150 Consecutive Patients
| CSF Leak Quality | |||||||
|---|---|---|---|---|---|---|---|
| Total | High Flow | Low Flow | |||||
| Intraoperative CSF leak | 150 | 59 | (39%) | 91 | (61%) | ||
| Large dural opening (>2 cm2) | 36/150 | (24%) | 36/59 | (61%) | 0/91 | (0%) | |
| Lumbar drainage | 57/150 | (38%) | 55/59 | (93%) | 2*/91 | (2%) | |
| Prior radiation | 18/150 | (12%) | 13/59 | (22%) | 5/91 | (5%) | |
| Postoperative CSF leak | 6/150 | (4%) | 4/59 | (7%) | 2/91 | (2%) | |
| Corridor of resection | |||||||
| Anterior cranial fossa | 26 | 20 | 6 | ||||
| Postoperative CSF leak | 1/26 | (4%) | 1/20 | (5%) | 0/6 | (0%) | |
| Transphenoidal | 114 | 29 | 85 | ||||
| Postoperative CSF leak | 4/114 | (3%) | 2/29 | (7%) | 2/85 | (2%) | |
| Transclival | 10 | 10 | 0 | ||||
| Postoperative CSF leak | 1/10 | (10%) | 1/10 | (10%) | 0/10 | (0%) | |
CSF, cerebrospinal fluid.
Lumbar drain placed after repair, not in operating room at the time of the procedure.
The remaining 91 patients were classified as having low-flow intraoperative CSF leaks. We define low-flow leaks as one that is suspected after dural opening, but does not involve opening of the ventricle and arachnoid cistern (such as the basilar or suprasellar cistern). Examples of such low-flow leak cases include a thinned diaphragma after adenoma removal with CSF weeping or a transcribriform encephalocele repair with CSF weeping from the olfactory groove defect. If this encephalocele defect extended back through the planum and into the suprasellar cistern, then such a defect would be considered high flow.
Of the group with low-flow leaks, 2.1% (2 of 91) had a postoperative CSF leak after reconstruction with the NSF. Pituitary adenoma was the final tumor pathology for the two cases of CSF leak in this group. The CSF leak, in both cases, was managed and resolved after postoperative placement of a lumbar drain, avoiding a return trip to the operating room.
There were 16 patients that had no NSF available for reconstruction. A vascular pedicled PCF (n = 10), TPFF (n = 2), or ITF (n = 4) was incorporated to reconstruct anterior or posterior skull base defects. The postoperative CSF leak rate for each of the three reconstructive procedures was 0%.
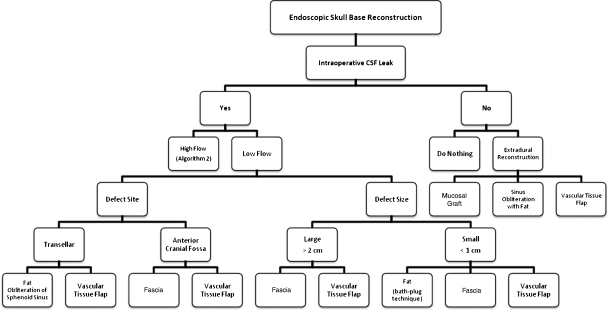
Based on these results and a comparison to various methods of managing CSF leaks as described in the literature (see discussion), an algorithm was designed based on defect location and type and nature of intraoperative CSF leak (Figs. 1 and 2). When there is no intraoperative CSF leak, vascular tissue flap reconstruction is recommended for cases where the diaphragma is thin or cases where the dura is subject to stress and less likely to heal, as seen in patients scheduled for postoperative radiation therapy. If there is an intraoperative CSF leak, the quality of the leak must be determined. If the leak is a low-flow leak, then the defect site and size will determine the vascular tissue flap needed. If the leak is a high-flow leak, then the defect site alone guides reconstruction.
Figure 1.
Algorithm for endoscopic skull base reconstruction.
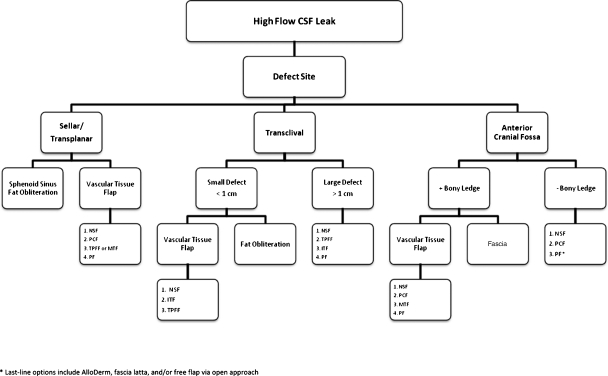
Figure 2.
Skull base reconstruction algorithm for high-flow intraoperative CSF leaks. ITF, inferior turbinate flap; MTF, middle turbinate flap; NSF, nasoseptal flap; PLF, pericranial flap; PF, palatal flap; TPFF, temporoparietal fascia flap.
The advantages and limitations of all vascular tissue flap options for endoscopic skull base reconstruction based on the size and site are discussed below and outlined in Table 2. The NSF may be applied to any skull base defect and size. The ITF excels in the reconstruction of small clival defects. If the surgeon is forced to reconstruct defects greater than 1 cm, then a fat bolster may be needed. Due to limitations in pedicle length, the ITF cannot reach the anterior cranial fossa or suprasellar area. The PCF has an extended pedicle that supports reconstruction from the anterior cranial fossa to the sella. The posterior skull base may be difficult to reach with a PCF. The temporoparietal fascia flap (TPF) is ideal for clival and parasellar defects. The TPFF is an inadequate choice for anterior skull base defects due to limitations in the arc of rotation necessary to tunnel the pedicle through the pterygopalatine fossa. The remaining two vascular tissue flaps are more difficult to harvest and have specific indications. The first is the middle turbinate flap (MTF). The flap is small, difficult to elevate, and is composed of a thin mucosa layer. Nevertheless, the flap is good for reconstruction of small (<1 cm) transphenoidal and anterior cranial fossa defects. The pedicled palatal flap (PF) has been described in cadaveric studies. In theory, the PF can reach all areas of the skull base given the 3-cm pedicle; however, it is difficult to dissect the palatine canals. This flap is a last line vascular tissue option due to inexperience as well as potential oral cavity donor site morbidity.
Table 2.
Intranasal and Regional Vascular Flaps Available for Skull Base Reconstruction
| Location | Vascular Tissue Flap | Pedicle | Comments/Limitations |
|---|---|---|---|
| Intranasal vascular tissue flap | NSF7 | Posterior nasoseptal from sphenopalatine artery | • Ideal for all skull base reconstruction |
| ITF6 | Inferior turbinate artery* | • Good for small clival defects | |
| • Cannot reach ACF or sella | |||
| MTF6 | Middle turbinate artery* | • Good for small ACF or transphenoidal defects | |
| • Small in size | |||
| • Thin mucosa | |||
| • Difficult to elevate | |||
| Regional vascular tissue flap | PCF4 | Supraorbital and supratrochlear artery | • Hardy flap with versatile dimensions |
| • Extends from ACF to sella, but not to posterior skull base | |||
| TPFF5 | Superficial temporal artery | • Good for clival or parasellar defects | |
| • Ninety-degree pedicle rotation limits reconstruction of ACF | |||
| PF17 | Greater palatine artery | • Theoretical flap that reaches all areas of skull base | |
| • Three-centimeter pedicle, but difficult to dissect |
ACF, anterior cranial fossa; ITF, inferior turbinate flap; MTF, middle turbinate flap; NSF, nasoseptal flap; PCF, pericranial flap; PF, palatal flap; TPFF, temporoparietal fascia flap.
Terminal branch of posterior lateral nasal artery.
DISCUSSION
The NSF has become our primary option for skull base reconstruction after EEA for intradural tumors.9 To date, this is the largest report evaluating CSF leak rate for reconstruction after EEA with vascularized tissue. The overall CSF leak rate for 225 (150 patients reported here plus 75 from reference 7) patients reconstructed with the NSF is 6.2%. Although the overall CSF leak rate using vascularized flaps is low in our series, keep in mind that since there is no comparison group available for this cohort of patients, it is not possible to describe this as an objectively superior approach to other techniques. The overall leak rate reflects a technical learning curve that endoscopic skull base surgeons must surmount to achieve successful reconstruction. Analyzing the first 25 patients that were reconstructed with the NSF7 revealed a 24.0% CSF leak rate, whereas the last 200 patients (after the learning curve) reconstructed with an NSF have a combined leak rate of 4.0%. Part of the learning curve for successful reconstruction involves understanding the nuances of reconstruction during preoperative evaluation and planning. Anticipating the skull base defect location, the size and type of intraoperative CSF leak after resection should guide reconstructive options. Below we present a reconstructive algorithm considering these factors to help outline successful reconstruction.
Anterior Cranial Fossa Defects
Transcribiform skull base reconstructions that do not extend back to the suprasellar cistern can be repaired by AlloDerm or the NSF with comparable CSF leak rates. In most cases, successful repair may be performed regardless of defect size. Germani et al10 reconstructed the anterior skull base with AlloDerm alone in 12 cases without any CSF leaks in defects larger than 2.0 cm. The remaining 30 patients reconstructed with AlloDerm for skull base defects of various size, ranging from 0.4 cm to 2.0 cm, were in combination with a mucosal graft, bone graft, and/or cartilage.10 The overall leak rate of this group was 3.4%. Germani et al10 explained “a single piece of acellular dermal allograft is positioned intracranially, with the margins of the graft extending extracranially, to overlay the bony margins of the defect.”
Successful repair of favorable defects, one with bony ledges, using AlloDerm is not limited to anterior cranial defects. In general, the success of the AlloDerm inlay–onlay technique is dependent on having bony ledges available to support the graft regardless of location: cribriform, planum, sella, or clivus. In our experience, we found that successful reconstruction with AlloDerm is dependent on all the mucosa being removed from the bone onto which the onlay is placed and have adequate boney and dural edges for inlay. This permits the critical, direct contact that is required for revascularization.8 In our practice (UNC and UPMC), the majority of EEA skull base defects have limited bony or dural edges that allow for inlay grafts (i.e., all sphenoid and clival defects). This has necessitated the use of onlay vascularized flaps to maximize healing and minimize leak rates. Now this has been the standard of care for all intradural skull base defects in our practices. Even though AlloDerm inlay is a successful option for reconstruction of some skull base defects,10,11 it is now used as an adjunct (if the flap is too small, etc.) or as last line option after vascular options have been exhausted in our practices.
Sella Defects
Size of the skull base defect does play a role in determining reconstructive material options when addressing lesions in the sella. In situations where there is limited opening across the planum and tuberculum, a fat “bath plug” or inlay–onlay strategy with AlloDerm or fascia lata will suffice.12 Lorenz et al13 reported a CSF leak rate of 8.3% after resection of 24 hypophyseal tumors (23 of which were greater than 1 cm). We found that lesions of the ventral skull base (i.e., planar meningiomas) tend to occupy a larger portion of the parasellar area and are best reconstructed with an NSF. Sonnenburg et al14 looked at endoscopic pituitary resection without intra-operative CSF leaks and found no need for intranasal reconstruction in these cases.
Again, the problem with AlloDerm parasellar reconstruction arises when the size of the skull base lesions expands to the limits of the optic nerve and the carotid artery. In this particular situation, there are no bony ledges to support an inlay graft, and the NSF is the preferred choice for successful reconstruction with minimal risk of CSF leak because it does not require an inlay bony buttress. It is critical to make sure the flap covers and overlaps the entire defect to lie over denuded bone or soft tissue surrounding the nasal side of the defect.
High-Flow CSF Leak
Although tumor removal after EEA typically results in larger defects based on the size of intracranial lesions, we found that preoperative planning based on tumor size alone does not predict successful reconstruction. This is because the size of the tumor does not necessarily correlate with the incidence of CSF leak after an EEA. We observed that cases with wide opening of the arachnoid cisterns (i.e., high-flow intraoperative leak) proved to be the factor most consistently predicting the likelihood of CSF leak, and simply dural defect size. CSF leak rates at UPMC for lesions that incited high-flow leaks and were reconstructed using AlloDerm was initially 40 to 50%, which was unacceptable. After variations of reconstruction and improvements on the leak rate to 30%, we realized that the technique of using an inlay–onlay graft was the limiting factor of skull base reconstruction. Although Sautter et al11 and Ismail et al15 described repair of skull base defects with 11.1% and 9.5% leak rate, respectively, there is no discussion of the quality of intraoperative CSF flow rate. With respect to the algorithm, the surgical team must determine if the lesion will result in a resection that opens the cistern, and if so, an NSF will provide the best outcome and the lowest CSF leak rates.
It is imperative to determine preoperatively if an NSF will be available for reconstruction in the cases that require its use for skull base reconstruction. If the NSF is not available due to prior surgery or secondary to tumor involvement, then the location of the anticipated skull base defect should guide reconstructive options in the algorithm. In our cohort, there were 16 patients that did not have an NSF available. For skull base lesions in the anterior skull base, we used an endoscopically harvested PCF.4 The PCF is a hardy, well-vascularized flap that has enormous surface area potential. If harvested properly, the PCF will potentially cover any size defect of the anterior skull base up to the clivus.16 If the anticipated skull base defect is transsellar, there are a few vascular reconstructive options. In particular, the TPFF and the MTF are viable choices.5 Defects that are further posterior in the skull base would require the use of a TPFF, ITF, or PF.6,17 Although the number of these various flaps is fewer than the NSF, their success rate is telling (0% failure), in part, due to the same principles that guide open reconstruction and make vascular reconstruction the standard for open skull base operations.
Principles of open reconstruction in any region of the body typically select and even prefer vascularized tissue to promote theories of more efficient wound healing. One study, based on an animal model, demonstrated that vascularized reconstruction for tumors involving bone have qualitative healing advantages over AlloDerm alone.18 In fact, several articles that incorporate AlloDerm into skull base reconstruction use vascularized tissue as an adjunct to reconstruction.10,11,15 The use of vascularized tissue as an adjunct to AlloDerm was even shown to provide benefit in promoting healing over the use of AlloDerm alone, though not as efficient as vascularized tissue alone.18 Another advantage of the NSF is the ability to characterize the flap postoperatively for potential flap failure.19 Characteristic appearance on MRI allows the treating surgeon to monitor changes in the flap, such as tumor recurrence.18 Unfortunately, AlloDerm and nonvascular reconstructive options that result in a less vascular or predictable scar may mask changes in the postoperative skull base.20
CONCLUSION
This review provides a comprehensive overview of the endoscopic reconstructive options available for reconstruction after EEA. Although acellular grafts have proven successful and can be applied in case specific scenarios that take into account select surgical and anatomical conditions, the NSF provides a universally applicable reconstructive option with an excellent success rate (96%) after overcoming the learning curve. The NSF, as well as other vascularized flaps, provides hardy closure with a pedicled blood supply that promotes wound healing. For this reason, the skull base reconstruction algorithm points to the increased use of various vascularized reconstructions for larger and higher complexity skull base defects.
References
- Hegazy H M, Carrau R L, Snyderman C H, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110(7):1166–1172. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Senior B A, Jafri K, Benninger M. Safety and efficacy of endoscopic repair of CSF leaks and encephaloceles: a survey of the members of the American Rhinologic Society. Am J Rhinol. 2001;15(1):21–25. doi: 10.2500/105065801781329356. [DOI] [PubMed] [Google Scholar]
- Kassam A, Thomas A J, Snyderman C, et al. Fully endoscopic expanded endonasal approach treating skull base lesions in pediatric patients. J Neurosurg. 2007;106(2, Suppl):75–86. doi: 10.3171/ped.2007.106.2.75. [DOI] [PubMed] [Google Scholar]
- Zanation A M, Snyderman C H, Carrau R L, Kassam A B, Gardner P A, Prevedello D M. Minimally invasive endoscopic pericranial flap: a new method for endonasal skull base reconstruction. Laryngoscope. 2009;119(1):13–18. doi: 10.1002/lary.20022. [DOI] [PubMed] [Google Scholar]
- Fortes F S, Carrau R L, Snyderman C H, et al. Transpterygoid transposition of a temporoparietal fascia flap: a new method for skull base reconstruction after endoscopic expanded endonasal approaches. Laryngoscope. 2007;117(6):970–976. doi: 10.1097/MLG.0b013e3180471482. [DOI] [PubMed] [Google Scholar]
- Fortes F S, Carrau R L, Snyderman C H, et al. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117(8):1329–1332. doi: 10.1097/mlg.0b013e318062111f. [DOI] [PubMed] [Google Scholar]
- Hadad G, Bassagasteguy L, Carrau R L, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- Kassam A, Carrau R L, Snyderman C H, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005;19(1):E8. [PubMed] [Google Scholar]
- Pinheiro-Neto C D, Prevedello D M, Carrau R L, et al. Improving the design of the pedicled nasoseptal flap for skull base reconstruction: a radioanatomic study. Laryngoscope. 2007;117(9):1560–1569. doi: 10.1097/MLG.0b013e31806db514. [DOI] [PubMed] [Google Scholar]
- Germani R M, Vivero R, Herzallah I R, Casiano R R. Endoscopic reconstruction of large anterior skull base defects using acellular dermal allograft. Am J Rhinol. 2007;21(5):615–618. doi: 10.2500/ajr.2007.21.3080. [DOI] [PubMed] [Google Scholar]
- Sautter N B, Batra P S, Citardi M J. Endoscopic management of sphenoid sinus cerebrospinal fluid leaks. Ann Otol Rhinol Laryngol. 2008;117(1):32–39. doi: 10.1177/000348940811700108. [DOI] [PubMed] [Google Scholar]
- Tabaee A, Anand V K, Brown S M, Lin J W, Schwartz T H. Algorithm for reconstruction after endoscopic pituitary and skull base surgery. Laryngoscope. 2007;117(7):1133–1137. doi: 10.1097/MLG.0b013e31805c08c5. [DOI] [PubMed] [Google Scholar]
- Lorenz R R, Dean R L, Hurley D B, Chuang J, Citardi M J. Endoscopic reconstruction of anterior and middle cranial fossa defects using acellular dermal allograft. Laryngoscope. 2003;113(3):496–501. doi: 10.1097/00005537-200303000-00019. [DOI] [PubMed] [Google Scholar]
- Sonnenburg R E, White D, Ewend M G, Senior B. Sellar reconstruction: is it necessary? Am J Rhinol. 2003;17(6):343–346. [PubMed] [Google Scholar]
- Ismail A S, Costantino P D, Sen C. Transnasal transsphenoidal endoscopic repair of CSF leakage using multilayer acellular dermis. Skull Base. 2007;17(2):125–132. doi: 10.1055/s-2007-970556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M R, Shah R N, Snyderman C H, et al. Pericranial flap for endoscopic anterior skull-base reconstruction: clinical outcomes and radioanatomic analysis of preoperative planning. Neurosurgery. 2010;66(3):506–512. discussion 512. doi: 10.1227/01.NEU.0000365620.59677.FF. [DOI] [PubMed] [Google Scholar]
- Oliver C L, Hackman T G, Carrau R L, et al. Palatal flap modifications allow pedicled reconstruction of the skull base. Laryngoscope. 2008;118(12):2102–2106. doi: 10.1097/MLG.0b013e318184e719. [DOI] [PubMed] [Google Scholar]
- Chang D W, Satterfield W C, Son D, et al. Use of vascularized periosteum or bone to improve healing of segmental allografts after tumor resection: an ovine rib model. Plast Reconstr Surg. 2009;123(1):71–78. doi: 10.1097/PRS.0b013e3181904baf. [DOI] [PubMed] [Google Scholar]
- Kang M D, Escott E, Thomas A J, et al. The MR imaging appearance of the vascular pedicle nasoseptal flap. AJNR Am J Neuroradiol. 2009;30(4):781–786. doi: 10.3174/ajnr.A1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler R W, Mariani L. Sellar reconstruction with resorbable vicryl patches, gelatin foam, and fibrin glue in transsphenoidal surgery: a 10-year experience with 376 patients. J Neurosurg. 2000;93(5):762–765. doi: 10.3171/jns.2000.93.5.0762. [DOI] [PubMed] [Google Scholar]