Abstract
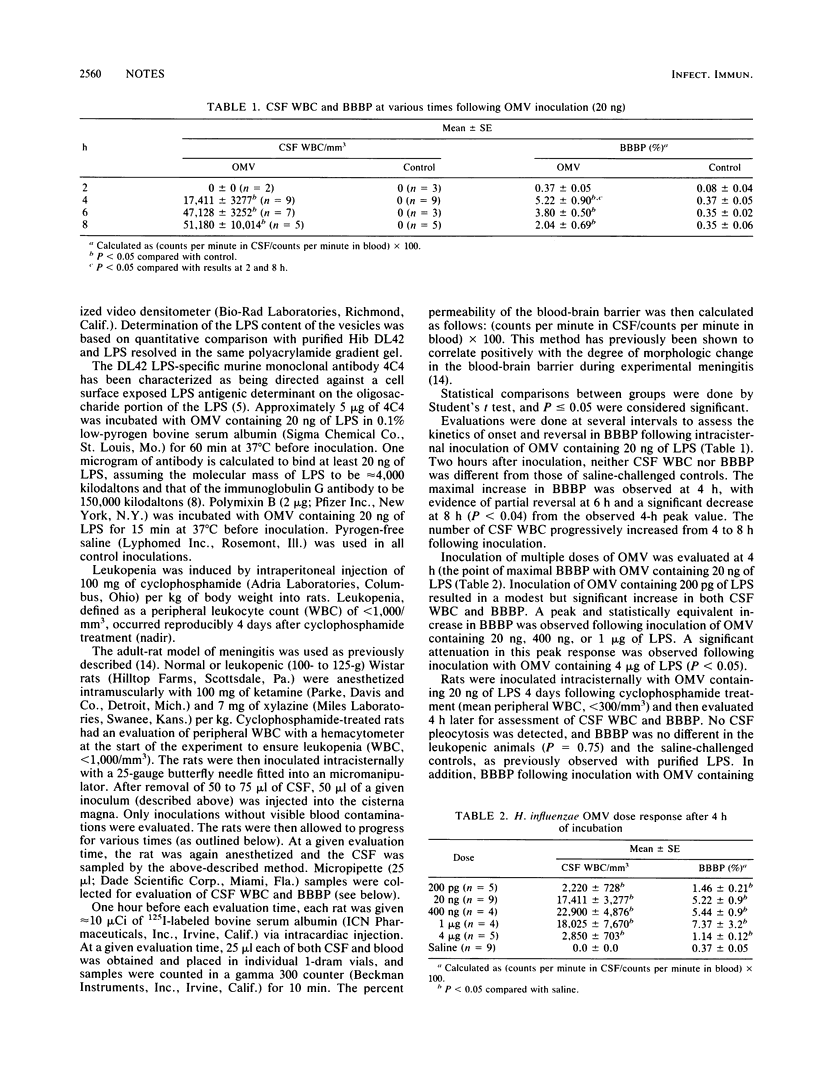
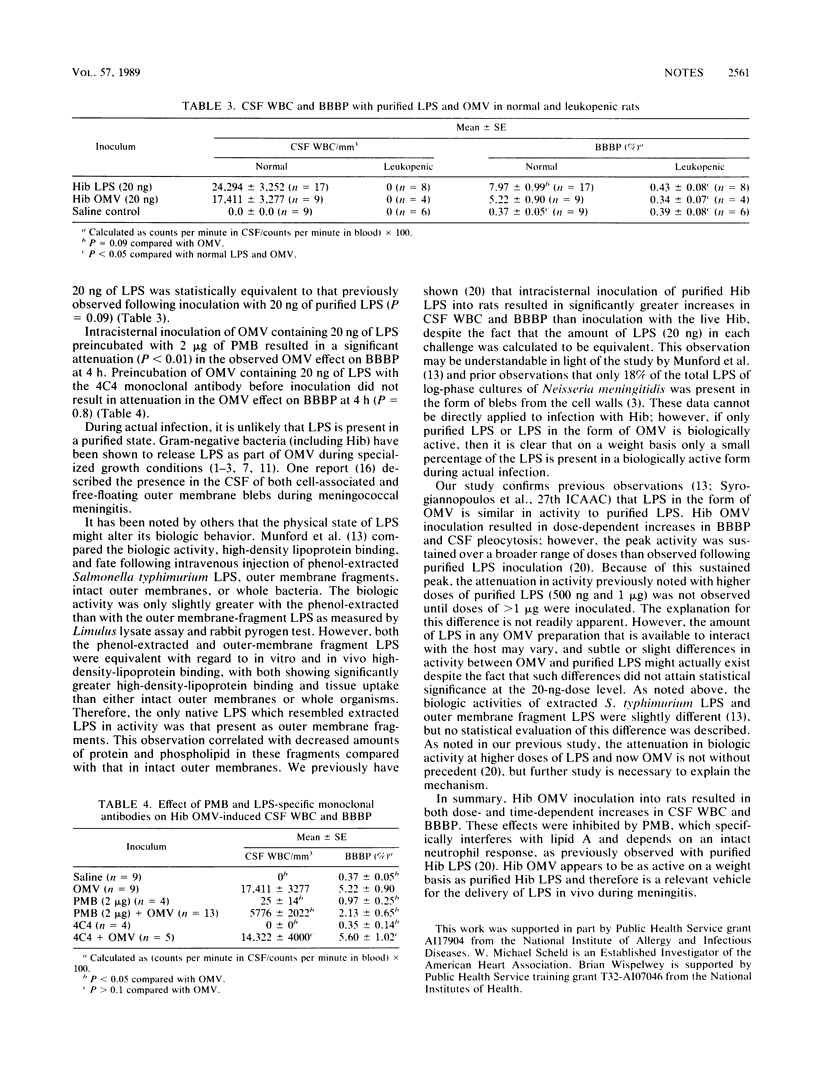
Haemophilus influenzae type b (Hib) lipopolysaccharide (LPS) may be present in the cerebrospinal fluid largely as part of outer membrane vesicles (OMV), which could possibly alter its activity. Similar to inoculation of purified Hib LPS, intracisternal inoculation of Hib OMV into adult rats resulted in dose- and time-dependent increases in blood-brain barrier permeability. Polymyxin B, but not an oligosaccharide-specific monoclonal antibody, significantly inhibited the activity of Hib OMV. No change in blood-brain barrier permeability occurred in leukopenic rats inoculated with Hib OMV. Hib OMV was as active as purified Hib LPS on a weight basis and therefore appears to be a relevant vehicle for the delivery of LPS during meningitis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. G., Work E. An extracellular glycolipid produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1965 Aug;96(2):567–576. doi: 10.1042/bj0960567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deich R. A., Hoyer L. C. Generation and release of DNA-binding vesicles by Haemophilus influenzae during induction and loss of competence. J Bacteriol. 1982 Nov;152(2):855–864. doi: 10.1128/jb.152.2.855-864.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., McCracken G. H., Jr, Frisch C. F., Johnston K. H., Hansen E. J. Antibody response of infants to cell surface-exposed outer membrane proteins of Haemophilus influenzae type b after systemic Haemophilus disease. Infect Immun. 1982 Jul;37(1):82–88. doi: 10.1128/iai.37.1.82-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulig P. A., Patrick C. C., Hermanstorfer L., McCracken G. H., Jr, Hansen E. J. Conservation of epitopes in the oligosaccharide portion of the lipooligosaccharide of Haemophilus influenzae type b. Infect Immun. 1987 Mar;55(3):513–520. doi: 10.1128/iai.55.3.513-520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J. M., Cheng K. J., Costerton J. W. Alkaline phosphatase of Pseudomonas aeruginosa: the mechanism of secretion and release of the enzyme from whole cells. Can J Microbiol. 1973 Nov;19(11):1407–1415. doi: 10.1139/m73-227. [DOI] [PubMed] [Google Scholar]
- Inzana T. J., Seifert W. E., Jr, Williams R. P. Composition and antigenic activity of the oligosaccharide moiety of Haemophilus influenzae type b lipooligosaccharide. Infect Immun. 1985 May;48(2):324–330. doi: 10.1128/iai.48.2.324-330.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Lesse A. J., Moxon E. R., Zwahlen A., Scheld W. M. Role of cerebrospinal fluid pleocytosis and Haemophilus influenzae type b capsule on blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest. 1988 Jul;82(1):102–109. doi: 10.1172/JCI113556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S. S., Wheeler B., Sanderson K. E., Costerton J. W., Cheng K. J. The release of alkaline phosphatase and of lipopolysaccharide during the growth of rough and smooth strains of Salmonella typhimurium. Can J Microbiol. 1973 Mar;19(3):335–343. doi: 10.1139/m73-056. [DOI] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Lipton J. M., Dietschy J. M. Biological activity, lipoprotein-binding behavior, and in vivo disposition of extracted and native forms of Salmonella typhimurium lipopolysaccharides. J Clin Invest. 1982 Oct;70(4):877–888. doi: 10.1172/JCI110684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliarello V. J., Long W. J., Scheld W. M. Morphologic alterations of the blood-brain barrier with experimental meningitis in the rat. Temporal sequence and role of encapsulation. J Clin Invest. 1986 Apr;77(4):1084–1095. doi: 10.1172/JCI112407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlech W. F., 3rd, Ward J. I., Band J. D., Hightower A., Fraser D. W., Broome C. V. Bacterial meningitis in the United States, 1978 through 1981. The National Bacterial Meningitis Surveillance Study. JAMA. 1985 Mar 22;253(12):1749–1754. [PubMed] [Google Scholar]
- Stephens D. S., Edwards K. M., Morris F., McGee Z. A. Pili and outer membrane appendages on Neisseria meningitidis in the cerebrospinal fluid of an infant. J Infect Dis. 1982 Oct;146(4):568–568. doi: 10.1093/infdis/146.4.568. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Täuber M. G., Borschberg U., Sande M. A. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J Infect Dis. 1988 Mar;157(3):456–464. doi: 10.1093/infdis/157.3.456. [DOI] [PubMed] [Google Scholar]
- Wispelwey B., Lesse A. J., Hansen E. J., Scheld W. M. Haemophilus influenzae lipopolysaccharide-induced blood brain barrier permeability during experimental meningitis in the rat. J Clin Invest. 1988 Oct;82(4):1339–1346. doi: 10.1172/JCI113736. [DOI] [PMC free article] [PubMed] [Google Scholar]



