Abstract
The developed standardized human cell based in vitro angiogenesis assay was intra-laboratory pre-validated to verify that the method is reliable and relevant for routine testing of modulators of angiogenesis, e.g., pharmaceuticals and industrial chemicals. This assay is based on the earlier published method but it was improved and shown to be more sensitive and rapid than the previous assay. The performance of the assay was assessed by using six reference chemicals, which are widely used pharmaceuticals that inhibit angiogenesis: acetyl salicylic acid, erlotinib, 2-methoxyestradiol, levamisole, thalidomide, and anti-vascular endothelial growth factor. In the intra-laboratory pre-validation, the sensitivity of the assay (upper and lower limits of detection and linearity of response in tubule formation), batch to batch variation in tubule formation between different Master cell bank batches, and precision as well as the reliability of the assay (reproducibility and repeatability) were tested. The pre-set acceptance criteria for the intra-laboratory pre-validation study were met. The relevance of the assay in man was investigated by comparing the effects of reference chemicals and their concentrations to the published human data. The comparison showed a good concordance, which indicates that this human cell based angiogenesis model predicts well the effects in man and has the potential to be used to supplement and/or replace of animal tests.
Keywords: angiogenesis, intra-laboratory method pre-validation, FGF-2, VEGF, tubule formation, in vitro assay
Introduction
Angiogenesis, the formation of new blood vessels, is a multistep process regulated by an interplay of pro- and anti-angiogenic factors. The steps involved are: endothelial cell proliferation, migration, differentiation, and tubule formation, as well as stabilization of newly formed blood vessels by a layer of pericytes and smooth muscle cells (Beilmann et al., 2004; Folkman, 2006; Nillesen et al., 2007). Angiogenesis is involved in critical physiological processes such as in embryonic development, wound healing, and female reproductive cycle (Friis et al., 2003; Berthod et al., 2006; Norrby, 2006), as well as in pathologic processes such as in tumor development and macular degeneration (Friis et al., 2003), rheumatoid arthritis (Friis et al., 2003; Middleton et al., 2004), ischemic diseases (van Weel et al., 2008; Cao, 2009), endometriosis (Rogers et al., 2009), and psoriasis (Folkman, 2006).
Due to complex interactions during angiogenesis, the evaluation of factors that affect angiogenesis would optimally be studied in vivo (Auerbach et al., 2003). The most commonly used in vivo angiogenesis assays include chick chorioallantoic membrane (CAM) assay, Matrigel plug assay, zebrafish embryo system, corneal micropocket assay, rat/mouse hind limb ischemia model, rat aortic ring assay, intradermal angiogenesis assays, and Xenopus tadpole assay (Auerbach et al., 2003). These in vivo assays are used to measure new blood vessel formation by pro- and anti-angiogenic factors and compounds (Akhtar et al., 2002; Auerbach et al., 2003; Norrby, 2006; Ishikane et al., 2008; Ziche and Morbidelli, 2009), and to study tumor angiogenesis (Auerbach et al., 2003; Norrby, 2006) or embryonic, and organogenic angiogenesis (Norrby, 2006). Despite the advantage of providing more information on complex cellular and molecular interactions compared to in vitro models (Norrby, 2006), animal models are burdened by several disadvantages such as variability, animal-specificity, and unethical procedures (Norrby, 2006; Ishikane et al., 2008). The current in vivo assays are not pertinent to human diseases as regards both efficacy and relevance (Norrby, 2006). Therefore, human cell based (in vitro) assays would have potential to be more predictive than animal models when investigating the effects in man.
Current in vitro angiogenesis assays include cell proliferation and cell migration assays, tubule formation assays, and organ culture assays (Auerbach et al., 2003; Ucuzian and Greisler, 2007). Three-dimensional in vitro assays permit cell-to-cell interactions, but responses are often difficult to evaluate and quantitate. Most cell culture assays (e.g., tubule formation assays in collagen or Matrigel, cell motility assays, trans-membrane assays, or cell proliferation assays) only reflect one single step of angiogenesis (Auerbach et al., 2003). An ideal in vitro assay would measure both stimulation and inhibition (Bishop et al., 1999; Norrby, 2006) and provide quantitative measurement of the tubule formation (Norrby, 2006). Although tubule formation in vitro does not cover the whole angiogenesis process, it effectively mimics the key steps, i.e., migration and differentiation of endothelial cells (Donovan et al., 2001).
The objective of the study was to intra-laboratory pre-validate the optimized test method for testing of angiogenesis modulators in routine use to replace and/or supplement animal experiments. The co-culture assay published by Bishop et al. (1999) and Donovan et al. (2001) was further developed, optimized and finally pre-validated in the laboratory. All critical steps including cell isolation procedure, cell number, cell passage, culture time, and tubule formation procedure as well as microscopic analysis were thoroughly investigated and optimized before the intra-laboratory pre-validation. During the intra-laboratory pre-validation, the performance of the assay was assessed by using six reference chemicals, inhibitors of angiogenesis, with animal and human data available; levamisole hydrochloride (levamisole), acetyl salicylic acid (ASA), thalidomide, erlotinib hydrochloride (erlotinib), anti-vascular endothelial growth factor (anti-VEGF), and 2-methoxyestradiol (2-ME). The relevance of the method to man was shown by comparing the obtained results to the literature data from the clinical studies with the same compounds. We here show that through thorough optimization and intra-laboratory pre-validation, it is possible to develop cell a culture assay into a reproducible and repeatable routine method with minimal variation and easy and fast semi-quantitative analysis of the effects.
Materials and Methods
Materials and chemicals
The BJ human foreskin fibroblasts were purchased from American Type Culture Collection (ATCC, LGC Promochem AB, Boras, Sweden, Cat. No. CRL-2522, Lot No. 57632920). Fetal bovine serum (FBS, Cat. No. 10106), l-glutamine (Glu, Cat. No. 25030), non-essential amino acids (NEAA, Cat. No. 11140), and TrypLE Express (Cat. No. 12604) were obtained from Gibco, Invitrogen, Carlsbad, CA, USA. DAB Substrate Kit (Cat. No. 00-2014) was from Zymed Laboratories Inc, Invitrogen, Carlsbad, CA, USA. EGM-2 bullet kit including SingleQuots-supplements (Cat. No. CC-3162), endothelial basal medium (EBM; Cat. No. CC-3156), EGM-2 SingleQuots-supplements (Cat. No. CC-4176), Gentamicin sulfate 50 mg/ml (Cat. No. 17-518Z), and Amphotericin B 250 μg/ml (Cat. No. 17-836E) were obtained from Lonza Group Ltd., Basel, Switzerland. Recombinant Human Fibroblast Growth Factor-basic 146 AA (FGF-2, Cat. No. 233-FB, Lot. No. HKW3809032, ED50 for inducing proliferation of NR6R-3T3 mouse fibroblasts is typically 0.1–0.6 ng/ml) and Recombinant human vascular endothelial growth factor 165 (VEGF, Cat. No. 293-VE, Lot. No. II2209012, ED50 for inducing proliferation of human umbilical vein endothelial cells (HUVEC) is typically 2.0–6.0 ng/ml) were obtained from R&D Systems, Abingdon, UK. 1,4-Ditiotreitol (DTT, Cat. No. 233156, Molecular Biology Grade) was obtained from Calbiochem, San Diego, CA, USA. Bovine serum albumin fraction V (BSA, Cat. No. 107350940019) was from Roche, Indianapolis, IN, USA. Erlotinib (Lot. No. BS06110030) was a kind gift from Roche Diagnostics GmbH, Mannheim, Germany. Anti-von Willebrand Factor (Anti-vWf) antibody produced in rabbit (Cat. No. F-3520), Neutral Red (NR) Solution (Cat. No. N2889), anti-VEGF (Cat. No. V6627, Lot. No. 088K1260), thalidomide (Cat No. T144, Lot. No. 097K4601), levamisole (tetramisole hydrochloride, Cat. No. L9756, Lot. No. 088K0753), 2-ME (Cat. No. M6383, Lot. No. 108K4087), and ASA (Cat. No. A5376, Lot. No. 048K0015) were from Sigma Aldrich, Manassas, VA, USA. Biotinylated anti-rabbit IgG (H + L) made in goat (Cat. No. BA-1000) and Vectastain Elite ABC Kit, Standard (Cat. No. PK-6100) were purchased from Vector laboratories Inc, Burlingame, CA, USA. Triton X-100 was from JT Baker, Phillipsburg, NJ, USA. Collagenase I was purchased from Invitrogen, Paisley, Scotland, UK. A 75-cm2 filtered cell culture flasks (Nunc EasyFlask™) were from Nunc, Roskilde, Denmark. MycoAlert® Mycoplasma Detection Kit (LT07-118) was purchased from Lonza Group Ltd., Basel, Switzerland.
Positive and negative controls
The tubule formation potency of each reference chemical was quantified based on the tubule formation potency of the positive control. The negative control compound induced no tubule formation. The positive control was a cocktail of commercial, well known angiogenic factors, VEGF, and fibroblast growth factor 2 (FGFβ or FGF-2), that are widely used to mimic the human tubule formation in vitro (Bishop et al., 1999; Cross and Claesson-Welsh, 2001; Donovan et al., 2001; Friis et al., 2003; Ai et al., 2007). The positive control medium consisted of 10 ng/ml VEGF and 1 ng/ml FGF-2 dissolved in endothelial basal medium (EBM). The optimal positive control was chosen by testing different concentrations of VEGF and FGF-2 (Table 1). As the negative control compound, which induced no tubule formation, endothelial basal medium (EBM) without any growth factors was used. Negative control gave the same response as the solvent. Positive control was used in four parallels and negative control in two parallels throughout the procedure in each 48-well plate to ensure the technical validity of the tests. Additionally, the dimethyl sulfoxide (DMSO) control was used in three of the reference chemicals (thalidomide, erlotinib, and 2-ME) as they were dissolved in DMSO. DMSO concentration never exceeded 0.5%.
Table 1.
The growth factor cocktails used in investigation of the optimal positive control, linearity, upper and lower limits and batch to batch variation.
| Growth factor cocktail no. | VEGF/FGF-2 concentration (ng/ml) |
|---|---|
| 1 | 75/7.5 |
| 2 | 50/5.0 |
| 3 | 25/2.5 |
| 4 | 10/1.0 |
| 5 | 7.5/0.75 |
| 6 | 5.0/0.5 |
| 7 | 2.5/.25 |
| 8 | 1.0/0.1 |
Reference chemicals
The six reference chemicals and their concentrations used for pre-validation of an in vitro angiogenesis assay are shown in Table 2. The rationale behind selection of the reference chemicals were that they are consistent with the defined objectives of the study (known inhibitors of angiogenesis), they represent different types of chemicals and reliable and relevant reference data in animals and man is available. No other inducers of angiogenesis than the positive control were included. The concentration range for each chemical was selected prior to the angiogenesis assay. The criteria for the selection of the chemical were non-toxicity, solubility in the test system, and that DMSO concentration not exceeding 0.5% in the test system. Reference chemicals were dissolved according to manufacturer's instructions and further diluted in stimulation medium (=endothelial basal medium supplemented with 10 ng/ml VEGF and 1 ng/ml FGF-2) prior to use.
Table 2.
Reference chemicals tested in the intra-laboratory pre-validation of an in vitro angiogenesis assay.
| Chemical name | CAS-RN | Chemical class | Product class | Concentrations tested | Purity | Supplier | Physical and chemical characteristics | |
|---|---|---|---|---|---|---|---|---|
| Acetyl salicylic acid | 50-78-2 | Salicylate | Non-steroidal anti-inflammatory drug (NSAID) | 10, 100, 500, 1000, 1500, and 2000 μM | 99.9% | Sigma Aldrich | Powder | |
| Erlotinib | 183319-69-9 | Quinazoline | HER1/EGFR tyrosine kinase inhibitor | 0.0005, 0.001, 0.01, 0.1, 1, 10, 25, and 50 μM | 99.9% | Roche Diagnostics | Powder | |
| Levamisole | 16595-80-5 | Imidazothiazole | Alkaline phosphatase inhibitor | 0.01, 0.1, 1, 10, 50, 100, 250, 500, 750, 1000, and 2000 μM | 99% | Sigma Aldrich | Powder | |
| 2-Methoxyestradiol | 363-07-2 | Estradiol metabolite | Tubulin inhibitor | 0.01, 0.1, 0.2, 0.4, 0.6 0.8, 1, and 2 μM | 99.5% | Sigma Aldrich | Powder | |
| Thalidomide | 50-35-1 | Phthalimide | Immunomodulator, TNF-α inhibitor | 10, 100, 200, 300, 400, and 500 μM | >99% | Sigma Aldrich | Powder | |
| Anti-VEGF | n/a | Human VEGF165 and VEGF121 antibody, IgG fraction of antiserum | Growth factor antibody | 0.5, 1, 2.5, 5, 7.5, 10, 25, and 50 μg/ml | n/a | Sigma Aldrich | Lyophilized powder |
These reference chemicals are widely studied angiogenesis inhibitors and pharmaceuticals. Anti-VEGF is the first angiogenesis inhibitor that has been approved by the U.S. Food and Drug Administration under trade name Avastin (bevacizumab) for the treatment of colon cancer (Folkman, 2006). According to Folkman (2006) VEGF antibody (trade name Lucentis) and aptamer of VEGF (trade name Macugen) are used for the treatment of macular degeneration; erlotinib (trade name Tarceva), an epidermal growth factor receptor (EGFR) tyrose kinase inhibitor is used for the treatment of lung cancer; thalidomide is used for the treatment of multiple myeloma. ASA has protective effects against colon cancer and cardiovascular disease (Yan et al., 2010). 2-ME is approved by FDA for the treatment of ovarian cancer under trade name Panzem. 2-ME has also been studied in the treatment of rheumatoid arthritis (Brahn et al., 2008), cardiovascular diseases (Dubey and Jackson, 2009), multiple myeloma (Rajkumar et al., 2007), breast cancer (Greenberg and Rugo, 2010), and prostate cancer (Harrison et al., 2010). Levamisole (trade name Ergamisol) has been used to treat worm infestations in both humans and animals and it is tested in clinical trials for the treatment of chronic idiopathic urticaria (Zhang et al., 2009), for colorectal cancer (Quasar Collaborative et al., 2007; Dahl et al., 2009), for malignant melanoma (Verma et al., 2006), and for malaria (Dondorp et al., 2007).
Methods
Setting up master cell banks
Quality control (QC) criteria for setting up Master cell banks were as follows: cell cultures were pure with high proliferation capacity and contained no mycoplasma. Cell viability was over 90%. Cell culture was quality-controlled microscopically and always prior to Master cell bank establishment. The cells were tested for mycoplasma contamination with MycoAlert® Mycoplasma Detection Kit according to manufacturer's instructions. The cell lines (human foreskin fibroblasts) were not passaged over 10 times and the passaging of primary cells (HUVEC) was investigated and optimized. No antibiotics were used in cell culture.
Human foreskin fibroblasts
Human foreskin fibroblasts were obtained from ATCC and cultured in Minimum Essential Medium with Earle's salts, w/o l-Glutamine supplemented with 10% FBS, 1% l-glutamine, 1% NEAA, and 1% antibiotic–antimycotic mixture in 75 cm2 cell culture flasks in 10 ml of medium at 37°C in 5% CO2 incubator. The medium was changed and the cells observed microscopically for their morphology and cell proliferation every 3 days. When confluent, the cells were detached with Tryple Express and subcultured in a ratio of 1:9. The cells were cryopreserved in liquid nitrogen, 500 000 cells per vial to create the Master cell bank.
Human umbilical vein endothelial cells
The human umbilical cords were obtained from scheduled cesarean sections with informed consent from Tampere University Hospital (permission No. R08028 from the Ethics Committee of the Pirkanmaa Hospital District, Tampere, Finland). The isolation of umbilical vein endothelial cells (HUVEC) from human umbilical cord veins was performed as described by Jaffe et al. (1973) but the process was further optimized. The umbilical cord was separated from the placenta and the umbilical vein was cannulated with a 20G needle. The needle was secured by clamping the cord over the needle with a surgical clamp. The vein was perfused with Umbilical cord buffer solution (UBS; 0.1 M phosphate buffer solution containing 0.14 M NaCl, 0.004 M KCl, and 0.011 M glucose) to wash out blood after which the other end of the umbilical vein was clamped with a surgical clamp. The vein was infused with 0.05% collagenase I. The umbilical cord was incubated in a water bath at 37°C for up to 20 min. After incubation, the collagenase solution containing HUVEC was flushed from the cord by infusing the vein with UBS. The suspension was flushed out into a 50-ml polypropylene tube. The cells were centrifuged at 250×g for 10 min, resuspended in EGM-2 BulletKit medium, seeded into 75 cm2 filtered cell culture flasks, and cultured at 37°C in 5% CO2 incubator.
The isolated HUVEC were daily observed microscopically for their morphology, cell culture purity, and cell proliferation. The medium was changed every 2–3 days. When confluent, the cells were detached with Tryple Express. Pure HUVEC cultures with good proliferation capacity were subcultured at primary culture (p0) in the ratio of 1:2–1:4 and at passages 1 (p1) upward in a ratio of 1:3–1:5. The isolated HUVEC were tested for their tubule formation capacity in the angiogenesis assay up to passage 10. At passage 2 (p2), the cells were cryopreserved in liquid nitrogen, 500 000 cells per vial to create the Master cell bank. The donor-specific batch number was given to each batch stored in the Master cell bank.
Co-culture establishment
BJ fibroblasts were taken from the Master cell bank, thawed, and cultured as above (see Human Foreskin Fibroblasts) for 3 days. On day 3, the BJ fibroblasts were plated into 48-well plates at a cell density of 20 000 cells/cm2, and grown for an additional 3 days. On day 3, the HUVEC were taken from the Master cell bank, thawed, and cultured as above (see Human Umbilical Vein Endothelial Cells), separately from BJ fibroblasts, for 3 days. On day 6, the HUVEC cells were carefully seeded on the top of BJ fibroblasts into 48-well plates at a cell density of 4000 cells/cm2. The co-culture was then further used for cytotoxicity test and for angiogenesis assay.
Cytotoxicity test
To find out the highest concentration for each reference chemical for the angiogenesis assay, a cytotoxicity test was performed. Both technicians performed individually the cytotoxicity test for each reference chemical. As the cytotoxicity assay, the neutral red uptake (NRU) cell viability assay was used. The co-culture of fibroblasts and HUVEC was first established as described above (see Co-Culture Establishment). Twenty-four hours after co-culture establishment, the test system was treated by exposing with positive or negative controls (see Positive and Negative Controls), or reference chemicals (see Reference Chemicals and Table 1) for 24 h. After exposure for 24 h, the exposure medium was removed and the cells were washed with preheated PBS. Two hundred fifty microliters of NR-medium (25 mg NR/1 ml medium) was added into the wells and incubated for 2 h at 37°C. After incubation, NR-medium was removed and the cells were washed with 250 μl PBS. After that, 100 μl NR-desorption medium (50% EtOH, 1% acetic acid in H2O) was added into the wells and incubated in a shaker for 20 min, protected from light. After shaking, the cells were allowed to settle down for 5 min. The absorbance was measured at 540 nm with Thermo Scientific Varioskan Flash Spectral Scanning Multimode Reader (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Angiogenesis assay
The angiogenesis assay was performed independently by two technicians and two analysts. The technicians performed the whole assay independently, except that the microscopic analysis was performed independently and blinded by two analysts. For microscopic analysis the samples were coded by a person not directly involved in the study.
The co-culture of fibroblasts and HUVEC was first established as described above (see Co-Culture Establishment). The day after co-culture creation, the co-culture was exposed to the positive or negative controls (see Positive and Negative Controls), the reference chemical treatments (see Table 1) or growth factor cocktails (see Table 2). The cells were cultured at 37°C for 6 days prior to immunocytochemical staining. The media containing either reference chemicals, or growth factor cocktail, or positive control or negative control, were changed once during the assay, on the third day.
Immunocytochemical staining
At day 6 from the onset of the experiment, the tubules were immunostained with anti-vWF. The media were removed and the cells were washed three times with PBS, fixed with ice-cold 70% ethanol for 20 min, permeabilized with 0.5% Triton X-100 (JT Baker, Phillipsburg, NJ, USA) for 15 min and blocked for unspecific staining with 10% BSA (Roche Diagnostics Corporation, Indianapolis, IN, USA) for 30 min. After blocking, the cells were incubated with primary antibody (anti-vWf, 1:5000) at 4°C overnight or for 1–2 h in room temperature (RT). Cells were then washed three times with PBS, incubated for 30 min with the secondary antibody (biotinylated anti-rabbit IgG, H + L made in goat) and washed again three times with PBS. The cells were then incubated with enzyme conjugate solution (Vectastain Elite ABC Kit) for 30 min, after which substrate was added (DAB Substrate Kit). The color development was followed under microscope for 5–10 min and the reaction was stopped with 0.5 M Tris buffer. After staining, 500 μl of Tris buffer was pipetted into each cell culture well and the plates were sealed with parafilm for storage at 4°C until microscopic analysis.
Microscopic analysis
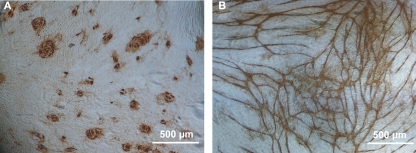
After immunocytochemical staining, the tubules were analyzed with Nikon Eclipse TS100 microscope (Nikon, Tokyo, Japan). The extent of tubules and their branching was quantified using predetermined visually inspected semi-quantitative grading scale from 0 to 8. The analysis and grading required expertise and therefore were performed by scientists. As the microscopic analysis was based on semi-quantitative visual analysis, we especially wanted to test the repeatability of the analysis. Therefore all plates and wells were coded and analyzed individually by two scientists. Figure 1 shows the tubule network formation from negative and positive control.
Figure 1.
Angiogenesis in vitro assay. The BJ fibroblast HUVEC co-culture was immunostained with anti-vWf antibody (1:5000) and with DAB Substrate kit. (A) Negative control, no tubule development (value 0 in tubule formation grading). Endothelial cells remain as epithelial-like round areas in co-culture. (B) Positive control (cocktail of 10 ng/ml VEGF and 1 ng/ml FGF-2) inducing tubule network formation (value 7 in tubule formation grading). Cells form tubule-like structures connecting to each other. Extensive branching of cells and long structures that cover the whole area of the well. Scale bar 500 μM.
Statistics
One-way analysis of variance with Dunnett's post test was used for the statistical analysis of the reference chemical results. The linearity of tubule formation was tested with linear regression and precision with coefficient of variation (CV). The day to day variation of technicians was tested with one-way analysis of variance, person to person variation between technicians with unpaired t-test and person to person variation between analysts with paired t-test. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Test acceptance criteria
Technical criteria
Three wells of the positive control had to give values 6–7 at each testing time. One well could give value 5. Then, the calculated minimum value for positive control is 5.75. The negative control had to be always negative (0). Only one out of two parallels in one combination of each reference chemical during each testing time could be discarded based on visual inspection in case the cells were found to be dead or detached.
Sensitivity of detection
For sensitivity of detection, linearity as well as upper and lower limits of detection, were investigated. Eight different growth factor cocktail concentrations (VEGF and FGF-2, 10:1) were used (Table 1) and investigated in order to find the optimal cocktail for positive control.
Linearity
Linearity was tested in order to find out the growth factor cocktail concentration range that induces tubule formation in a linear manner. For linearity testing, the negative control, and different growth factor cocktails (VEGF and FGF-2, 10:1) were used (see Table 1). Each cocktail was tested in six parallels.
Upper and lower limits of detection
The dose–response effect of the growth factor cocktails were investigated, and the combination of growth factors (see Table 1 for the growth factor cocktails used) giving the maximum value in the linear part of the response curve was selected to be the upper limit of detection. This was also chosen as the fixed positive control for the test (see Positive and Negative Controls). For the lower limit of detection, the growth factor cocktail that gives value 1 in the linear range of the tubule formation scale was selected. Each cocktail was tested in six parallels.
Batch to batch variation
Testing of the batch to batch variation between different HUVEC batches (different umbilical cords, i.e., donor samples) was performed to confirm that each of the Master cell bank batches set up in our laboratory gives comparable results. The batch to batch variation testing was performed in the angiogenesis assay with the growth factor cocktails shown in Table 1. Three HUVEC batches of passage 4 with 6 parallels were investigated. All of the batches had to follow the criteria for upper and lower limits of detection and to follow linearity.
Precision
To find out the maximal variation due to technicians and microscopic analysts, two technicians performed the positive and negative control tests (each technician performed one 48-well plate of negative control and one 48-well plate of positive control). The negative control was tested in the angiogenesis assay with endothelial basal medium (EBM) without any growth factors. The positive control was tested in the angiogenesis assay with one VEGF and FGF-2 dilution, i.e., positive control (10 ng/ml VEGF and 1 ng/ml FGF-2). Total variation in the test had to be ≤15% analyzed with CV. It is known, that cell culture conditions may be affected by the position of the well in the plate. Therefore, the whole plate was used to test the maximal variation including technicians, microscopic analysts, and cell culture conditions within plate.
Reliability
To test the reliability, two technicians repeated the test three times in unchanged conditions for all the reference chemicals. Reliability included repeatability, i.e., the positive control and the reference chemicals must give comparable results regardless of the testing time, and reproducibility, i.e., the effect of each reference chemical must be the same regardless of the technician. The positive control included altogether 24 parallels performed at three consecutive days by two technicians and each testing time included four parallels. The positive control contained four parallels and was always placed at the same position in a 48-well plate.
Repeatability
The test was performed by two technicians – microscopic analyst – pairs three times using identical protocol (day to day variation).
Reproducibility
Two technician – microscopic analyst – pairs repeated the same identical test protocol (person to person variation). In addition, each analyst analyzed all plates.
Performance
The overall performance of the assay was tested by repeating the identical protocol with six different reference chemicals three times by two technician – microscopic analyst – pairs.
Test method data quality
All work was performed according to the Good Laboratory Practice Regulations as set forth in OECD [ENV/MC/CHEM (98)17], in accordance with OECD guidelines [OECD Guidance document on the Validation and international Acceptance of New or Updated Test Methods for Hazard Assessment (OECD, 2005, No 34) and CPMP/ICH/281/95] and ECVAM (European Centre for the Validation of Alternative Methods) guidance documents (http://ecvam.jrc.it/) and according to the standard operating procedures of FICAM.
Results
Primary huvec master cell bank optimization
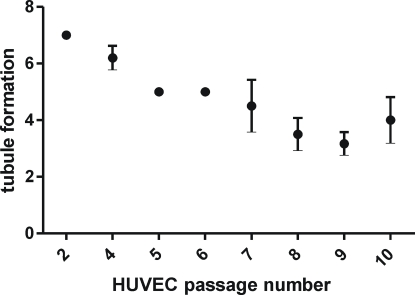
The tubule formation potency of HUVEC (treated with the positive control) was studied up to passage 10. The results of the passage optimization are seen in Figure 2. It was seen that up to passage 4 the tubule formation remained quite constant and high. During cultivation, when the passage number increased, the tubule formation decreased. Thus, the passage 4 was chosen as the fixed passage to be used in the angiogenesis assay.
Figure 2.
Tubule formation potency of the positive control in the angiogenesis assay at different HUVEC passages. The results are given as mean ± SD. The results are averages of at least three separate experiments.
Cytotoxicity test
The concentration giving the viability of 80% or higher was taken as the highest concentration to be used in the angiogenesis assay. Consequently, the concentration ranges were as follows: levamisole 0.01–2000 μM, thalidomide 10–500 μM, erlotinib 0.0005–50 μM, 2-ME 0.01–2 μM, and anti-VEGF 0.5–50 μg/ml. Only ASA showed toxicity. The toxicity was found at 2000 μM. Therefore the concentration range for ASA used in angiogenesis assay was 10–1500 μM.
Angiogenesis assay
Technical validity of the test
Positive control in each testing time gave the values 6–7 from 3 out of 4 parallels. Negative control always gave 0. No wells needed to be discarded.
Intra-laboratory pre-validation parameters
Sensitivity of detection
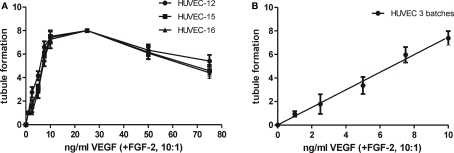
The sensitivity of detection was evaluated by studying linearity and the upper and lower limits of detection. Figure 3A shows the results obtained with HUVEC in the angiogenesis assay (three batches). The response was linear up to the growth factor cocktail of 10 ng/ml VEGF and 1 ng/ml FGF-2 with all three batches. At cocktails with higher growth factor concentrations, the response decreased. The growth factor cocktail that caused maximal tubule formation in the linear part of the response curve was a combination of 10 ng/ml VEGF and 1 ng/ml FGF-2 (Figure 3B) and was the upper limit of detection. This combination was also chosen as the fixed positive control for the test (see Positive and Negative Controls). The lowest VEGF and FGF-2 cocktail combination that induced tubule formation (value 1 in microscopic analysis of tubule formation grading) was found to be a combination of 1 ng/ml VEGF and 0.1 ng/ml FGF-2. This was the lower limit of detection.
Figure 3.
(A) Upper and lower limits of detection of tubule formation for three different HUVEC batches (HUVEC-12, HUVEC-15, HUVEC-16, n = 12 in each curve; (B) the linearity of tubule formation as tested from three different HUVEC batches with linear regression (n = 36). The growth factor concentrations are shown as VEGF concentrations. The concentrations of VEGF and FGF-2 used are seen in detail in Table 2. VEGF/FGF-2 ratio was always 10:1. The results are given as mean ± SD.
Batch to batch variation
The batch to batch variation was tested between three HUVEC batches treated with positive control (each batch with six parallels). The results are shown in Table 3. It was seen, that the criteria for positive control value were met with all batches and that the variation between the batches was extremely small (CV = 1.72%).
Table 3.
Batch to batch variation in tubule formation between three HUVEC batches (HUVEC-12, HUVEC-15, HUVEC-16, n = 12 with each batch).
| Criteria | HUVEC-12 | HUVEC-15 | HUVEC-16 | |
|---|---|---|---|---|
| Positive control (mean) | ≥5.75 | 7.417 | 7.500 | 7.250 |
| Mean (three batches) | ≥5.75 | 7.389 | ||
| Variation (CV%) | ≤15% | 1.72% |
CV%, coefficient of variation (%).
Precision
To find out the maximal variation, including that caused by technicians and microscopic analysts, the angiogenesis assay with positive and negative controls was performed. The results of the positive and negative control and their CV% are shown in Table 4. Negative control was always found to give the value 0 however; the CV% of positive control was found to be between 6.27 and 7.82%.
Table 4.
The precision of the in vitro angiogenesis assay. Maximal variation in the angiogenesis assay was tested with positive and negative controls (Two pairs of microscopic analyst–technician performing each one 48-well plate of negative control and one 48-well plate of positive control).
| Criteria | Technician 1–microscopic analyst 1 | Technician 1–microscopic analyst 2 | Technician 2–microscopic analyst 1 | Technician 2–microscopic analyst 2 | |
|---|---|---|---|---|---|
| Positive control (mean) | ≥5.75 | 6.354 | 6.771 | 6.896 | 6.958 |
| Negative control | 0 | 0 | 0 | 0 | 0 |
| Variation (CV%) | ≤15 | 7.61 | 6.27 | 6.85 | 7.82 |
CV%, coefficient of variation (%).
Reliability
Repeatability
The results of day to day variation between technicians and microscopic analysts are presented in Table 5. No significant differences were observed among technicians (p = 0.906 and p = 0.064). The microscopic analyst–technician pairs had following day to day variation: microscopic analyst 1 technician 1 CV = 0.59%, microscopic analyst 2 technician 1 CV = 0.67%, microscopic analyst 1 technician 2 CV = 6.38%. Microscopic analyst 2 technician 2 CV = 5.02%. We further studied whether the variation was due to analysis or due to technical performance (see Reproducibility).
Table 5.
Day to day variation (repeatability) and person to person variation (reproducibility) between the technicians and the microscopic analysts. Criteria set and the positive control values obtained.
| Reproducibility and repeatability | |||||
|---|---|---|---|---|---|
| Criteria set for positive control/CV%/p–value | Obtained positive control value | ||||
| Technician 1 –microscopic analyst 1 mean (n = 24) | Technician 1 – microscopic analyst 2 mean (n = 24) | Technician 2 –microscopic analyst 1 mean (n = 24) | Technician 2 –microscopic analyst 2 mean (n = 24) | ||
| Day 1 (n = 48) | ≥5.75 | 5.79 | 5.833 | 5.75 | 5.75 |
| Day 2 (n = 48) | ≥5.75 | 5.833 | 5.833 | 6.583 | 6.083 |
| Day 3 (n = 48) | ≥5.75 | 5.875 | 5.75 | 6.625 | 6.5 |
| Mean between days | ≥5.75 | 5.833 | 5.805 | 6.319 | 6.111 |
| Day to day variation among technicians (CV%) | ≤15% | 0.59% | 0.67% | 6.38% | 5.02% |
| Day to day variation among technicians (one-way ANOVA) | p < 0.05 | ns, p = 0.906 | ns, p = 0.064 | ||
| Day to day variation between analysts (CV%) | ≤15% | 0.34% | %2.37 | ||
| Person to person variation between technicians | 5.63% | 0.64% | |||
| Person to person variation between technicians (statistical significance, unpaired t-test) | p < 0.05 | p = 0.007 | |||
| Person to person variation between analysts (statistical significance, paired t-test) | p < 0.05 | ns, p = 0.2084 | |||
| Total mean of positive control | ≥5.75 | 6.017 | |||
| Total variation of positive control (CV%) | ≤15% | 1.39% | |||
CV, coefficient of variation, ns, non-significant.
Reproducibility
When the person to person variation was studied, each analyst analyzed all plates. Overall there was only a minor difference in CV% between technicians [the results of technician 1 or technician 2 were analyzed by both (two) analysts], being 0.64% with technician 1 and 5.63% with technician 2. The person to person variation is seen in Table 5. The results showed that the CV% between microscopic analysts was very low; CV% was 0.34% when the results of technician 1 were analyzed and 2.37%, when the results of technician 2 were analyzed. No statistically significant difference (p = 0.2084) was observed between the analysts. When comparing the technicians to each other (person to person variation), a statistically significant difference was observed (p = 0.007), whereas no person to person variation between analysts was seen However, the total CV, i.e., the total variation in the assay was as low as CV = 1.39%.
Performance
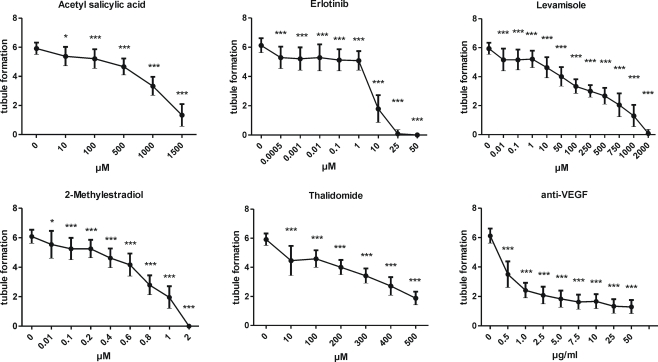
The performance of the assay was assessed by six different reference chemicals with several concentrations of each. The results of the effects of reference chemicals on tubule formation are seen in Figure 4. All the reference chemicals inhibited tubule formation as expected. Although the response was reference chemical specific, the phenomenon was repeatable, and variation remained constant throughout the study. The tubule inhibition was calculated to be mild (20% inhibition from positive control), moderate (40–60% inhibition from positive control or strong (75–85% inhibition from control). The severity of inhibition and the concentrations causing mild, moderate, or strong inhibition are summarized in Table 6, where the reference chemical results of our in vitro assay are compared to other in vitro angiogenesis test methods, to the relevant plasma concentrations in clinical studies and to effective doses from animal studies.
Figure 4.
The performance of the assay. The effects of the reference chemicals on tubule formation. The results are given as mean ± SD, n = 24 including three testing times with two technicians. Microscopic analysis of all wells was performed individually by two analysts. The results were tested statistically by using one-way ANOVA with Dunnett's post test. *p < 0.05, **p < 0.01 and ***p < 0.001.
Table 6.
The comparison of the results from intra-laboratory pre-validated assay to the results from other in vitro methods, clinical studies and animal models.
| Reference chemical | Results from intra-laboratory pre-validated angiogenesis test method | Results using other in vitro angiogenesis test methods | Results from clinical trials | Results using animal models | ||
|---|---|---|---|---|---|---|
| Mild inhibition (% of control) <20% | Moderate inhibition (% of control) 40–60% | Strong inhibition (% of control) 75–85% | Inhibitory effect and concentration | Cmax | Effective dose (ED) | |
| Acetyl salicylic acid | 1.8–8 μg/ml (10–100 μM) | 180 μg/ml (1000 μM) | 270 μg/ml (1500 μM) | Moderate 500 μM (Borthwick et al., 2006) | • 260–1026 μM (Juárez Olguín et al., 2004) | • 26–300 μM, CAM model (Sharma et al., 2001) |
| • 0–25 μg/ml (Maalouf et al., 2009) | ||||||
| • 170 ± 96.7 ng/ml (Bae et al., 2008) | ||||||
| Erlotinib (EGF receptor tyrosine kinase inhibitor) | 0.04–40 ng/ml (0.5 nM–0.1 μM) | 4 μg/ml (10 μM) | 22 μg/ml (50 μM) | Mild 1–20 μM (Birle and Hedley, 2006), Moderate 10 μM (Jimeno et al., 2007) | • 0.3–1.13 μg/ml (Herbst et al., 2005) | • 50 mg/kg, Mouse carcinoma model (Jimeno et al., 2007) |
| • 0.251–10.7 μg/ml (Ranson et al., 2010a) | • 50 mg/kg, Mouse tumor model (Cerniglia et al., 2009) | |||||
| • 2.93 ± 1.3 μg/ml (Ranson et al., 2010b) | ||||||
| • 0.3 μM (Clarke et al., 2010) | ||||||
| • 0.56–4 μM (Kraut et al., 2010) | ||||||
| Levamisole | 2–240 ng/ml (0.01–1 μM) | 25–120 μg/ml (100–500 μM) | 240–500 μg/ml (1000–2000 μM) | Mild 500 μM, moderate 750–1000 μM, strong 2000 μM (Friis et al., 2005), strong 2000 μM (Sylvest et al., 2010) | • 0.62 μg/ml–1.27 μg/ml (Reid et al., 1998) | • 1.2–12 mg/kg, Nude mouse tumor model (Friis et al., 2005) |
| • 0.716 ± 0.217 μg/ml (Kouassi et al., 1986) | • Cmax 0.37 μg/ml calf parasite infection (Taylor et al., 1988 | |||||
| 2-Methoxyestradiol | 3–60 ng/ml (0.01–0.2 μM) | 300 ng/ml (1 μM) | 600 ng/ml (2 μM) | Mild 10 μM, moderate 50 μM (Kang et al., 2006), Mild 0.5 μM, moderate 1 μM (Dobos et al., 2004) | • 3.3 ng/ml (Tevaarwerk et al., 2009) | • 100 mg/kg Murine rheumatoid arthritis model (Plum et al., 2009) |
| • 30.27 ± 20.18 ng/ml (Matei et al., 2009) | • 7.5 mg/kg, 75 mg/kg Mouse tumor model (Dobos et al., 2004) | |||||
| • 3.0–21.4 ng/ml (Dahut et al., 2006) | ||||||
| • 1.4–13.2 ng/ml (James et al., 2007) | ||||||
| • 2.2–9.6 ng/ml (Sweeney et al., 2005) | ||||||
| Thalidomide | 2–25 μg/ml (10–100 μM) | 77–100 μg/ml (300–400 μM) | - | - | • 2 μg/ml (Kakimoto et al., 2002) | • 100 mg/kg, Rat Alzheimer model (Ryu and McLarnon, 2008) |
| • 1.68 ± 0.41 μg/ml (Murakami et al., 2009) | • 19–1000 μM, CAM model (Sharma et al., 2001) | |||||
| • 1.44 ± 0.50 μg/ml (Kamikawa et al., 2006) | ||||||
| • 0.43–1.03 μg/ml (Vieira and Valente Mdo, 2009) | ||||||
| Anti-VEGF | 0.01–0.1 μg/ml | 0.5–1 μg/ml | 25–50 μg/ml | Strong 0.1 mg/ml-10 mg/ml (Sims et al., 2008), Strong 5 μg/ml (Friis et al., 2006), Strong 10 μg/ml (Friis et al., 2003) | • 363 μg/ml (Herbst et al., 2005) | • 2–4 mg/kg, Mouse carcinoma model (Sims et al., 2008) |
| • 123.2 ± 16.4 μg/ml (Wu et al., 2010) | • Cmax 104.46 ± 1.44 ng/ml, injection in rabbit (Kim et al., 2010) | |||||
| • 11.94–194.08 μg/ml (Ning et al., 2010) | • Cmax 1430 ± 186 ng/ml, vitreous injection macaque eyes (Miyake et al., 2010) | |||||
| • 20.7–24.2 ng/ml (Sharma et al., 2010) | • Cmax 676 ± 100 μg/ml, cynomolgus monkeys (Xu et al., 2008) | |||||
| • 16.6–42.5 μg/ml (Krohne et al., 2008) | ||||||
| • 2.63–165 μg/ml (Zhu et al., 2008) | ||||||
Discussion
The present intra-laboratory pre-validation study showed that a standardized in vitro angiogenesis assay is reliable and reproducible for testing the modulators of angiogenesis, and this human primary cell based in vitro assay mimics well the effects in man. Thus, it has great potency to supplement and/or replace animal tests.
Appropriately pre-validated human cell in vitro assays are urgently needed for reducing and replacing animal tests. In vitro angiogenesis assays are regarded as useful tools for screening compounds and their effective concentrations, but due to complex interactions during angiogenesis, they often need to be followed by in vivo studies (Auerbach et al., 2003). Animal assays, although not necessarily predictive for effects in human, are widely used as they provide information on complex cellular and molecular interactions (Norrby, 2006). The disadvantages of the current in vitro assays are that they lack metabolism and are rarely completely based on human biology (Auerbach et al., 2003).
We further developed, optimized and finally intra-laboratory pre-validated the previously published angiogenesis assay (Bishop et al., 1999). Our assay is based on quality-controlled primary HUVEC that have been minimally expanded in vitro. The optimization of the HUVEC passage number was found to be one crucial factor for the adequate performance of the assay. The tubule formation ability decreased significantly after serial passaging. Based on the optimization phase, the use of one, fixed passage of HUVEC was found to be optimal. This shows, that the effect of passage number on the biological activity of cells has to be investigated and the passage number fixed in order to maintain repeatability of the assay. Through optimization, we could obtain a routine assay with minimal variation (overall CV% between the two technicians and microscopic analysts was 1.39%). However, statistically significant difference was found between technicians. The effect of this on the final results is avoided by using positive and negative controls as there was no significant difference in day to day variation among technicians. They are used to calculate the response of unknown substances, as well as used as internal controls in the assay.
The optimal positive control concentration, i.e., VEGF/FGF-2 growth factor cocktail was obtained by investigating the biological response of several growth factor concentrations in several HUVEC batches. The positive control optimization resulted in stable and repeatable effect. The batch to batch variation for HUVEC was minimal due to the optimized passage and pre-set QC criteria for the cells stored in Master cell bank. Moreover, the test duration was shortened from previous methods (Bishop et al., 1999; Donovan et al., 2001) from 14 to 6 days of co-culture and the assay setup was optimized for 48-well plate to minimize the use of cells and to increase the capacity. A semi-quantitative microscopic analysis method, based on tubule formation, their connections and the complexity of the developed network, was developed. The prerequisite for the semi-quantitative grading was that the analysis covered the whole area of each well, contrary to the method of Bishop et al. (1999), where images taken from only five random fields in larger wells (24-well plates) were analyzed by computer analysis program. In optimization phase, the performance assay was tested with 19 reference chemicals; endostatin, interleukin-1 soluble receptor 1 (IL-1 SR1), suramin, mevinolin, paclitaxel, fumagillin, anti-epidermal growth factor receptor (anti-EGFR) cyclooxygenase-2-specific inhibitor NS-398, indomethacin, VEGF, anti-VEGF, FGF-2, anti-FGF-2, platelet-derived growth factor beta (PDGF-BB), levamisole, erlotinib, ASA, 2-ME, and thalidomide.
The performance of the in vitro angiogenesis assay was proven in intra-laboratory pre-validation with six different reference chemicals (levamisole, ASA, thalidomide, erlotinib, anti-VEGF, and 2-ME). The obtained results were compared to the published data from other in vitro angiogenesis assays (Table 6). The table shows that comparable results were detected with ASA (Borthwick et al., 2006), with erlotinib (Birle and Hedley, 2006; Jimeno et al., 2007), and with 2-ME (Dobos et al., 2004). However, 2-ME had previously shown low inhibition at 10 μM and moderate inhibition at 50 μM (Kang et al., 2006), whereas our assay showed low and moderate inhibition at 0.01–0.2 and 1 μM, respectively. Moreover, compared to the method by Friis et al. (2003, 2005), which is a similar assay to ours, levamisole was reported to have mild inhibition at 500 μM (in our study 0.01–1 μM), moderate at 750–1000 μM (in our study 100–500 μM), and strong at 2000 μM (in our study 1000–2000 μM). The anti-VEGF was reported to have moderate to strong inhibition at 0.1–10 mg/ml in an in vitro model with different study setup (Sims et al., 2008) compared to our assay (inhibition at 0.5–50 μg/ml). There are known to be marked differences in tubule formation depending on the in vitro angiogenesis assay used (Donovan et al., 2001; Auerbach et al., 2003). However, compared to several previous in vitro studies, our assay shows improvement of sensitivity.
The relevance of the assay to man was investigated by comparing the obtained reference chemical results with data from clinical studies. The effective concentrations observed in our assay showed very good concordance with the respective therapeutic plasma concentrations (Table 6). The plasma concentrations of anti-VEGF obtained from different clinical trials varied extensively. One explanation is that the endogenous plasma concentrations of VEGF and FGF-2 differ among individuals and are also dependent on the disease (Kakimoto et al., 2002).
In conclusion, we here show that through thorough optimization, it is possible to develop a cell culture assay into a reproducible and repeatable routine method with minimal variation and with easy and fast semi-quantitative quantification of the end points. The intra-laboratory pre-validation study was completed successfully, the test was technically accepted and the pre-set acceptance criteria were met. In comparison of the results to the data from clinical trials shows that this human cell based in vitro angiogenesis assay mimics very well the effects in man, and thus it can be used to replace and/or supplement animal tests when testing angiogenesis modulators. The applicability domain contains so far pharmaceuticals. In addition to the chemical testing, the method has potency to test conditioned media of cells or even the effect of different cells (normal or cancer cells) with regard to their effect on angiogenesis.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Acknowledgments
We thank Ms. Paula Helpiölä, Ms. Mirja Hyppönen, and Ms. Hilkka Mäkinen for excellent technical assistance. The English language was checked by Virve Kajaste, MA, USA. Erlotinib was a kind gift from Roche Diagnostics GmbH. Funding for the project was provided by Pirkanmaa Centers for Economic Development, Transport and the Environment, City of Tampere, Ministry of Education and Culture and Ministry of Agriculture and Forestry.
References
- Ai S., Cheng X. W., Inoue A., Nakamura K., Okumura K., Iguchi A., Murohara T., Kuzuya M. (2007). Angiogenic activity of bFGF and VEGF suppressed by proteolytic cleavage by neutrophil elastase. Biochem. Biophys. Res. Commun. 364, 395–401 [DOI] [PubMed] [Google Scholar]
- Akhtar N., Dickerson E. B., Auerbach R. (2002). The sponge/Matrigel angiogenesis assay. Angiogenesis 5, 75–80 10.1023/A:1021507031486 [DOI] [PubMed] [Google Scholar]
- Auerbach R., Lewis R., Shinners B., Kubai L., Akhtar N. (2003). Angiogenesis assays: a critical overview. Clin. Chem. 49, 32–40 [DOI] [PubMed] [Google Scholar]
- Bae S. K., Seo K. A., Jung E. J., Kim H. S., Yeo C. W., Shon J. H., Park K. M., Liu K. H., Shin J. G. (2008). Determination of acetylsalicylic acid and its major metabolite, salicylic acid, in human plasma using liquid chromatography-tandem mass spectrometry: application to pharmacokinetic study of Astrix in Korean healthy volunteers. Biomed. Chromatogr. 22, 590–595 [DOI] [PubMed] [Google Scholar]
- Beilmann M., Birk G., Lenter M. C. (2004). Human primary co-culture angiogenesis assay reveals additive stimulation and different angiogenic properties of VEGF and HGF. Cytokine 26, 178–185 10.1016/j.cyto.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Berthod F., Germain L., Tremblay N., Auger F. A. (2006). Extracellular matrix deposition by fibroblasts is necessary to promote capillary-like tube formation in vitro. J. Cell. Physiol. 207, 491–498 [DOI] [PubMed] [Google Scholar]
- Birle D. C., Hedley D. W. (2006). Signaling interactions of rapamycin combined with erlotinib in cervical carcinoma xenografts. Mol. Cancer Ther. 5, 2494–2502 10.1158/1535-7163.MCT-05-0504 [DOI] [PubMed] [Google Scholar]
- Bishop E. T., Bell G. T., Bloor S., Broom I. J., Hendry N. F., Wheatley D. N. (1999). An in vitro model of angiogenesis: basic features. Angiogenesis 3, 335–344 10.1023/A:1026546219962 [DOI] [PubMed] [Google Scholar]
- Borthwick G. M., Johnson A. S., Partington M., Burn J., Wilson R., Arthur H. M. (2006). Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. FASEB J. 20, 2009–2016 10.1096/fj.06-5987com [DOI] [PubMed] [Google Scholar]
- Brahn E., Banquerigo M. L., Lee J. K., Park E. J., Fogler W. E., Plum S. M. (2008). An angiogenesis inhibitor, 2-methoxyestradiol, involutes rat collagen-induced arthritis and suppresses gene expression of synovial vascular endothelial growth factor and basic fibroblast growth factor. J. Rheumatol. 35, 2119–2128 10.3899/jrheum.080302 [DOI] [PubMed] [Google Scholar]
- Cao Y. (2009). Monotherapy versus combination therapy of angiogenic and arteriogenic factors for the treatment of ischemic disorders. Curr. Mol. Med. 9, 967–972 [DOI] [PubMed] [Google Scholar]
- Cerniglia G. J., Pore N., Tsai J. H., Schultz S., Mick R., Choe R., Xing X., Durduran T., Yodh A. G., Evans S. M., Koch C. J., Hahn S. M., Quon H., Sehgal C. M., Lee W. M., Maity A. (2009). Epidermal growth factor receptor inhibition modulates the microenvironment by vascular normalization to improve chemotherapy and radiotherapy efficacy. PLoS ONE 4, e6539. 10.1371/journal.pone.0006539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. L., Pao W., Wu N., Miller V. A., Lassman A. B. (2010). High dose weekly erlotinib achieves therapeutic concentrations in CSF and is effective in leptomeningeal metastases from epidermal growth factor receptor mutant lung cancer. J. Neurooncol. 99, 283–286 10.1007/s11060-010-0128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross M. J., Claesson-Welsh L. (2001). FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 22, 201–207 [DOI] [PubMed] [Google Scholar]
- Dahl O., Fluge O., Carlsen E., Wiig J. N., Myrvold H. E., Vonen B., Podhorny N., Bjerkeset O., Eide T. J., Halvorsen T. B., Tveit K. M. (2009). Final results of a randomised phase III study on adjuvant chemotherapy with 5 FU and levamisol in colon and rectum cancer stage II and III by the Norwegian Gastrointestinal Cancer Group. Acta Oncol. 48, 368–376 10.1080/02841860902755244 [DOI] [PubMed] [Google Scholar]
- Dahut W. L., Lakhani N. J., Gulley J. L., Arlen P. M., Kohn E. C., Kotz H., McNally D., Parr A., Nguyen D., Yang S. X., Steinberg S. M., Venitz J., Sparreboom A., Figg W. D. (2006). Phase I clinical trial of oral 2-methoxyestradiol, an antiangiogenic and apoptotic agent, in patients with solid tumors. Cancer Biol. Ther. 5, 22–27 [DOI] [PubMed] [Google Scholar]
- Dobos J., Timar J., Bocsi J., Burian Z., Nagy K., Barna G., Petak I., Ladanyi A. (2004). In vitro and in vivo antitumor effect of 2-methoxyestradiol on human melanoma. Int. J. Cancer 112, 771–776 [DOI] [PubMed] [Google Scholar]
- Dondorp A. M., Silamut K., Charunwatthana P., Chuasuwanchai S., Ruangveerayut R., Krintratun S., White N. J., Ho M., Day N. P. (2007). Levamisole inhibits sequestration of infected red blood cells in patients with falciparum malaria. J. Infect. Dis. 196, 460–466 [DOI] [PubMed] [Google Scholar]
- Donovan D., Brown N. J., Bishop E. T., Lewis C. E. (2001). Comparison of three in vitro human “angiogenesis” assays with capillaries formed in vivo. Angiogenesis 4, 113–121 10.1023/A:1012218401036 [DOI] [PubMed] [Google Scholar]
- Dubey R. K., Jackson E. K. (2009). Potential vascular actions of 2-methoxyestradiol. Trends Endocrinol. Metab. 20, 374–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J. (2006). Angiogenesis. Annu. Rev. Med. 57, 1–18 [DOI] [PubMed] [Google Scholar]
- Friis T., Engel A. M., Klein B. M., Rygaard J., Houen G. (2005). Levamisole inhibits angiogenesis in vitro and tumor growth in vivo. Angiogenesis 8, 25–34 10.1007/s10456-005-3588-0 [DOI] [PubMed] [Google Scholar]
- Friis T., Hansen A. B., Houen G., Engel A. M. (2006). Influence of angiogenesis inhibitors on endothelial cell morphology in vitro. APMIS 114, 211. [DOI] [PubMed] [Google Scholar]
- Friis T., Kjaer Sorensen B., Engel A. M., Rygaard J., Houen G. (2003). A quantitative ELISA-based co-culture angiogenesis and cell proliferation assay. APMIS 111, 658–668 10.1034/j.1600-0463.2003.1110609.x [DOI] [PubMed] [Google Scholar]
- Greenberg S., Rugo H. S. (2010). Triple-negative breast cancer: role of antiangiogenic agents. Cancer J. 16, 33–38 10.1097/PPO.0b013e3181d38514 [DOI] [PubMed] [Google Scholar]
- Harrison M. R., Hahn N. M., Pili R., Oh W. K., Hammers H., Sweeney C., Kim K., Perlman S., Arnott J., Sidor C., Wilding G., Liu G. (2010). A phase II study of 2-methoxyestradiol (2ME2) NanoCrystal(R) dispersion (NCD) in patients with taxane-refractory, metastatic castrate-resistant prostate cancer (CRPC). Invest. New Drugs [Epub ahead of print]. 10.1007/s10637-010-9455-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R. S., Johnson D. H., Mininberg E., Carbone D. P., Henderson T., Kim E. S., Blumenschein G., Jr., Lee J. J., Liu D. D., Truong M. T., Hong W. K., Tran H., Tsao A., Xie D., Ramies D. A., Mass R., Seshagiri S., Eberhard D. A., Kelley S. K., Sandler A. (2005). Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J. Clin. Oncol. 23, 2544–2555 [DOI] [PubMed] [Google Scholar]
- Ishikane S., Ohnishi S., Yamahara K., Sada M., Harada K., Mishima K., Iwasaki K., Fujiwara M., Kitamura S., Nagaya N., Ikeda T. (2008). Allogeneic injection of fetal membrane-derived mesenchymal stem cells induces therapeutic angiogenesis in a rat model of hind limb ischemia. Stem Cells 26, 2625–2633 10.1634/stemcells.2008-0236 [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. (1973). Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J. Clin. Invest. 52, 2745–2756 10.1172/JCI107470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J., Murry D. J., Treston A. M., Storniolo A. M., Sledge G. W., Sidor C., Miller K. D. (2007). Phase I safety, pharmacokinetic and pharmacodynamic studies of 2-methoxyestradiol alone or in combination with docetaxel in patients with locally recurrent or metastatic breast cancer. Invest. New Drugs 25, 41–48 10.1007/s10637-006-9008-5 [DOI] [PubMed] [Google Scholar]
- Jimeno A., Kulesza P., Wheelhouse J., Chan A., Zhang X., Kincaid E., Chen R., Clark D. P., Forastiere A., Hidalgo M. (2007). Dual EGFR and mTOR targeting in squamous cell carcinoma models, and development of early markers of efficacy. Br. J. Cancer 96, 952–959 10.1038/sj.bjc.6603656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez Olguín H., Flores Pérez J., Lares Asseff I., Loredo Abdalá A., Carbajal Rodriguez L. (2004). Comparative pharmacokinetics of acetyl salicylic acid and its metabolites in children suffering from autoimmune diseases. Biopharm. Drug Dispos. 25, 1–7 10.1002/bdd.379 [DOI] [PubMed] [Google Scholar]
- Kakimoto T., Hattori Y., Okamoto S., Sato N., Kamata T., Yamaguchi M., Morita K., Yamada T., Takayama N., Uchida H., Shimada N., Tanigawara Y., Ikeda Y. (2002). Thalidomide for the treatment of refractory multiple myeloma: association of plasma concentrations of thalidomide and angiogenic growth factors with clinical outcome. Jpn. J. Cancer Res. 93, 1029–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikawa R., Ikawa K., Morikawa N., Asaoku H., Iwato K., Sasaki A. (2006). The pharmacokinetics of low-dose thalidomide in Japanese patients with refractory multiple myeloma. Biol. Pharm. Bull. 29, 2331–2334 [DOI] [PubMed] [Google Scholar]
- Kang S. H., Cho H. T., Devi S., Zhang Z., Escuin D., Liang Z., Mao H., Brat D. J., Olson J. J., Simons J. W., Lavallee T. M., Giannakakou P., Van Meir E. G., Shim H. (2006). Antitumor effect of 2-methoxyestradiol in a rat orthotopic brain tumor model. Cancer Res. 66, 11991–11997 10.1158/0008-5472.CAN-06-1320 [DOI] [PubMed] [Google Scholar]
- Kim M. J., Han E. S., Kim J., Kim T. W. (2010). Aqueous humor concentration of bevacizumab after subconjunctival injection in rabbit. J. Ocul. Pharmacol. Ther. 26, 49–53 [DOI] [PubMed] [Google Scholar]
- Kouassi E., Caille G., Lery L., Lariviere L., Vezina M. (1986). Novel assay and pharmacokinetics of levamisole and p-hydroxylevamisole in human plasma and urine. Biopharm. Drug Dispos. 7, 71–89 10.1002/bdd.2510070110 [DOI] [PubMed] [Google Scholar]
- Kraut E. H., Rhoades C., Zhang Y., Cheng H., Aimiumu J., Chen P., Lang J., Young D. C., Agrawal A., Dancey J., Chan K. K., Grever M. R. (2010). Phase I and pharmacokinetic study of erlotinib (OSI-774) in combination with docetaxel in squamous cell carcinoma of the head and neck (SSCHN). Cancer Chemother. Pharmacol. [Epub ahead of print]. 10.1007/s00280-010-1332-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krohne T. U., Eter N., Holz F. G., Meyer C. H. (2008). Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am. J. Ophthalmol. 146, 508–512 [DOI] [PubMed] [Google Scholar]
- Maalouf R., Mosley M., James Kallail K., Kramer K. M., Kumar G. (2009). A comparison of salicylic acid levels in normal subjects after rectal versus oral dosing. Acad. Emerg. Med. 16, 157–161 [DOI] [PubMed] [Google Scholar]
- Matei D., Schilder J., Sutton G., Perkins S., Breen T., Quon C., Sidor C. (2009). Activity of 2 methoxyestradiol (Panzem NCD) in advanced, platinum-resistant ovarian cancer and primary peritoneal carcinomatosis: a Hoosier Oncology Group trial. Gynecol. Oncol. 115, 90–96 [DOI] [PubMed] [Google Scholar]
- Middleton J., Americh L., Gayon R., Julien D., Aguilar L., Amalric F., Girard J. P. (2004). Endothelial cell phenotypes in the rheumatoid synovium: activated, angiogenic, apoptotic and leaky. Arthritis Res. Ther. 6, 60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T., Sawada O., Kakinoki M., Sawada T., Kawamura H., Ogasawara K., Ohji M. (2010). Pharmacokinetics of bevacizumab and its effect on vascular endothelial growth factor after intravitreal injection of bevacizumab in macaque eyes. Invest. Ophthalmol. Vis. Sci. 51, 1606–1608 [DOI] [PubMed] [Google Scholar]
- Murakami H., Shimizu K., Sawamura M., Suzuki K., Sugiura I., Kosugi H., Shimazaki C., Taniwaki M., Abe M., Takagi T. (2009). Phase II and pharmacokinetic study of thalidomide in Japanese patients with relapsed/refractory multiple myeloma. Int. J. Hematol. 89, 636–641 [DOI] [PubMed] [Google Scholar]
- Nillesen S. T., Geutjes P. J., Wismans R., Schalkwijk J., Daamen W. F., van Kuppevelt T. H. (2007). Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials 28, 1123–1131 10.1016/j.biomaterials.2006.10.029 [DOI] [PubMed] [Google Scholar]
- Ning Y. M., Gulley J. L., Arlen P. M., Woo S., Steinberg S. M., Wright J. J., Parnes H. L., Trepel J. B., Lee M. J., Kim Y. S., Sun H., Madan R. A., Latham L., Jones E., Chen C. C., Figg W. D., Dahut W. L. (2010). Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 2070–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrby K. (2006). In vivo models of angiogenesis. J. Cell Mol. Med. 10, 588–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum S. M., Park E. J., Strawn S. J., Moore E. G., Sidor C. F., Fogler W. E. (2009). Disease modifying and antiangiogenic activity of 2-methoxyestradiol in a murine model of rheumatoid arthritis. BMC Musculoskelet. Disord. 10, 46. 10.1186/1471-2474-10-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quasar Collaborative G., Gray R., Barnwell J., McConkey C., Hills R. K., Williams N. S., Kerr D. J. (2007). Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 370, 2020–2029 10.1016/S0140-6736(07)61866-2 [DOI] [PubMed] [Google Scholar]
- Rajkumar S. V., Richardson P. G., Lacy M. Q., Dispenzieri A., Greipp P. R., Witzig T. E., Schlossman R., Sidor C. F., Anderson K. C., Gertz M. A. (2007). Novel therapy with 2-methoxyestradiol for the treatment of relapsed and plateau phase multiple myeloma. Clin. Cancer Res. 13, 6162–6167 10.1158/1078-0432.CCR-07-0807 [DOI] [PubMed] [Google Scholar]
- Ranson M., Shaw H., Wolf J., Hamilton M., McCarthy S., Dean E., Reid A., Judson I. (2010a). A phase I dose-escalation and bioavailability study of oral and intravenous formulations of erlotinib (Tarceva, OSI-774) in patients with advanced solid tumors of epithelial origin. Cancer Chemother. Pharmacol. 66, 53–58 10.1007/s00280-009-1133-3 [DOI] [PubMed] [Google Scholar]
- Ranson M., Reck M., Anthoney A., Hanauske A. R., Dean E., Melezinek I., Klingelschmitt G., Kletzl H., Blatter J., Twelves C. (2010b). Erlotinib in combination with pemetrexed for patients with advanced non-small-cell lung cancer (NSCLC): a phase I dose-finding study. Ann. Oncol. 21, 2233–2239 [DOI] [PubMed] [Google Scholar]
- Reid J. M., Kovach J. S., O'Connell M. J., Bagniewski P. G., Moertel C. G. (1998). Clinical and pharmacokinetic studies of high-dose levamisole in combination with 5-fluorouracil in patients with advanced cancer. Cancer Chemother. Pharmacol. 41, 477–484 [DOI] [PubMed] [Google Scholar]
- Rogers P. A., Donoghue J. F., Walter L. M., Girling J. E. (2009). Endometrial angiogenesis, vascular maturation, and lymphangiogenesis. Reprod. Sci. 16, 147–151 [DOI] [PubMed] [Google Scholar]
- Ryu J. K., McLarnon J. G. (2008). Thalidomide inhibition of perturbed vasculature and glial-derived tumor necrosis factor-alpha in an animal model of inflamed Alzheimer's disease brain. Neurobiol. Dis. 29, 254–266 [DOI] [PubMed] [Google Scholar]
- Sharma S., Abhyankar V., Burgess R. E., Infante J., Trowbridge R. C., Tarazi J., Kim S., Tortorici M., Chen Y., Robles R. L. (2010). A phase I study of axitinib (AG-013736) in combination with bevacizumab plus chemotherapy or chemotherapy alone in patients with metastatic colorectal cancer and other solid tumors. Ann. Oncol. 21, 297–304 [DOI] [PubMed] [Google Scholar]
- Sharma S., Ghoddoussi M., Gao P., Kelloff G. J., Steele V. E., Kopelovich L. (2001). A quantitative angiogenesis model for efficacy testing of chemopreventive agents. Anticancer Res. 21, 3829–3837 [PubMed] [Google Scholar]
- Sims T. L., Williams R. F., Ng C. Y., Rosati S. F., Spence Y., Davidoff A. M. (2008). Bevacizumab suppresses neuroblastoma progression in the setting of minimal disease. Surgery 144, 269–275 10.1016/j.surg.2008.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney C., Liu G., Yiannoutsos C., Kolesar J., Horvath D., Staab M. J., Fife K., Armstrong V., Treston A., Sidor C., Wilding G. (2005). A phase II multicenter, randomized, double-blind, safety trial assessing the pharmacokinetics, pharmacodynamics, and efficacy of oral 2-methoxyestradiol capsules in hormone-refractory prostate cancer. Clin. Cancer Res. 11, 6625–6633 10.1158/1078-0432.CCR-05-0440 [DOI] [PubMed] [Google Scholar]
- Sylvest L., Bendiksen C. D., Houen G. (2010). Phosphatase inhibitors with anti-angiogenic effect in vitro. APMIS 118, 49–59 10.1111/j.1600-0463.2009.02561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. M., Mallon T., Carrol B. (1988). Efficacy of a levamisole bolus in ostertagia and cooperia infections. Ann. Rech. Vet. 19, 107–110 [PubMed] [Google Scholar]
- Tevaarwerk A. J., Holen K. D., Alberti D. B., Sidor C., Arnott J., Quon C., Wilding G., Liu G. (2009). Phase I trial of 2-methoxyestradiol nanoCrystal dispersion in advanced solid malignancies. Clin. Cancer Res. 15, 1460–1465 10.1158/1078-0432.CCR-08-1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucuzian A. A., Greisler H. P. (2007). In vitro models of angiogenesis. World J. Surg. 31, 654–663 [DOI] [PubMed] [Google Scholar]
- van Weel V., van Tongeren R. B., van Hinsbergh V. W., van Bockel J. H., Quax P. H. (2008). Vascular growth in ischemic limbs: a review of mechanisms and possible therapeutic stimulation. Ann. Vasc. Surg. 22, 582–597 [DOI] [PubMed] [Google Scholar]
- Verma S., Quirt I., McCready D., Bak K., Charette M., Iscoe N. (2006). Systematic review of systemic adjuvant therapy for patients at high risk for recurrent melanoma. Cancer 106, 1431–1442 10.1002/cncr.21760 [DOI] [PubMed] [Google Scholar]
- Vieira J. L., Valente Mdo S. (2009). Thalidomide levels in patients with erythema nodosum leprosum. Ther. Drug Monit. 31, 602–603 10.1097/FTD.0b013e3181b46c1e [DOI] [PubMed] [Google Scholar]
- Wu J. Y., Wu X. N., Ding L., Zhao Y. B., Ai B., Li Y., Hu X., Cheng G. (2010). Phase I safety and pharmacokinetic study of bevacizumab in Chinese patients with advanced cancer. Chin. Med. J. 123, 901–906 [PubMed] [Google Scholar]
- Xu L., Zuch C. L., Lin Y. S., Modi N. B., Lum B. L. (2008). Pharmacokinetics and safety of bevacizumab administered in combination with cisplatin and paclitaxel in cynomolgus monkeys. Cancer Chemother. Pharmacol. 61, 607–614 [DOI] [PubMed] [Google Scholar]
- Yan K. H., Yao C. J., Chang H. Y., Lai G. M., Cheng A. L., Chuang S. E. (2010). The synergistic anticancer effect of troglitazone combined with aspirin causes cell cycle arrest and apoptosis in human lung cancer cells. Mol. Carcinog. 49, 235–246 [DOI] [PubMed] [Google Scholar]
- Zhang H., Shan C., Hua Z., Zhao P., Zhang H. (2009). Treatment of chronic idiopathic urticaria with levamisole: a multicentre, randomized, double-blind, controlled trial. J. Int. Med. Res. 37, 1167–1172 [DOI] [PubMed] [Google Scholar]
- Zhu Q., Ziemssen F., Henke-Fahle S., Tatar O., Szurman P., Aisenbrey S., Schneiderhan-Marra N., Xu X., Grisanti S. (2008). Vitreous levels of bevacizumab and vascular endothelial growth factor-A in patients with choroidal neovascularization. Ophthalmology 115, 1750–1755 10.1016/j.ophtha.2008.04.023 [DOI] [PubMed] [Google Scholar]
- Ziche M., Morbidelli L. (2009). The corneal pocket assay. Methods Mol. Biol. 467, 319–329 [DOI] [PubMed] [Google Scholar]