Abstract
It has become increasing clear that alterations in cellular metabolism have a key role in the generation and maintenance of cancer. Some of the metabolic changes can be attributed to the activation of oncogenes or loss of tumor suppressors. Here, we show that the mitochondrial sirtuin, SirT3, acts as a tumor suppressor via its ability to suppress reactive oxygen species (ROS) and regulate hypoxia inducible factor 1α (HIF-1α). Primary mouse embryo fibroblasts (MEFs) or tumor cell lines expressing SirT3 short-hairpin RNA exhibit a greater potential to proliferate, and augmented HIF-1α protein stabilization and transcriptional activity in hypoxic conditions. SirT3 knockdown increases tumorigenesis in xenograft models, and this is abolished by giving mice the anti-oxidant N-acetyl cysteine. Moreover, overexpression of SirT3 inhibits stabilization of HIF-1α protein in hypoxia and attenuates increases in HIF-1α transcriptional activity. Critically, overexpression of SirT3 decreases tumorigenesis in xenografts, even when induction of the sirtuin occurs after tumor initiation. These data suggest that SirT3 acts to suppress the growth of tumors, at least in part through its ability to suppress ROS and HIF-1α.
Keywords: SirT3, ROS, HIF, mitochondria, hypoxia
Introduction
Oncogenic transformation is accompanied by a variety of cellular changes, including changes in metabolism. It is not known whether the alterations in metabolism are a cause or an affect of transformation, but they can help cancer cells to survive various stresses such as hypoxia and glucose limitation. Some of these changes are facilitated by the transcription factor hypoxia inducible factor 1α (HIF-1α). HIF-1α is a heterodimer of two basic helix-loop-helix/PAS proteins, HIF-1α and the aryl hydrocarbon nuclear trans-locator (ARNT or HIF-1β) (Wang et al., 1995). An alternate isoform, HIF-2α has both overlapping and non-redundant functions with HIF-1α (Webb et al., 2009). Both HIFα subunits and ARNT are ubiquitously expressed, however, the alpha subunit is labile in conditions of normal oxygen (5–21% O2). Under hypoxic conditions (0.5–5% O2) the HIFα subunit is stabilized, dimerizes with ARNT, translocates to the nucleus, and subsequently binds to HIF response elements (HRE) within target genes. Among HIF-1α, transcription targets are genes involved in glucose metabolism, angiogenesis and metastasis (Semenza, 2003), thereby tightly linking HIF-1α-mediated transcription to tumorigenesis.
The stability of HIF-1α protein is based on the hydroxylation of proline residues within the oxygen-dependent degradation domain. This hydroxylation reaction is catalyzed by a family of proline hydroxylation enzymes (Bruick and McKnight, 2001; Epstein et al., 2001). Hydroxylated prolines serve as a binding site for the tumor suppressor Von-Hippel Lindeu protein (pVHL), the substrate recognition component of the VBC-CUL-2 E3 ubiquitin ligase complex (Ivan et al., 2001; Jaakkola et al., 2001). In hypoxic conditions, HIF-1α is stabilized by inhibition of the proline hydroxylation enzymes through reactive oxygen species (ROS) generated from the Qo-site of complex III in the mitochondrial electron transport chain (Bell et al., 2007a). These ROS are necessary for hypoxic activation, as when a mitochondrial-targeted antioxidant is added during hypoxia HIF-1α protein is not stabilized. Aberrant stabilization/activation of HIF-1α is associated with different types of cancers. For example, loss of pVHL results in normoxic stabilization of HIF-1α, and causes renal clear cell carcinoma, phaeochromocytomas, and paragangliomas (Kaelin, 2008). In addition to pVHL, loss of succinate dehydrogenase, fumarate hydratase and REDD1 have been demonstrated to stabilize and activate HIF-1α in normoxic conditions, due to induction of ROS and mitochondrial metabolites (Isaacs et al., 2005; Selak et al., 2005; Guzy et al., 2008; Horak et al., 2010). Thus, tight regulation of HIF-1α stability/activity is important to maintain cells in a non-transformed state.
Sirtuins are NAD-dependent deactylases that share homology to the yeast Sir2 protein. Their enzymatic activity is regulated by the ratio of NAD to NADH; high NAD levels activate sirtuins and conversely high NADH levels inhibit activity (Lin and Guarente, 2003). There are seven mammalian homologs of Sir2 with SirT1 having the most conserved sequence to Sir2. SirT1 localizes primarily in the nucleus along with SirT6 and SirT7. SirT2 is in the cytoplasm whereas SirT3, SirT4, and SirT5 are localized in the mitochondria (Haigis and Guarente, 2006). SirT1 was recently demonstrated to negatively regulate HIF-1α activity in hypoxia by direct deacetylation (Lim et al., 2010). This regulation is dependent on the abundance of NAD and NADH, as when NADH increases in hypoxia, SirT1 deacetylation of HIF-1α is inhibited thereby allowing for sustained activation of HIF-1α. SirT1 can also deacetylate HIF-2α to regulate transcriptional activity (Dioum et al., 2009). In addition, SirT6 has been shown to repress HIF-1α-mediated transcription by binding to chromatin on HREs and attenuating the activation of glycolytic genes via deacetylating histones around the promoters (Zhong et al., 2010).
Of the three mitochondrial sirtuins, SirT3 is the most studied to date and has been linked to longevity in humans (Bellizzi et al., 2005). Recently SirT3 was described to act as a mitochondrial localized tumor suppressor (Kim et al., 2010). Specifically, SirT3 deficiency alone is sufficient for the transformation of primary MEFs with Myc and/or RAS to from tumors in xenograft models. These data indicate that deletion of SirT3 replaces the need for the loss of a tumor suppressor required for transformation of primary cells with an oncogene. Kim and colleagues also demonstrated that SirT3−/− Myc/Ras MEFs exhibit increased glycolysis, decreased oxidative phosphorylation and increased ROS. The authors propose that loss of SirT3 promotes transformation through an increase in chromosomal instability via increased production of ROS and altered intracellular metabolism. In this study we further investigated how SirT3 can act as a tumor suppressor and determine whether the increased ROS in the absence of SirT3 can effect HIF-1α activation. We demonstrate that loss of SirT3 increases the number of population doublings of primary MEFs and stabilizes HIF-1α protein to activate HIF-1α target genes in hypoxia. Moreover, we demonstrate that the absence of SirT3 increases tumorigenesis of established cancer cells and that this increase is dependent on ROS. Critically, we also show that SirT3 overexpression is sufficient to impede HIF-1α stabilization in hypoxia and to inhibit tumorigenesis, indicating a novel role of SirT3 in the maintenance and progression of cancer.
Results
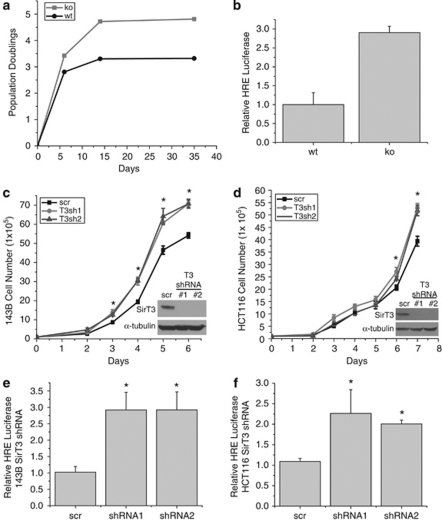
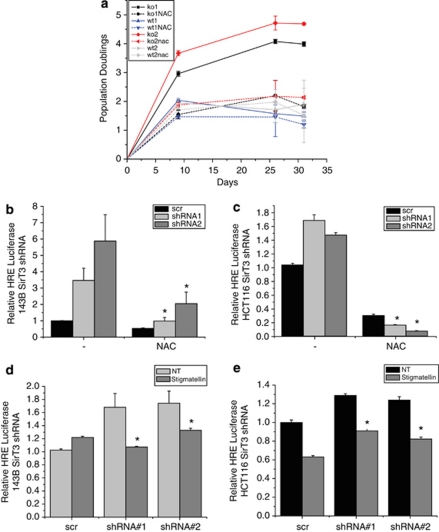
Loss of SirT3 increases ROS and allows for transformation (Kim et al., 2010). Increases in ROS are also associated with senescence and apoptosis in primary cells. We thus set out to determine the effect of SirT3 deletion on replicative life span of primary MEFs. Surprisingly, we found that SirT3−/− MEFS have increased population doublings compared with wt controls (Figure 1a). Another example of increased replicative life span is found when cells are incubated in hypoxic conditions (Parrinello et al., 2003) thereby generating ROS, and this increase is dependent on HIF activity (Bell et al., 2007b). Therefore, we explored whether loss of SirT3 activates HIF. Using a synthetic HIF-1α reporter construct (HRE-luc) that consists of three putative HRE sites upstream of firefly luciferase, we found that MEFs displayed mildly elevated HIF-1α transcriptional activity in the absence of SirT3 (Figure 1b). Next we examined whether SirT3 has a role in the maintenance and progression of cancer by using established cancer cell lines. We knocked down SirT3 in the human osteosarcoma cell line 143B (Figure 1c) and the human colon carcinoma cell line HCT116 with two different short-hairpin (sh)RNAs (Figure 1d), and found that both knockdown cell lines displayed increased proliferation (Figures 1c and d). These data indicate that SirT3 has tumor suppressor functions in already established cancer cell lines in addition to its previously described role in inhibiting transformation of primary cells (Kim et al., 2010). Both the 143B and HCT116 SirT3 knockdown cell lines showed a mild increase in luciferase activity when transfected with HRE-luc (Figures 1e and f). These data demonstrate that deleting SirT3 results in higher growth rates in normal and established cancer cell lines, and may increase normoxic HIF-1α activity.
Figure 1.
SirT3 loss of function increases proliferation and HIF-1α activity. (a) Population doublings in primary SirT3 wild type (wt) and SirT3−/− (KO) MEFs cultured in normal oxygen conditions (21% O2). (b) Luciferase values from immortalized wt and KO MEFs transfected with HRE-luciferase in normoxia. SirT3 protein levels in 143B (c) and HCT116 (d) cells stably expressing a scrambled control shRNA (scr) or two different shRNAs that target SirT3. Both shRNA sequences increase the rate of cellular proliferation. Relative luciferase values of 143B (e) and HCT116 (f) cells in SirT3 stable knockdown cell lines (shRNA) or a scrambled control vector (scr) line transfected with HRE-luciferase. Error bars are s.e.m. and * indicates a P value <0.05 with two-tailed Student's t-test.
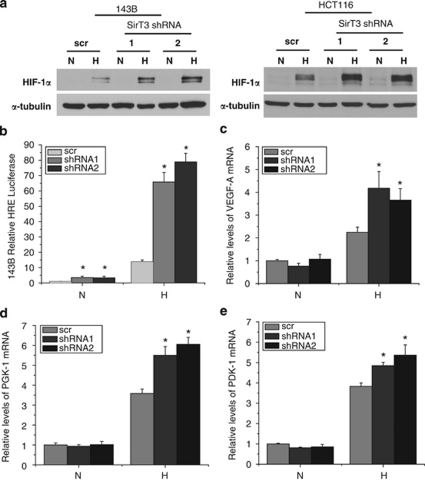
We next explored whether SirT3 knockdown affected the hypoxic activation of HIF-1α. Culturing the SirT3 knockdown cells in hypoxic conditions (1% O2) resulted in a striking increase in the amount of HIF-1α protein stabilized when compared with the expected increase in the scrambled shRNA control (Figure 2a). To determine whether there is a concomitant increase in HIF-1α transcriptional activity we utilized the HRE-luc construct. In hypoxic conditions there was a 13-fold increase in luciferase activity in the scramble control compared with normoxic conditions, whereas the SirT3 knockdown cells demonstrated a 70-fold increase in luciferase activity under the same conditions (Figure 2b). To determine the effects on endogenous HIF-1α targets, we performed quantitative PCR on RNA isolated from cells incubated at ambient O2 levels or 1% O2. Levels of all putative HIF-1α targets tested, VEGF-A, PGK-1 and PDK-1 were increased in hypoxia in the control samples, and the loss of SirT3 further increased these HIF-1α targets, albeit to a lesser extent than HRE-luc (Figures 2c–e). Interestingly, there was no increase of these targets in the SirT3 knockdown cells cultured under normoxic conditions. This may be due to a requirement of co-activators that are only activated in hypoxia.
Figure 2.
Knockdown of SirT3 augments the hypoxic response. (a) HIF-1α protein levels from whole cell lysates of indicated tumor cell lines incubated at normoxic (N, 21% O2) or hypoxic (H, 1% O2) conditions for 4 h. (b) Relative luciferase values of 143B cells in SirT3 stable knockdown cell lines (shRNA) or a scrambled control vector (scr) line transfected with HRE-luciferase cultured in normoxic (N, 21% O2) or hypoxic (H, 1% O2) conditions for 16 h. Quantitative PCR for VEGF-A (c), PGK-1 (d) and PDK-1 (e) on RNA isolated from 143B cells stably expressing SirT3 shRNA or a scrambled control vector (scr) cultured in normoxic (N, 21% O2) or hypoxic (H, 1% O2) conditions for 16hrs. Error bars are s.e.m. and * indicates a P value <0.05 with two-tailed Student's t-test.
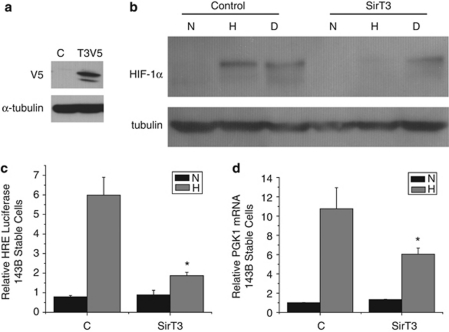
Since loss of SirT3 augments hypoxic activation of HIF-1α, we wished to know whether SirT3 gain of function attenuates HIF-1α in the hypoxic response. Thus we stably expressed V5-tagged SirT3 in 143B cells (Figure 3a), incubated cells in 1% O2 and analyzed HIF-1α expression. The control cells had a significant increase of HIF-1α protein in hypoxic conditions whereas the cells expressing SirT3 failed to stabilize HIF-1α protein during hypoxia (Figure 3b). However, both control and SirT3-expressing cells stabilized HIF-1α protein in the presence of DMOG, a competitive inhibitor of the proline hydroxylation enzymes. The decrease in HIF-1α protein stability in hypoxic SirT3-expressing cells corresponded to an attenuated transcriptional response of HRE-luc (Figure 3c), as well as the endogenous HIF-1α responsive target gene PGK1 (Figure 3d). These data demonstrate that increased SirT3 can prevent HIF-1α stabilization in hypoxia, reciprocal to the phenotype of SirT3 knockdown cells.
Figure 3.
SirT3 gain of function inhibits hypoxic activation of HIF-1α. (a) Western blot of 143B cells stably overexpressing SirT3 tagged with V5. (b) Western blots of HIF-1α using total cell lysates from 143B control (c) and SirT3 overexpressing cells in normoxic (N, 21% O2) or hypoxic (H, 1% O2) conditions or treated with DMOG (d) for 16 h. (c) Relative luciferase values in 143B SirT3 overexpressing cells transfected with HRE-luciferase. (d) PGK1 mRNA from 143B SirT3 overexpressing cells in normoxic (N21% O2) or hypoxic (H, 1% O2) conditions. Error bars are s.e.m. and * indicates a P value <0.05 with two-tailed Student's t-test.
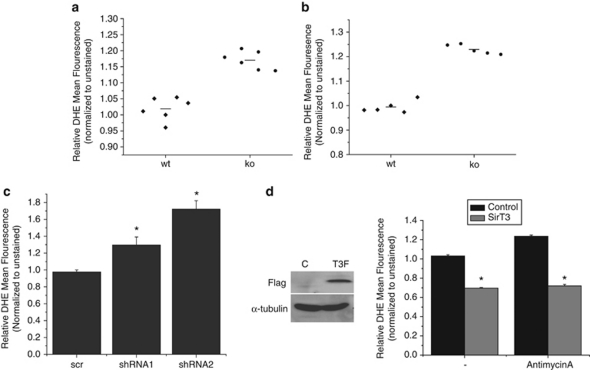
Previous work has demonstrated that addition of exogenous ROS is sufficient to stabilize and activate HIF-1α in normoxic conditions, and ROS is necessary for its hypoxic stabilization (Chandel et al., 2000). As has been previously published, we observed an increase in reactive oxygen species in SirT3−/− primary and immortalized MEFs (Figures 4a and b). Interestingly, when we knocked down SirT3 in established cancer cell lines we also observed increased ROS (Figure 4c and Supplementary Figure S1). Further, overexpression of SirT3 decreased basal ROS levels, as well as the increased ROS levels mediated by antimycin A (Figure 4d). These data suggest that the alteration of ROS levels may be the main mechanism by which SirT3 regulates HIF-1α. To determine whether the activation of HIF-1α in the absence of SirT3 is due to ROS, we treated cells with antioxidants and determined the extent of HIF-1α activation. The 143B and HCT116 cells that have SirT3 stably knocked down were treated with N-acetyl-cysteine (NAC), and the increase in ROS was prevented (Supplementary Figure S1). Strikingly, addition of NAC to primary SirT3−/− MEFs abolished the increase in population doublings observed in the primary SirT3−/− MEFs (Figure 5a). NAC treatment also decreased the normoxic increase of HIF-1α transcriptional activity in 143B and HCT116 SirT3 knockdown cells (Figures 5b and c).
Figure 4.
ROS levels are regulated by SirT3. Relative levels of dihydroethidium (DHE) fluorescence in wild type (wt) or SirT3 −/− (KO) primary MEFs (a) and immortalized MEFs (b) treated with 10 μ DHE in normal oxygen conditions and then analyzed by flow cytometry. Each data point represents an independent culture of cells. (c) Relative fluorescence of 143B cells with SirT3 stably knocked down with two different SirT3 shRNAs or a scrambled control after incubation with DHE. (d) Western blots of whole cell lysates isolated from 143B cells stably overexpressing SirT3 Flag and their relative fluorescence after incubation with DHE with or without antimycin A (2 μg/ml). Error bars are s.e.m. and * indicates a P value <0.05 with two-tailed Student's t-test.
Figure 5.
Increased HIF-1α activity in the absence of SirT3 is mediated by ROS from complex III. (a) Population doublings of two wild-type and two SirT3−/− (KO) primary MEFs in the presence or absence of NAC (5 m). The 143B (b) and HCT116 (c) SirT3 stable knockdown cell lines transfected with HRE-luciferase with or without 24 h NAC (10 m) pretreatment. Luciferase activity in stigmatellin (1 μ) treated or not treated (NT) 143B (d) and HCT116 (e) stable cell lines with scrambled or SirT3 shRNA and transfected with HRE-luciferase. Error bars are s.e.m. and * indicates a P value <0.05 with two-tailed Student's t-test.
Hypoxia or treatment with antimycin A induces ROS production from the Qo site of mitochondrial complex III. The fact that SirT3 overerxpression inhibits hypoxic activation of HIF-1α as well as antimycin A induced ROS, thus indicates that SirT3 may be acting on complex III. To determine if the source of the increased ROS in the absence of SirT3 is complex III of mitochondria, we measured HIF-1α activity in the presence or absence of the complex III inhibitor stigmatellin. Stigmatellin binds to the Qo site of complex III to inhibit electron transfer and therefore the ability of ROS to be generated from the Qo site (Breyton, 2000). Addition of stigmatellin attenuated the increase in HRE-luciferase in the SirT3 knockdown cells (Figures 5d and e), thereby demonstrating that the increase in HIF-1α activity is due to ROS generated by complex III. Knocking down SirT3 in both the 143B- and HCT116-established cancer cell lines did not alter protein levels of MnSOD or the cytosolic antioxidants catalase and SOD1 (Supplementary Figure S2).
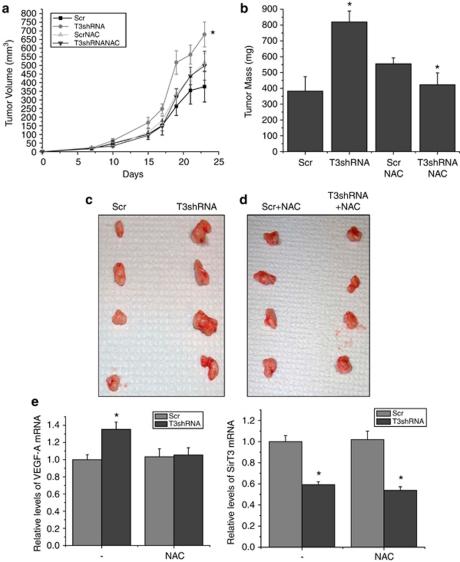
To determine whether SirT3 modulates the progression and maintenance of tumors, we preformed studies taking advantage of the fact that HCT116 cells are dependent on HIF for the generation of tumors in xenograft models (Dang et al., 2006). When HCT116 cells with stable knockdown of SirT3 were injected into the flanks of Nu/Nu mice, they formed tumors with an increased rate of growth and increased final mass compared with control HCT116 cells expressing a scrambled shRNA injected in the same mouse on the opposite flank (Figures 6a–d). The tumors formed by the injected cells maintained the SirT3 knockdown level of expression and also had increased mRNA levels of the pro-angiogenic HIF-1α target VEGF-A (Figure 6e), indicating that HIF-1α activity is upregulated in these tumors. To determine whether this difference in tumorigenesis is due to increased ROS in the SirT3 knockdown cells, we supplied NAC to the drinking water. In the presence of NAC, the tumors derived from the SirT3 shRNA cells did not display an increase in the rate of tumor growth (Figure 6c), final mass (Figure 6d) or VEGF-A mRNA (Figure 6e). This data implies that SirT3 deficiency increases ROS to activate HIF-1α and facilitate tumorigenesis.
Figure 6.
Loss of SirT3 in established human cancer cell lines increases tumor growth and is dependent on ROS. Tumor volume (a) and tumor mass (b) 24 days after subcutaneous injection of HCT116 scramble control and SirT3 knockdown cells in Nu/Nu mice with or without 40 m NAC supplementation in the drinking water. (c, d) Pictures of tumors from a and b after mice were euthanized. (e) Quantitative PCR for VEGF-A and SirT3 on RNA isolated from the tumors. Error bars are s.e.m. and * indicates a P value <0.05 with two-tailed Student's t-test.
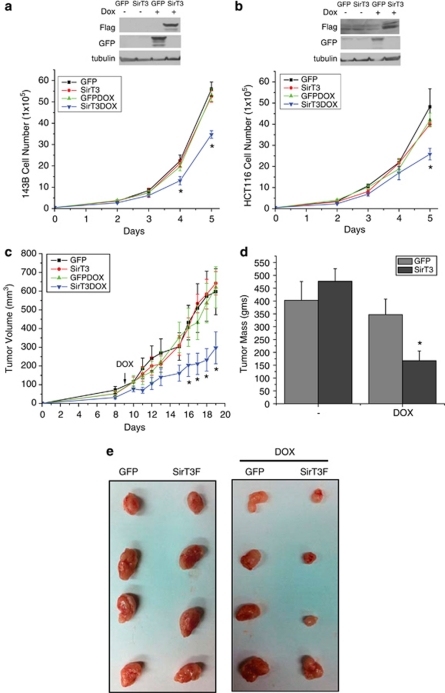
The data from Figure 3 suggests that overexpression of SirT3 might decrease the ability of cells to form tumors. However, we were not successful in generating HCT116 cell lines overexpressing SirT3, suggesting that constitutive SirT3 overexpression is selected against in this cell line. Therefore we turned to an inducible system in order to analyze the role of SirT3 gain of function on proliferation and tumorigenesis. We generated 143B and HCT116 cells stably expressing doxycycline-inducible SirT3, or GFP as a control (Figures 7a and b). When induced by the addition of doxycycline, both the 143B and HCT116 cells that expressed SirT3 had decreased proliferation compared with the GFP cells and the un-induced cells (Figures 7a and b). Next, we performed xenograft experiments with the HCT116 GFP or SirT3 inducible cell lines. Nine days after injecting the cells the food was switched in half the cages to food containing doxycycline. Induction of SirT3 led to smaller tumors compared with the induction of GFP or no induction (Figure 7e). The rate of tumorigenesis was decreased, as well as the final tumor mass, with the cells that stably induced SirT3 expression (Figures 7c and d). These data demonstrate for the first time that overexpression of SirT3 indeed can inhibit the growth of established cancer cells in xenograft models of tumorigenesis.
Figure 7.
Overexpression of SirT3 negatively regulates proliferation and tumorigenesis. Inducible overexpression of GFP and SirT3 in 143B (a) and HCT116 (b) decreases proliferation. Tumor volume (c) and tumor mass (d) of HCT116 GFP or SirT3 cells injected into the flanks of Nu/Nu mice. Doxycycline was added on day 9 to induce expression of GFP or SirT3. Error bars are s.e.m. and * indicates a P value <0.05 with two-tailed Student's t-test. (e) Pictures of tumors from c and d.
Discussion
Mitochondria are known to act as signaling organelles through the generation of ROS produced by the electron transport chain (Starkov, 2008). High levels of ROS results in DNA damage as well as damage to proteins and lipids, thereby promoting cell death, senescence or aging (Martindale and Holbrook, 2002). At lower levels, electron transport chain-generated ROS can promote cell division, modulate MAP kinase signaling cascades through regulation of phosphatases and activate transcription factors (Adler et al., 1999; Havens et al., 2006). Here we described a novel tumor suppressor function of SirT3 due to the suppression of electron transport chain-generated ROS. We demonstrate that as a consequence of increased ROS in the absence of SirT3, the pro-tumorigenic, ROS responsive transcription factor HIF-1α is slightly activated in normoxia and hyper-activated in hypoxia. The normoxic activation is inhibited by the antioxidant NAC and stigmatellin, an inhibitor of mitochondrial complex III, indicating that ROS from complex III is involved in this activation. Recently, two enzymes that have a role in the detoxification of ROS were demonstrated to be direct targets of SirT3 (Qiu et al., 2010; Someya et al., 2010; Tao et al., 2010). These enzymes, MnSOD and IDH2, are localized to the matrix of the mitochondria; therefore they probably do not have a role in the increased cytosolic ROS we demonstrate to activate HIF. Interestingly, four of the eleven proteins that make up complex III have been demonstrated to be acetylated (Schwer et al., 2009). It will be interesting to determine if acetylation of these subunits alters ROS generation from complex III and if any of these subunits are direct targets of SirT3.
Our data showing that SirT3 stimulates greater HIF-1α protein stabilization in hypoxic conditions suggests that SirT3 modulates HIF through ROS-mediated alteration of proline hydroxylation enzyme function, thereby altering hydroxylation and subsequent proteosomal degradation of HIF-1α. Previous reports have demonstrated that the increase in ROS in the absence of SirT3 inhibits FOXO3a activity (Sundaresan et al., 2009) and FOXO3a regulates expression of the HIF-1α transcriptional inhibitor CITED2 (Bakker et al., 2007), thus it is possible that CITED2 may have a role in our results. However, the 143B SirT3 knockdown cells have only slightly decreased levels of CITED2 whereas the HCT116 knockdown cells have increased levels of CITED2 (Supplementary Figure S3) in normoxic conditions, indicating that affects on HIF cannot be fully explained by this mechanism.
Using the HCT116 colon carcinoma cell line, an established human cancer cell line, which form xenografts in a HIF-1α dependent manner, we demonstrate that knockdown of SirT3 increases tumor growth. Further we demonstrate that administration of the antioxidant NAC normalizes this increase to the level of tumor formation of control cells. Previously it was reported that NAC administration completely inhibits tumor formation in xenograft models (Gao et al., 2007). Interestingly, our NAC treated scramble shRNA expressing cells did form tumors, which is not consistent with the aforementioned study. This discrepancy may be explained by the fact that in that study they pretreated mice with NAC one week before injection whereas we started treating with NAC on the day of injection. Previously, SirT3 has been demonstrated to act as a tumor suppressor by negatively regulating MnSOD/ROS-mediated genomic instability (Kim et al., 2010). This has major consequences for the initiation of tumorigenesis, but is less important for the maintenance and progression of cancers, which would be promoted by the survival and metastasis of primary tumor cells. Activation of HIF-1α will directly augment the transcription of genes involved in angiogenesis and metastasis, such as VEGF, MMP-9, lysyl oxidase and carbonic anhydrase IX (Rankin and Giaccia, 2008). HIF-1α also activates metabolic genes, such as PGK-1 and PFK1A, which increase glycolysis for ATP production during hypoxia (Semenza, 2009). In the absence of SirT3, HIF-1α activation may thus promote the growth and spread of tumors. The dual action of SirT3 to inhibit HIF-1α and prevent ROS-mediated genomic instability makes loss of SirT3 a potent oncogenic event.
If the role of SirT3 as a tumor suppressor acts reciprocally, then activation or gain of function should act as an inhibitor of tumorigenesis. Indeed, we demonstrate for the first time that overexpression of SirT3 is able to decrease proliferation of established cancer cell lines. Moreover, induction of SirT3 inhibits the ability of established tumors to grow in xenograft models. Chemical compounds that activate SirT3 may thus be exploited to treat cancers. In addition to the above considerations, it has been demonstrated that hypoxic activation of HIF-1α protects tumor cells from chemotherapeutics (Rohwer et al., 2010). Our finding that SirT3 gain of function is sufficient to suppress the hypoxic activation of HIF-1α also indicates that SirT3 activation may also provide therapeutic benefits by increasing the susceptibility of tumors to chemotherapeutics.
The tumor suppressing contributions of SirT3 could partially explain why a polymorphism in a variable number tandem repeat regions within intron 5 of the human SirT3 gene, which results in higher than normal expression of SirT3, is associated with increased longevity in humans (Rose et al., 2003; Bellizzi et al., 2005). A second link between SirT3 and longevity may relate to ROS, one of the modulators of aging via damage of DNA, proteins and lipids. Increased expression of SirT3 could protect from aging by decreasing ROS and increasing efficiency of metabolism. Numerous reports have indicated that the HIF pathway has a role in life span of C. elegans (Chen et al., 2009; Zhang et al., 2009). The recent connections between SirT1, SirT6 and HIF (Dioum et al., 2009; Lim et al., 2010; Zhong et al., 2010) indicate that three of the seven mammalian sirtuins regulate the HIF pathway, representing extensive metabolic cross-talk between pathways that respond to food and oxygen availability. The interactions among these sirtuins on HIF allow for the integration of responses to dietary (sirtuins) and hypoxic (HIF-1α) stresses, and may lead to new approaches to age-related diseases.
Materials and methods
Cell culture
All cells were incubated in 37 °C humidified incubator with 5% CO2. HCT116 (ATCC) were cultured in McCoy's5A (ATCC) whereas MEFs and 143B cells were cultured in Dulbecco's modified Eagle medium (Sigma, St Louis, MO, USA). All the media were supplemented with 10% fetal bovine serum (Hyclone, South Logan, UT, USA), 100 U/ml penicillin/streptomycin (Mediatech, Manassas, VA, USA) and 20 m HEPES (Mediatech). MEFs were isolated from E13.5 embryo from a SirT3+/− x SirT3+/− cross. In all experiments using NAC (Sigma), it was added the day before the experiment was started. Except for the population doublings (5 m), NAC was used at a concentration of 10 m. Stable cells were generated using lentivirus or retrovirus. To monitor population doublings, cells were split at approximately 70% confluence, counted, and seeded at a density of 4 × 104 cells. Population doubling was calculated using the following formula: PD=log(number of cells counted/number of cells seeded)/log2+previous PD. Representative curves are displayed from three independent experiments. Hypoxic conditions (1% O2) were obtained using humidified variable aerobic workstation InVivo2 400 (Ruskinn, Pencoed, UK).
Constructs and cloning
Full length SirT3 was cloned from mouse liver complementary DNA as previously described (Cooper et al., 2009). To generate a lentiviral transfer vector with V5-tagged SirT3, we PCR amplified SirT3 and ligated it into pENTR-D-TOPO (Invitrogen) and then recombined into pLenti4-TO-V5-DEST (Invitrogen). Flag tagged SirT3 was generated by PCR amplification with flanking EcoRI sites and a Flag tag in the reverse primer. The PCR product was gel purified (Qiagen, Valencia, CA, USA) and ligated into EcoRI digested pMSCV PIG (Addgene plasmid 21645, Addgene, Cambridge, MA, USA) or EcoRI digested pLVX-tight puro (Clontech, Mountain View, CA, USA). The pMA2640 (Addgene plasmid 25434) was used to stably express rtTA. SirT3 pLKO.1 shRNA vectors were purchased from Sigma (sequence 1 is TRCN0000038892 and sequence 2 is TRCN0000038893) and the control pLKO.1 scramble vector was obtained from Addgene (plasmid 1864). We used pMD2.G (Addgene plasmid 12259) and psPAX2 (Addgene plasmid 12260) to generate virus using the above described transfer vectors.
Gene reporter assay
HIF-mediated transcriptional activity was measured using HRE-luc plasmid. HRE-luc is three copies of a HIF response element (3 × HRE) from the pgk-1 gene cloned into the pGL2-Basic plasmid (Promega, Madison, WI, USA). Cells (2 × 105) were plated into each well of a six-well plate and the next day cells were transfected with 1 μg HRE-luc and 0.05 μg of pRL-TK plasmid using Fugene HD (Roche, Indianapolis, IN, USA) according to the manufacturer's protocol. After 24 h, cells were subjected to conditions for 16 h before lysates were collected. Luciferase values were determined using a dual-luciferase reporter assay kit (Promega) according to the manufacturer's protocol. Values for firefly luciferase were normalized to Renilla luciferase under the control of the thymidine kinase promoter in the pRLTK vector.
ROS determination
Cells (105) were plated into each well of a 24-well plate and the next day the cells were washed once with phosphate-buffered saline and labeled at 37 °C for 30 min in phenol red-free Dulbecco's Modified Eagle Medium (Sigma) containing 10μ dihydroethidium (Invitrogen). After 30 min, plates were placed on ice and cells were trypsinized and resuspended in dihydroethidium free labeling media. Fluorescence was measured using FACScan cytometer (BD Biosciences, Woburn, MA, USA) and the geometric mean fluorescence intensity of a minimum of 10 000 cells was analyzed in each sample. Mean fluorescence intensity values were corrected for autofluorescence by normalizing to unlabeled cells.
SDS–polyacrylamide gel electrophoresis and western blots
Total cell lysates were prepared by washing cells with cold phosphate-buffered saline and then the cells were lysed with RIPA Buffer (50 m Tris-HCL pH8, 150 m NaCl, 1 m EDTA, 0.1% SDS, 0.1% NaDeOC, 1% NP40, complete protease inhibitor (Roche)). At least 50 μg of total cell lysates was run on a SDS–polyacrylamide gel electrophoresis and then proteins were transferred to polyvinylidene difluoride (Millipore, Billerica, MA, USA). Membranes were incubated with appropriate primary antibodies overnight at 4 °C. Antibodies used are as follows: human HIF-1α (BD Biosciences), α-tubulin (Sigma), Flag (Sigma), V5 (Abcam, Cambridge, MA, USA) and Flag (Sigma).
Quantitative real time PCR
RNA was isolated using Aurum Total RNA Mini Kit (Biorad, Hercules, CA, USA) and complementary DNA was generated from 1μg RNA using Retroscript Kit (Ambion, Austin, TX, USA) according to manufacturer's protocols. Complementary DNA was analyzed using the Light Cycler 480 II (Roche) with SYBR Green master mix from Biorad. Primers sequences were obtained from Primer Bank (http://pga.mgh.harvard.edu/ primerbank/citation.html).
Xenografts
A volume of 200 μl of phosphate-buffered saline containing 5 × 106 HCT116 cells were injected into either flank of 7-week-old Nu/Nu mice (Jackson Labs, Bar Harbor, ME, USA). The control scr expressing cells were injected on the left or right side of the mouse and the SirT3 knockdown cells were injected on the other side of the same mouse. When tumors surpassed 2 mm we measured them with calipers in two dimensions (width W and length L) 2–3 times a week. The average tumor volume was calculated as V=L × W2 × 0.52. At the end of the experiment mice were euthanized and tumors were harvested and weighed. Animals treated with NAC had their drinking water supplemented with 40 m NAC as previously described (Gao et al., 2007). Doxycycline treatment was performed by feeding animals 0.625 g/kg doxycycline (Rodent Diet 2018, 625 Doxycycline, Harlan laboratories, South Easton, MA, USA). All animal care followed approved institutional guidelines of MIT. All animal experiments were approved by the Committee on Animal Care at MIT.
Acknowledgments
We would like to thank Allyson Evans, Eric Williams, and Matt Vander Heiden for helpful comments on the manuscript and data, as well as Marcia Haigis and Lydia Finley for sharing unpublished information. This work is supported by NRSA postdoctoral fellowship to ELB, an American Cancer Society postdoctoral fellowship to BME and grants from the NIH and a gift from the Paul F Glenn Foundation to LG.
Dr Guarente has received compensation as a member of the scientific advisory board of Sirtris and owns stock in the company. ELB, BME, and SJHR declare no potential conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- Adler V, Yin Z, Tew KD, Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene. 1999;18:6104–6111. doi: 10.1038/sj.onc.1203128. [DOI] [PubMed] [Google Scholar]
- Bakker WJ, Harris IS, Mak TW. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol Cell. 2007;28:941–953. doi: 10.1016/j.molcel.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GRS, et al. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007a;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell EL, Klimova TA, Eisenbart J, Schumacker PT, Chandel NS. Mitochondrial reactive oxygen species trigger hypoxia-inducible factor-dependent extension of the replicative life span during hypoxia. Mol Cell Biol. 2007b;27:5737–5745. doi: 10.1128/MCB.02265-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, et al. A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 2005;85:258–263. doi: 10.1016/j.ygeno.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Breyton C. The cytochrome b(6)f complex: structural studies and comparison with the bc(1) complex. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 2000;1459:467–474. doi: 10.1016/s0005-2728(00)00185-7. [DOI] [PubMed] [Google Scholar]
- Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–1340. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- Chen D, Thomas EL, Kapahi P. HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet. 2009;5:e1000486. doi: 10.1371/journal.pgen.1000486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper HM, Huang JY, Verdin E, Spelbrink JN. A new splice variant of the mouse SIRT3 gene encodes the mitochondrial precursor protein. PLoS One. 2009;4:e4986. doi: 10.1371/journal.pone.0004986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang DT, Chen F, Gardner LB, Cummins JM, Rago C, Bunz F, et al. Hypoxia-inducible factor-1alpha promotes nonhypoxia-mediated proliferation in colon cancer cells and xenografts. Cancer Res. 2006;66:1684–1693. doi: 10.1158/0008-5472.CAN-05-2887. [DOI] [PubMed] [Google Scholar]
- Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cancer Cell. 2007;12:230–238. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Guzy RD, Sharma B, Bell E, Chandel NS, Schumacker PT. Loss of the SdhB, but Not the SdhA, subunit of complex II triggers reactive oxygen species-dependent hypoxia-inducible factor activation and tumorigenesis. Mol Cell Biol. 2008;28:718–731. doi: 10.1128/MCB.01338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP. Mammalian sirtuins—emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20:2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- Havens CG, Ho A, Yoshioka N, Dowdy SF. Regulation of late G1/S phase transition and APC Cdh1 by reactive oxygen species. Mol Cell Biol. 2006;26:4701–4711. doi: 10.1128/MCB.00303-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak P, Crawford AR, Vadysirisack DD, Nash ZM, DeYoung MP, Sgroi D, et al. Negative feedback control of HIF-1 through REDD1-regulated ROS suppresses tumorigenesis. Proc Natl Acad Sci USA. 2010;107:4675–4680. doi: 10.1073/pnas.0907705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell. 2005;8:143–153. doi: 10.1016/j.ccr.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- Kim HS, Patel K, Muldoon-Jacobs K, Bisht KS, Aykin-Burns N, Pennington JD, et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JH, Lee YM, Chun YS, Chen J, Kim JE, Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Guarente L. Nicotinamide adenine dinucleotide, a metabolic regulator of transcription, longevity and disease. Curr Opin Cell Biol. 2003;15:241–246. doi: 10.1016/s0955-0674(03)00006-1. [DOI] [PubMed] [Google Scholar]
- Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- Parrinello S, Samper E, Krtolica A, Goldstein J, Melov S, Campisi J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwer N, Dame C, Haugstetter A, Wiedenmann B, Detjen K, Schmitt CA, et al. Hypoxia-inducible factor 1alpha determines gastric cancer chemosensitivity via modulation of p53 and NF-kappaB. PLoS One. 2010;5:e12038. doi: 10.1371/journal.pone.0012038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Dato S, Altomare K, Bellizzi D, Garasto S, Greco V, et al. Variability of the SIRT3 gene, human silent information regulator Sir2 homologue, and survivorship in the elderly. Exp Gerontol. 2003;38:1065–1070. doi: 10.1016/s0531-5565(03)00209-2. [DOI] [PubMed] [Google Scholar]
- Schwer B, Eckersdorff M, Li Y, Silva JC, Fermin D, Kurtev MV, et al. Calorie restriction alters mitochondrial protein acetylation. Aging Cell. 2009;8:604–606. doi: 10.1111/j.1474-9726.2009.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009;19:12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkov AA. The role of mitochondria in reactive oxygen species metabolism and signaling. Ann N Y Acad Sci. 2008;1147:37–52. doi: 10.1196/annals.1427.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Coleman MC, Pennington JD, Ozden O, Park S-H, Jiang H, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb J, Coleman M, Pugh C. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cell Mol Life Sci. 2009;66:3539–3554. doi: 10.1007/s00018-009-0147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Shao Z, Zhai Z, Shen C, Powell-Coffman JA. The HIF-1 hypoxia-inducible factor modulates lifespan in C. elegans PLoS One. 2009;4:e6348. doi: 10.1371/journal.pone.0006348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.