Abstract
The osteoinduction of porous biphasic calcium phosphate ceramics (BCP) has been widely reported and documented, but little research has been performed on rodent animals, e.g., mice. In this study, we report osteoinduction in a mouse model. Thirty mice were divided into two groups. BCP materials (Sample A) and control ceramics (Sample B) were implanted into the leg muscle, respectively. Five mice in each group were killed at 15, 30, and 45 d after surgery. Sample A and Sample B were harvested and used for hematoxylin and eosin (HE) staining, immunohistochemistry (IHC) staining, and Alizarin Red S staining to check bone formation in the biomaterials. Histological analysis showed that no bone tissue was formed 15 d after implantation (0/5) in either of the two groups. Newly-formed bone tissues were observed in Sample A at 30 d (5/5) and 45 d (5/5) after implantation; the average amounts of newly-formed bone tissues were approximately 5.2% and 8.6%, respectively. However, we did not see any bone tissue in Sample B until 45 d after implantation. Bone-related molecular makers such as bone morphogenesis protein-2 (BMP-2), collagen type I, and osteopontin were detected by IHC staining in Sample A 30 d after implantation. In addition, the newly-formed bone was also confirmed by Alizarin Red S staining. Because this is the report of osteoinduction in the rodent animal on which all the biotechnologies were available, our results may contribute to further mechanism research.
Keywords: Osteoinduction, Hydroxyapatite/β-tricalcium phosphate, Biphasic calcium phosphate, Osteogenesis
1. Introduction
In the past few decades, osteoinduction has been widely reported and documented. Ripamonti et al. (1989) reported bone induction in a composite allogeneic bone/alloplastic implant; Zhang et al. (1991), Osborn (1991), and Ripamonti (1991a) discovered bone formation at ectopic sites after implantation of porous calcium phosphate ceramic materials in dogs and baboons. Later, osteoinductive bioceramics began to be reported by different labs in pigs, sheep, rabbits, and other large animal models all over the world (Damien and Parsons, 1991; Toth et al., 1993; Li et al., 1994; Yuan et al., 2000; 2001a; Nihouannen et al., 2005; 2008; Ye et al., 2007; Fellah et al., 2008). Since then, generous attention was paid to these kinds of biomaterials, such as synthetic hydroxyapatite ceramics (HA), porous biphasic calcium phosphate ceramics (BCP), tricalcium phosphate ceramics (TCP), calcium pyrophosphate ceramics, and coral-derived hydroxyapatite. These ceramic materials have been shown to have intrinsic osteoinduction (Ducheyne and Cuckler, 1992; Toth et al., 1993; Yang et al., 1997; Yuan et al., 1998a; 1998b; Nihouannen et al., 2005; 2008; Ye et al., 2007; Fellah et al., 2008). However, the main evidence of bone formation in these biomaterials was based on histological analysis and there were few reports focusing on the molecular mechanism.
Based on the extensive research work surrounding these biomaterials, the intriguing phenomena have been categorized into two aspects: material factors and biological factors (Zhang et al., 2000; Yuan et al., 2002; Habibovic et al., 2005; Fan et al., 2007; Fellah et al., 2008; Nihouannen et al., 2008; Ripamonti et al., 2009). The majority of research performed on these types of bioceramic materials was focused on the material factors including porosity, porous structure, phase composition, crystallinity, and sintering temperature, etc. (Yuan et al., 1999; Zhang et al., 2000). The osteoinduction of these biomaterials was described as material-dependent. The quantity and quality of the newly-formed bone tissue were affected by a series of material factors. Fellah et al. (2008) found that high micro-porosity and a small crystal size were essential for the adhesion, proliferation, and differentiation of the osteogenic cells which produced the bone extracellular matrix. Fan et al. (2007) and Kasten et al. (2008) reported that the biological function of osteoinduction depended on the micro/nano structural surface characteristics of the biomaterials. Habibovic et al. (2005) investigated a 3D microenvironment of osteoinductive biomaterials and discovered that the presence of micropores within macropore walls was an essential prerequisite for osteoinduction. On the other hand, for the biological factors, these osteoinductive Ca-P biomaterials were found to be animal-species dependent (Ripamonti, 1991c; Ducheyne and Cuckler, 1992; Yamasaki and Sakai, 1992; Green et al., 1995; Nihouannen et al., 2005; 2008; Fellah et al., 2008). Based on these studies, osteogenesis was reported in baboons, monkeys, pigs, dogs, goats, and rabbits after undergoing different types of implantation (Ripamonti, 1991b; 1996; Yang et al., 1997; Yuan et al., 2002; 2006; Ye et al., 2007; Nihouannen et al., 2008). Overall, the majority of the animals were quite large but included few rodent animals (rats or mice).
However, further mechanism research was difficult to perform on large animals, because most of the molecular biotechniques were specifically based for rodent animals. Owing to this restriction, the mechanism of osteoinduction was not well explored; e.g., the source of stem cells, the types of signalling molecules and transcription factors, as well as the regulatory mechanism remained unknown. Thus, osteoinduction in the mouse model was considered to be the prerequisite for the above mentioned studies.
Our study was designed to investigate osteoinduction in a mouse model with the use of two types of bioceramic materials. Immunohistochemistry (IHC) staining and Alizarin Red S staining (a bone chelating fluorescent markers for calcium) were used to confirm the newly-formed bone at the ectopic sites in the muscles of mice.
2. Materials and methods
2.1. Porous BCP biomaterials
Two different BCP biomaterials (Sample A and Sample B) were provided by the National Engineering Research Center for Biomaterials (Sichuan University, China). Both of them were composed of HA and β-TCP with a 70/30 ratio. The starting apatite powers with a Ca/P ratio of 1.50 were wet-synthesized. Porous green bodies were foamed by 5%–10% H2O2 under 70–80 °C. Sample A was sintered at 1 100 °C and Sample B was sintered at 1 200 °C for 2 h, respectively, then cooled naturally. Microporosities of Sample A and Sample B were approximately 50%–60% and 20%–30%, respectively, both with pore dimensions ranging from 100 to 500 μm. The total porosity, including macropores and micropores, in Sample A was considerably more than that in Sample B. The chemical characteristics and surface structures of the two samples were measured by X-ray diffraction (XRD) and scanning electron microscopy (SEM). The ceramics were fabricated into Φ3 mm×5 mm cylinders and steam-sterilized at 121 °C for 30 min before implantation.
2.2. Animals
Thirty mice were obtained from the Sichuan University Laboratory Animal Center (strain name: Balb/C; body weight: 28–32 g; gender: male), and were maintained in ventilated air-filtered cages in a temperature and light-controlled environment. All of the experiments were approved by the Animal Care and Use Committee of Sichuan University. All of the animals were anaesthetized by intraperitoneal injection of 0.02 g/ml pentobarbital sodium (40 mg/kg body weight; Sigma Chemical, St. Louis, MO, USA) before implantation and injected with penicillin at 2.5 U/time twice a day for 3 d continuously post-operation to prevent infection. All procedures were performed according to the Guideline for the Care and Use of Laboratory Animals of the National Institutes of Health (Publication No. 85-23, Revision, 1985) under the supervision of a licensed veterinarian.
2.3. Surgical procedure
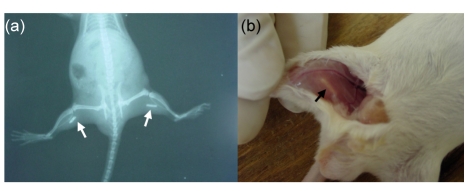
Thirty mice were divided into two groups. Each animal was implanted with different ceramics in the muscles of both hind legs (Sample A: Group 1; Sample B: Group 2). X-ray pictures were taken to confirm the positions of the samples in the muscles (Fig. 1a). Five mice of each group were sacrificed with overdose of pentobarbital sodium at 15, 30, and 45 d after implantation. All of the implants were traced at the implanted sites (Fig. 1b). The implants were harvested with surrounding soft tissues together and then fixed in 10% neutral formalin (pH=7.2) for 24–48 h at room temperature.
Fig. 1.
Detection of implanted sites of BCP biomaterials
(a) X-ray picture was taken to show the position of the implanted site; (b) All the implants were traced in the implanted site. Arrows: the implanted materials
2.4. Histological preparation
The fixed samples were decalcified in a fast-decalcifying fluid (hydrochloric acid 8 ml, methanoic acid 8 ml, and distilled water 184 ml) for 24 h, washed with phosphate buffer solution (PBS), dehydrated with gradient of ethanol solutions at 70%, 80%, 90%, 95% and 100%, and then embedded in paraffin (melting point 56–58 °C). Continuous 5-μm sections were made, and transferred onto slides. The sections were then stained with hematoxylin and eosin (HE) for histological analysis. Sequential sections were also prepared for IHC staining and Alizarin Red S staining.
2.5. IHC staining
The sections of 30 d in Group 1 were deparaffinized, and rehydrated in water. The endogenous peroxidase was blocked with 3% H2O2 and epitope was retrieved under pressure sterilizer. Then, the sections were further incubated with primary antibody of anti-mouse collagen type I (1:500; Santa Cruz, CA, USA) or anti-mouse osteopontin (1:500; Santa Cruz) or anti-mouse bone morphogenesis protein-2 (BMP-2) (1:500; Santa Cruz) over night at 4 °C. After being washed with PBS five times, the sections were incubated with proper horseradish peroxidase (HRP)-labeled second antibodies for 1 h at 37 °C. Subsequently, the sections were developed with 3,3′-diaminobenzidine (DAB; Pierce Biotechnology, USA) and counterstained with hematoxylin.
2.6. Alizarin Red S staining
Alizarin Red S staining was conducted under the description by Sontag (1980), which was applied by Qu et al. (2004). Briefly, the serial sections were deparaffinized, and rehydrated in water. Immersion solutions (0.01 g/ml) were prepared by dissolving 1 g of Alizarin S (Sigma Chemical, St. Louis, MO, USA) in 90 ml deionized water plus 10 ml of 0.01 g/ml NH4OH. The sections were then immersed in the solutions for 10 min. After the immersion, the sections were rinsed in fresh water for several minutes, and then allowed to stand for 10 min to allow the unbound Alizarin S to diffuse out of the tissues. After being quickly immersed in 0.05 g/ml acetic acid, the sections were dehydrated in ethanol. Observations were carried out at 400× magnification by using an Olympus IX 71 microscope with green and blue fluorescence rays.
3. Results
3.1. Characterization of BCP biomaterials
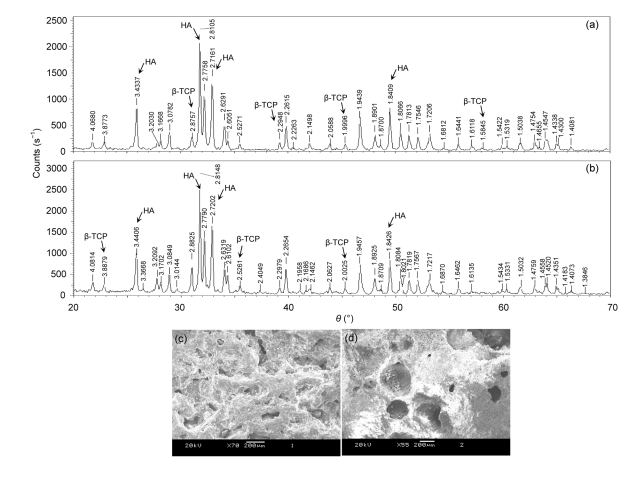
As shown in Fig. 2, there was significant difference in the surface structure and morphology between the two types of BCP biomateials. All of them contained 70% HA and 30% β-TCP, but the porosity of each group was obviously different. Microporosity of Sample A was approximately 50%–60%, while that of Sample B was approximately 20%–30%. The total porosity in Sample A was more than that in Sample B. For SEM observations, the architectures of the two biomaterials possess a trabeculae-like structure and interconnected micropores. But, Sample A had a rough surface with small crystals and uniform distribution macropores, while the surfaces of the Sample B had less pores (Figs. 2c and 2d).
Fig. 2.
XRD (a, b) and SEM (c, d) images of the two different BCP matierals sintered at different temperatures
(a, c) Sample A: BCP bioceramics sintered at 1 100 °C; (b, d) Sample B: BCP biomaterials sintered at 1 200 °C
3.2. Histology observation
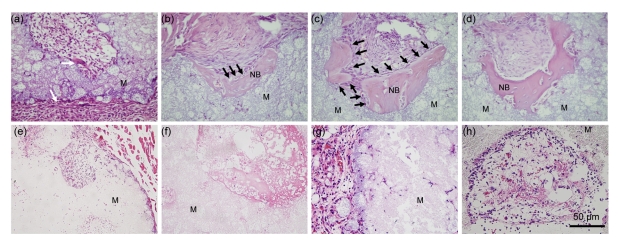
In Group 1, the bioceramic rods were observed to be encapsulated with a layer of dense connective tissues 15 d after implantation (Fig. 3a). Monocytes and lymphocytes were found infiltrated in these dense connective tissues. A large amount of active mesenchymal tissues were found outside of these porous BCP biomaterials. Macrophages were also observed on the interface between the biomaterials and mesenchymal tissues (Fig. 3a). Thirty days after implantation, the bioceramic rods were also encapsulated with a layer of dense connective tissues. A large number of irregular arrangements of bone tissues were found outer of the collected biomaterials in all animals (5/5) (Figs. 3b and 3c). Some osteoblasts were observed to be excreting bone matrix and embedding themselves to form bone lacuna (Fig. 3b). The osteoblasts were observed linearly on the interface between the induced bone and the mesenchymal tissues (Fig. 3c). Forty-five days after implantation, more bone tissues were observed in the micropores of the bioceramics in all animals (5/5), the arrangement was more regular compared with the bone tissues harvested at 30 d. Some calcification lines could be observed in the bone matrix (Fig. 3d). Overall, after implantation of Sample A, the average amounts of newly-formed bone tissues were about 5.2% and 8.6% in 30 and 45 d, respectively. However, bone tissue was not found until 45 d after implantation in Sample B (Figs. 3e–3h). Only inflammatory cells, such as monocytes, macrophages and lymphocytes, were observed in the implanted biomaterials (Figs. 3g and 3h).
Fig. 3.
HE staining of two different types of BCP biomaterials harvested from mice
Histological features of Sample A (a–d) and Sample B (e–h) were observed after 15, 30, and 45 d. (a, e) Histological features of BCP biomaterials implanted in the muscles of mice after 15 d. (b, c, f, g) Histological features of BCP biomaterials implanted in the muscles of different mice after 30 d; (d, h) Histological features of BCP biomaterials implanted in the muscles of mice after 45 d. M: decalcified biomaterials; NB: induced bone. White arrow: dense connective layer; Black arrow: induced bone
3.3. Immunohistochemistry
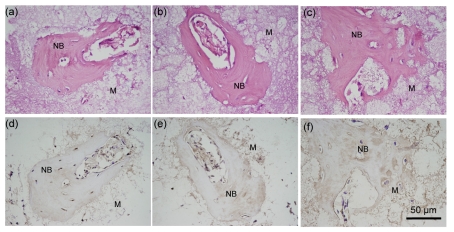
Thirty days after implantation, serial sections of Sample A were used for HE and IHC stainings. The brown color indicated the expression of the specific antigen BMP-2. As shown in microscopic pictures, BMP-2 was positive within all the osteocytes in the lacunae (Fig. 4d), and collagen type I was located within the osteocytes and in the bone matrix of the harvested bone tissue (Fig. 4e). The linearly-arranged osteoblasts were positively identified with the presence of collagen type I. Also, the collagen type I in the surrounding connective tissues was in brown color. Osteopontin was also tested to be positive within the osteocytes and in the bone matrix (Fig. 4f).
Fig. 4.
HE staining (a–c) and IHC staining (d–f) of serial sections of Sample A after 30-d implantation
(a, d) Primary antibody of anti-mouse BMP-2; (b, e) Primary antibody of anti-mouse collagen type I; (c, f) Primary antibody of anti-mouse osteopontin. M: decalcified biomaterials; NB: induced bone. Brown colored area: positive region
3.4. Alizarin Red S staining
Serial sections were used for HE staining and Alizarin S staining after 30 d of implantation in Group 1. For the Alizarin Red S section, the observation was conducted under the excitations of green and blue fluorescence rays. The fluorescent marker chelated to the induced bone was excited to emit red ray under the excitation of green fluorescence ray (Fig. 5b), but no signal was detected under the excitation of blue ray (data not shown).
Fig. 5.
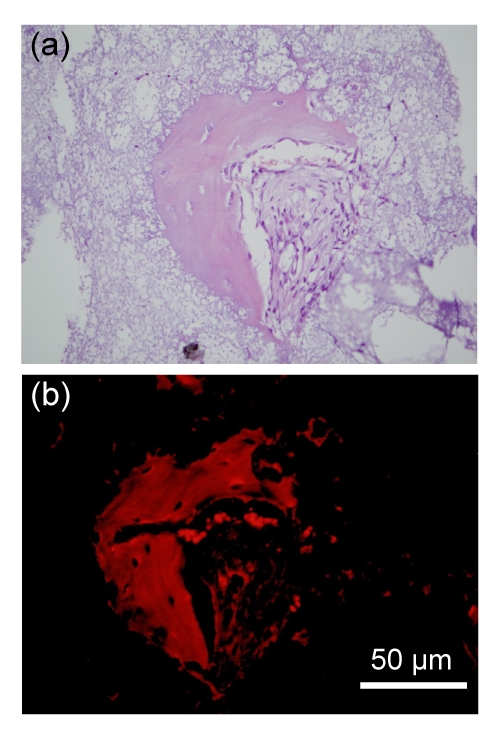
Induced bone of Sample A after 30-d implantation
(a) HE staining; (b) Alizarin Red S staining. The fluorescence marker chelated to calcium emitted red ray
4. Discussion
This research addressed the bone formation at ectopic sites in a mouse model by the specific bioceramic materials. Previously, a large number of studies have reported osteoinduction of bioceramic materials mainly in dogs, pigs, monkeys, baboons, goats, and rabbits (Ripamonti, 1991a; Ripamonti, 1996; Yang et al., 1997; Yuan et al., 2002; 2006; Ye et al., 2007; Nihouannen et al., 2008; Kasten et al., 2008). As indicated in the reports of Fellah’s team, osteoinduction by bioceramic materials is a complex biological phenomenon which is not fully understood, but has shown that ectopic bone formation is both material and animal dependant (Nihouannen et al., 2005; 2008; Fellah et al., 2008). Cheng et al. (2010) reported osteoinduction in the fractured fibula of mice by certain calcium phosphate ceramics containing 60% HA and 40% β-TCP. Consistently, we found bone tissue could be induced in mice muscles without any specific treatment on the animals. More importantly, the common mouse was available for all of the modern biotechniques. Based on our results, the achievements in molecular biology could be used for the further research in this area.
In this study, we implanted two different BCP biomaterials into the leg muscles of mice. A large number of newly-formed bone tissues were found outer of the collected biomaterials after implantation with BCP materials sintered at 1 100 °C in 30 and 45 d. However, only a large amount of monocytes and lymphocytes were observed after implantation with BCP materials sintered at 1 200 °C within 45 d. These results were confirmed by labelling bone-related molecular makers, such as BMP-2, collagen type I, and osteopontin, as well as Alizarin Red S staining. Previous research indicated that bone formation was observed in contact with the BCP granules in ectopic sites after six weeks, but this research was performed on a large-bodied animal model (Fellah et al., 2008). In addition, the preparation of the bioceramic materials was not very identical (Cheng et al., 2010). It has been reported that the sintering temperature, microstructure, and porosity of the materials seemed to play a critical role in the bone-induction mechanism in ectopic sites, and ectopic bone formation was firstly observed not only inside the macropores, but also between the ceramic particles (Nihouannen et al., 2005; 2008; Fellah et al., 2008). Our results also showed that specific BCP ceramics were osteoinductive in mice, and this osteoinduction was closely related to the properties of the biomaterials. Therefore, the distributions and sizes of the macro- and micro-pores, sintering temperature, as well as the surface structure, might play an important role in osteogenic differentiation in vivo (Habibovic et al., 2005; Fellah et al., 2007; 2010; Kasten et al., 2008).
Previous reports on the bone formation at ectopic sites induced by these biomaterials were mainly based on histological analysis. Decalcified and undecalcified sections were made for histological observation under ordinary optical microscopes and back-scattered scanning electron microscopy (BSEM) (Nihouannen et al., 2005; Yuan et al., 2006; Fan et al., 2007; Fellah et al., 2008). Fellah et al. (2007; 2010) recently reported that the inflammatory cytokines, e.g., IL-6 and TNF-α, released by macrophages stimulated by BCP microparticles, may have both positive and negative effects on new bone formation. However, the very little related research that labelled the marker molecules expressed by the osteocytes or osteoblasts has been performed and was most likely due to the limitation of animal models to some extent (Hennessy et al., 2009). In this study, we observed bone formation in the mice model and our results may contribute to further mechanism studies based on the modern biotechnologies developed in the mice model.
This type of biomaterial was expected to be used in orthopaedic, maxillofacial surgery and dental devices as bone substitute, but the prerequisite is safety. Wang et al. (2004a) compared the bone-related gene expression of human primary osteogenic sarcoma cell line SaOS-2 cultured on calcium phosphate ceramics with different phase compositions and different sintering temperatures in vitro. The gene expression patterns from his and other researches supported the biocompatibility and bioactivity potentials of calcium phosphate ceramics (Wang C. et al., 2004b; Wang H. et al., 2007; Wang J.J. et al., 2009; Guo et al., 2009). Previous research on safety was mainly focused on histological analysis of long-term tissue response after the biomaterials were implanted in the animals. Yuan et al. (2001b) reported that the quality and quantity of the induced bone by BCP bioceramics were normal with bone marrow after 2.5-year tissue response in dogs. Ye et al. (2007) reported that based on observation of 4.5-year tissue response in pigs, the newly-formed bone after implantation of BCP bioceramics neither disappeared nor gave rise to autonomous growth, and the surrounding soft tissues were normal with no presence of tumor cells. Owing to all, the results regarding osteoinduction were based on histological analysis and we thought that further research should be directed towards a better understanding at molecular levels. In addition, the research on the osteoinduction was still limited, and many questions needed to be clarified, e.g., the origin of the osteoblasts, signaling molecules, and the transcriptional factors involved in the process of the stem-cell differentiation.
5. Conclusions
In this study we reported osteoinduction with the specific BCP biomaterials in mice. Our research indicated that bone tissues could be induced in the muscles of mice in 30 d after implantation of specific BCP biomaterials sintered at 1 100 °C at a 70/30 ratio of HA and β-TCP. The average amounts of newly-formed bone tissues were approximately 5.2% and 8.6% in 30 and 45 d, respectively. The results were confirmed by bone-related molecular makers, such as BMP-2, collagen type I, and osteopontin, and also confirmed by Alizarin Red S staining. Based on these data, we concluded that specific BCP biomaterials could induce bone formation in the mice model. Our results may contribute to further mechanism research benefiting from the modern biotechnologies developed on a mouse model.
Acknowledgments
We would like to thank Dr. Shu XU (Department of Pathology, Guiyang Medical Collage, Guiyang, China), Dr. Hong-ying ZHANG (Department of Pathology, West China Hospital, Sichuan University, Chengdu, China), Miss Ming-xia QIN, Dr. Zhi-dan TU, Mr. Ji BAO, Dr. Li ZHANG (Key Laboratory of Transplant Engineering and Immunology, Ministry of Health, West China Hospital, Sichuan University, Chengdu, China), and Mr. Fei-wu LONG (Chengdu Second People Hospital, Chengdu, China) for their help during the research.
Footnotes
Project (No. 2005CB623901) supported by the National Basic Research Program (973) of China
References
- 1.Cheng LJ, Ye F, Yang RN, Lu XF, Shi YJ, Li L, Fan HS, Bu H. Osteoinduction of hydroxyapatite/β-tricalcium phosphate bioceramics in mice with a fractured fibula. Acta Biomater. 2010;6(4):1569–1574. doi: 10.1016/j.actbio.2009.10.050. [DOI] [PubMed] [Google Scholar]
- 2.Damien CJ, Parsons JR. Bone graft and bone graft substitutes: a review of current technology and applications. J Appl Biomater. 1991;2(3):187–208. doi: 10.1002/jab.770020307. [DOI] [PubMed] [Google Scholar]
- 3.Ducheyne P, Cuckler JM. Bioactive ceramic prosthetic coatings. Clin Orthop Relat Res. 1992;276:102–114. [PubMed] [Google Scholar]
- 4.Fan HS, Ikoma T, Tanaka J, Zhang XD. Surface structural biomimetics and the osteoinduction of calcium phosphate biomaterials. J Nanosci Nanetechnol. 2007;7(3):808–813. doi: 10.1166/jnn.2007.501. [DOI] [PubMed] [Google Scholar]
- 5.Fellah BH, Josselin N, Chappard D, Weiss P, Layrolle P. Inflammatory reaction in rats muscle after implantation of biphasic calcium phosphate micro particles. J Mater Sci Mater Med. 2007;18(2):287–294. doi: 10.1007/s10856-006-0691-8. [DOI] [PubMed] [Google Scholar]
- 6.Fellah BH, Gauthier O, Weiss P, Chappard D, Layrolle P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials. 2008;29(9):1177–1188. doi: 10.1016/j.biomaterials.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 7.Fellah BH, Delorme B, Sohier J, Magne D, Hardouin P, Layrolle P. Macrophage and osteoblast responses to biphasic calcium phosphate microparticles. J Biomed Mater Res Part A. 2010;93A(4):1588–1595. doi: 10.1002/jbm.a.32663. [DOI] [PubMed] [Google Scholar]
- 8.Green JP, Wojno TH, Wilson MW, Grossniklaus HE. Bone formation in hydroxyapatite orbital implants. Am J Ophthalmol. 1995;120(5):681–682. doi: 10.1016/s0002-9394(14)72222-6. [DOI] [PubMed] [Google Scholar]
- 9.Guo H, Su J, Wei J, Kong H, Liu C. Biocompatibility and osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. Acta Biomater. 2009;5(1):268–278. doi: 10.1016/j.actbio.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Habibovic P, Yuan H, van der Valk CM, Meijer G, van Blitterswijk CA, de Groot K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials. 2005;26(17):3565–3575. doi: 10.1016/j.biomaterials.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 11.Hennessy KM, Pollot BE, Clem WC, Phipps MC, Sawyer AA, Culpepper BK, Bellis SL. The effect of collagen I mimetic peptides on mesenchymal stem cell adhesion and differentiation, and on bone formation at hydroxyapatite surfaces. Biomaterials. 2009;30(10):1898–1909. doi: 10.1016/j.biomaterials.2008.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kasten P, Beyen I, Niemeyer P, Luginbuhl R, Bohner M, Richter W. Porosity and pore size of β-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: an in vitro and in vivo study. Acta Biomater. 2008;4(6):1904–1915. doi: 10.1016/j.actbio.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Li YB, Klein CP, Zhang X, de Groot K. Formation of a bone apatite-like layer on the surface of porous hydroxyapatite ceramics. Biomaterials. 1994;15(10):835–841. doi: 10.1016/0142-9612(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 14.Nihouannen DL, Daculsi G, Saffarzadeh A, Gauthier O, Delplace S, Pilet P, Layrolle P. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone. 2005;36(6):1086–1093. doi: 10.1016/j.bone.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 15.Nihouannen DL, Saffarzadeh A, Gauthier O, Moreau F, Pilet P, Spaethe R, Layrolle P, Daculsi G. Bone tissue formation in sheep muscles induced by a biphasic calcium phosphate ceramic and fibrin glue composite. J Mater Sci Mater Med. 2008;19(2):667–675. doi: 10.1007/s10856-007-3206-3. [DOI] [PubMed] [Google Scholar]
- 16.Osborn JF. The Biological Profile of Hydroxyapatite Ceramic with Respect to the Cellular Dynamics of Animal and Human Soft Tissue and Mineralized Tissue under Unloaded and Loaded Conditions. In: Barbosa MA, editor. Biomaterials Degradation. New York: Elsevier Science Publishers; 1991. pp. 185–225. [Google Scholar]
- 17.Qu SX, Guo X, Weng J, Cheng JC, Feng B, Yeung HY, Zhang XD. Evaluation of the expression of collagen type I in porous calcium phosphate ceramics implanted in an extra-osseous site. Biomaterials. 2004;25(4):659–667. doi: 10.1016/S0142-9612(03)00577-5. [DOI] [PubMed] [Google Scholar]
- 18.Ripamonti U. Bone induction in nonhuman primates. An experimental study on the baboon. Clin Orthop Relat Res. 1991;269:284–294. [PubMed] [Google Scholar]
- 19.Ripamonti U. The induction of bone in osteogenic composites of bone matrix and porous hydroxyapatite replicas: an experimental study on the baboon (Papio ursinus) J Oral Maxillofac Surg. 1991;49(8):817–830. doi: 10.1016/0278-2391(91)90010-J. [DOI] [PubMed] [Google Scholar]
- 20.Ripamonti U. The morphogenesis of bone in replicas of porous hydroxyapatite obtained from conversion of calcium carbonate exoskeletons of coral. J Bone Joint Surg Am. 1991;73(5):692–703. [PubMed] [Google Scholar]
- 21.Ripamonti U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials. 1996;17(1):31–35. doi: 10.1016/0142-9612(96)80752-6. [DOI] [PubMed] [Google Scholar]
- 22.Ripamonti U, Schnitzler CM, Cleaton-Jones PC. Bone induction in a composite allogeneic bone/alloplastic implant. J Oral Maxillofac Surg. 1989;47(9):963–969. doi: 10.1016/0278-2391(89)90381-9. [DOI] [PubMed] [Google Scholar]
- 23.Ripamonti U, Jean C, Lerato K, Laura R. The induction of bone formation by coral-derived calcium carbonate/hydroxyapatite constructs. Biomaterials. 2009;30(7):1428–1439. doi: 10.1016/j.biomaterials.2008.10.065. [DOI] [PubMed] [Google Scholar]
- 24.Sontag W. An automatic microspectrophotometric scanning method for the measurement of bone formation rates in vivo. Calcif Tissue Int. 1980;32(1):63–68. doi: 10.1007/BF02408522. [DOI] [PubMed] [Google Scholar]
- 25.Toth JM, Lynch KL, Hackbarth DA. Ceramic-induced osteogenesis following subcutaneous implantation of calcium phosphates. Bioceramics. 1993;6:9–13. [Google Scholar]
- 26.Wang C, Duan Y, Markovic B, Barbara J, Howlett CR, Zhang X, Zreiqat H. Phenotypic expression of bone-related genes in osteoblasts grown on calcium phosphate ceramics with different phase compositions. Biomaterials. 2004;25(13):2507–2514. doi: 10.1016/j.biomaterials.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 27.Wang C, Duan Y, Markovic B, Barbara J, Howlett CR, Zhang X, Zreiqat H. Proliferation and bone-related gene expression of osteoblasts grown on hydroxyapatite ceramics sintered at different temperature. Biomaterials. 2004;25(15):2949–2956. doi: 10.1016/j.biomaterials.2003.09.088. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Li Y, Zuo Y, Li J, Sansi M, Cheng L. Biocompatibility and osteogenesis of biomimetic nano-hydroxyapatite/polyamide composite scaffolds for bone tissue engineering. Biomaterials. 2007;28(22):3338–3348. doi: 10.1016/j.biomaterials.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Wang JJ, Ye F, Cheng LJ, Shi YJ, Bao J, Sun HQ, Wang W, Zhang P, Bu H. Osteogenic differentiation of mesenchymal stem cells promoted by overexpression of connective tissue growth factor overexpression of connective tissue growth factor. J Zhejiang Univ Sci B. 2009;10(5):355–367. doi: 10.1631/jzus.B0820252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamasaki H, Sakai H. Osteogenic response to porous hydroxyapatite ceramics under the skin of dogs. Biomaterials. 1992;13(5):308–312. doi: 10.1016/0142-9612(92)90054-R. [DOI] [PubMed] [Google Scholar]
- 31.Yang ZJ, Yuan H, Zou P, Tong W, Qu S, Zhang XD. Osteogenic responses to extraskeletally implanted synthetic porous calcium phosphate ceramics: an early stage histomorphological study in dogs. J Mater Sci Mater Med. 1997;8(11):697–701. doi: 10.1023/A:1018540024082. [DOI] [PubMed] [Google Scholar]
- 32.Ye F, Lu XF, Lu B, Wang JJ, Shi YJ, Zhang L, Chen JQ, Li YP, Bu H. A long-term evaluation of osteoinductive HA/beta-TCP ceramics in vivo: 4.5 years study in pigs. J Mater Sci Mater Med. 2007;18(11):2173–2178. doi: 10.1007/s10856-007-3215-2. [DOI] [PubMed] [Google Scholar]
- 33.Yuan H, Zou P, Yang Z, Zhang X, de Bruijn JD, de Groot K. Bone morphogenetic protein and ceramic-induced osteogenesis. J Mater Sci Mater Med. 1998;9(12):717–721. doi: 10.1023/A:1008998817977. [DOI] [PubMed] [Google Scholar]
- 34.Yuan H, Yang Z, Li Y, Zhang X, de Bruijn JD, de Groot K. Osteoinduction by calcium phosphate biomaterials. J Mater Sci Mater Med. 1998;9(12):723–726. doi: 10.1023/A:1008950902047. [DOI] [PubMed] [Google Scholar]
- 35.Yuan H, Kurashina K, de Bruijn JD, Li Y, de Groot K, Zhang X. A preliminary study on osteoinduction of two kinds of calcium phosphate ceramics. Biomaterials. 1999;20(19):1799–1806. doi: 10.1016/S0142-9612(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 36.Yuan H, Li Y, de Bruijn JD, de Groot K, Zhang X. Tissue responses of calcium phosphate cement: a study in dogs. Biomaterials. 2000;21(12):1283–1290. doi: 10.1016/S0142-9612(00)00016-8. [DOI] [PubMed] [Google Scholar]
- 37.Yuan H, de Bruijn JD, Li Y, Feng J, Yang Z, de Groot K, Zhang X. Bone formation induced by calcium phosphate ceramics in soft tissue of dogs: a comparative study between porous α-TCP and β-TCP. J Mater Sci Mater Med. 2001;12(1):7–13. doi: 10.1023/A:1026792615665. [DOI] [PubMed] [Google Scholar]
- 38.Yuan H, Yang Z, de Bruijn JD, de Groot K, Zhang X. Material-dependent bone induction by calcium phosphate ceramics: a 2.5-year study in dog. Biomaterials. 2001;22(19):2617–2623. doi: 10.1016/S0142-9612(00)00450-6. [DOI] [PubMed] [Google Scholar]
- 39.Yuan H, van den Doel M, Li S, van Blitterswijk CA, de Groot K, de Bruijn JD. A comparison of the osteoinductive potential of two calcium phosphate ceramics implanted intramuscularly in goats. J Mater Sci: Mater Med. 2002;13(12):1271–1275. doi: 10.1023/A:1021191432366. [DOI] [PubMed] [Google Scholar]
- 40.Yuan H, van Blitterswijk CA, de Groot K, de Bruijn JD. A comparison of bone formation in biphasic calcium phosphate (BCP) and hydroxyapatite (HA) implanted in muscle and bone of dogs at different time periods. J Biomed Mater Res Part A. 2006;78A(1):139–147. doi: 10.1002/jbm.a.30707. [DOI] [PubMed] [Google Scholar]
- 41.Zhang XD, Zhou P, Wu C, et al. Bioceramics and the Human Body. London: Elsevier Applied Science; 1991. p. 408. [Google Scholar]
- 42.Zhang XD, Yuan H, de Groot K. Calcium Phosphate Biomaterials with Intrinsic Osteoinductivity; The 6th World Biomaterials Congress ; Kamuela, Hawaii, USA. 2000. pp. 1–13. [Google Scholar]