Abstract
Background and Purpose
Delayed cerebral ischemia (DCI) is a major complication after aneurysmal subarachnoid hemorrhage (aSAH) that is manifested by changes in cerebral blood flow (CBF) accompanied by neurological decline and results in long-term functional and neuropsychological (NP) impairment. Preclinical evidence has demonstrated that the arachidonic acid metabolite, 20-hydroxyeicosatetraenoic acid (20-HETE), affects cerebral microvascular tone and CBF after aSAH. The purpose of this study was to determine if CSF 20-HETE levels were associated with DCI and long term NP outcomes in aSAH patients.
Methods
CSF samples collected twice daily through 14 days after hemorrhage on 108 acute, adult aSAH patients. Samples were analyzed for 20-HETE via HPLC MSQ single quadrupole mass spectrometry. DCI was defined as the presence of impaired CBF (angiographic vasospasm, elevated transcranial Dopplers, abnormal CT or MR perfusion scans) accompanied by neurological deterioration. Outcomes including death and neuropsychological testing were completed at 3 months after hemorrhage.
Results and Conclusions
Detectible 20-HETE levels were observed in 31% of patient samples and were associated with severity of hemorrhage (Hunt&Hess p=0.04; Fisher p=0.05). Detection of 20-HETE was not associated with angiographic vasospasm (p=0.34), however, detectible 20-HETE was significantly associated with DCI (p=0.016). Our data also suggests that detectable 20-HETE was associated with decreased performance in 5 NP domains. These results provide the first clinical evidence that CSF 20-HETE concentrations are associated with DCI and poor outcomes and provide impetus for future studies to elucidate the clinical utility of inhibiting 20-HETE formation as a novel therapeutic intervention in patients with aSAH.
Keywords: subarachnoid hemorrhage, delayed cerebral ischemia, 20-HETE, neuropsychological outcome, fatty acid, arachidonic acid
Aneurysmal subarachnoid hemorrhage (aSAH) is a devastating illness that strikes healthy individuals during active and productive years of their life and results in significant death and disability. Delayed cerebral ischemia (DCI) has been well accepted as a leading complication of aSAH that contributes to the overall morbidity and mortality, and is known to occur in approximately 20–60% of the patients who survive the initial hemorrhage.1–4 While the definition of DCI continues to be refined, the development of DCI after aSAH is thought to result from a mismatch between available cerebral blood flow (CBF) and the metabolic needs of the brain tissue and is manifested by changes in CBF accompanied by neurological decline.5–8 This ischemic complication results in exacerbated long-term functional and neuropsychological (NP) impairment that interferes with resumption of previously held familial, social, and employment roles.8
Historically, cerebral vasospasm was considered the primary cause of DCI. Approximately 50–70% of aSAH patients have angiographic evidence of cerebral vascular constriction (angiographic vasospasm) and one third of these experience symptoms of DCI.1,8 However, not all patients that develop neurologic decline with perfusion deficits have angiographic evidence of cerebral vasospasm.4,8–13 This clinical reality suggests that other causes of DCI in aSAH patients warrant exploration. DCI is most likely to occur 3–15 days after the initial bleeding thereby allowing sufficient timing for therapeutic intervention. Unfortunately, to date, no therapeutic intervention has been developed that significantly reduces the incidence of DCI.4
The exact mechanisms of DCI are not established although recent evidence has linked metabolic regulators of cerebral microvascular blood flow as important mediators in its development.6,7,12 To date, therapies such as endothelin receptor antagonists have been effective in reducing angiographic vasospasm; however their effect on DCI has not been significant.3,11 Standardized care including the use of calcium channel antagonists and triple H therapy (hypertension, hypervolemia, and hemodilution) may be responsible for a lower incidence or impact of DCI, but do not provide insight in to risk or early identification of the problem.3,9,12
Recent evidence has suggested that a metabolite of arachidonic acid, known as 20-hydroxyeicosatetraenoic acid (20-HETE) may be influential in reduced CBF. 20-HETE is formed by enzymes of CYP4A and 4F families via ω-hydroxylation of arachidonic acid (AA) in cerebral arteries.14 Stimulated by angiotensin II, endothelin and norepinephrine; 20-HETE is a potent microvascular vasoconstrictor in renal, mesenteric and cerebral vascular beds. While the majority of evidence is pre-clinical, 20-HETE has been implicated in changes in the cerebral vascular tone including the development of delayed cerebral vasospasm and ischemia.15 Administration of inhibitors of 20-HETE synthesis and 20-HETE antagonists have been shown to reverse delayed vasospasm and prevent acute decreases in CBF.14–19 Likewise, inhibition of 20-HETE formation was found to be neuroprotective in a temporary focal ischemia SAH animal model20 and attenuated the post-injury CBF that typically accompanies ischemic and hemorrhagic stroke.21 And while 20-HETE has been associated with microvascular constriction in the cerebral vascular beds, it has also been associated with dilation in larger cerebral vessels.22
More recently, elevated levels of 20-HETE in CSF and plasma have been reported in a small number of aSAH patients with documented evidence of cerebral vasospasm and neurological deficits.16;23–24 Based on the evidence implicating 20-HETE in the development of both cerebral vasospasm and cerebral ischemia the purpose of this study was to determine the time course of 20-HETE concentrations in the CSF of patients with aSAH and to determine if 20-HETE CSF levels were associated with DCI and/or NP outcomes in a larger cohort of adult patients with aSAH.
METHODS
Patient Sample
Adult patients age 18–75 years, diagnosed with aSAH via cerebral angiogram or head computed tomography (CT) were recruited from the neurovascular intensive care unit. Criteria for enrollment also included Fisher grade >1 and CSF access (ventriculostomy or lumbar drain). Patients were not enrolled if they had a history of debilitating neurological disease or SAH from trauma, mycotic aneurysm or arteriovenous malformation. The protocol was approved by the Institutional Review Board and informed consent was obtained from the patient or proxy prior to data collection.
Data Collection
Socio-demographics, including gender, age, race, severity of injury (Fisher Grade and Hunt & Hess (HH) Score) as well as clinical data were collected from the medical record. CSF was withdrawn by registered nurses directly from the CSF tubing each morning (8am+/−1 hour) and evening (7pm+/−1 hour) through 14 days after initial hemorrhage (contingent on CSF access). Specimens were frozen and stored at −80 degrees for batch analysis. NP data was obtained through 1.5–2 hour face to face interviews conducted at 3 months after initial hemorrhage by an experienced clinicians trained in NP assessment.
20-HETE Measurement
CSF aliquots of 1ml were extracted via solid phase extraction using Oasis® HLB 1cc extraction cartridges (Waters, Milford, MA). 20-HETE was separated using HPLC with a 5 mm Beta Basic-C18 (150×2.1; ThermoHypersil, Bellefonte, PA) column and quantified using a MSQ single quadrupole mass spectrometer (ThermoFinnigan, San Jose, CA) using negative electrospray and single ion mode detection at mass m/z 319.5 for 20-HETE and m/z 325.5 for deuterated (d6)-20-HETE. Data were acquired and analyzed using Xcalibur software (version 1.0.0.1). 20-HETE concentrations were quantified from the standard curve of the ratio of 20-HETE to internal standard peak areas as previously described.24,25
DCI
DCI was defined as the presence of impaired CBF accompanied by neurological deterioration (simultaneously or within 12 hours pre or post determination of impaired CBF). Neurologic deterioration was determined by the presence of any of the following; a change in level of consciousness, presence of a new focal neurologic deficit, pupil changes or worsening Glasgow Coma Scale (GCS) or National Institutes of Health Stroke Scale (NIHSS) scores documented by the bedside practitioner in the absence of medication administration. Cerebral blood flow was assessed by cerebral angiography, transcranial Doppler (TCD) and CT or MR perfusion scans. Angiographic vasospasm was determined from cerebral angiograms read and coded by neurosurgeons blinded to participant identity and dichotomized as either ‘negative’ (0–24% narrowing of cerebral blood vessels) or ‘positive’ (≥25% narrowing of cerebral blood vessels). Daily TCDs were coded as abnormal flows when there was a systolic middle cerebral artery velocity > 200 ml/sec and/or a Lindegaard ratio > 3.0.1,2 Finally, head CT/MR and head CT/MR perfusion scans were reviewed for the presence of ischemia, infarction or low blood flow. All patients received standard therapy for the study institution for SAH patients including strict blood pressure and central venous pressure parameters, nimodipine and triple H therapy.
NP Function
Seven domains of NP function were assessed (Table 1). The selected tests have excellent psychometric properties and have been used in large clinical trials studying the effects of multiple disorders on NP outcomes.26–28 To control for co-morbidities level of education was obtained from the participant or proxy and pre-morbid intelligence was estimated using the North American Adult Reading Test (NAART), which provides a valid estimate of pre-morbid verbal intelligence resistant to the effects of acquired brain damage29 and levels of depressive symptoms were assessed utilizing the Beck Depression Inventory (BDI), a widely used measure with well-established psychometrics.30
Table 1.
Tests of NP function by domain
| Domain of NP Function | Individual Test(s) |
|---|---|
| Attention | Trail Making Test A |
| Verbal learning and memory | Digit Span Forward and Backward Rey Auditory Verbal Learning Test Wechsler Memory Scale III Logical Memory Immediate and Delayed Recall |
| Psychomotor speed | Grooved Pegboard (Dominant and Non-dominant) |
| Mental flexibility | Trail Making Test B |
| Executive function | Stroop Color Word Test |
| Visuospatial ability | Rey and Taylor Complex Figure-Copy |
| Language | Controlled Oral Word Association Test |
Data Analysis
20-HETE measurements in CSF were dichotomized into groups of detectable and non-detectable 20-HETE concentrations at baseline. Fisher’s exact tests and Mann-Whitney U-tests were used to analyze the association between detectable and non-detectable levels of 20-HETE with socio-demographic, clinical, angiographic vasospasm and DCI factors. To analyze the effect of detectable and non-detectable 20-HETE levels on NP outcome scores, multivariable regression models were created using backwards linear regression, adjusting for age, gender, years of education, race, HH, and depression at baseline. All statistical analyses were performed using SPSS version 16 (SPSS Inc., Chicago, USA). To adjust for multiple comparisons, a Bonferroni correction was applied, which meant that in order to be considered statistically significant, a relationship had to have a p< 0.002.
RESULTS
Patient Characteristics
A total of 108 aSAH patients were included in this analysis. The mean age (54 years; SD 11.2) and the predominance of female patients (73%, n=55) reflect the known population of aSAH patients and the higher numbers of Caucasians (85%, n=64) are representative of the population of the study site (Table 2). Overall impact of initial insult as graded by HH(3–5) as well as Fisher(3–4) was moderate to severe. Mean duration of ventriculostomy placement was 7±3.4 days; yielding 11.7±6.5 CSF samples obtained per patient. Of the patients with DCI (n=54), a combination of vasospasm demonstrated by cerebral angiography and/or TCD along with neurological deterioration was present in all but one patient. The presence of new cerebral ischemia or infarct along with neurological decline was used in the other patient with DCI. DCI could not be determined on nine patients (8%) due to poor neurological condition which excluded the ability to derive a neurologic deterioration (Table 3).
Table 2.
Association of Socio-demographic and Clinical Characteristics on 20-HETE Detectable and Non-detectable levels in aSAH Patients * denotes p < 0.05.
| Characteristics | 20-HETE Group | p-value | ||||
|---|---|---|---|---|---|---|
| Non-detectable (N=75) | Detectable (N=33) | |||||
| N (%) | N (%) | |||||
| Gender | Female | 55 (73%) | 20 (61%) | 0.26 | ||
| Male | 20 (27%) | 13 (39%) | ||||
| Race | White | 64 (85%) | 29 (88%) | 1.00 | ||
| Non-white | 11 (15%) | 4 (12%) | ||||
| HH Score | Low(1–2) | 27 (36%) | 5 (15%) | 0.04* | ||
| High (3–5) | 48 (64%) | 28 (85%) | ||||
| Fisher Grade | 2 | 22 (29%) | 3 (9%) | 0.05 | ||
| 3 | 36 (48%) | 18 (55%) | ||||
| 4 | 17 (23%) | 12 (36%) | ||||
| Glasgow Coma Scale | Mild | 51 (68%) | 23 (69%) | 0.28 | ||
| Moderate | 9 (12%) | 1 (3%) | ||||
| Severe | 15 (20%) | 9 (27%) | ||||
| Mortality 3 months after aSAH | Alive | 57 (76%) | 24 (73%) | 0.81 | ||
| Dead | 18 (24%) | 9 (27%) | ||||
| Characteristics | Non-detectable | Detectable | p-value | |||
| N | Mean (SD) | N | Mean SD) | |||
| Age | 75 | 54.6 (11.2) | 3 3 |
54.1 (9.7) | 0.90 | |
| Years of Education | 36 | 13 (1.9) | 1 6 |
13.3 (1.8) | 0.60 | |
Table 3.
Determination of delayed cerebral Ischemia (DCI)
| Delayed cerebral ischemia | 20-HETE Group | |
|---|---|---|
| Non-detectable (N=75) | Detectable (N=33) | |
| None | 37 (49%) | 8 (24%) |
| Cerebral angiography and neurological deterioration | 1 (1%) | 2 (6%) |
| TCD and neurological deterioration | 15 (20%) | 8 (24%) |
| Cerebral angiography, TCD and neurological deterioration | 16 (21%) | 11 (33%) |
| Cannot identify - missing variable or poor exam | 6 (8%) | 3 (9%) |
| Cerebral ischemia (CTP/MRI) | 0 (0%) | 1 (1%) |
CSF 20-HETE Levels Following aSAH
20-HETE was detected in 33 (31%) aSAH patients (Figure 1). There was no significant association between demographic variables (age, race, gender) and detectable 20-HETE concentrations (Table 2). There was a difference in detectable levels of 20-HETE concentration based on severity of injury graded by both HH score and Fisher grade. About 85% (n=28) of patients with detectable levels of 20-HETE concentration had high HH scores, while 64% (n=48) of patients with non detectable levels of 20-HETE concentrations had high HH scores (p=0.04). Similarly, only 9% (n=3) of patients with detectable levels of 20-HETE concentration had a Fisher Grade 2 compared to 29% (n=22) of patients with non detectable levels (p=.05) (Table 2).
Fig 1.
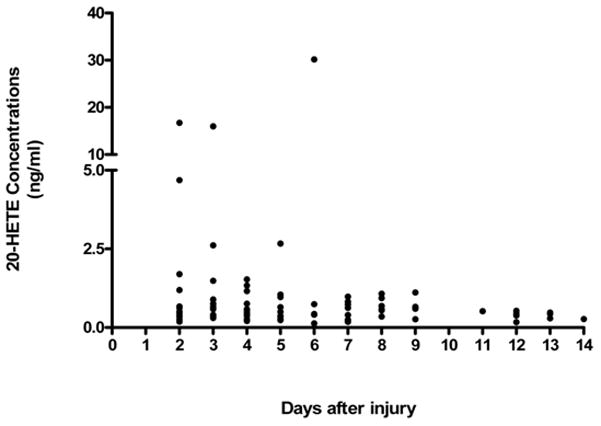
The overall profile of detectable 20-HETE concentrations in CSF at any time during 14 days inpatient stay of aSAH patients (N=33).
CSF 20-HETE Levels and DCI
In this analysis, the presence of angiographic cerebral vasospasm alone was not associated with detectable levels of CSF 20-HETE (p=.34) (Table 4). However, there was a significant relationship between the development of DCI and the presence of detectable CSF 20-HETE (p=.016). In patients with detectable CSF 20-HETE 73% (n=22) developed DCI while only 46% (n=32) of patients with non detectable levels of 20-HETE had DCI (Table 3).
Table 4.
Association of Detectable and Non-detectable 20-HETE Levels and Angiographic Vasospasm and Delayed Cerebral Ischemia
| Outcome | Category | 20-HETE Group | p-value | |
|---|---|---|---|---|
| Non-detectable (n=48) | Detectable (n=25) | |||
| Angiographic Vasospasm | No | 27 (56%) | 11 (44%) | 0.34 |
| Yes | 21 (44%) | 14 (56%) | ||
| Delayed Cerebral Ischemia | No | Non-detectable (n=69) | Detectable (n=30) | 0.016 |
| 37 (54%) | 8 (27%) | |||
| Yes | 32 (46%) | 22 (73%) | ||
CSF 20-HETE Levels and NP Outcomes
The effect of CSF 20-HETE levels on NP outcomes at 3 months post injury was assessed with multivariable linear regression models using a backwards selection procedure. Results indicated poorer function in multiple NP domains for patients with detectable 20-HETE concentrations after controlling for age, gender, years of education race, injury (HH) and depression. These domains included visuospatial ability (p=.03), learning and memory (p<.01, p=.03), language (p=.02), attention (p=.02) and mental flexibility (p<.01) (Table 5). Due to the limited power of these preliminary findings statistical significance was not maintained after Bonferroni adjustment. There was no association between mortality at3 months and detectable 20-HETE levels (Table 2).
Table 5.
Effect of Detectable and Non-detectable 20-HETE levels on NP Outcome Measures at 3 Months Post-injury
| Outcome Measurement | 20-HETE Group | p-value | ||||
|---|---|---|---|---|---|---|
| Non-detectable | Detectable | |||||
| NP Domain | NP Test | N | Adjusted Mean (SE) | N | Adjusted Mean (SE) | |
| Visuospatial Ability | Rey Complex Figure Test Copy | 30 | 28.4 (1.3) | 13 | 22.8 (2.0) | 0.03 |
| Learning and Memory | Rey Immediate Recall | 32 | 13.5 (1.2) | 15 | 7.4 (1.8) | <0.01 |
| Rey Delayed Recall | 29 | 13.8 (1.3) | 17 | 8.6 (1.9) | 0.03 | |
| Digit backward (Span) | 30 | 6.2 (0.4) | 13 | 5.2 (0.5) | 0.12 | |
| WMS-III Logical Memory Test (Story A 1st Recall) | 34 | 11.8 (0.8) | 15 | 11.1 (1.1) | 0.60 | |
| WMS-III Logical Memory Test (Story B 1st Recall) | 34 | 10.1 (0.7) | 15 | 10.0 (1.1) | 0.95 | |
| WMS-III Logical Memory Test (Story B 2nd Recall) | 32 | 12.9 (0.9) | 14 | 11.0 (1.4) | 0.27 | |
| Language | Controlled Oral Word Association (Combined F A S Score) | 31 | 30.6 (2.0) | 13 | 21.7 (3.1) | 0.02 |
| Animal Naming Test | 31 | 15.6 (0.9) | 13 | 14.5 (1.4) | 0.51 | |
| Attention | Trail Making Test A† | 30 | 38.8 (7.6) | 13 | 73.1 (11.5) | 0.02 |
| Mental Flexibility | Trail Making Test B† | 28 | 85.8 (7.8) | 13 | 131.8 (12.7) | <0.01 |
| Psychomotor Speed | Grooved Pegboard Test (Dominant hand) † | 30 | 97.6 (6.5) | 14 | 114.0 (9.5) | 0.16 |
| Grooved Pegboard Test (Non-Dominant hand) † | 29 | 109.8 (6.0) | 12 | 108.8 (9.4) | 0.93 | |
| Executive Function | Stroop Color/Word Test: Color | 28 | 40.3 (1.7) | 13 | 36.7 (2.5) | 0.25 |
| Stroop Color/Word Test: Color and Word | 28 | 42.6 (1.5) | 13 | 38.8 (2.3) | 0.18 | |
Higher scores indicate poorer outcome in NP tests noted by †
Discussion
This study demonstrates that 20-HETE CSF levels are associated with severity of injury and DCI in patients after aSAH. These results provide the first clinical evidence in a large cohort of patients that 20-HETE may be a pathogenic mediator of aSAH in humans. Previous smaller clinical studies by our laboratory and others have detected 20-HETE in CSF of aSAH patients23 and suggested that 20-HETE CSF levels are elevated in aSAH patients as compared to CSF from healthy subjects.24 Furthermore, multiple preclinical studies have demonstrated that 20-HETE is a mediator of reduced cerebral blood flow after aSAH in both rat and dog models.15,17–18 Our current findings builds on this previous work by demonstrating that 20-HETE is also associated with complications and outcomes in adult patients with aSAH.
Growing evidence has implicated 20-HETE in the pathogenesis of cardiovascular and neurovascular disease. 20-HETE has been shown to alter vascular smooth muscle ion flux upon activation by cytochrome P450 enzymes in the brain. The formation of 20-HETE is mediated by the CYP4F and CYP4A isoforms of the cytochrome P450 enzyme superfamily. In humans, CYP4F2 and CYP4A11 have demonstrated the greatest catalytic activity for 20-HETE formation. It has been suggested that 20-HETE is formed from arachidonic acid released during ischemia and cellular stress as opposing regulators of CBF and neuronal damage.14 In this human subject sample, severity of injury andDCI were significantly associated with the presence of detectable 20-HETE levels. In addition, certain single nucleotide polymorphisms (SNPs) associated with increased production of 20-HETE have been associated with cerebral ischemia and hypertension in humans.30–34 The potential association of these variants with hemorrhagic stroke and/or 20-HETE CSF levels is an important area for future work.
Animal studies by our laboratory and others have demonstrated that inhibition of 20-HETE formation is neuroprotective in temporary focal ischemia and subarachnoid hemorrhage models.20;35–36 These studies have also shown that 20-HETE inhibitors attenuate post-injury reductions in CBF that accompanies both ischemic and hemorrhagic stroke. The mechanism of this neuroprotection has not been fully elucidated, however it has been speculated that the protection is afforded by a decrease in 20-HETE production. 20-HETE has been shown to constrict blood vessels in the microvascular space, regulate new blood vessel growth, and has been shown to augment vascular remodeling.37–38 Of additional interest, while 20-HETE has associated with microvascular constriction, it may dilate larger cerebral blood vessels.22 This is of significant interest in this analysis since the presence of 20-HETE was significant with the development of DCI but not angiographic vasospasm. However, many authors have suggested that cerebral angiography is more representative of macrovascular lumen changes and may not adequately explain the ischemic changes after aSAH3,4,8,9,39. This limitation may explain the lack of significance between the presence of 20-HETE and angiographic evidence of vasospasm. Recent research related to outcomes after aSAH have moved away from cerebral vasospasm alone and focused on measures of abnormal perfusion to explain DCI including microvascular changes. In addition, there is a focused effort to accurately define the elements of cerebral ischemia after aSAH. 8 The findings of this analysis support the evidence that 20-HETE may adversely impact symptomatic ischemic events in aSAH patients possibly as a result of cerebral microvascular constriction.
Our study also provides preliminary evidence concerning the relationship between 20-HETE CSF concentrations and long term outcomes after aSAH. Our observation that detectible 20-HETE CSF levels are associated with poorer performance on NP outcomes in 5 domains (visuo-spatial, learning and memory, language, attention and mental flexibility) was anticipated based on the proposed role of 20-HETE as a pathogenic mediator of aSAH. However it is important to note that this study was powered for the evaluation of DCI and 20-HETE and therefore, evaluation of NP outcomes results should be considered preliminary; and as such, the significant relationships with outcomes did not withstand Bonferroni corrections; however, these preliminary results demonstrate the importance of additional exploration of these relationships with a larger cohort of patients.
Limitations
One limitation of this study is that our evaluated population requires access to cerebral spinal fluid. Patients who require placement of external ventricular catheters represent a subgroup of aSAH patients who may have higher risk of complications after aSAH due to extent of injury including DCI. A second limitation is due to the lack of consensus as to the definition of DCI. In this analysis we defined DCI via components of altered blood flow in the presence of neurological deterioration. Although the definitions are similar, more definitive criteria for DCI have been published since this analysis which will be vital in future studies.8 Not all patients in this analysis had angiographic data available, as it is performed as a result of clinical changes rather than research protocols, therefore the correlation with angiographic vasospasm is on a subset of this patient sample. Furthermore determining a clinical change on a patient with poor neurological condition from the onset of injury limits the use of neurological assessment used to determine whether the patient has symptomatic ischemia. Of note, development of neurological symptoms greater than one hour that has been suggested in more recent literature was not considered in this analysis, but may be an important consideration in future studies. Finally, the level of 20-HETE that we observed required statistical analysis of detectible versus non-detectible concentrations to assess the relationship with DCI and NP outcomes. Future studies will focus on full time profile and AUC analysis of 20-HETE using data from more sensitive triple quad mass spectroscopy including samples from time points that reflect initial levels of HETE expression in CSF.
Conclusions
In this study we observed that the presence of CSF 20-HETE was associated with DCI and poor neurological outcomes in aSAH patients. Based on this finding, we conclude that 20-HETE may be involved in the pathogenesis of aSAH in humans. These results support the need for continued investigation of the role of 20-HETE and aSAH. Specifically, future studies employing more sensitive analytical methods (i.e. triple quadrupole mass spectrometers) will allow for improved sensitivity and evaluation of the temporal profile of 20-HETE concentrations in patients after aSAH.
Acknowledgments
None
Sources of Funding: NIH National Institute of Nursing Research Grant R01NR004339
Footnotes
Conflict of Interest/Disclosures: None
References
- 1.Frontera J, Fernandez J, Schmidt M, Claassen J, Wartenberg K, Badjatia N, et al. Defining vasospasm after subarachnoid hemorrhage; what is the most clinically relevant definition? Stroke. 2009;40:1963–1968. doi: 10.1161/STROKEAHA.108.544700. [DOI] [PubMed] [Google Scholar]
- 2.Zubkov AY, Rabinstein AA. Medical management of cerebral vasospasm: present and future. Neurological Research. 2009;31:626–631. doi: 10.1179/174313209X382331. [DOI] [PubMed] [Google Scholar]
- 3.Laskowitz D, Kolls B. Neuroprotection in subarachnoid hemorrhage. Stroke. 2010;41:S79–S84. doi: 10.1161/STROKEAHA.110.595090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lanterna L, Lunchi A, Martchenko S, Gritti P, Bonaldi G, Biroli F. Cerebral watershed hypoperfusion in subarachnoid hemorrhage: computed tomography perfusion analysis. J Neurosurg. 2010 September 17; doi: 10.3171/2010.8.JNS091766. [DOI] [PubMed] [Google Scholar]
- 5.Gordon G, Mulligan S, MacVicar B. Astrocyte control of the cerebrovasculature. GLIA. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- 6.Losiniecki A, Zuccarello M. Subarachnoid hemorrhage: effect on cerebral blood flow and cerebral metabolism. Frontier of Bioscience. 2008;1:1845–56. doi: 10.2741/2804. [DOI] [PubMed] [Google Scholar]
- 7.Kolias AG, Sen J, Belli A. Pathogenesis of cerebral vasospasm following aneurysmal subarachnoid hemorrhage: putative mechanisms and novel approaches. Journal of Neuroscience Research. 2009;87:1–11. doi: 10.1002/jnr.21823. [DOI] [PubMed] [Google Scholar]
- 8.Vergouwen MD, Vermeulen M, van Gihn J, Rinkel G, Wijdicks E, Muizelaar J, et al. Stroke. 2010;41:2391–2395. doi: 10.1161/STROKEAHA.110.589275. [DOI] [PubMed] [Google Scholar]
- 9.Vergouwen MD, Vermeulen M, Roos YB. Delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage: is angiographic vasospasm an epiphenomenon? Stroke. 2009;40:e39. doi: 10.1161/STROKEAHA.108.537985. [DOI] [PubMed] [Google Scholar]
- 10.Aralasmak A, Akyuz M, Ozkaynak C, Sindel T, Tuncer R. CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology. 2009;51:85–93. doi: 10.1007/s00234-008-0466-7. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald RL, Kassel NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, et al. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–21. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- 12.Al-Tamimi Y, Orsi N, Quinn A, Homer-Vanniasinkam A, Ross S. A review of delayed ischemic neurologic deficit following aneurysmal subarachnoid hemorrhage: historical overview, current treatment and pathophysiology. World of Neurosurgery. 2010;73:654–667. doi: 10.1016/j.wneu.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Dankbaar J, Rijsdijk M, van der Schaar I, Velthuis B, Wermer M, Rinkel G. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia arter anuerysmal subarachnoid hemorrhage. Diagnostic Neuroradiology. 2009;51:813–819. doi: 10.1007/s00234-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roman R. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiology Review. 2002;82:131–85. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi K, Miyata N, Renic M, Harder D, Roman R. Hemoglobin, NO and 20-HETE interactions in medicating cerebral vasoconstriction following SAH. Am J Physiol Reful Integr Comp Physiol. 2006;290:R84–R89. doi: 10.1152/ajpregu.00445.2005. [DOI] [PubMed] [Google Scholar]
- 16.Renic M, Klaus J, Omura T, Kawashima N, Onishi M, Miyata M, et al. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. Journal of Cerebral Blood Flow Metabolism. 2009;29:629. doi: 10.1038/jcbfm.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kehl F, Cambj-Sapunar L, Maier KG, Miyata N, Kametani S, Okamoto H, et al. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. American Journal of Physiology Heart Circulation Physiology. 2002;282:1556–65. doi: 10.1152/ajpheart.00924.2001. [DOI] [PubMed] [Google Scholar]
- 18.Hacein-Bey L, Harder D, Meier H, Varelas P, Miyata N, Lauer K, et al. Reversal of delayed vasospasm by TS-011 in the dual hemorrhage dog model of subarachnoid hemorrhage. American Journal of Neuroradiology. 2006;27:1350–1354. [PMC free article] [PubMed] [Google Scholar]
- 19.Yu M, Cambj-Sapunar L, Kehl F, Maier K, Takeuchi K, Miyata N, et al. Effects of a 20-HETE antagonist and agonists on cerebral vascular tone. 2004;486:297–306. doi: 10.1016/j.ejphar.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Poloyac S, Zhang Y, Bies R, Kochanek P, Graham S. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. Journal of Cerebral Blood Flow Metabolism. 2006;26:1551–61. doi: 10.1038/sj.jcbfm.9600309. [DOI] [PubMed] [Google Scholar]
- 21.Omura T, Tanaka Y, Miyata N, Doizumi C, Sakurai T, Fukasawa M, et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke. 2006;37:1307–1313. doi: 10.1161/01.STR.0000217398.37075.07. [DOI] [PubMed] [Google Scholar]
- 22.Fang X, Faraci F Kaduce T, Harmon S, Modrick M, Hu S, et al. 20-Hydroxyeicosatetraenoic acid is a potent dilator of mouse artery: role of cyclooxygenase. 2006;291:H2301–2307. doi: 10.1152/ajpheart.00349.2006. [DOI] [PubMed] [Google Scholar]
- 23.Poloyac S, Reynold R, Yonas H, Kerr M. Identification and quantification of the hyeroxyeicosatetraenoic acids, 20-HETE and 12-HETE, in the cerebrospinal fluid after subarachnoid hemorrhage. Journal of Neuroscience Methods. 2005;144:257–263. doi: 10.1016/j.jneumeth.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Roman RJ, Renci M, Dunn KM, Takeuchi K, Hacein-Bey L. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurological Research. 2006;28:738–49. doi: 10.1179/016164106X152016. [DOI] [PubMed] [Google Scholar]
- 25.Bolocato C, Frye R, Zemaitis M, Poloyac S. Determination of 20-hydroxyeicosatetraenoic acid in microsmal incubates using high-performance liquid chromatography-mass spectrometry (HPLC-MS) J Chromatogr B Analyt Technol Biomed Life Sci. 2003;794:363–372. doi: 10.1016/s1570-0232(03)00496-3. [DOI] [PubMed] [Google Scholar]
- 26.Saykin A, Gur R, Gur R, Shtasel D, Flannery K, Mozley L, et al. Normative neuropsychological test performance: effects of age, education, gender and ethnicity. Applied Neuropsychology. 1995;2:79–88. doi: 10.1207/s15324826an0202_5. [DOI] [PubMed] [Google Scholar]
- 27.Salinsky M, Storzbach D, Dodrill C, Binder L. Test-retest bias, reliability and regression equations for neuropsychological measures repeated over a 12–16 week period. J Int Neuropsychol Soc. 2001;7:597–605. doi: 10.1017/s1355617701755075. [DOI] [PubMed] [Google Scholar]
- 28.Levine A, Miller E, Becker J, Seines O, Cohaen B. Normative data for determining significance of test-retest differences on eight common neuropsychological instruments. Clinical Neuropsychology. 2004;18:373–384. doi: 10.1080/1385404049052420. [DOI] [PubMed] [Google Scholar]
- 29.Blair C. How similar are fluid cognition and general intelligence? A developmental neuroscience perspective on fluid cognition as an aspect of human cognitive ability. Behavioral Brain Science. 2006;29:109–125. doi: 10.1017/S0140525X06009034. [DOI] [PubMed] [Google Scholar]
- 30.Segal D, Coolidge F, Cahill B, O’Riley A. Psychometric properties of the Beck Depression Inventory II (BDI-II) among community-dwelling older adults. Behavioral Modification. 2008;32:3–20. doi: 10.1177/0145445507303833. [DOI] [PubMed] [Google Scholar]
- 31.Gainer JV, Bellamine A, Dawson EP, Womble KE, Grant SW, Wang Y, et al. Functional variant of CYP4A11 20-hydroxyeicosatetraenoic acid synthase is associated with essential hypertension. Circulation. 2005;111:63–69. doi: 10.1161/01.CIR.0000151309.82473.59. [DOI] [PubMed] [Google Scholar]
- 32.Ward NC, Tsai I, Barden A, van Bockxmeer F, Puddey I, Hodgson J, et al. A single nucleotide polymorphism in the CYP4F2 but not CYP4A11 gene is associated with increased 20-HETE excretion and blood pressure. Hypertension. 2008;5:1393–8. doi: 10.1161/HYPERTENSIONAHA.107.104463. [DOI] [PubMed] [Google Scholar]
- 33.Fava C, Montagnana M, Almgren P, Rosberg L, Lippi G, Hedblad B, et al. The V433M variant of the CYP4F2 is associated with ischemic stroke in male Swedes beyond its effect on blood pressure. Hypertension. 2008;52:373–380. doi: 10.1161/HYPERTENSIONAHA.108.114199. [DOI] [PubMed] [Google Scholar]
- 34.Laffer C, Gainer J, Waterman M, Capdevila J, Laniado-Schwartzman M, Nasjletti A, et al. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension. 2008;51:767–772. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takeuchi K, Renic M, Bohman Q, Harder D, Miyata N, Roman R. Reversal of Delayed Vasospasm by an Inhibitor of the Synthesis of 20-HETE. Am J Physiol Heart Circ Physiol. 2005;289:H2203–11. doi: 10.1152/ajpheart.00556.2005. [DOI] [PubMed] [Google Scholar]
- 36.Omura T, Tanaka Y, Miyata N, Koizumi C, Sakurai T, Fukasawa M, et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke. 2006;37:1307–13. doi: 10.1161/01.STR.0000217398.37075.07. [DOI] [PubMed] [Google Scholar]
- 37.Harder D, Gebremedhin D, Narayanan J, Jefcoat C, Falck J, Campbell W, et al. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 38.Amaral S, Maier K, Schippers D, Roman R, Greene A. CYP4A metabolites of arachidonic acid and VEGF are mediators of skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2004;284:H1528–H1535. doi: 10.1152/ajpheart.00406.2002. [DOI] [PubMed] [Google Scholar]
- 39.Vergouwen M, Vermeulen M, Coert B, Stroes E, Roos Y. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism. 2008;28:1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]


